Abstract
Conventional radiotherapy, in addition to its well-established tumoricidal effects, can also activate the host immune system. Radiation therapy modulates tumour phenotypes, enhances antigen presentation and tumour immunogenicity, increases production of cytokines and alters the tumour microenvironment, enabling destruction of the tumour by the immune system. Investigating the combination of radiotherapy with immunotherapeutic agents, which also promote the host antitumour immune response is, therefore, a logical progression. As the spectrum of clinical use of stereotactic radiotherapy continues to broaden, the question arose as to whether the ablative radiation doses used also stimulate immune responses and, if so, whether we can amplify these effects by combining immunotherapy and stereotactic ablative radiotherapy (SABR). In this Perspectives article, we explore the preclinical and clinical evidence supporting activation of the immune system following SABR. We then examine studies that provide data on the effectiveness of combining these two techniques — immunotherapy and SABR — in an approach that we have termed ‘ISABR.’ Lastly, we provide general guiding principles for the development of future clinical trials to investigate the efficacy of ISABR in the hope of generating further interest in these exciting developments.
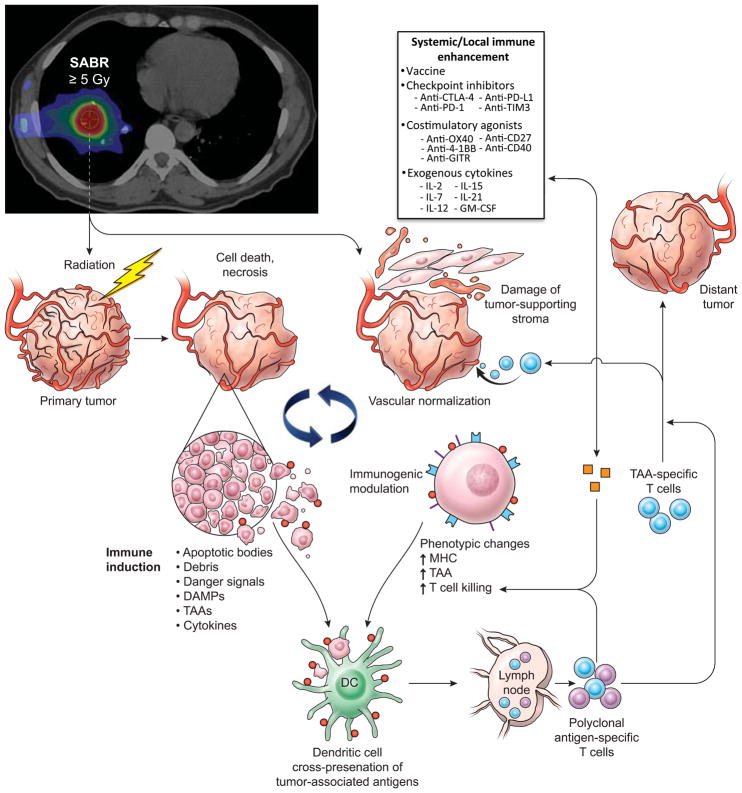
Radiation therapy has been used as a predominant treatment option for nearly all types of cancer in the definitive, adjuvant and palliative settings. Traditional medical teaching has focused on the ability of locally applied radiation to directly kill tumour cells within the target volume by causing irreparable DNA damage, which irreversibly damages the tumour cells and prevents them from engaging in further replication and division (FIG. 1). In 2010, data were published indicating that radiotherapy can damage epithelial cells of small blood vessels by reducing sprouting, migration and proliferative capacities, and causing premature senescence, thereby starving cancer cells of nutrients 1,2. More interestingly, a substantial amount of data have emerged showing that locally applied radiation can also stimulate systemic immune responses, thus leading to enhanced tumour cell recognition by the immune system and death of the tumour cells (FIG. 1). A number of investigators have reported that, following irradiation, tumour cells release a large amount of antigens, referred to as tumour-associated antigens (TAAs), in the form of necrotic and apoptotic tumour cells and debris3–5. The substantial increase in number and diversity of TAAs can enable antigen-presenting cells and dendritic cells to stimulate a tumour-specific immune response (FIG. 1). In addition to tumour cells acting as the trigger, the destruction of the tumour-supporting stroma that often results from radiotherapy can also potentiate immune recognition6. Other reports have focused on the release of ’danger’ signals following radiotherapy, which might promote the transition from nonspecific immune responses to adaptive immunity7,8. Several other mechanisms of tumour sensitization following radiotherapy, including increased expression of cytokines and modulation of tumour phenotypes, have also been associated with promising outcomes (FIG. 1)9–11. Termed ‘immunogenic modulation’, these processes encompass a spectrum of radiation-induced molecular alterations in the biology of the cancer cell that either independently or collectively make the tumour more amenable to cytotoxic-T-lymphocyte-mediated destruction. These mechanisms have been reviewed in detail elsewhere12, and include the following: downregulation of antiapoptotic and/or prosurvival genes 12,13; modulation of antigen-processing machinery components 14,15; and translocation of calreticulin to the cell surface of the tumour14,16,. These radiation-induced changes can be exploited to provide synergistic clinical benefits when the radiation treatment is followed by, or given concurrently with, an immunotherapy regimen.
Figure 1. Immune stimulation by SABR.
Antitumour effects of stereotactic ablative radiotherapy (SABR). SABR results in immune activation by inducing tumour-cell death, modulating tumour-cell phenotype and normalizing aberrant tumour vasculature to allow for improved oxygen and drug delivery. After cell death, the release of tumour debris with associated danger signals, tumour-associated antigens (TAAs), and inflammatory cytokines are recognized by and activate dendritic cells, promoting antigen presentation to cells of the immune system. Polyclonal antigen-specific T cells are then generated, some of which can attack tumours located within the radiation field, as well as distant tumours; this response can be augmented by the addition of systemic immune-enhancement measures. GM-CSF; granulocyte macrophage colony stimulating factor; IL, interleukin; MHC, major histocompatibility complex.
Technological advances that enable the delivery of higher doses of localized radiation to tumour targets with stereotactic ablative radiotherapy (SABR), also known as stereotactic body radiotherapy (SBRT), have been widely implemented in curing patients with early stage cancers of the lung and liver, and its role as a treatment for patients with metastatic disease is being actively investigated17–19. SABR involves treatment of tumours with radiation doses that often exceed 5 Gy per fraction with an exceedingly high level of conformality and sharp dose fall-off to spare the surrounding organs at risk. Investigators in many previous studies have focused on the effects of conventional fractionation regimens on the immune system; however, preliminary data suggest that radiation-induced immune responses might be dose-dependent 20,21. In fact, using radiation doses in the ‘ablative’ range can not only effectively destroy tumour cells directly, but might also encourage these SABR-killed cells to function as a vaccine in situ22,23.
Herein, we provide a definitive description of ISABR (immunotherapy and SABR), whereby exposure of tumour cells to higher doses of radiation delivered in a limited number of fractions promotes productive interactions between tumours and the immune system, which can be further exploited and/or augmented using active immunotherapy (FIG. 1, BOX 1). This Perspectives article is focused on the available data regarding the relationship between SABR and the initiation of antitumour immune responses. Furthermore, we discuss the early clinical benefits of incorporating immunotherapeutic strategies with SABR, and finally propose novel ways of bridging the gap from bench to bedside with this approach to cancer treatment.
Box 1. Overview of immune checkpoints in cancer treatment70.
Function
Inhibition of certain signaling pathways is necessary for maintaining self-tolerance and to prevent autoimmunity
This inhibition of certain pathways protects tissues from damage during activation of the immune system
Role in cancer
Dysregulation by tumours results in diminished cancer-cell recognition by the host immune system and evasion of an immune-mediated attack
As targets for cancer therapy
Targeted inhibition of these inhibitory receptors results in activation of the immune system and amplification of antigen-specific T-cell responses
Clinical application
CTLA-4 (cytotoxic-T-lymphocyte-associated antigen 4) counteracts the activity of T-cell stimulation by interfering with the co-stimulatory receptor CD28, thereby dampening the amplitude of T-cell activation
The FDA-approved agent Ipilimumab is an anti-CTLA-4 monoclonal antibody for treatment of patients with melanoma and is currently in phase II/III trials for efficacy as a treatment of various other forms of cancers
PD-1 (programmed cell death protein 1) limits T-cell activity in peripheral tissues, thus limiting the extent of inflammatory responses and autoimmune reactions. PD-1 is also expressed on regulatory T cells, which function as inhibitors of an immune response
PD-L1 (programmed cell death 1 ligand 1) is able to interact with CD80 on T cells, thereby delivering inhibitory signals, mainly owing to the PD-1:PD-L1 interaction
Pembrolizumab is an anti-PD-1 monoclonal antibody that acts as an immunomodulator by blocking activation of the PD-1 receptor on activated T-cells by PD-L1 on other cell types, including some cancer cells. This agent is approved by the FDA for the treatment of patients with advanced-stage melanoma and PD-L1-positive NSCLC, and is also undergoing extensive investigations for application in other forms of cancer.
Nivolumab is another anti-PD-1 monoclonal antibody that is approved by the FDA for treatment of patients with unresectable or metastatic melanoma, as well as for the treatment of non-small-cell lung cancer.
Preclinical evidence
Data from several preclinical studies have demonstrated activation of an immune response following treatment with SABR. In a mouse model, investigators demonstrated increased T-cell priming in draining lymph nodes, leading to CD8+ T-cell-dependent size reductions or eradication of primary tumours and distant metastases after a single fraction of radiation doses of between 15–25 Gy 24. Interestingly, the investigators observed that the radiation-induced immune responses and reductions in tumour burden following SABR were abrogated with use of conventional fractionation, thus mirroring the CD8+-depleted condition. In a similar study, antitumour immune responses were evaluated in mice after treatment of OVA-expressing B16–F0 tumours with single (15 Gy) or fractionated (3 Gy x 5 fractions) doses of radiation25. Use of either fractionation schedule facilitated antigen presentation and priming of T cells in draining lymph nodes. Once primed, these tumour-antigen-specific T cells had an enhanced ability to traffic to and infiltrate tumours. Both regimens were successful in stimulating the immune system, although use of 15 Gy single-dose irradiation resulted in a greater number of host immune cells infiltrating tumours, compared with the 3 Gy x 5 fractionated schedule25. This important difference in the immune response following irradiation with varying radiation fraction sizes was further highlighted elsewhere22: Mice bearing B16-OVA murine melanoma were treated up to 15 Gy radiation in various fraction sizes, and tumour growth followed. Researchers showed effective immune stimulation with doses of 7.5 Gy and 10 Gy, but not 5 Gy. Conversely, use of higher doses of radiation, namely ≥15 Gy, increased the fraction of splenic regulatory T (TREG) cells, which function to suppress tumour-specific immunity 26. The importance of the radiation dose and fractionation schedule used was further corroborated in a study showing activation of immune-response-related genes, radiation-induced damage-associated molecular pattern molecules (DAMPs), and inflammatory cytokines in human prostate cancer cells when exposed to radiation in the range of 8–10 Gy 27. Thus, these data suggest the existence of a threshold dose below which immune stimulation might be suboptimal and above which immunosuppression prevails. Lastly, data from a study by our group, published in 201029, further support not only the importance of fraction size with regards to activation of the immune system, but also the longevity of the immune response following irradiation. We analyzed changes in tumour-cell phenotype in prostate cancer cell lines following single-fraction SABR and found that co-stimulatory and co-inhibitory T-cell signalling molecules can be modulated to promote productive antitumour immune responses following treatment with at least 10 Gy doses of radiation. In an attempt to find a potential therapeutic window for the addition of immunotherapeutic treatments, we analysed changes in tumour phenotypes at several time points following SABR. Whereas increased expression of immunostimulatory markers, including OX-40 ligand and 41BB ligand, was evident 72 hours after SABR, decreased expression of PD-L1 (programmed cell death 1 ligand 1), an inhibitor of T-cell expansion and function28, for example, was detect up to 144 hours after SABR20.
These studies focused on the immunomodulatory effects of SABR; however, the successful combination of SABR with immunotherapy regimens, resulting in synergistic anti-tumour effects, has also been reported in the preclinical setting. Cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4), similarly to PD-L1, functions to inhibit T-cell activation and suppress antitumour immune responses29. Both of these molecules have prominent roles in immune-checkpoints pathways that, when active, maintain self-tolerance and inhibit autoimmune reactions. Data now confirm that many tumours activate certain immune-checkpoint pathways to evade the host immune response and promote resistance. Thus, the development of checkpoint inhibitors has emerged as a prominent treatment strategy that enables stimulation of antitumour immune responses (BOX 1). Data from one elegant study31 demonstrated regression of the primary irradiated tumour and distant metastases following radiotherapy (two fractions of 12 Gy) combined with CTLA-4 blockade. Not surprisingly, the substantially improved local and distant tumour control translated into longer survival. Further analyses confirmed that these effects were elicited by CD8+ T-cell-dependent antitumour immunity30. Data from a similar study by the same group demonstrated that the use of different SABR regimens (20 Gy x 1, 8 Gy x 3, or 6 Gy x 5) in combination with anti-CTLA-4 antibody therapy again resulted in enhanced or complete regression of the primary tumour compared with use of single-modality therapy. Interestingly, substantial inhibition of tumour growth outside of the radiation field was seen only when immunotherapy was added to the fractionated SABR schedule and not the single-dose regimen31. As seen in a previous study from the same research group, the amount of CD8+ T cells demostrating tumour-specific IFN-γ production was proportional to the inhibition of the secondary tumour. Lastly, in the previously mentioned report25, investigators observed that ablative radiotherapy (15–25 Gy x 1) alone generated robust CD8+ T-cell-dependent immunity, leading to reductions in tumour burden, reduced relapse of the primary tumour, and eradication of metastases. These investigators further showed that treatment combining two consecutive doses of 12 Gy radiation with ad-LIGHT, an immunotherapeutic agent and member of the tumour necrosis factor superfamily — composed of a ligand of the stromal-cell-expressed lymphotoxin β receptor and T-cell-expressed herpes viral entry mediator — resulted in prolonged survival compared with treatment with either modality alone24.
Treatment with other types of immunotherapy has also been shown to augment antitumour responses when combined with high-dose radiotherapy. Combinations of clinically relevant monoclonal antibodies designed to stimulate antitumour immunity (such as anti-CD137 and anti-CD40 antibodies) or relieve immunosuppression (anti-PD-1 antibodies) with single (12 Gy) or fractionated (4–5 Gy x 4) radiotherapy have also been investigated33. Single-fraction treatment combined with anti-CD137 and anti-PD-1 therapy was found to result in enhanced host immune responsiveness to tumours, with a tumour rejection rate of up to 40% in mouse models32. Similarly, the fractionated radiotherapy regimens in combination with anti-CD137 and/or anti-PD-1 antibodies were more effective in controlling tumour growth than either treatment alone. Notably, radiotherapy did not deplete, but rather enriched tumours for functionally active, tumour-specific immune effector cells32. Data from an elegant study, with results published in 201534, corroborated these observations that perhaps combining immunotherapy techniques that have different mechanisms of action might yield better outcomes. In this study34, despite an initial tumour response, resistance was common when radiation was combined with anti-CTLA-4 antibodies. Resistance correlated with upregulation of PD-L1 and T-cell exhaustion; however, the addition of PD-L1 blockade to this regimen reversed T-cell exhaustion, and, as also promoted by anti-CTLA-4, further improved the CD8+ T cell: TREG cell ratio, and further enhanced expansion of the T-cell population and diversification of the T-cell-receptor repertoire 33. Lastly, treatment with single fractions of 10 Gy radiation in combination with L19–IL-2, a fusion protein designed to selectively deliver IL-2 to cancer cells, targeting tumour neovascularization, resulted in 75% cure rates and increased the percentage of antigen-specific CD8+ cytotoxic T cells34.
Taken together, these data demonstrate not only that effective immune stimulation can be achieved following SABR monotherapy, but also that addition of immunotherapeutic strategies to SABR therapy results in improved outcomes compared with treatment with either modality alone. These promising preclinical results served as the basis for testing this combination in the clinical setting.
Clinical evidence for ISABR
The most well-known success story of combining SABR with immunotherapy was detailed in a case report published in 201236. In conjunction with ipilimumab, an anti-CTLA-4 monoclonal antibody, SABR (28.5 Gy delivered in three fractions) was successfully used to treat a painful metastatic paraspinal lesion in a patient suffering from metastatic melanoma. The findings of post-SABR CT scans confirmed not only a local response, but also substantial regression of distant lesions located outside of the radiation field35. Local radiation in combination with anti CTLA-4 immunotherapy resulted in systemic antitumor activity, termed the ‘abscopal effect’36, which seemed to be mediated by the immune system36. In a similar case study, authors reported clinically significant improvements in the outcome of a patient with metastatic melanoma. Following SABR (54 Gy in three fractions) treatment of two of seven metastatic liver lesions, a complete systemic response occurred, despite disease progression on ipilimumab alone37. A third case report of an abscopal effect of ipilimumab in a patient with metastatic, non-small-cell lung cancer (NSCLC) was published in 201339. While undergoing ipilimumab immunotherapy, the most metabolically-active liver metastasis was selected as the target for SABR and was treated with a total radiation dose of 30 Gy in five fractions. Post-treatment scans showed an objective response within the radiation field as well as resolution of non-irradiated foci in the liver, bone and lung 38. Lastly, in an intriguing retrospective study with results published in 201340, investigators analyzed clinical and radiographic records of patients with melanoma who were treated with ipilimumab and either whole-brain radiotherapy (WBRT) or stereotactic radiosurgery (SRS) for brain metastases. The median survival of patients who received WBRT and ipilimumab was 3.1 months compared with 19.9 months in patients who received SRS and ipilimumab therapy. Both treatment with ipilimumab and treatment with SRS were significant predictors of improved overall survival (HR 0.43 and HR 0.45, with P = 0.005 and 0.008, respectively. Neither SRS nor ipilimumab treatment individually appeared to account for the prolonged survival seen in the analysis39. These findings were corroborated in another case report of a patient with metastatic melanoma, in whom a systemic complete response in the skin and lymph nodes was observed following treatment with ipilimumab and SRS for brain metastases40. Additional studies investigating the combination of immunotherapies with SABR, with similar findings to those studies discussed in this section, have also been published (Table 1)41–44.
Table 1.
Selected examples of published studies of SABR and Immunotherapy combinations
| Study details | SABR dose (Gy)/fractions | SABR Target | Immunotherapy agent | Sequence of treatments | Location of response |
|---|---|---|---|---|---|
| Postow et al., (2012)36 | 28.5/3 | Paraspinal | Ipilimumab | immunotherapy, then SABR, then immunotherapy | IF and OF |
| Hiniker et al., (2012)38 | 54/3 | Liver | Ipilimumab | immunotherapy, then SABR, then immunotherapy | IF and OF |
| Golden et al., (2013)39 | 30/5 | Liver | Ipilimumab | Concurrent | IF and OF |
| Silk et al., (2013)40 | 14–24/1–5 | Brain | Ipilimumab | immunotherapy then SABR, or SABR then immunotherapy | IF |
| Stamell et al., (2014)41 | NR | Brain | Ipilimumab | Concurrent | IF and OF |
| Karbach et al., (2014)42 | 45/1 | Brain | Autologous tumor-lysate-loaded dendritic cells | SABR then immunotherapy | IF and OF |
| Kiess et al., (2014)43 | 15–24/1 | Brain | Ipilimumab | SABR then immunotherapy, or Concurrent treatment, or Immunotherapy then SABR | IF |
| Kwon et al., (2015)44 | 8/1 | Bone | Ipilimumab | SABR then immunotherapy | IF |
| Seung et al., (2012)45 | 20/1 | Any | IL-2 | SABR then immunotherapy | IF and OF |
SABR, stereotactic ablative radiotherapy; IF, in field; OF, out of field.
These remarkable results have set the stage for the initiation of several clinical trials investigating the combination of SABR with immunotherapy. Currently, investigators at Johns Hopkins University are enrolling patients with metastatic melanoma with newly diagnosed metastases to the brain or spine; patients will receive an intravenous dose of ipilimumab, followed by CyberKnife® (Accuray Ltd, California, USA) SABR a week later, and three more doses of ipilimumab, to test the safety of this combination46. A similar trial, named RADVAX and led by investigators of the Abramson Cancer Center at the University of Pennsylvania, is a stratified phase I/II dose-escalation trial designed to investigate SABR followed by ipilimumab, also in patients with previously treated or untreated metastatic melanoma47. A phase I/II trial at MD Anderson Cancer Center currently recruiting participants will investigate the safety and efficacy of the combination of ipilimumab and SABR in patients with advanced-stage solid tumours. Patients will be randomly assigned to receive either concurrent (early) SABR starting on day 1 of ipilimumab therapy, or sequential (late) SABR beginning on day 2948. Patients with metastatic cancer and at least one metastatic or primary lesion in the liver, lung or adrenal gland are eligible for enrolment. A range of other clinical studies in this area are currently published or ongoing (Tables 1 and 2)49. Results of the ongoing trials we have described, which are anticipated to become available in the next few years, will hopefully provide further insight into the appropriate selection of patients that will benefit from ISABR. Until then, the information in the subsequent section might provide some guiding principles for future investigations.
Table 2.
Selected ongoing clinical trials investigating the efficacy of ISABR
| Institution and study details | SABR dose (Gy)/fraction | SABR Target | Immunotherapy agent | Sequence of treatments | Phase |
|---|---|---|---|---|---|
| Johns Hopkins University, NCT0195019546 | NS | Brain, Spine | Ipilimumab | Immunotherapy, then SABR, then immunotherapy | I |
| University of Pennsylvania, NCT01497808 (RADVAX)47 | NS | NS | Ipilimumab | SABR then immunotherapy | I/II |
| MD Anderson Cancer Center, NCT0223990048 | 50/4 60/10 |
Liver, Lung, Adrenal | Ipilimumab | Concurrent, or immunotherpy then SABR | I/II |
| Chiles Research Institute, NCT0186290071 | 15/1 20/1 |
Lung, Liver | Anti-OX40 | Concurrent | I/II |
| Stanford University, NCT0176922272 | 20/2 | Any | Ipilimumab | Concurrent | I/II |
| New York University, NCT0140106273 | 22.5/3 | Any | Fresolimumab | Concurrent | I/II |
| NIH/NCI, NCT0229894674 | 8/1 24/3 |
Liver | PD-1 inhibitor | SABR then immunotherapy | I |
| Thomas Jefferson University, NCT0170350775 | 24/1 21/1 18/1 15/1 |
Brain | Ipilimumab | Concurrent | I |
| MD Anderson Cancer Center, NCT0244474176 | 50/4 | Lung, Liver | PD-1 inhibitor | Concurrent | I/II |
ISABR; Immunotherapy combined and stereotactic ablative radiotherapy; SABR, stereotactic ablative radiotherapy; NCI, National Cancer Institute; NS, not specified.
Future directions
On the basis of the preclinical and clinical data presented in this Perpsectives, sufficient evidence exists to support continued exploration of the combination of immunotherapy and SABR. Nevertheless, several considerations need to be adequately addressed prior to the development of a clinical trial designed to test the efficacy of ISABR.
Firstly, appropriate patient selection remains of paramount importance. In nearly all clinical scenarios, factors including tumour site, stage and type will all affect any relevant outcomes. Currently, SABR is most-frequently used in the setting of metastatic disease and in patients with stage I NSCLC. Findings of randomized trials have confirmed that for patients with stage I NSCLC, SABR alone results in ≥95% local tumour control within the irradiated field50,51. The rates of microscopic or distant spread in the early stage disease scenario are low: about 5–10% of patients will develop regional lymph-node recurrences, and up to 15% will have distant metastases52. SABR only targets primary lesions, although rates of lymph-node recurrence and distant failures are comparable to those seen following surgical resection of the affected lobe and regional lymph-node dissection. Despite the previous assumption that removal of the visible tumour burden in the lung and dissection of draining lymph nodes would result in lower incidences of regional and distant metastases, this theory has not held true based on results of phase II prospective studies53, randomized studies54 and a patient-population study55. Furthermore, in a phase III trial, treatment with an antigen-specific immunotherapeutic vaccine, termed MAGE-A3, did not result in any benefit compared with placebo for patients with resected stage IB, II, and IIIA NSCLC, thus failing to meet the primary outcome56. One can hypothesize, on the basis of these observations, that the combination of this tumour-specific therapeutic vaccine with SABR might result in the generation of the aforementioned in situ vaccine with subsequent stimulation of an effective systemic immune response (TABLE 2). Collectively, these findings suggest that localized SABR alone might stimulate the immune system to prevent tumour recurrence and/or metastases. Adding active immunotherapy to SABR might further reduce lymph-node involvement and distant disease, potentially leading to even higher cure rates.
In addition to the current patient groups, patients with advanced-stage disease might also achieve important clinical benefits from treatment with ISABR. Patients suffering from oligometastatic disease or those with locally advanced tumours that have a high propensity for metastasis frequently harbour disease that is not routinely detected during laboratory examinations or imaging work-up. Thus, inciting an immune response using a combination of SABR and an immunotherapeutic approach can address the visible disease burden and also target cancer cells that have, thus far, evaded detection using traditional diagnostic approaches. Building upon findings of basic research35, a clinical trial is currently ongoing, with the aim of investigating the effectiveness of combining high-dose radiotherapy and the L19–IL-2 fusion protein in patients with oligometastatic solid tumours57 (NCT02086721). In these clinical situations, it remains unclear whether all of the disease needs to be treated, or if SABR targeting just a fraction of the index lesion being treated can nevertheless incite an effective systemic immune response against all oligometastases.
Similarly, patients with a more substantial disease burden might also benefit from ISABR using the same approach. An accessible metastatic lesion targeted with SABR can initiate an immune response, thereby enabling an effective immune-based attack on other metastases located outside of the radiation field. In this scenario, the SABR-treated lesion acts as an in situ tumour vaccine. However, the subsequent immune response following radiation alone is often insufficient to address the distant macroscopic or microscopic disease burden. Additionally, patients with advanced-stage or metastatic disease are frequently treated with several systemic agents, most of which are immunosuppressive. In these instances, in which chemotherapy is used to combat oligometastatic and/or occult metastatic disease, an unanswered question exists concerning whether the use of upfront chemotherapy reduces the recruitment of effector T cells for activation within the irradiated tumour microenvironment — where antigen elaboration occurs. Thus, implementation of an immunotherapeutic strategy, in addition to SABR, might generate a more robust and effective immune response. IASBR relies on the SABR-treated tumour to stimulate a personalized, tumour-specific immune response; therefore, choosing patients with the most appropriate tumour histology, location, and stage might have a less important role in clinical trials investigating this approach.
The optimal timing of the two IASBR treatment modalities is a second important aspect that needs addressing before a trial is embarked upon. Some investigators have proposed that immunotherapeutic treatments should be administered after radiotherapy. One theory hypothesizes that the activation of an immune response and augmentation of this response by immunotherapy might be less effective if radiation has not already generated de novo tumour antigens and broken any pre-existing peripheral immune tolerance of the tumour58. Additionally, treatment with SABR following the activation of immune cells could be detrimental to an effective antitumour cellular response, owing to the cytotoxic and ablative nature of this radiotherapy technique58. Conversely, administration of SABR after immunotherapy does offer certain advantages: stimulating antigen-presenting cells and effector T cells prior to SABR will allow these cells to be readily available to respond to the efflux of tumour antigens generated as a result of radiation treatment; similarly, having an active immunoadjuvant within the tumour microenvironment at the time of SABR could maximize its therapeutic effects58.
Despite these general principles, the immunotherapy agent of choice most probably dictates the optimal sequencing of SABR. For instance, in the example of adoptive T-cell transfer immunotherapy, SABR as the latter therapy would, presumably, interfere with the immune response at the tumour site. Therapeutic cancer vaccine therapy, on the other hand, might require SABR in order to release tumour antigens, which are necessary for activation of antigen presentation and immune-mediated cell killing. To help shed further light on this issue, in a study with results published in abstract form in 2014, investigators administered an anti-CTLA-4 antibody or OX40 agonist antibody either before or after a single radiation dose of 20 Gy to subcutaneous colorectal adenocarcinomas in a mouse model59. SABR delivered to the altered tumour microenvironment created by anti-CTLA-4 antibody administration resulted in 100% tumour clearance as opposed to only 50% clearance when an anti-CTLA-4 antibody was sequenced after radiotherapy. Consistent with the notion that the optimal timing of treatment modalities might be determined by the immunotherapy agent selected, administration of the OX40 agonist antibody increased the numbers of activated CD8+ T cells and was optimal when delivered one day after single-fraction radiotherapy59. Collectively, taking into account the different specific mechanisms of action of immunotherapeutic strategies might help to dictate the most-appropriate timing of immunotherapuetic interventions in relation to SABR. Thus, these data suggest than an umbrella recommendation regarding the optimal sequencing of immunotherapy and SABR might be misleading, and possibly inappropriate. Rather, obtaining a solid understanding of the mechanism of action of the chosen agent and its role in either stimulating or suppressing a tumour-directed immune response will be more valuable.
A final point, which also requires consideration, is the ability to identify patients’ responses to therapy. As mentioned above, patients with obvious progressive disease in a single location yet potentially also harbouring tumour cells in other locations, which are undetectable using traditional methods, might derive the greatest clinical benefit from ISABR. Thus, measuring responses to treatment using standard laboratory tests or imaging modalities might falsely reveal a lack of systemic disease control. Soley choosing traditional clinical end points, such as tumour resectability, tumour response, disease-free survival, and/or overall survival to assess the efficacy of ISABR may not tell the complete story. Therefore, supplementing with immunological readouts as well to capture disease response is recommended, as they can establish proof-of-principle of activation of the immune system prior to exploration of clinical end points. For instance, measuring the production of inflammatory cytokines in a patient’s serum following administration of ISABR might act as a surrogate for the true efficacy of an antitumour immune response. Quantifying the generation of tumour-specific T-cells will help assess the ability of ISABR to elicit a tumour-directed immune response. Furthermore, measuring alterations in the number and function of TREG cells, natural killer cells, and circulating antigen–antibody complexes will provide an overall picture of the generation of a productive immune response. Similarly, the presence of neoantigens and a greater mutational load seems to correlate with the cytolytic activity of NK cells and CD8+ T cells and, therefore, might help to predict outcomes following ISABR60. Indeed, tumour mutational load, described as the predicted burden of deleterious alleles61, has been demonstrated to positively correlate with an improved objective response, durable clinical benefit and progression-free survival in patients with NSCLC who received treatment with anti-PD-1 therapy62. Lastly, reports published in 2014 indicate that CTLA-4 blockade induces evolution and diversification of the T-cell repertoire, thereby increasing the number of unique T-cell-receptor clonotypes. In this study63, improved clinical outcomes were associated with less clonotype loss and maintenance of high-frequency clonotypes during treatment63; perhaps these features could act as surrogates for clinically-relevant antitumour responses. In fact, these changes in T-cell clonality have been associated with increased overall survival in clinical trials with cohorts of patients with prostate64 or breast 65 cancer. These, along with other immunological tests, should be used to supplement standard examination criteria in order to more accurately determine the extent of disease response66. Data from another study67 highlight the challenges in choosing the most-appropriate readouts in clinical immunotherapy studies. The investigators reported tumour progression despite induction of very high levels of tumour-antigen-specific CD8+ T cells in patients with melanoma, following vaccination with altered peptide immunogens67. Efforts to identify such immune biomarkers in addition to the use of more-traditional measures of disease response should be undertaken so that rational treatment combinations can be designed in terms of intensity, sequencing and maintenance of immune stimulation after combination with SABR. This approach will also enable the possibility of enriching treatment populations in clinical trials with patients who are most likely to respond to treatment and/or tailoring therapy specifically for distinct subsets of patients.
Conclusions
The proposal to combine immunotherapy and radiotherapy is not novel. Many investigators have shown that this bimodality therapeutic approach is not only feasible, but also effective, and that the doses of radiation required fall within the window of conventional fractionation schedules. Within the last two decades, clinicians have taken advantage of technological breakthroughs that enable treatment with higher doses of radiation while maintaining acceptable levels of exposure of the surrounding organs at risk. The popularity of SABR has risen drastically over the past few years and its full potential, no doubt, remains to be realized. Owing to the local efficacy and ablative qualities of SABR as a single modality, SABR is infrequently combined with other treatments, especially immunotherapy. Thus, the goal of this Perspectives was to present the relevant literature supporting the combination of immunotherapy with SABR, described as ‘ISABR’, in the preclinical and clinical settings. Currently, a few clinical trials of this approach are underway, although the results are not expected to become available for several years. The aim of the final section of this Perspectives was, therefore, to provide some general guiding principles to consider regarding the development of, and to promote interest in, future research efforts as we believe the synergistic relationship between SABR and immunotherapy is just beginning to blossom.
Table 3.
Proposed ISABR studies
| Patient population | SABR dose (Gy)/number of fractions | SABR target | Type of immunotherapy | Sequence | Readout |
|---|---|---|---|---|---|
| Stage I NSCLC | 50–60/3–5 60–70/8–10 |
Primary tumor | Vaccine-MAGE* PD-L1 |
Immunotherapy followed by SABR Concurrent ISABR |
PET/CT scan, PD-L1, TIL, Treg, CD8/CD4, Exome micro-RNA, cytokine production, CEA-specific T cells |
| Early stage hepatocellular carcinoma | 40–60/3–5 50–70/8–10 |
Primary tumor | PD-L1 | Concurrent ISABR | MRI scan, PD-L1, TIL, Treg, CD8/CD4, Exome micro-RNA, cytokine production |
| Stage IV CRC | 50–60/3–5 60–70/8–10 |
Dominant Liver or lung metastasis | Vaccine-CEA PD-1 |
Immunotherapy followed by SABR Concurrent ISABR |
CT scan, MRI of the abdomen, cytokine production, CEA-specific T cells, TILs in treated and off-target metastases, inflammatory cytokine production, PD-L1, Treg, CD8/CD4, Exome micro-RNA |
| Stage IV NSCLC with spinal/brain metastases | 12–25/1 15–24/1 |
Spinal metastases Brain metastases |
PD-L1 PD-1 + ipilumumab |
Immunotherapy then SABR Concurrent ISABR |
PET/CT, brain MRI, tumour-specific T cells, PD-L1 expression levels, inflammatory cytokine production, TIL, Treg, CD8/CD4, Exome micro-RNA |
| Stage IV NSCLC | Organ-dependent dose regimen | Oligometastasis | PD-L1 PD-1 + ipilumumab |
SABR then immunotherapy Concurrent ISABR |
PET/CT scan to monitor regression at distant metastatic sites, PD-L1 expression levels, TILs in treated primary and untreated metastases, Treg, CD8/4, Exome micro-RNA |
CEA, carcinoembryonic antigen; CRC, colorectal cancer; ISABR; Immunotherapy combined and stereotactic ablative radiotherapy; MAGE, melanoma antigen E; NSCLC, non-small-cell lung cancer; SABR, stereotactic ablative radiotherapy; TILs, tumour-infiltrating lymphocytes; T-reg, regulatory T-cells; RNA, ribonucleic acid.
Acknowledgments
Supported in part by Cancer Center Support (Core) Grant CA016672 from the National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Biographies
Dr. Michael B. Bernstein earned his medical degree from Albert Einstein College of Medicine, NY, USA. During medical school, he participated in a two-year research fellowship program studying cancer immunotherapy at the National Cancer Institute, Bethesda, USA. He completed his Radiation Oncology residency at Montefiore Medical Center, NY, USA and a fellowship focusing on stereotactic radiotherapy at The University of Texas MD Anderson Cancer Center, Houston, USA. He has authored several papers on the topic of radiation therapy and immunotherapy and continues to actively pursue these research interests.
Dr. James W. Hodge received his PhD in Comparative and Experimental Medicine from the University of Tennessee, Tennessee, USA and an MBA from The George Washington University, Washington DC, USA. He currently heads the Recombinant Vaccine Group in the Laboratory of Tumor Immunology and Biology of the National Cancer Institute, Bethesda, USA. He has made significant contributions to the design and development of novel recombinant vaccines for cancer immunotherapy and has authored over 100 papers on the topic. He is considered a leader in the field of immunotherapy.
Dr. Sunil Krishnan gained his MD in 1993 from Christian Medical College, Vellore, India. Currently, Dr. Krishnan is the Director of the Center for Radiation Oncology Research at M. D. Anderson Cancer Center and the John E. and Dorothy J. Harris Professor of Gastrointestinal Cancer in the Department of Radiation Oncology. He has co-authored over 150 peer-reviewed scientific publications, co-authored 17 book chapters, and co-edited 2 books, mostly related to radiation therapy. Over the last decade, Dr. Krishnan has developed and advanced novel strategies to improve radiation treatment outcomes of gastrointestinal cancers. This has included combination of radiation therapy with targeted agents, botanicals, and metallic nanoparticles as well as delivery of higher doses of radiation therapy more precisely to tumors via image guidance.
Dr. Joe Y. Chang received his medical degree from Shanghai Medical College, Fudan University, Shanghai, China and holds an MS in Immunology. He later earned his PhD in Cancer Biology from MD Anderson Cancer Center. He completed his Radiation Oncology residency training at Rush-Presbyterian St. Luke Medical Center, Chicago. He is Professor and the Director of Stereotactic Ablative Radiotherapy at MD Anderson Cancer Center. He published more than 200 research articles/books chapters in the field of stereotactic radiotherapy, proton therapy, image-guided radiotherapy, and gene therapy/immunotherapy. He is recognized as a leading authority in the field of stereotactic radiotherapy and proton therapy.
Footnotes
Author contributions
All authors researched data for this manuscript, made a substantial contribution to discussions of content, wrote the manuscript and reviewed and/or edited the manuscript prior to submission.
Competing interests statement
The authors declare no competing interests.
References
- 1.Imaizumi N, Monnier Y, Hegi M, Mirimanoff RO, Ruegg C. Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting. PloS one. 2010;5:e11084. doi: 10.1371/journal.pone.0011084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamrava M, Bernstein MB, Camphausen K, Hodge JW. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Molecular bioSystems. 2009;5:1262–1270. doi: 10.1039/b911313b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Z, et al. Efficient antitumor immunity derived from maturation of dendritic cells that had phagocytosed apoptotic/necrotic tumor cells. International journal of cancer. Journal international du cancer. 2001;93:539–548. doi: 10.1002/ijc.1365. [DOI] [PubMed] [Google Scholar]
- 4.Kotera Y, Shimizu K, Mule JJ. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer research. 2001;61:8105–8109. [PubMed] [Google Scholar]
- 5.Melcher A, Gough M, Todryk S, Vile R. Apoptosis or necrosis for tumor immunotherapy: what’s in a name? J Mol Med (Berl) 1999;77:824–833. doi: 10.1007/s001099900066. [DOI] [PubMed] [Google Scholar]
- 6.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Current opinion in immunology. 2006;18:226–231. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Aymeric L, Locher C, Kroemer G, Zitvogel L. The dendritic cell-tumor cross-talk in cancer. Current opinion in immunology. 2011;23:146–152. doi: 10.1016/j.coi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 8.McBride WH, et al. A sense of danger from radiation. Radiation research. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 9.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. International journal of radiation oncology, biology, physics. 2005;63:655–666. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Current pharmaceutical design. 2002;8:1765–1780. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 11.Kwilas AR, Donahue RN, Bernstein MB, Hodge JW. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Frontiers in oncology. 2012;2:104. doi: 10.3389/fonc.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Seminars in Oncology. 2012;39:323–339. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunology, Immunotherapy. 2011;60:1227–1242. doi: 10.1007/s00262-011-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodge JW, et al. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. International Journal of Cancer. 2013;133:624–636. doi: 10.1002/ijc.28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reits EA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gameiro SR, et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–416. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JY, et al. Stereotactic ablative radiotherapy: a potentially curable approach to early stage multiple primary lung cancer. Cancer. 2013;119:3402–3410. doi: 10.1002/cncr.28217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palma DA, et al. The oligometastatic state - separating truth from wishful thinking. Nature reviews. Clinical oncology. 2014;11:549–557. doi: 10.1038/nrclinonc.2014.96. [DOI] [PubMed] [Google Scholar]
- 19.Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2847–2854. doi: 10.1200/JCO.2014.55.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein MB, et al. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer biotherapy & radiopharmaceuticals. 2014;29:153–161. doi: 10.1089/cbr.2013.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. International journal of radiation oncology, biology, physics. 2012;83:1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. International journal of radiation oncology, biology, physics. 2012;84:879–880. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, et al. An in situ autologous tumor vaccination with combined radiation therapy and TLR9 agonist therapy. PloS one. 2012;7:e38111. doi: 10.1371/journal.pone.0038111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lugade AA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. International journal of cancer. Journal international du cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 27.Aryankalayil MJ, et al. Defining molecular signature of pro-immunogenic radiotherapy targets in human prostate cancer cells. Radiation research. 2014;182:139–148. doi: 10.1667/RR13731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Said EA, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nature medicine. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nature immunology. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 30.Demaria S, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:728–734. [PubMed] [Google Scholar]
- 31.Dewan MZ, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbrugge I, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer research. 2012;72:3163–3174. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 33.Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zegers CM, et al. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res. 2015;21:1151–1160. doi: 10.1158/1078-0432.CCR-14-2676. [DOI] [PubMed] [Google Scholar]
- 35.Postow MA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. The New England journal of medicine. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer biotherapy & radiopharmaceuticals. 2012;27:12–22. doi: 10.1089/cbr.2012.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiniker SM, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Translational oncology. 2012;5:404–407. doi: 10.1593/tlo.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer immunology research. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer medicine. 2013;2:899–906. doi: 10.1002/cam4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. International journal of radiation oncology, biology, physics. 2013;85:293–295. doi: 10.1016/j.ijrobp.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karbach J, et al. Long-term complete remission following radiosurgery and immunotherapy in a melanoma patient with brain metastasis: immunologic correlates. Cancer Immunol Res. 2014;2:404–409. doi: 10.1158/2326-6066.CIR-13-0200. [DOI] [PubMed] [Google Scholar]
- 42.Kiess AP, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92:368–375. doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwon ED, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seung SK, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Sci Transl Med. 2012;4:137ra174. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 45.US National Library of Science. ClinicalTrials.gov [online] 2014 https://clinicaltrials.gov/ct2/show/NCT01950195?term=NCT01950195&rank=1.
- 46.US National Library of Science. ClinicalTrials.gov [online] 2014 https://clinicaltrials.gov/ct2/results?term=NCT01497808&Search=Search.
- 47.US National Library of Science. ClinicalTrials.gov [online] 2014 https://clinicaltrials.gov/ct2/show/NCT02239900?term=NCT02239900&rank=1.
- 48.Crittenden M, et al. Current clinical trials testing combinations of immunotherapy and radiation. Semin Radiat Oncol. 2015;25:54–64. doi: 10.1016/j.semradonc.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang JY, et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage I non-small cell lung cancer. Radiat Oncol. 2012;7:152. doi: 10.1186/1748-717X-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Timmerman R, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13:802–809. doi: 10.1016/S1470-2045(12)70242-5. [DOI] [PubMed] [Google Scholar]
- 52.Timmerman R, et al. RTOG 0618: stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. J Clin Oncol. 2013;31:S7523. [Google Scholar]
- 53.Chang JY, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. An important study showing SABR as an option for therapy in operable stage I non-small cell lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shirvani SM, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg. 2014;149:1244–1253. doi: 10.1001/jamasurg.2014.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vansteenkiste JF, et al. MAGRIT, a double-blind, randomized, placebo-controlled Phase III study to assess the efficacy of the recMAGE-A3 + AS15 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small cell lung cancer (NSCLC). Presented at the European Society for Medical Oncology; Madrid Spain. 2014. [Google Scholar]
- 56.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02086721?term=NCT02086721&rank=1.
- 57.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123:2756–2763. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young K, Cottam B, Baird JR, Gough MJ, Crittenden M. Ideal timing of immunotherapy with radiation in murine tumor models. Int J Radiat Oncol. 2014;90:S58. [Google Scholar]
- 59.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Henn BM, Botigue LR, Bustamante CD, Clark AG, Gravel S. Estimating the mutation loa in human genomes. Nat Rev Genet. 2015;16:333–343. doi: 10.1038/nrg3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rizvi NA, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cha E, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Science Transl Med. 2014;6:238ra270. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwek SS, et al. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J Immunol. 2012;189:3759–3766. doi: 10.4049/jimmunol.1201529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Disis ML, et al. HER-2/neu vaccine-primed autologous T-cell infusions for the treatment of advanced stage HER-2/neu expressing cancers. Cancer Immunol Immunother. 2014;63:101–109. doi: 10.1007/s00262-013-1489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gulley JL, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res. 2014;2:133–141. doi: 10.1158/2326-6066.CIR-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg SA, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 67.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. This review discusses the promising approach of utilizing immune checkpoint blockade to enhance antitumour immunity with the potential to produce durable clinical responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01862900?term=NCT01862900&rank=1.
- 69.US National Library of Science. ClinicalTrials.gov [online] 2013 https://clinicaltrials.gov/ct2/show/NCT01769222?term=NCT01769222&rank=1.
- 70.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01401062?term=NCT01401062&rank=1.
- 71.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02298946?term=NCT02298946&rank=1.
- 72.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT01703507?term=NCT01703507&rank=1.
- 73.US National Library of Science. ClinicalTrials.gov [online] 2015 https://clinicaltrials.gov/ct2/show/NCT02444741?term=NCT02444741&rank=1.
- 74.Bolli M, et al. Tissue microarray evaluation of Melanoma antigen E (MAGE) tumor-associated antigen expression: potential indications for specific immunotherapy and prognostic relevance in squamous cell lung carcinoma. Ann Surg. 2002;236:785–793. doi: 10.1097/01.SLA.0000036266.09823.6C. discussion 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jungbluth AA, et al. Expression of MAGE-antigens in normal tissues and cancer. Int J Cancer. 2000;85:460–465. [PubMed] [Google Scholar]