Abstract
Philadelphia chromosome-like acute lymphoblastic leukemia (Ph-like ALL) is a recently identified high risk disease subtype characterized by a gene expression profile similar to that observed in Philadelphia chromosome-positive (Ph-positive) ALL, but without an underlying BCR-ABL1 translocation. Adults and children with Ph-like ALL harbor a diversity of alterations that all lead to activated kinase signaling. Outcomes for patients with Ph-like ALL are poor, which has prompted investigation into the role of tyrosine kinase inhibitor (TKI)-based therapies for this disease. Several clinical trials are now ongoing that include screening for the Ph-like signature and treatment of patients with Ph-like ALL with TKI therapy. This review examines how testing for Ph-like ALL is being incorporated into clinical trials.
Keywords: Acute lymphoblastic leukemia, Philadelphia chromosome-like, tyrosine kinase inhibitor, Janus kinase inhibitor, Imatinib, Dasatinib, Ruxolitinib
1. Introduction
Leukemia was introduced into the medical lexicon in the mid-1840s by Rudolf Virchow, but the earliest descriptions of the disease date back to the early 1800s [1]. These earliest reports consist of physical descriptions of patients with particular focus on the gross and microscopic appearance of their blood [2–4]. Over the past centuries, several essential milestones have been achieved in defining unique subsets of leukemia and understanding underlying disease biology. These advances have ranged from phenotypic classification of lymphoid and myeloid leukemias in the early 1900s to the initial descriptions of leukemia cytogenetics in the 1950s and the discovery of the Philadelphia (Ph) chromosome in 1959 [5–8]. In the ensuing decades, continued improvements in acute lymphoblastic leukemia (ALL) survival were attributed largely to successive refinements in risk classification and treatment stratification based on sentinel cytogenetic lesions, early response and other clinical variables.
In recent years, genome-wide profiling studies have led to further refinements in disease classification by defining novel ALL subtypes. Philadelphia chromosome-like (Ph-like) ALL, which has a gene expression profile (GEP) similar to that of Philadelphia chromosome-positive (Ph-positive) disease, but without the presence of the BCR-ABL1 translocation [9,10], is an example of one of these recently identified poor risk subtypes. In this article, we review the distinctive clinical features of Ph-like ALL and describe how real-time assessment for the Ph-like signature is being incorporated into contemporary ALL treatment protocols.
2. Clinical and biological features of Ph-like ALL
There are approximately 5000 new cases of ALL in the United States every year with over half arising in children and adolescents ages 0–19 years [11]. ALL is the most common cancer diagnosis in this age group with an incidence rate of 4.4 per 100,000 persons [11]. Age at diagnosis has continued to be one of the strongest prognostic factors [12,13]. While children ages 1–9 years have 5-year overall survival (OS) rates of greater than 90%, this number decreases to less than 70% in adolescents ages 15–19 years and continues to decline to approximately 35% in the young adult population ages 25–39 years [14]. The reasons behind these survival differences are multifactorial and include variability in treatment, unique toxicity profiles, complex psychosocial factors, and biological differences in disease [15,16].
Recent genomic discoveries have enabled more precise disease classification and provided insight into why inferior responses to traditional chemotherapeutic regimens may be observed in some disease subtypes. One of these most recent discoveries has been the identification of Ph-like ALL [9,10]. This high risk subtype has a peak prevalence in adolescents and young adults and may account, in part, for their inferior outcomes compared to younger children. In addition to older age at diagnosis, the Ph-like GEP is associated with other adverse clinical features. Several studies have established that children and adults with Ph-like ALL have higher white blood cell (WBC) counts at presentation [10,17,18]. Additionally, patients with Ph-like ALL have high rates of minimal residual disease (MRD) at the end of induction [17,19–21]. Ph-like ALL is also more common in patients of Hispanic and Native American ethnicity [20,22]. Notably, the frequency of self-declared Hispanic ethnicity was 37% among 284 children, adolescents, and young adults with Ph-like ALL in a recent study [18].
2.1. Prevalence and outcome
The prevalence of Ph-like ALL varies significantly across the age spectrum (Table 1). Increasing prevalence of Ph-like ALL with age has been reported in one large pediatric and adolescent/young adult (AYA) study, ranging from 10% in younger children (1–10 years) to 28% in young adults (21–39 years) [21,23]. Although there have been conflicting reports regarding the frequency in the older adult population (age ≥40 years), recent studies have identified the Ph-like signature in 13–33% of older adults [19,20,23–25]. These variations in the reported prevalence are attributed, in part, to differences in the race and ethnicities of the populations studied and differences in the GEPs used to classify Ph-like disease [20,26].
Table 1.
Frequency and outcomes of Ph-like ALL in children, adolescents and adults.
| Study | Age (years) | Total (Ph-like) | Frequency Ph-like | Outcomes (Ph-like vs. B-other) |
|---|---|---|---|---|
| Jain et al. [20] | 15–39 | 80 (33) | 42% | 5-yr OS (all ages) 23% vs. 59% (p = 0.006) |
| 40–84 | 68 (16) | 24% | ||
| Herold et al. [19] | 16–20 | 26 (5) | 19% | 5-yr DFS (all ages) 19% vs. 57% (p = 0.001) |
| 21–39 | 68 (12) | 18% | 5-yr OS (all ages) 22% vs. 64% (p = 0.006) | |
| 40–55 | 45 (4) | 9% | ||
| 55–84 | 67 (5) | 7% | ||
| Boer et al. [24] | 16–20 | 24 (6) | 25% | 5-yr EFS (all ages) 24% vs. 42% (NR) |
| 21–39 | 48 (9) | 19% | 5-yr OS (all ages) 33% vs. 50% (NR) | |
| 40–71 | 55 (6) | 11% | ||
| Roberts et al. [23] | 21–39 | 344 (96) | 28% | 5-yr EFS 24% vs. 61% (p < 0.0001) |
| 40–59 | 304 (62) | 20% | 5-yr EFS 21% vs. 39% (p = 0.0021) | |
| 60–86 | 150 (36) | 24% | 5-yr EFS 8% vs. 33% (p = 0.47) | |
| Roberts et al. [21] | 1–15 | 853 (108) | 13% | 5-yr EFS 58% vs. 84% (p < 0.001) |
| 16–20 | 372 (77) | 21% | 5-yr EFS 41% vs. 83% (p < 0.001) | |
| 21–39 | 168 (46) | 27% | 5-yr EFS 24% vs. 63% (p < 0.001) | |
| Loh et al. [17] | 1–31 | 572 (81) | 14% | 5-yr EFS 63% vs. 86% (p < 0.0001) |
| Reshmi et al. [18] | 1–31 | 1389 (284) | 20% | NR |
Jain B-other: non-BCR-ABL1. Herold, Boer, Roberts B-other: KMT2A-wild type (WT) and non-BCR-ABL1. Loh B-other: non-BCR-ABL1. Roberts and Loh limited to NCI high risk patients. Reshmi includes patients eligible for Children’s Oncology Group high risk B-ALL study AALL1131. OS = overall survival, 5-yr EFS = event-free survival at 5 years, NR = not reported.
Ph-like ALL has been consistently associated with decreased OS and event-free survival (EFS) among all age groups [9,10,17,20–24,26–29] (Table 1). One study demonstrated non-inferior outcomes in a cohort of Ph-like ALL patients with risk-directed therapy. This study, however, included younger patients with an allocation to hematopoietic stem cell transplantation for many patients in complete first remission based on initial MRD response [30].
2.2. Genetic characterization
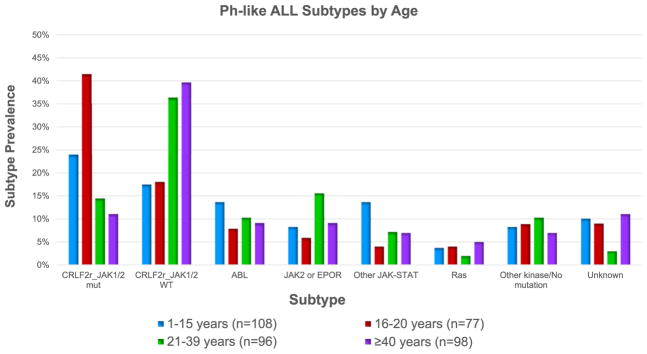
Rearrangements in genes involved in cytokine receptor and kinase signaling are a hallmark of Ph-like ALL, identified in 80–90% of cases. Although the frequency and spectrum of underlying genetic alterations varies among different age groups and populations, most involve activation of ABL and/or JAK-STAT signaling pathways as a common mechanism of transformation. These alterations can be grouped into six categories: 1) CRLF2 rearrangements with or without JAK1 or JAK2 mutations, 2) ABL-class fusions (rearrangements in ABL1, ABL2, CSF1R or PDGFRB), 3) JAK2 or EPOR rearrangements, 4) other mutations or alterations involved in JAK-STAT signaling, 5) Ras pathway alterations, and 6) other kinase alterations [21,23] (Fig. 1). As in (Ph-positive) ALL, deletions in the IKZF1 (Ikaros) lymphoid transcription factor have been reported in approximately 70% of children and adults with Ph-like ALL [9,10,23].
Fig. 1. Ph-like ALL subtypes by age.
CRLF2 rearrangements separated by JAK status, mutant (mut) or wild-type (WT). ABL-class fusions: ABL1, ABL2, CSF1R, and PDGFRB. JAK2 or EPOR rearrangements. Other JAK-STAT: FLT3 (1–15 years), IL7R, SH2B3, JAK1/3, TYK2, IL2RB (1–15 years), and TSLP (1–15 years). Ras pathway: KRAS, NRAS, NF1, PTPN11 and BRAF (1–15 years). Other kinase/No mutation: Assorted kinase lesions or no kinase-activating alteration identified. Unknown: lacked material for genomic analysis. Data adapted from references 21 and 23.
Specific genetic alterations have been associated with age at diagnosis. CRLF2 rearrangements are the most common finding in Ph-like ALL. The frequency of these rearrangements also increases with age, occurring in up 60% of adolescents and adults [20,21]. CRLF2 rearrangements are accompanied by JAK1 or JAK2 mutations in approximately half of all cases [18]. Regardless of JAK mutational status, however, CRLF2-rearranged leukemias appear susceptible to JAK pathway inhibition in preclinical studies [31,32]. Herold et al. reported that the frequency of JAK2 mutations and IGH-CRLF2 rearrangements increase significantly with age ≥16 years in adults [19], a finding in agreement with a recent report from the MD Anderson Cancer Center (MDACC) [20]. In another recent report of Ph-like ALL in adults, approximately 70% harbored genetic alterations that may be targetable with JAK inhibitors, (e.g., CRLF2 rearrangements, JAK2or EPOR rearrangements, and IL7R mutations) [23].
ABL-class fusions are the second most common alteration in Ph-like ALL, occurring in approximately 10–15% of children and adults with Ph-like ALL [18,21,23]. JAK2 and EPOR rearrangements and other JAK-STAT signaling pathway alterations are observed most frequently in the young adult population (age 21–39 years) [21,23]. Isolated Ras pathway alterations make up a minority of alterations in all populations [21,23]. Currently, 10–11% of children and adults with Ph-like ALL have unknown alterations [21,23].
While patients with Ph-like ALL experience inferior outcomes compared to those with non-Ph-like B-ALL, specific alterations appear to have different prognostic importance [21,22,29]. Patients with IKZF1 alterations, regardless of other expression profiles, may have inferior outcomes with a 5-year overall survival (OS) of 20% in young adults [9,10,21,23]. Among children and AYAs (ages 1–39 years), JAK2 and EPOR rearrangements portend poor outcomes with 5 year EFS of 26.1% [21]. In contrast, favorable outcomes with 5-year EFS of 86% were observed in patients with isolated Ras pathway alterations [21]. CRLF2 overexpression in adults has been associated with significantly worse outcomes with 5-year survival <20% [20].
3. Diagnostic algorithms
Given the prevalence of Ph-like ALL and the poor outcomes that have been observed with conventional therapy, screening algorithms for the Ph-like signature have now been developed to facilitate real-time treatment stratification. Adult and pediatric treating groups have used a number of different approaches ranging from selected screening of patients with high risk clinical features, such as high mid- and end of induction disease burdens [33–35], to the use of more comprehensive gene expression profiling or next-generation sequencing in all newly diagnosed patients. While the strategies used to define the Ph-like signature vary [26], the overall goal of diagnostic testing is accurate identification of the underlying genetic alterations such that treatment can be modified accordingly.
3.1. Tiered approaches
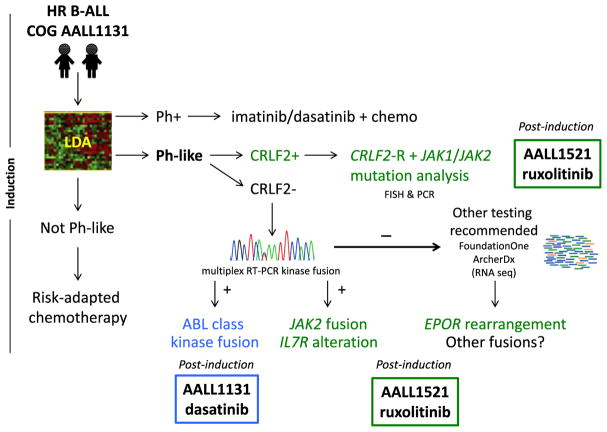
The Children’s Oncology Group (COG) is now screening all National Cancer Institute (NCI) high risk (HR) patients with newly diagnosed B-ALL for the Ph-like GEP with a quantitative reverse transcriptase polymerase chain reaction (RT-PCR)-based low density array (LDA) platform [18]. The GEP of Ph-like ALL was used to design an LDA card where the expression of 8 or 15 genes is highly predictive of the Ph-like ALL signature. Patients with an LDA coefficient of 0.5–1 are designated positive for Ph-like ALL [18]. A series of downstream tests are performed in dedicated reference laboratories in those patients who are LDA screen positive. CRLF2 is among the genes assessed in the LDA classifier, which can also directly assess for the presence of P2RY8-CRLF2 fusion. In CRLF2-overexpressing cases without this fusion, fluorescence in situ hybridization (FISH) is then performed to identify IGH-CRLF2 rearrangements. JAK1 and JAK2 mutation testing is next completed in all patients with confirmed CRLF2 rearrangements. In Ph-like cases without CRLF2 rearrangements, RT-PCR assays are done to detect ABL class, JAK2, and other known kinase fusions with confirmation by Sanger sequencing. Finally, RNA sequencing or alternate testing is performed on remaining LDA-positive cases without otherwise defined genetic alterations. This testing for Ph-like ALL is now being performed in real-time for patients with HR B-ALL enrolled on the COG AALL1131 phase 3 clinical trial (NCT02883029) within the first 4 weeks of therapy so that patients with confirmed targetable mutations or fusions can be allocated to TKI-containing treatment after completion of induction therapy (Fig. 2). Other groups have adopted a similar approach. While tiered screening and testing approaches have practical advantages in clinical trials that accrue large numbers of patients, some alterations can be missed, especially as a number of new fusions and novel breakpoints continue to be discovered [18]. Further, timely completion of all necessary testing by the end of induction can be challenging in some cases with this staged approach.
Fig. 2. Diagnostic algorithm for identifying children with Ph-like ALL in real-time.
Patients with confirmed ABL class (blue) or JAK pathway (green) alterations are eligible to receive dasatinib (COG AALL1131) or ruxolitinib (COG AALL1521), respectively, in combination with chemotherapy post-induction.
3.2. Alternative approaches
Recent advances in next-generation sequencing technologies have led to development of DNA- and RNA-based assays capable of highly sensitive detection of leukemia-associated genetic mutations and fusions. In addition to the above RT-PCR assays currently used by the COG reference laboratories [18], several commercially-available platforms (e.g., ArcherDx FusionPlex, NanoString Vantage, FoundationOne Heme, others) can also be used to identify Ph-like ALL-associated alterations. One advantage of these newer technologies is their potential ability to identify fusions involving previously-unknown 5′ partners, as there are a relatively small number of known 3′ partners in Ph-like ALL [18,21]. Finally, unbiased RNA sequencing (RNA-seq; whole transcriptome sequencing) is likely the most comprehensive method for identifying Ph-like ALL fusions, including those unable to be detected by other assays. However, RNA-seq is not widely available at this time as a clinical test and remains relatively costly and labor-intensive with respect to bioinformatic analysis.
4. Treatment of Ph-like ALL
Adult and childhood cancer cooperative groups are now actively developing clinical trials to test the efficacy of addition of kinase inhibitors ruxolitinib or dasatinib to chemotherapy for patients with Ph-like ALL harboring JAK pathway alterations or ABL class fusions, respectively (Table 2).
Table 2.
Current clinical trials of kinase inhibitor-based therapies for children, adolescents, and adults with Ph-like ALL.
| Patient population | Age | Disease status | Inhibitor | Trial |
|---|---|---|---|---|
| Ph-like with ABL class alterations | ≥10 years | relapse | dasatinib | NCT02420717 |
| Ph-like with ABL class alterations | 1–30 years | de novo | dasatinib | NCT01406756 |
| Ph-like with ABL class alterations | 1–18 years | de novo | dasatinib | NCT03117751 |
| Ph-like with CRLF2/JAK pathway alterations | ≥10 years | relapse | ruxolitinib | NCT02420717 |
| Ph-like with CRLF2/JAK pathway alterations | 1–21 years | de novo | ruxolitinib | NCT02723994 |
| Ph-like with CRLF2/JAK pathway alterations | 1–18 years | de novo | ruxolitinib | NCT03117751 |
4.1. JAK pathway alterations
Preclinical studies to date have demonstrated constitutive activation of kinase signaling networks in subsets of Ph-like ALL harboring JAK pathway alterations (described in detail in Chapter 4) [21,31,36,37]. These alterations are the most common finding in Ph-like ALL across the age spectrum. Additional studies have reported activity of various JAK inhibitors in patient-derived xenograft (PDX) models of JAK pathway-mutant Ph-like ALL, providing rationale for testing of JAK inhibitor-based therapies in the clinic [32,37–41]. To date, many preclinical studies in ALL models have sought to elucidate anti-leukemia effects of the JAK1/JAK2 inhibitor ruxolitinib, which is approved by the Food and Drug Administration for treatment of patients with JAK2-mutant myeloproliferative neoplasms [41–43]. As an initial investigation, the first-in-child phase 1 COG trial ADVL1011 recently demonstrated the safety of ruxolitinib monotherapy in children with relapsed or refractory cancers without a maximum tolerated dose of ruxolitinib identified [44].
The COG is now investigating the potential efficacy of combining ruxolitinib with augmented Berlin-Frankfurt-Münster (BFM)-based post-induction chemotherapy (consolidation through maintenance) in children, adolescents, and young adults with newly-diagnosed HR JAK pathway-mutant Ph-like ALL via the phase 2 trial AALL1521 (NCT02723994). Patients with relevant mutations are identified during induction therapy via the same LDA and molecular analyses as for patients with ABL class-mutant Ph-like ALL treated with dasatinib on COG AALL1131 (Fig. 2). In AALL1521, patients are stratified by genetic alterations (e.g., CRLF2 rearrangements versus JAK2 or EPOR fusions) and by end-induction MRD status to elucidate potential differential efficacy of combination therapy in each subset. An embedded dose-finding part of this trial will establish safe and tolerable dosing of ruxolitinib with augmented BFM-based chemotherapy prior to efficacy analyses at the recommended phase 2 dose in larger statistically-powered cohorts of patients. The primary endpoint of this non-randomized phase 2 trial is 3 year EFS versus survival of historic control patients treated with an identical chemotherapy backbone without ruxolitinib [45].
A stratum of the Total XVII trial at St. Jude Children’s Research Hospital will also assess the safety of ruxolitinib addition to chemotherapy in children with JAK-mutant Ph-like ALL (NCT03117751). A phase 2 trial of ruxolitinib in combination with multi-agent chemotherapy [hyper-CVAD, high-dose methotrexate and cytarabine, POMP maintenance] in children ≥10 years and adults with relapsed/refractory JAK-mutant ALL is also ongoing at MDACC (NCT02420717) [46]. In this study, patients with JAK pathway-mutant Ph-like ALL are initially treated with up to three weeks of ruxolitinib monotherapy with subsequent addition of multi-agent chemotherapy for those patients with suboptimal response to single-agent ruxolitinib. The primary outcome measurement is complete response rate after six weeks of reinduction therapy. A separate arm of this trial is similarly assessing the efficacy of dasatinib with chemotherapy in patients with Ph-like ALL harboring ABL class alterations.
4.2. ABL class rearrangements
The second most common subgroup of Ph-like ALL is defined by ABL-class rearrangements involving ABL1, ABL2, CSF1R and PDGFRB. Cell lines and human cells expressing these gene fusions, as well as patient-derived xenograft models have demonstrated marked sensitivity to the ABL inhibitors imatinib and dasatinib [21,47]. Following the successful paradigm of combining a TKI with chemotherapy for the treatment of Ph+ ALL in children [48–50], anecdotal reports of favorable responses to TKI therapy in patients of Ph-like ALL have also been described [21,30,51,52].
Several groups are now investigating the addition of dasatinib to conventional augmented BFM-based or hyper-CVAD treatment platforms to determine if this will improve outcomes in patients with Ph-like ALL and ABL class fusions. In the current COG AALL1131 trial, patients with confirmed ABL class fusions begin dasatinib daily in combination with chemotherapy at the start of the consolidation phase and continue until the end of maintenance therapy. The EFS rates of children and AYA treated with dasatinib in combination with chemotherapy will be compared to a historical group from the same protocol treated with chemotherapy alone, before uniform screening for the Ph-like signature was implemented. Similar studies investigating dasatinib in combination with chemotherapy for children with ABL class fusions are underway at St. Jude Children’s Research Hospital and the MDACC (Table 2). Given the relative overall rarity of Ph-like ALL, randomized studies to assess the potential benefit of TKI addition will likely require broader collaborations. While a number of questions remain, including the role for emerging immunotherapies and hematopoietic stem cell transplantation in Ph-like ALL, as well as the potential for emergence of TKI resistance mutations with prolonged daily exposure, the implementation of real-time screening procedures and precision medicine treatment approaches has been a promising advance.
5. Summary
Ph-like ALL is a recently described high risk subtype of ALL caused by rearrangements and mutations that activate cytokine receptor and tyrosine kinase signaling. Ph-like ALL occurs in up to one quarter of adolescents and adults with B-ALL, warranting efficient diagnostic screening in these patient populations. While a tiered diagnostic approach may be most cost effective and pragmatic in large clinical trials, ongoing discovery of new genetic alterations may warrant broader and unbiased testing such as RNA-seq. Preclinical evidence and anecdotal reports support a precision medicine treatment approach for Ph-like ALL. However, optimal therapy has not yet been defined, and prospective trials evaluating TKIs in combination with chemotherapy are currently underway.
Practice Points.
Ph-like ALL has a gene expression profile similar to Ph-positive ALL, but lacks BCR-ABL1 and has been independently associated with a poor prognosis.
Ph-like ALL occurs in up to one quarter of adolescents and adults with B-ALL.
The majority of patients with Ph-like ALL have underlying genetic alterations in kinase or cytokine receptor signaling pathways targetable with available TKIs.
Given the prevalence, prognostic significance, and potential treatment implications, testing for Ph-like ALL should be considered in high risk populations.
Research agenda.
There is a need for ongoing investigation to further characterize the underlying genetic alterations in Ph-like ALL.
High risk patient populations with B-ALL should have access to testing for the Ph-like signature.
Given the rarity of this disease subtype, research collaborations will help to define the optimal treatment approach for Ph-like ALL.
Acknowledgments
This work was supported in part by NIH/NCI K08CA184418 (SKT) and COG Chair’s grant CA98543. The authors acknowledge the NCI TARGET project, the COG ALL Committee, the University of New Mexico, Nationwide Children’s Hospital and University of Alabama at Birmingham for development of the testing algorithm for Ph-like ALL.
Footnotes
Conflict of interest
SKT receives research funding from Gilead Sciences and Incyte Corporation.
References
- 1.Kampen KR. The discovery and early understanding of leukemia. Leukemia Res. 2012;36(1):6–13. doi: 10.1016/j.leukres.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 2.Cullen P. Case of splenitis acutus, in which the serum of the blood drawn from the arm had the appearance of milk. Edinb Med Surg J Clin Oncol. 1811;7:169–71. [PMC free article] [PubMed] [Google Scholar]
- 3.Velpeau A. Altération du sang Archives Générales de Medecine. J publié par une société de médecins Paris. 1825:462–3. [Google Scholar]
- 4.Virchow RLK. Gesammelte Abhandlungen zur wissenschaftlichen medicin. Vol. 1856. Frankfurt: Meidinger Sohn & comp; 1845. Weisses blut; pp. 149–54. [Google Scholar]
- 5.Ford CE, Jacobs PA, Lajtha LG. Human somatic chromosomes. Nature. 1958;181(4623):1565–8. doi: 10.1038/1811565a0. [DOI] [PubMed] [Google Scholar]
- 6.Nowell PC, Hungerford DA. Chromosome studies in human leukemia. II. Chronic granulocytic leukemia. J Natl Cancer Inst. 1961;27:1013–35. [PubMed] [Google Scholar]
- 7.Piller G. Leukaemia - a brief historical review from ancient times to 1950. Br J Haematol. 2001;112(2):282–92. doi: 10.1046/j.1365-2141.2001.02411.x. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan RJM. In: The Blood in health and disease. Frowde H, editor. Oxford University Press; 1909. [Google Scholar]
- 9.Mullighan CG, Su X, Zhang J, Radtke I, Phillips LA, Miller CB, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–80. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–34. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Cancer Statistics Working Group. United States cancer statistics: 1999–2013 incidence and mortality web-based report. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2016. Available at: www.cdc.gov/uscs. [Google Scholar]
- 12.Mastrangelo R, Poplack D, Bleyer A, Riccardi R, Sather H, D’angio G. Report and recommendations of the rome workshop concerning poor-prognosis acute lymphoblastic leukemia in children: biologic bases for staging, stratification, and treatment. Pediatr Blood Cancer. 1986;14(3):191–4. doi: 10.1002/mpo.2950140317. [DOI] [PubMed] [Google Scholar]
- 13.Hammond D, Sather H, Nesbit M, Miller D, Coccia P, Bleyer A, et al. Analysis of prognostic factors in acute lymphoblastic leukemia. Pediatr Blood Cancer. 1986;14(3):124–34. doi: 10.1002/mpo.2950140305. [DOI] [PubMed] [Google Scholar]
- 14.Lewis DR, Seibel NL, Smith AW, Stedman MR. Adolescent and young adult cancer survival. J Natl Cancer Inst Monogr. 2014;2014(49):228–35. doi: 10.1093/jncimonographs/lgu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stock W. Adolescents and young adults with acute lymphoblastic leukemia. ASH Educ Program Book. 2010;2010(1):21–9. doi: 10.1182/asheducation-2010.1.21. [DOI] [PubMed] [Google Scholar]
- 16.Kent EE, Sender LS, Largent JA, Anton-Culver H. Leukemia survival in children, adolescents, and young adults: influence of socioeconomic status and other demographic factors. Cancer Causes Control. 2009;20(8):1409–20. doi: 10.1007/s10552-009-9367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loh ML, Zhang J, Harvey RC, Roberts K, Payne-Turner D, Kang H, et al. Tyrosine kinome sequencing of pediatric acute lymphoblastic leukemia: a report from the Children’s Oncology Group TARGET project. Blood. 2013;121(3):485–8. doi: 10.1182/blood-2012-04-422691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reshmi SC, Harvey RC, Roberts KG, Stonerock E, Smith A, Jenkins H, et al. Targetable kinase gene fusions in high risk B-ALL: a study from the Children’s Oncology Group. Blood. 2017 Apr 13; doi: 10.1182/blood-2016-12-758979. . pii: blood-2016-12-758979, [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 19.Herold T, Schneider S, Metzeler KH, Neumann M, Hartmann L, Roberts KG, et al. Adults with Philadelphia chromosome-like acute lymphoblastic leukemia frequently have IGH-CRLF2 and JAK2 mutations, persistence of minimal residual disease and poor prognosis. Haematologica. 2017;102(1):130–8. doi: 10.3324/haematol.2015.136366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain N, Roberts KG, Jabbour E, Patel K, Eterovic AK, Chen K, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood. 2017;129(5):572–81. doi: 10.1182/blood-2016-07-726588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–15. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey RC, Mullighan CG, Chen I-M, Wharton W, Mikhail FM, Carroll AJ, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–21. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts KG, Gu Z, Payne-Turner D, McCastlain K, Harvey RC, Chen I-M, et al. High frequency and poor outcome of Philadelphia chromosome–like acute lymphoblastic leukemia in adults. J Clin Oncol 2016:JCO. 2016;69:0073. doi: 10.1200/JCO.2016.69.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boer JM, Koenders JE, van der Holt B, Exalto C, Sanders MA, Cornelissen JJ, et al. Expression profiling of adult acute lymphoblastic leukemia identifies a BCR-ABL1-like subgroup characterized by high non-response and relapse rates. Haematologica. 2015;100(7):e261–4. doi: 10.3324/haematol.2014.117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herold T, Baldus CD, Gökbuget N. Ph-like acute lymphoblastic leukemia in older adults. N Engl J Med. 2014;371(23):2235. doi: 10.1056/NEJMc1412123#SA1. [DOI] [PubMed] [Google Scholar]
- 26.Boer JM, Marchante JR, Evans WE, Horstmann MA, Escherich G, Pieters R, et al. BCR-ABL1-like cases in pediatric acute lymphoblastic leukemia: a comparison between DCOG/Erasmus MC and COG/St. Jude signatures. Haematologica. 2015;100(9):e354–7. doi: 10.3324/haematol.2015.124941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvestri D, Vendramini E, Fazio G, Locatelli F, Conter V, Borga C, et al. Philadelphia-like signature in childhood acute lymphoblastic leukemia: the AIEOP experience. Blood. 2013;122(21):353. [Google Scholar]
- 28.Imamura T, Kiyokawa N, Kato M, Imai C, Okamoto Y, Yano M, et al. Characterization of pediatric Philadelphia-negative B-cell precursor acute lymphoblastic leukemia with kinase fusions in Japan. Blood Cancer J. 2016;6(5):e419. doi: 10.1038/bcj.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Veer A, Waanders E, Pieters R, Willemse ME, Van Reijmersdal SV, Russell LJ, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122(15):2622–9. doi: 10.1182/blood-2012-10-462358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts KG, Pei D, Campana D, Payne-Turner D, Li Y, Cheng C, et al. Outcomes of children with BCR-ABL1–like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol. 2014;32(27):3012–20. doi: 10.1200/JCO.2014.55.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tasian SK, Doral MY, Borowitz MJ, Wood BL, Chen IM, Harvey RC, et al. Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood. 2012;120(4):833–42. doi: 10.1182/blood-2011-12-389932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maude SL, Tasian SK, Vincent T, Hall JW, Sheen C, Roberts KG, et al. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood. 2012;120(17):3510–8. doi: 10.1182/blood-2012-03-415448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Connor D, Moorman AV, Wade R, Hancock J, Tan RM, Bartram J, et al. Use of minimal residual disease assessment to redefine induction failure in pediatric acute lymphoblastic leukemia. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(6):660–7. doi: 10.1200/JCO.2016.69.6278. [DOI] [PubMed] [Google Scholar]
- 34.Schwab C, Ryan SL, Chilton L, Elliott A, Murray J, Richardson S, et al. EBF1-PDGFRB fusion in pediatric B-cell precursor acute lymphoblastic leukemia (BCP-ALL): genetic profile and clinical implications. Blood. 2016;127(18):2214–8. doi: 10.1182/blood-2015-09-670166. [DOI] [PubMed] [Google Scholar]
- 35.Stadt UZ, Escherich G, Indenbirken D, Alawi M, Adao M, Horstmann MA. Rapid capture next-generation sequencing in clinical diagnostics of kinase pathway aberrations in B-Cell precursor ALL. Pediatr Blood Cancer. 2016;63(7):1283–6. doi: 10.1002/pbc.25975. [DOI] [PubMed] [Google Scholar]
- 36.Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, Rodig SJ, et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2010;107(1):252–7. doi: 10.1073/pnas.0911726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iacobucci I, Li Y, Roberts KG, Dobson SM, Kim JC, Payne-Turner D, et al. Truncating erythropoietin receptor rearrangements in acute lymphoblastic leukemia. Cancer Cell. 2016;29(2):186–200. doi: 10.1016/j.ccell.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weigert O, Lane AA, Bird L, Kopp N, Chapuy B, van Bodegom D, et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J Exp Med. 2012;209(2):259–73. doi: 10.1084/jem.20111694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suryani S, Bracken LS, Harvey RC, Sia KC, Carol H, Chen IM, et al. Evaluation of the in vitro and in vivo efficacy of the JAK inhibitor AZD1480 against JAK-mutated acute lymphoblastic leukemia. Mol Cancer Ther. 2015;14(2):364–74. doi: 10.1158/1535-7163.MCT-14-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu SC, Li LS, Kopp N, Montero J, Chapuy B, Yoda A, et al. Activity of the type II JAK2 inhibitor CHZ868 in B Cell acute lymphoblastic leukemia. Cancer Cell. 2015;28(1):29–41. doi: 10.1016/j.ccell.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tasian SK, Teachey DT, Li Y, Shen F, Harvey RC, Chen IM, et al. Potent efficacy of combined PI3K/mTOR and JAK or ABL inhibition in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood. 2017;129(2):177–87. doi: 10.1182/blood-2016-05-707653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–35. doi: 10.1056/NEJMoa1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loh ML, Tasian SK, Rabin KR, Brown P, Magoon D, Reid JM, et al. A phase 1 dosing study of ruxolitinib in children with relapsed or refractory solid tumors, leukemias, or myeloproliferative neoplasms: a Children’s Oncology Group phase 1 consortium study (ADVL1011) Pediatr Blood Cancer. 2015;62(10):1717–24. doi: 10.1002/pbc.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-Acute lymphoblastic leukemia: a report from Children’s oncology group study AALL0232. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(20):2380–8. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curran E, Stock W. How I treat acute lymphoblastic leukemia in older adolescents and young adults. Blood. 2015;125(24):3702–10. doi: 10.1182/blood-2014-11-551481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22(2):153–66. doi: 10.1016/j.ccr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz KR, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(31):5175–81. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schultz KR, Carroll A, Heerema NA, Bowman WP, Aledo A, Slayton WB, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: children’s Oncology Group study AALL0031. Leukemia. 2014;28(7):1467–71. doi: 10.1038/leu.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Biondi A, Schrappe M, De Lorenzo P, Castor A, Lucchini G, Gandemer V, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13(9):936–45. doi: 10.1016/S1470-2045(12)70377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lengline E, Beldjord K, Dombret H, Soulier J, Boissel N, Clappier E. Successful tyrosine kinase inhibitor therapy in a refractory B-cell precursor acute lymphoblastic leukemia with EBF1-PDGFRB fusion. Haematologica. 2013;98(11):e146–8. doi: 10.3324/haematol.2013.095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weston BW, Hayden MA, Roberts KG, Bowyer S, Hsu J, Fedoriw G, et al. Tyrosine kinase inhibitor therapy induces remission in a patient with refractory EBF1-PDGFRB-positive acute lymphoblastic leukemia. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31(25):e413–6. doi: 10.1200/JCO.2012.47.6770. [DOI] [PubMed] [Google Scholar]