Abstract
Urbanization represents an unintentional global experiment that can provide insights into how species will respond and interact under future global change scenarios. Cities produce many conditions that are predicted to occur widely in the future, such as warmer temperatures, higher carbon dioxide (CO2) concentrations and exacerbated droughts. In using cities as surrogates for global change, it is challenging to disentangle climate variables—such as temperature—from co-occurring or confounding urban variables—such as impervious surface—and then to understand the interactive effects of multiple climate variables on both individual species and species interactions. However, such interactions are also difficult to replicate experimentally, and thus the challenges of cities are also their unique advantage. Here, we review insights gained from cities, with a focus on plants and arthropods, and how urban findings agree or disagree with experimental predictions and historical data. We discuss the types of hypotheses that can be best tested in cities, caveats to urban research and how to further validate cities as surrogates for global change. Lastly, we summarize how to achieve the goal of using urban species responses to predict broader regional- and ecosystem-level patterns in the future.
Keywords: city, global change, multi-trophic interactions, urban, warming
1. Species interactions in cities: getting ahead of the curve
Cities are the future. Urban populations are expected to increase by 2.5 billion in the next 30 years [1] and urbanization will affect the physical environment, responses and interactions of all organisms that live in cities. Already, cities have warmer temperatures, higher carbon dioxide (CO2) levels and exacerbated ‘droughts’ due to less infiltration and greater runoff of precipitation [2–6]. In relation to global change, species in cities—in particular, plant and arthropod taxa that have underscored research North America and Europe—have responded to urban and climate variables with phenological shifts, physiological changes and adaptive evolution (e.g. [7–9]). Relationships between species have been altered by abiotic and biotic interactions, ecological niches have shifted and new communities have developed (e.g. [10–12]). Similar climate conditions and impacts may occur outside of cities in future global change scenarios [5,13] (figure 1). Cities have thus been recognized for their potential importance as surrogates for global changes happening at larger scales, such as global warming, and for their potential use in observing and predicting broader ecological and evolutionary processes [9,14–20].
Figure 1.
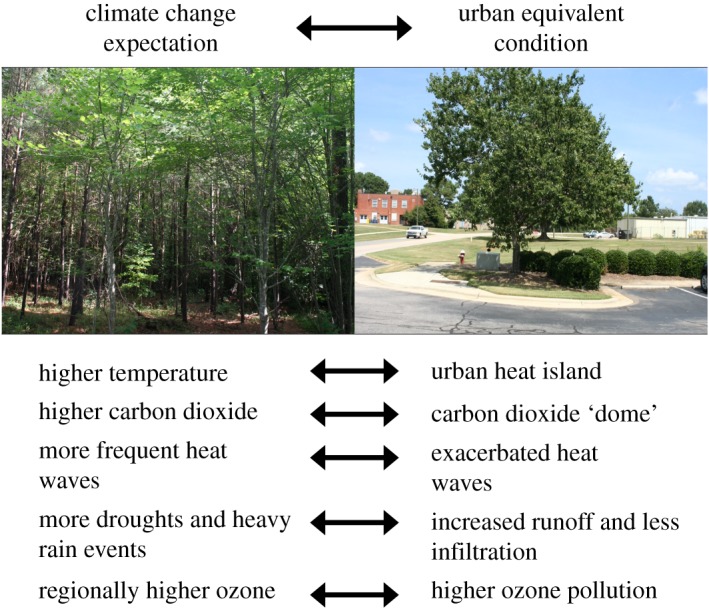
Climate change expectations and urban equivalent conditions. Pictured are a suburban forest (left) and an urban street tree (right) in the southeastern USA, located 5 km apart. (Online version in colour.)
A critical question in moving forward with the use of cities as surrogates for global change is whether and, perhaps more usefully, when results from any one city or study organism are generalizable. To date, research has been performed disproportionately in North America and Europe, in fields ranging from atmospheric and earth sciences (e.g. [21,22]) to evolutionary biology (e.g. [23])—disciplines that are not traditionally well integrated—and has focused on the responses of plants and arthropods [24]. Even within the spectrum of ecological research, which is itself broad, separating the effects of global warming and urban warming, by, for example, comparing cities across latitudes [9,25], or comparing functional groups among cities [26,27], are relatively recent initiatives. In this review, we will (i) examine insights that have been gained specifically from cities, regarding phenological, physiological, population, community and latitudinal responses by and interactions among species (electronic supplementary material, table S1). Where possible, we will discuss how urban results do or do not agree with experimental predictions or historical data, and how combinations of climate and urban variables, current and historical data or other approaches may be used to isolate effects of interest from confounding urban variables in cities. Then, we will (ii) consider the types of hypotheses that can be best addressed in cities, caveats to urban research and what can be done to further validate cities as surrogates for global change. Lastly, we will (iii) summarize the potential for urban species responses to inform broader predictions. Owing to a much greater amount of research on plants and arthropods, our review will focus on these taxa and their responses to warming; however, we emphasize that cities may ultimately present the only globally accessible and cost-effective means to observe the interactions of other taxa—from microbes to vertebrates—in response to multiple interactive climate variables, in different regions of the world. With their potential as ‘space for time’ substitutions, species responses in cities have occurred over a time scale and geographical scope that will complement our understanding of global change gained from historical, experimental and modelling data.
2. Global change insights gained from cities
(a). Phenological responses
Within cities, along urban–rural gradients and among cities at different latitudes (figure 2), studies have demonstrated phenological adjustments by urban species, sometimes in response to measured changes in urban temperature and sometimes in response to urban variables (such as impervious surfaces) that correlate with temperature. For example, long-term records show that the phenology of multiple flowering plants has advanced in 10 central European cities relative to rural areas, with stronger trends in recent years [7]. Earlier plant phenology and urbanization are similarly correlated in remote sensing studies [30–33]. The combined effects of climate variables—specifically temperature—and urban variables on phenology have been observed via long-term records or on-the-ground observations for other flowering plants [34,35], urban insects [27,36] and urban birds [37,38]. Phenological advances in response to warming in cities are generally supported by experimental studies (e.g. reviews in [39,40]) and historical datasets from herbarium or museum collections [41–44].
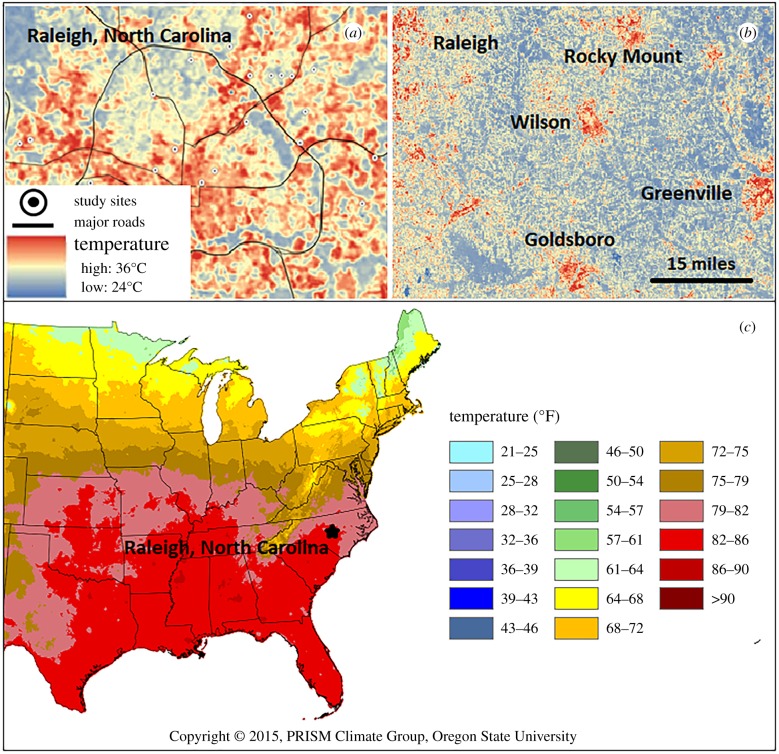
Figure 2.
Examples of how within-city, urban–rural and latitudinal comparisons can be used to study species responses to differences in temperature. (a) Within-city differences in temperature in Raleigh, NC, USA (adapted with permission from Dale & Frank [28,29]). (b) Urban–rural differences in temperature in eastern North Carolina (image provided by Elsa Youngsteadt). The Landsat thermal images in (a,b) are from 18 August 2007. (c) Average monthly temperature for August 2007 and latitudinal differences in temperature across the eastern USA, with Raleigh, NC indicated for reference (image from the PRISM Climate Group; www.prism.oregonstate.edu; data accessed 15 January 2017).
Interactions between urban species are also affected by changes in phenology. In Raleigh, NC, USA, a herbivorous scale insect was not only more abundant on oak trees in warmer relative to cooler areas [45], but the phenology of the herbivore became decoupled from the phenology of the parasitoid wasps that previously formed a natural biocontrol, leading to decreased parasitism at a critical stage of herbivore development and increased herbivory on trees in warmer parts of the city [11] (electronic supplementary material, figure S3). Similarly, reduced parasitism of cereal leaf beetles, a herbivore pest of grain crops, was observed during multiple warmer springs in an agricultural setting [46]. However, field warming experiments may have context-dependent effects on plant and insect phenology [47]. For example, warming advanced the phenology of host trees more than the phenology of a herbivorous caterpillar [48], but in another study, warmed caterpillar eggs had high survival on host trees despite the lag period between egg hatch and bud burst [49]. Overall, there are increasing trends towards phenological asynchrony between plants and herbivores or pollinators [50,51], and asynchrony in multi-trophic interactions [24,52,53].
Intriguingly, historical collections have demonstrated that the phenology of multiple wild bee species advanced in parallel with that of bee-pollinated plants [42]. The use of historical data in combination with urban sampling is potentially a very powerful approach in understanding phenological responses. For example, Primack et al. [41] observed current flowering times of arboretum plants in Boston, MA, USA, relative to flowering times of historical specimens of the same plants. Natural history collections or long-term records of invertebrate and vertebrate sightings could be used to complement herbarium studies and shed light on how phenological shifts have influenced species interactions over longer periods of time than are feasible to measure experimentally. Understanding how urban species have responded to gradual changes in climate variables over time will improve our ability to validate cities as surrogates for global change.
(b). Physiological responses
Physiological responses by species in cities include changes in rates of photosynthesis and growth of plants and changes in abundance and thermal tolerance of insects. Urban red maples had higher rates of photosynthesis [54] and growth in warmer relative to cooler sites within a city [28,55], and red maple seedlings in a warming experiment were also able to acclimate photosynthetically [56]. However, while temperate forest trees generally respond positively to increases in temperature in warming experiments [57–60] (but see [61,62]), urban trees are more variable and have demonstrated lower rates of photosynthesis with warming [21,63] or no difference in rates of photosynthesis [64]. Explanations for these inconsistent physiological responses by trees could relate to the interactive effects of multiple climate variables in cities (such as relative humidity, CO2 or soil moisture), urban variables (such as impervious surface, soil compaction or building architecture), choice of focal species and/or biotic interactions (with herbivores and pathogens, pollinators or natural enemies). Gregg et al. [65] found that increased growth of poplar seedlings in New York City was not due to warming, soil alteration or other urban variables, but, instead, was related to the negative effect of higher rural ozone concentrations on rural trees. Where urban species responses agree with warming experiments and/or historical data, we can be more confident in the robustness of cities as surrogates for warming. The challenge, in moving forward, is determining why responses diverge. For example, the likelihood that air pollutants produced in cities may ultimately have a greater impact on trees in rural environments [65,66], or the possibility that warmer nighttime temperatures have greater effect than more commonly modelled daytime temperatures [64,67], may explain divergence between urban and experimental results.
Physiological changes may include acclimation or adaptive evolution in response to climate or urban variables in cities. Plasticity allows a genotype to acclimate or express a different phenotype under altered environmental conditions, while adaptive evolution is a change in the frequency of a genetically based trait, due to selective pressure, that results in higher reproductive success [18,68]. In an example of adaptation, Cheptou et al. [8] observed selection in favour of non-dispersing seeds in an annual plant in Montpellier, France, which improved urban fitness. Selection on functional traits also occurred in the city; urban plants retained physiological changes in size, rates of photosynthesis and concentrations of leaf nitrogen when grown together in a greenhouse with plants from rural sites [69].
The ability to cope with a broader range of temperatures and also acclimate to warmer overall temperatures may result in trade-offs between traits. For example, colder winter temperatures at ground level, due to snow removal in cities, selected against cyanogenesis in urban clover [23]. This otherwise beneficial anti-herbivory trait was selected against because of the greater potential for freezing to damage plant cells, and subsequent auto-toxicity, without an insulating cover of snow in cities [23]. The effects of warmer temperatures are more commonly studied in the context of global change, and while the frequency of cold days and cold nights are expected to decrease in the future, occasional cold winter extremes will continue to occur [70]. It is important to understand physiological responses of species to hot and cold temperatures, because more limited exposure to cold temperatures could result in more negative impacts when such temperatures do occur. Historical records may provide additional insights into how species balance general thermal tolerance with an ability to respond to cold and hot temperature extremes.
Plasticity in physiological responses to urban warming has also been demonstrated for insects, other invertebrates and fungi. Urban leaf cutter ants had higher thermal tolerance relative to rural ants in São Paolo, Brazil [71], as did urban chitinolytic fungi [72], and the abundance, survival or fecundity of multiple species of scale insects increased with temperature in Raleigh, NC, USA [11,28,29,45,55,73]. However, hatching success of land snail (Arianta arbustorum) eggs decreased in warmer urban areas [74], and recent studies have shown that solitary bees and bumble bees are more sensitive to warming [75,76], and that a more impervious surface, which is generally correlated with temperature, may increase the susceptibility of honey bee (Apis mellifera) colonies to disease [77]. The direct effects of warming on arthropods and other ectotherms are frequently mediated by habitat architecture and geometry, which may be more heterogeneous in cities but also more predictable, as buildings remain the same shape and size throughout the year [78]. Thus, for modelling the effects of climate variables that depend, to an extent, on arthropod behaviour in relationship to habitat, physiological responses of arthropods may potentially be more predictable in cities relative to warming experiments.
(c). Population and community-level responses
Plant and arthropod populations and community structure and function respond to both climate and urban variables in cities. Higher urban temperatures and CO2 concentrations in Baltimore, MD, USA, relative to a rural site, closely matched short-term (approx. 50 year) climate predictions [5] and affected the structure of plant communities that were germinated from the same soil and seed bank. Urban plant productivity was higher after 1 year in Baltimore [14], relative to rural plant productivity, and urban plant succession reflected a greater ratio of perennials to annuals after 5 years [10], which could have multiple impacts on plant community function by affecting pollinator resources, habitat for beneficial or pest insects and multi-trophic interactions.
Arthropods may be affected directly by climate and urban variables in cities, and also via indirect effects on their predators and host plants [79]. Meineke et al. [80] found that spider abundance in urban trees in a mid-latitude city in the USA did not increase with warming, even though herbivore (prey) abundance did, and Turrini et al. [81] observed limited top-down control of aphids on urban relative to rural plants in Switzerland, but also found that plant biomass mitigated the benefit of aphid predators. Experimental warming affected plant community composition in China due to competitive interactions between plant species, but only in the presence of a beetle herbivore [82]. In other experiments, the combined effects of elevated warming, CO2 and drought on an insect herbivore were mediated by changes in plant secondary compounds and nitrogen content [83], and elevated warming, CO2 and nitrogen deposition affected floral attractiveness and nectar chemistry for bees [84]. In a herbarium collection, the abundance of scale insects preserved on red maples tracked historical temperature fluctuations [12], representing direct responses of scale insects to temperature but also indirect responses to changes in the condition of their host trees [28,55,73]. Thus, insect responses to the direct effects of temperature (or CO2) are often difficult to separate from indirect effects on herbivores via the responses of their plant hosts [79,85].
Overall, studies of interactions among species or trophic levels are still outweighed by individual studies of plants or arthropods [24,47], and there is a general consensus that long-term and larger-scale experimental studies are needed to understand how interactive climate variables will affect population-level responses and community- and ecosystem-level processes [51,53,85,86]. Using cities as surrogates for some effects of global change is an opportunity to add to our understanding of the effects of multiple climate variables and the responses and interactions of understudied taxa like vertebrates, worldwide, at potentially lower cost in terms of time, money and infrastructure relative to traditional experimental manipulation of climate variables.
(d). Latitudinal responses
Species responses to urban temperature may vary with respect to differences in background temperature due to latitude [17]. More positive effects of global change are generally predicted for species at higher latitudes [87,88]. Indeed, over a latitudinal range from 35.8° to 42.4°, arthropod abundance was positively correlated with increased within-city temperature in a high-latitude city in the eastern USA [25]. However, responses were of a greater magnitude and were more variable from positive to negative in lower latitude cities. Heterogeneous responses may ultimately cause greater disruptions of ecological dynamics than consistent directional responses—whether positive or negative—because species that currently interact with each other may respond differently to future warming [25] (electronic supplementary material, figure S3).
Latitude and urbanization may also have non-additive effects on species. Although shifts towards earlier phenology are common in response to latitude or urbanization alone, the interaction of these two factors led to delays in emergence and changes in the abundance of multiple species of butterflies in OH, USA, suggesting that butterflies responded to multiple environmental (climate and/or urban) variables [36], or that butterflies had a nonlinear response to temperature [89,90]. Long-term phenologies of flowering trees across the UK have been shown to be influenced by latitude and urbanization, with more pronounced advances occurring in northern areas [35] (but see [90]), and similarly over a large latitudinal gradient, urbanization and temperature had increasing effects on phenology for more than half of China's 32 major cities [33]. Recurrent adaptive evolution of cyanogenesis in clover was also strongly associated with both urbanization and latitude [23,91], while reflecting different selective pressures in different world regions [92].
3. Future directions in cities
(a). Hypothesis testing and study species in cities
Observing the effects of multiple climate variables over long periods of time and at a large spatial scale is a unique advantage in the use of cities as surrogates for global change. Cities are ideal for observing the effects of gradual changes in temperature and CO2 on long-lived plants and animals that may be difficult to grow and manipulate experimentally. The responses of mature trees to global change are critical to ecosystem health and services, and long-lived arthropods or those with an overwinter diapause—which is often regulated by temperature—may also be ideally studied in cities, rather than in short-term experiments, to fully capture multi-trophic interactions over a species' entire lifespan. Cities can likewise be used to observe and test responses of insects that are difficult to confine experimentally, such as wild bees, which require a large amount of space and a variety of habitat and floral resources, or temperature-dependent outbreaks of insects such as defoliating caterpillars (e.g. [93,94]). Lastly, there are intriguing examples of vertebrate responses to urban variables, such as anoles with longer limbs that better suit their city habitat [95], and understanding these responses alongside corresponding climate variables and trophic interactions in cities would provide valuable insights into future regional and ecosystem-level patterns.
Cities are also an ideal location to study the formation and function of novel no-analogue communities. Intentional and unintentional species introductions in cities, habitat fragmentation and more challenging abiotic conditions have resulted in urban communities with no current rural analogues. Overall changes in biodiversity and species interactions are a major prediction of global change scenarios (e.g. [13,96,97]); however, changes in community assemblages do not necessarily mean a loss of biodiversity [98]. Thus, unique relationships between species in cities may predict future community interactions in rural environments. For example, urban corridors may facilitate the movement and persistence of heat-tolerant species of ants [99], butterflies [36,100] and spiders [101], as well as the establishment of non-native anoles [102] and the migration of birds [103]. Street trees and other amenity plants are often established outside of their native range, so comparisons can be made on the same species in hotter or cooler regions where they may exist naturally in the future [104]. Herbarium, entomological and natural history collections of other taxa can shed light on when new species were introduced in cities and how species have responded and interacted since their introductions. Such collections are frequently made at urban and rural locations and can be compared across space and time [41,43,44,105–107].
(b). Validating climate variables and evolutionary potential of species in cities
In the context of using cities as surrogates for the effects of global change, the measurement of multiple terrestrial climate variables such as temperature, relative humidity, precipitation and soil moisture, and atmospheric climate variables such as CO2 and ozone (‘essential climate variables’ as defined by the NOAA Global Observing Systems Information Center, https://www.ncdc.noaa.gov/gosicas; accessed 30 January 2018) will contribute greatly to understanding species responses in cities and their relevance at the regional and ecosystem level. For example, increased evapotranspiration, such as in Madison, WI, USA [22], is influenced by soil moisture and urban warming, and may play a significant role in tree physiology [108–110], condition and herbivory [55,73]. Simultaneous measurement of climate variables and urban variables such as land cover, population density and impervious surface is crucial in addressing confounding factors associated with urban environments, such as air quality [65,66], water or soil pollution [111–114], soil compaction and invasive species [115–117].
Understanding the potential for acclimation or adaptive evolution by urban species (and the potential for non-adaptive genetic drift and gene flow) is also important in validating cities as surrogates for global change. Given concerns that many organisms will be unable to keep pace with environmental change via migration or adaptation [68,118–120], the responses of urban species that are unable to move beyond city limits or that experience delayed reproductive maturity—such as trees—may inform our understanding of the potential for natural populations to cope with global change and/or to experience non-adaptive processes [20,120]. Plastic and evolved responses to climate and urban variables have been demonstrated for individual species in cities (e.g. reviews in [18,20,68,121]), but the repeatability of such patterns among cities remains relatively untested (but see [23]), as does the degree to which plasticity facilitates or constrains evolution in cities, or whether genetic drift of gene flow may explain divergence in cities [121]. In addition to their direct effects on species responses to climate variables, trait changes at the organism level may have cascading effects on multi-trophic interactions and on critical ecosystem services. Identifying drivers of urban adaptation and understanding the short- relative to long-term role of plasticity in urban species responses are important steps in making generalizable predictions based on urban species responses and interactions.
4. Conclusion: making predictions from cities
In this review, we summarize insights gained from cities into how climate and urban variables influence species responses and interactions. We discuss phenological and physiological responses at population, community and latitudinal scales of interaction, we examine how insights gained from cities compare with experimental studies and historical data, and we consider future directions for urban research in terms of hypothesis testing and validating cities as effective surrogates for effects of global change. The overall goal of this review is to determine how and when urban species responses can be used to predict broader regional- and ecosystem-level patterns in the future, and to demonstrate that using cities as surrogates for global change can improve our geographic and taxonomic understanding of species responses and interactions.
Overall, the ability to study mature organisms that are ahead of the curve, experiencing the effects of multiple climate variables, and undergoing biotic and abiotic interactions in situ are significant advantages of urban research. These advantages will complement the understanding we have gained from field experiments, growth chamber studies and modelling efforts. While care must be taken to disentangle multiple driving variables and confounding effects in cities, difficulties also exist in experimental manipulation of climate variables, in the cost of manipulating multiple variables or species over long periods and in the geographic restriction of many experimental systems to temperate latitudes [122,123]. Addressing these caveats in cities will produce results that are more generalizable between individual study systems, cities and ecosystems. Thus, while studies using cities as surrogates for the effects of global change have been primarily limited to the effects of warming on plants or arthropods, cities potentially represent an accessible and cost-effective means to observe the interactions of diverse taxa in response to multiple interactive climate variables and to make use of complementary experimental and historical data to define testable hypotheses. Species responses and interactions in cities are currently an underused resource in making broader ecological predictions.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Michael Just, Elsa Youngsteadt and three anonymous reviewers for feedback on previous versions of this manuscript. This manuscript is submitted for publication with the understanding that the US Government is authorized to reproduce and distribute reprints for governmental purposes.
Data accessibility
This article has no additional data.
Authors' contributions
All authors wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project was supported by Cooperative Agreement no. G11AC20471, G13AC00405 and G15AP00153 from the United States Geological Survey. The North Carolina State University Department of Entomology also contributed support for this research. This study was also funded by an Agriculture and Food Research Initiative Competitive grant no. (2013-02476) and an ARPD grant no. (2016-70006-25827 from the USDA National Institute of Food and Agriculture to S.D.F.).
Disclaimer
The contents of this article are solely the responsibility of the authors and do not necessarily represent the views of the Department of the Interior Southeast Climate Science Center or the USGS.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division. 2014. World urbanization prospects: the 2014 revision, Highlights. ST/ESA/SER.A/352. New York, NY: UN DESA.
- 2.Oke TR. 1973. City size and the urban heat island. Atmos. Environ. 7, 769–779. ( 10.1016/0004-6981(73)90140-6) [DOI] [Google Scholar]
- 3.Idso CD, Idso SB, Balling RC. 2001. An intensive two-week study of an urban CO2 dome in Phoenix, Arizona, USA. Atmos. Environ. 35, 995–1000. ( 10.1016/S1352-2310(00)00412-X) [DOI] [Google Scholar]
- 4.Pataki DE, Xu Y, Luo Q, Ehleringer JR. 2007. Inferring biogenic and anthropogenic carbon dioxide sources across an urban to rural gradient. Oecologia 152, 307–322. ( 10.1007/s00442-006-0656-0) [DOI] [PubMed] [Google Scholar]
- 5.George K, Ziska LH, Bunce JA, Quebedeaux B. 2007. Elevated atmospheric CO2 concentration and temperature across an urban–rural transect. Atmos. Environ. 41, 7654–7665. ( 10.1016/j.atmosenv.2007.08.018) [DOI] [Google Scholar]
- 6.Gill SE, Handley JF, Ennos AR, Pauleit S. 2007. Adapting cities for climate change: the role of green infrastructure. Built Environ. 33, 115–133. ( 10.2148/benv.33.1.115) [DOI] [Google Scholar]
- 7.Roetzer T, Wittenzeller M, Haeckel H, Nekovar J. 2000. Phenology in central Europe—differences and trends of spring phenophases in urban and rural areas. Int. J. Biometerol. 44, 60–66. ( 10.1007/s004840000062) [DOI] [PubMed] [Google Scholar]
- 8.Cheptou PO, Carrue O, Rouifed S, Cantarel A. 2008. Rapid evolution of seed dispersal in an urban environment in the weed Crepsis sancta. Proc. Natl Acad. Sci. USA 105, 3796–3799. ( 10.1073/pnas.0708446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pretzsch H, et al. 2017. Climate change accelerates growth of urban trees in metropolises worldwide. Sci. Rep. 7, 15403 ( 10.1038/s41598-017-14831-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George K, Ziska LH, Bunce JA, Quebedeaux B, Hom JL, Wolf J, Teasdale JR. 2009. Macroclimate associated with urbanization increases the rate of secondary succession from fallow soil. Oecologia 159, 637–647. ( 10.1007/z00442-008-1238-0) [DOI] [PubMed] [Google Scholar]
- 11.Meineke EK, Dunn RR, Frank SD. 2014. Early pest development and loss of biological control are associated with urban warming. Biol. Lett. 10, 20140586 ( 10.1098/rsbl.2014.0586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youngsteadt EK, Dale AG, Terando AJ, Dunn RR, Frank SD. 2014. Do cities simulate climate change? A comparison of herbivore response to urban and global warming. Glob. Change Biol. 21, 97–105. ( 10.1111/gcb.12692) [DOI] [PubMed] [Google Scholar]
- 13.Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM. 2008. Global change and the ecology of cities. Science 319, 756–760. ( 10.1126/science.1150195) [DOI] [PubMed] [Google Scholar]
- 14.Ziska LH, Bunce JA, Goins EW. 2004. Characterization of an urban-rural CO2/temperature gradient and associated changes in initial plant productivity during secondary succession. Oecologia 139, 454–458. ( 10.1007/s00442-004-1526-2) [DOI] [PubMed] [Google Scholar]
- 15.Carreiro MM, Tripler CE. 2005. Forest remnants along urban-rural gradients: examining their potential for global change research. Ecosystems 8, 568–582. ( 10.1007/s10021-003-0172-6) [DOI] [Google Scholar]
- 16.Calfapietra C, Peñuelas J, Niinements Ü. 2015. Urban plant physiology: adaptation-mitigation strategies under permanent stress. Trends Plant Sci. 20, 72–76. ( 10.1016/j.tplants.2014.11.001) [DOI] [PubMed] [Google Scholar]
- 17.Diamond SE, Dunn RR, Frank SD, Haddad NM, Martin RA. 2015. Shared and unique responses of insects to the interaction of urbanization and background climate. Curr. Opin. Insect Sci. 11, 71–77. ( 10.1016/j.cois.2015.10.001) [DOI] [PubMed] [Google Scholar]
- 18.Donihue CM, Lambert MR. 2015. Adaptive evolution in urban ecosystems. Ambio 44, 194–203. ( 10.1007/s13280-014-0547-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell C, Szota C, Arndt SK. 2015. Urban plantings: ‘living laboratories’ for climate change response. Trends Plant Sci. 20, 597–599. ( 10.1016/j.tplants.2015.08.006) [DOI] [PubMed] [Google Scholar]
- 20.Johnson MTJ, Munshi-South J. 2017. Evolution of life in urban environments. Science 358, eaam8327 ( 10.1126/science.aam8327) [DOI] [PubMed] [Google Scholar]
- 21.Lahr EC, Schade GW, Crossett CC, Watson MR. 2015. Photosynthesis and isoprene emission from trees along an urban–rural gradient in Texas. Glob. Change Biol. 21, 4221–4236. ( 10.1111/gcb.13010) [DOI] [PubMed] [Google Scholar]
- 22.Zipper SC, Schatz J, Kucharik CJ, Loheide SP. 2017. Urban heat island-induced increases in evapotranspirative demand. Geophys. Res. Lett. 44, 873–881. ( 10.1002/2016GL072190) [DOI] [Google Scholar]
- 23.Thompson KA, Renaudin M, Johnson MT. 2016. Urbanization drives the evolution of parallel clines in plant populations. Proc. R. Soc. B 283, 20162180 ( 10.1098/rspb.2016.2180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamieson MA, Trowbridge AM, Raffa KR, Lindroth RL. 2012. Consequences of climate warming and altered precipitation patterns for plant–insect and multitrophic interactions. Plant Physiol. 160, 1719–1727. ( 10.1104/pp.112.206524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Youngsteadt EK, Ernst AF, Dunn RR, Frank SD. 2016. Responses of arthropod populations to warming depend on latitude: evidence from urban heat islands. Glob. Change Biol. 23, 1436–1447. ( 10.1111/gcb.13550) [DOI] [PubMed] [Google Scholar]
- 26.Baldock KCR, et al. 2015. Where is the UK's pollinator biodiversity? The importance of urban areas for flower-visiting insects. Proc. R. Soc. B 282, 20142849 ( 10.1098/rspb.2014.2849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis EB, Morgan BJT, Roy DB, Brereton TM. 2017. Urban indicators for UK butterflies. Ecol. Indic. 76, 184–193. ( 10.1016/j.ecolind.2017.01.009) [DOI] [Google Scholar]
- 28.Dale AG, Frank SD. 2014. The effects of urban warming on herbivore abundance and street tree condition. PLoS ONE 9, e102996 ( 10.1371/journal.pone.0102996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dale AG, Frank SD. 2014. Urban warming trumps natural enemy regulation of herbivorous pests. Ecol. Appl. 24, 1596–1607. ( 10.1890/13-1961.1) [DOI] [PubMed] [Google Scholar]
- 30.White MA, Nemani RR, Thornton PE, Running SW. 2002. Satellite evidence of phenological differences between urbanized and rural areas of the eastern United States deciduous broadleaf forest. Ecosystems 5, 260–277. ( 10.1007/s10021-001-1170-8) [DOI] [Google Scholar]
- 31.Zhang X, Friedl MA, Schaaf CB, Strahler AH. 2004. Climate controls on vegetation phenological patterns in norther mid- and high latitudes inferred from MODIS data. Glob. Chang. Biol. 10, 1133–1145. ( 10.1111/j.1365-2486.2004.00784x) [DOI] [Google Scholar]
- 32.Briber BM, Hutyra LR, Dunn AL, Raciti SM, Munger JW. 2013. Variations in atmospheric CO2 mixing ratios across a Boston. MA urban to rural gradient. Land 2, 304–327. ( 10.3390/land2030304) [DOI] [Google Scholar]
- 33.Zhou D, Zhao S, Zhang L, Liu S. 2016. Remotely sensed assessment of urbanization effects on vegetation phenology in China's 32 major cities. Remote Sens. Environ. 176, 272–281. ( 10.1016/j.rse.2016.02.010) [DOI] [Google Scholar]
- 34.Luo Z, Sun OJ, Ge Q, Xu W, Zheng J. 2006. Phenological responses of plants to climate change in an urban environment. Ecol. Res. 22, 507–514. ( 10.1007/s11284-006-0044-6) [DOI] [Google Scholar]
- 35.Comber A, Brunsdon C. 2015. A spatial analysis of plant phenophases and the impact of increases in urban land use. Int. J. Climatol. 35, 972–980. ( 10.1002/joc.4030) [DOI] [Google Scholar]
- 36.Diamond SE, Cayton H, Wepprich T, Jenkins CN, Dunn RR, Haddad NM, Ries L. 2014. Unexpected phenological responses of butterflies to the interaction of urbanization and geographic temperature. Ecology 95, 2613–2621. ( 10.1890/13-1848.1) [DOI] [Google Scholar]
- 37.Partecke J, Van't Hof TJ, Gwinner E. 2005. Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus mercula. J. Avian Biol. 36, 295–305. ( 10.1111/j.0908-8857.2005.03344.x) [DOI] [Google Scholar]
- 38.Møller AP, et al. 2015. Effects of urbanization on bird phenology: a continental study of paired urban and rural populations. Clim. Res. 66, 185–199. ( 10.3354/cr01344) [DOI] [Google Scholar]
- 39.Wolkovich EM, et al. 2012. Warming experiments underpredict plant phenological responses to climate change. Nature 485, 494–497. ( 10.1038/nature11014) [DOI] [PubMed] [Google Scholar]
- 40.Chung H, Muraoka H, Nakamura M, Han S, Muller O, Son Y. 2013. Experimental warming studies on tree species and forest ecosystems: a literature review. J. Plant Res. 126, 447–460. ( 10.1007/s10265-013-0565-3) [DOI] [PubMed] [Google Scholar]
- 41.Primack D, Imbres C, Primack RB, Miller-Rushing AJ, Del Tredici P. 2004. Herbarium specimens demonstrate earlier flowering times in response to warming in Boston. Am. J. Bot. 91, 1260–1264. ( 10.3732/ajb.91.8.1260) [DOI] [PubMed] [Google Scholar]
- 42.Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R. 2011. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc. Natl Acad. Sci. USA 51, 20 645–20 649. ( 10.1073/pnas.1115559108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lavoie C. 2013. Biological collections in an ever changing world: herbaria as tools for biogeographical and environmental studies. Perspect. Plant Ecol. Syst. 15, 68–76. ( 10.1016/j.ppees.2012.10.002) [DOI] [Google Scholar]
- 44.Meineke EK, Davis CC, Davies TJ. In press The unrealized potential of herbaria for global change biology. Ecol. Monogr. ( 10.1002/ecm.1307) [DOI] [Google Scholar]
- 45.Meineke EK, Dunn RR, Sexton JO, Frank SD. 2013. Urban warming drives insect pest abundance on street trees. PLoS ONE 8, e59687 ( 10.1371/journal.pone.0059687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans EW, Carlile NR, Innes MB, Pitigala N. 2013. Warm springs reduce parasitism of the cereal leaf beetle through phenological mismatch. J. Appl. Entomol. 137, 383–391. ( 10.1111/jen.12028). [DOI] [Google Scholar]
- 47.Forrest JRK. 2016. Complex responses of insect phenology to climate change. Curr. Opin. Insect Sci. 17, 49–54. ( 10.1016/j.cois.2016.07.002) [DOI] [PubMed] [Google Scholar]
- 48.Schwartzberg EG, Jamieson MA, Raffa FK, Reigh PB, Montgomery RA, Lindroth RL. 2014. Simulated climate warming alters phenological synchrony between an outbreak insect herbivore and host trees. Oecologia 175, 1041–1049. ( 10.1007/s00442-014-2960-4) [DOI] [PubMed] [Google Scholar]
- 49.Abarca M, Lill JT. 2015. Warming affects hatching time and early season survival of eastern tent caterpillars. Oecologia 179, 901–912. ( 10.1007/s00442-015-3371-x) [DOI] [PubMed] [Google Scholar]
- 50.DeLucia EH, Nabity PD, Zavala JA, Berenbaum MR. 2012. Climate change: resetting plant–insect interactions. Plant Physiol. 160, 1677–1685. ( 10.1104/pp.112.204750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Facey SL, Ellsworth DS, Staley JT, Wright DJ, Johnson SN. 2014. Upsetting the order: how climate and atmospheric change affects herbivore–enemy interactions. Curr. Opin. Insect Sci. 5, 66–74. ( 10.1016/j.cois.2014.09.015) [DOI] [PubMed] [Google Scholar]
- 52.Barton BT, Beckerman AP, Schmitz OJ. 2009. Climate warming strengthens indirect interactions in an old-field food web. Ecology 90, 2346–2351. ( 10.1890/08-2254.1) [DOI] [PubMed] [Google Scholar]
- 53.Rosenblatt AE, Smith-Ramesh LM, Schmitz OJ. 2016. Interactive effects of multiple climate change variables on food web dynamics: modeling the effects of changing temperature, CO2, and water availability on a tri-trophic food web. Food Webs 13, 98–108. ( 10.1016/j.fooweb.2016.10.002) [DOI] [Google Scholar]
- 54.Dale AG. 2015. The effects of urban habitats on the fitness and abundance of an herbivorous pest of street trees, and their subsequent effects on tree condition and ecosystem services. PhD thesis North Carolina State University, Raleigh, NC: See http://catalog.lib.ncsu.edu/record/NCSU3535816. [Google Scholar]
- 55.Dale AG, Frank SD. 2017. Warming and drought combine to increase pest insect fitness on urban trees. PLoS ONE 12, 0173844 ( 10.1371/journal.pone.0173844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sendall KM, Reich PB, Zhao C, Jihua H, Wei X, Stefanski A, Rice K, Rich RL, Montgomery RA. 2015. Acclimation of photosynthetic temperature optima of temperate and boreal tree species in response to experimental forest warming. Glob. Change Biol. 21, 1342–1257. ( 10.1111/gcb.12781) [DOI] [PubMed] [Google Scholar]
- 57.Gunderson CA, Norby RJ, Wullschleger SD. 2000. Acclimation of photosynthesis and respiration to simulated climatic warming in northern and southern populations of Acer saccharum: laboratory and field evidence. Tree Physiol. 20, 87–96. ( 10.1093/treephys/20.2.87) [DOI] [PubMed] [Google Scholar]
- 58.Lewis JD, Lucash M, Olszyk D, Tingey DT. 2001. Seasonal patterns of photosynthesis in Douglas fir seedlings during the third and fourth year of exposure to elevated CO2 and temperature. Plant Cell Environ. 24, 539–548. ( 10.1046/j.1365-3040.2001.00700.x) [DOI] [Google Scholar]
- 59.Hikosaka K, Nabeshima E, Hiura T. 2007. Seasonal changes in the temperature response of photosynthesis in canopy leaves of Quercus crispula in a cool-temperate forest. Tree Physiol. 27, 1035–1041. ( 10.1093/treephys/27.7.1035). [DOI] [PubMed] [Google Scholar]
- 60.Gunderson CA, O'Hara KH, Campion CM, Walker AV, Edwards NT. 2010. Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Glob. Change Biol. 16, 2272–2286. ( 10.1111/j.1365-2486.2009.02090.x) [DOI] [Google Scholar]
- 61.Crous KY, Quentin AG, Lin Y-S, Medlyn BE, Williams DG, Barton CVM, Ellsworth DS. 2013. Photosynthesis of temperate Eucalyptus globulus trees outside their native range has limited adjustment to elevated CO2 and climate warming. Glob. Chang. Biol. 19, 3790–3807. ( 10.1111/gcb.12314) [DOI] [PubMed] [Google Scholar]
- 62.Krause GH, Cheesman AW, Winter K, Krause B, Virgo A. 2013. Thermal tolerance, net CO2 exchange and growth of a tropical tree species Ficus insipida, cultivated at elevated daytime and nighttime temperatures. J. Plant Physiol. 170, 822–827. ( 10.1016/j.jplph.2013.01.005) [DOI] [PubMed] [Google Scholar]
- 63.Meineke EK, Youngsteadt EK, Dunn RR, Frank SD. 2016. Urban warming reduces aboveground carbon storage. Proc. R. Soc. B 283, 20161574 ( 10.1098/rspb.2016.1574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Searle SY, Turnbull MH, Boelman NT, Schuster WSF, Yakir D, Griffin KL. 2012. Urban environment of New York City promotes growth in northern red oak seedlings. Tree Physiol. 32, 389–400. ( 10.1093/treephys/tps027) [DOI] [PubMed] [Google Scholar]
- 65.Gregg JW, Jones CG, Dawson TE. 2003. Urbanization effects on tree growth in the vicinity of New York City. Nature 424, 183–187. ( 10.1038/nature01728) [DOI] [PubMed] [Google Scholar]
- 66.Paoletti E, De Marco A, Beddows DCS, Harrison RM, Manning WJ. 2014. Ozone levels in European and USA cities are increasing more than at rural sites, while peak values are decreasing. Environ. Pollut. 192, 295–299. ( 10.1016/j.envpol.2014.04.040) [DOI] [PubMed] [Google Scholar]
- 67.Peng S, Huang J, Sheehy JE, Laza RC, Visperas RM, Zhong X, Centeno GS, Kush GS, Cassman KG. 2004. Rice yields decline with higher night temperature from global warming. Proc. Natl Acad. Sci. USA 101, 9971–9975. ( 10.1073/pnas.0403720101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diamond SE, Martin RA. 2016. The interplay between plasticity and evolution in response to human-induced environmental change. F1000 Res. 5, 2835 ( 10.12688/f1000research.9731.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lambrecht SC, Mahieu S, Cheptou P. 2016. Natural selection on plant physiological traits in an urban environment. Acta Oecol. 77, 67–74. ( 10.1016/j.actao.2016.09.002) [DOI] [Google Scholar]
- 70.Stocker TF, et al. 2013. Technical summary. In Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker TF. et al. ). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 71.Angilletta MJ, Wilson RS, Niehaus AC, Sears MC, Navas CA, Ribeiro PL. 2007. Urban physiology: urban ants possess higher heat tolerance. PLoS ONE 2, e258 ( 10.1371/journal.pone.0000258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McLean MA, Angilletta MJ, Williams KS. 2005. If you can't stand the heat, stay out of the city: thermal reaction norms of chitinolytic fungi in an urban heat island. Therm. Biol. 30, 384–391. ( 10.1016/j.jtherbio.2005.03.002) [DOI] [Google Scholar]
- 73.Meineke EK, Frank SD. 2018. Water availability drives tree growth responses to herbivory and warming. J. Appl. Ecol. 55, 1701–1713. ( 10.1111/1365-2664.13130) [DOI] [Google Scholar]
- 74.Baur B, Baur A. 1993. Climatic warming due to thermal radiation from an urban area as a possible cause for the local extinction of a land snail. J. Appl. Ecol. 30, 333–340. ( 10.2307/2404635) [DOI] [Google Scholar]
- 75.Hamblin AL, Youngstead E, Lopez-Uribe MM, Frank SD. 2017. Physiological thermal limits predict differential responses of bees to urban heat island effects. Biol. Lett. 13, 20170125 ( 10.1098/rsbl.2017.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamblin AL, Youngsteadt E, Frank SD. 2018. Wild bee abundance declines with urban warming, regardless of floral density. Urban Ecosyst. 21, 419–428. ( 10.1007/s11252-018-0731-4) [DOI] [Google Scholar]
- 77.Youngstead EK, Appler RH, Lopez-Uribe MM, Tarpy DR, Frank SD. 2015. Urbanization increases pressure on feral and managed honey bees. PLoS ONE 10, e0142031 ( 10.1371/journal.pone.0142031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pincebourde S, Murdock CC, Vickers M, Sears MW. 2016. Fine-scale microclimate variation can shape the responses of organisms to global change in both natural and urban environments. Integr. Comp. Biol. 56, 45–61. ( 10.1093/icb/icw016) [DOI] [PubMed] [Google Scholar]
- 79.Bale JS, et al. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob. Change Biol. 8, 1–16. ( 10.1046/j.1365-2486.2002.00451.x) [DOI] [Google Scholar]
- 80.Meineke EK, Holmquist AJ, Wimp GM, Frank SD. 2017. Changes in spider community composition are associated with urban temperature, not herbivore abundance. J. Urb. Ecol. 3, 1–8. ( 10.1093/jue/juw010) [DOI] [Google Scholar]
- 81.Turrini T, Sanders D, Knop E. 2016. Effects of urbanization on direct and indirect interactions in a tri-trophic system. Ecol. Appl. 26, 664–675. ( 10.1890/14-1787) [DOI] [PubMed] [Google Scholar]
- 82.Lu X, Siemann E, He M, Wei H, Shao X, Ding J. 2016. Warming benefits a native species competing with an invasive congener in the presence of a biocontrol beetle. New Phytol. 211, 1371–1381. ( 10.1111/nph.13976) [DOI] [PubMed] [Google Scholar]
- 83.Scherber C, et al. 2013. Multi-factor climate change effects on insect herbivore performance. Ecol. Evol. 3, 1449–1460. ( 10.1002/ece3.564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoover SER, Ladley JJ, Shchepetkina AA, Tisch M, Gieseg SP, Tylianakis JM. 2012. Warming, CO2, and nitrogen deposition interactively affect a plant–pollinator mutualism. Ecol. Lett. 15, 227–234. ( 10.1111/j.1461-0248.2011.01729.x) [DOI] [PubMed] [Google Scholar]
- 85.Zvereva EL, Kozlov MV. 2006. Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: a metaanalysis. Glob. Change Biol. 12, 27–41. ( 10.1111/j.1365-2486.2005.01086.x) [DOI] [Google Scholar]
- 86.Rosenblatt AE, Schmitz OJ. 2014. Interactive effects of multiple climate change variables on trophic interactions: a meta-analysis. Clim. Change Res. 1 ( 10.1186/s40665-014-0008-y) [DOI] [Google Scholar]
- 87.Parmesan C. 2006. Ecological and evolutionary responses to climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 88.Parmesan C. 2007. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 13, 1860–1872. ( 10.1111/j.1365-2486.2007.01404.x) [DOI] [Google Scholar]
- 89.Menzel A, Sparks TH, Estrella N, Roy DB. 2006. Altered geographic and temporal variability in phenology in response to climate change. Glob. Ecol. Biogeogr. 15, 498–504. ( 10.1111/j.1466-822X.2006.00247.x) [DOI] [Google Scholar]
- 90.Jochner S, Sparks TH, Laube J, Menzel A. 2016. Can we detect nonlinear responses to temperature in European plant phenology? Int. J. Biometerol. 60, 1551–1561. ( 10.1007/s0048) [DOI] [PubMed] [Google Scholar]
- 91.Kooyers NJ, Olsen KM. 2012. Rapid evolution of an adaptive cyanogenesis cline in introduced North American white clover (Trifolium repens L.). Mol. Ecol. 21, 2455–4268. ( 10.1111/j.1365-294X.2012.05486.x) [DOI] [PubMed] [Google Scholar]
- 92.Kooyers NJ, Olsen KM. 2013. Searching for the bull's eye: agents and targets of selection vary among geographically disparate cyanogenesis clines in white clover (Trifolium repens L.). Heredity 111, 495–504. ( 10.1038/hdy.2013.71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams DS, Liebhold AM. 1995. Forest defoliators and climate change: potential changes in spatial distribution of outbreaks of western spruce budworm (Lepidoptera: Tortricidae) and gypsy moth (Lepidoptera: Lymantriidae). Environ. Entomol. 24, 1–9. ( 10.1093/ee/24.1.1) [DOI] [Google Scholar]
- 94.Roques A, et al. et al. 2015. Climate warming and past and present distribution of the processionary moths (Thaumentopoea spp.) in Europe, Asia Minor, and North Africa. In Processionary Moths: an Update (ed. Roques A.), pp. 81–161. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 95.Winchell KM, Reynolds RG, Prado-Irwin SR, Puente-Rolón AR, Revell LJ. 2016. Phenotypic shifts in urban areas in the tropical lizard Anolis cristatellus. Evolution 70, 1009–1022. ( 10.1111/evo.12925) [DOI] [PubMed] [Google Scholar]
- 96.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 97.Bálint M, Domisch S, Engelhardt CHM, Haase P, Lehrian S, Sauer J, Theissinger K, Pauls SU, Nowak C. 2011. Cryptic biodiversity loss linked to global climate change. Nat. Clim. Change 1, 313–318. ( 10.1038/NCLIMATE1191) [DOI] [Google Scholar]
- 98.Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, Sievers C, Magurran AE. 2014. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299. ( 10.1126/science.1248484) [DOI] [PubMed] [Google Scholar]
- 99.Menke SB, Guénard B, Sexton JO, Weiser MD, Dunn RR, Silverman J. 2011. Urban areas may serves as habitat and corridors for dry-adapted, heat tolerant species: an example from ants. Urban Ecosyst. 14, 135–163. ( 10.1007/s11252-010-0150-7) [DOI] [Google Scholar]
- 100.Kaiser A, Merckx T, VanDyck H. 2016. The urban heat island and its spatial scale dependent impact on survival and development in butterflies of different thermal sensitivity. Ecol. Evol. 6, 4129–4140. ( 10.1002/ece3.2166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lowe EC, Wilder SM, Hochuli DF. 2014. Urbanization at multiple scales is associated with larger size and higher fecundity of an orb-weaving spider. PLoS ONE 9, e105480 ( 10.1371/journal.pone.0105480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tiatragul S, Kurniawan A, Kolbe JJ, Warner DA. 2017. Embroys of non-native anoles are robust to urban thermal environments. J. Therm. Biol. 65, 119–124. ( 10.1016/j.jtherbio.2017.02.021) [DOI] [PubMed] [Google Scholar]
- 103.Wikelski W, Tarlow EM, Raim A, Diehl RH, Larkin RP, Visser GH. 2003. Avian metabolism: costs of migration in free-flying songbirds. Nature 423, 704 ( 10.1038/423704a) [DOI] [PubMed] [Google Scholar]
- 104.Van der Veken S, Hermy M, Velland M, Knapen A, Verheyen K. 2008. Garden plants get a head start on climate change. Front. Ecol. Environ. 6, 212–216. ( 10.1890/070063) [DOI] [Google Scholar]
- 105.Krishtalka L, Humpfrey PS. 2000. Can natural history museums capture the future? BioScience 50, 611–617. ( 10.1641/0006-3568(2000)050%5B0611:CNHMCT%5D2.0.CO;2) [DOI] [Google Scholar]
- 106.Pyke GH, Erlich PR. 2010. Biological collections and ecological/environmental research: a review, some observations and a look to the future. Biol. Rev. 85, 247–266. ( 10.1111/j.1469-185X.2009.00098.x) [DOI] [PubMed] [Google Scholar]
- 107.Johnson KG, et al. 2011. Climate change and biosphere responses: unlocking the collections vault. BioScience 61, 147–153. ( 10.1525/bio.2011.61.2.10) [DOI] [Google Scholar]
- 108.Pataki DE, McCarthy HR, Litvak E, Pincetl S. 2011. Transpiration of urban forests in the Los Angeles metropolitan area. Ecol. Appl. 21, 661–677. ( 10.1890/09-1717.1) [DOI] [PubMed] [Google Scholar]
- 109.Chen L, Zhang Z, Li Z, Tang J, Caldwell P, Zhang W. 2011. Biophysical control of whole tree transpiration under an urban environment in Northern China. J. Hydrol. 402, 388–400. ( 10.1016/j.jhydrol.2011.03.034) [DOI] [Google Scholar]
- 110.Savi T, Bertuzzi S, Branca A, Tretiach M, Nardini A. 2015. Drought-induced xylem cavitation and hydraulic deterioration: risk factors for urban trees under climate change? New Phytol. 205, 1106–1116. ( 10.1111/nph.13112) [DOI] [PubMed] [Google Scholar]
- 111.White CS, McDonnell MJ. 1988. Nitrogen cycling processes and soil characteristics in an urban versus rural forest. Biogeochemistry 5, 243–262. ( 10.1007/BF02180230) [DOI] [Google Scholar]
- 112.Pouyat RV, McDonnell MJ. 1991. Heavy metal accumulations in forest soils along an urban–rural gradient in southeastern New York, USA. Water Air Soil Pollut. 57–58, 797–807. ( 10.1007/BF00282943) [DOI] [Google Scholar]
- 113.Gardiner MM, Harwood JD. 2017. Influence of heavy metal contamination on urban natural enemies and biological control. Curr. Opin. Insect Sci. 20, 45–53. ( 10.1016/j.cois.2017.03.007) [DOI] [PubMed] [Google Scholar]
- 114.Sivakoff FS, Gardiner MM. 2017. Soil lead contamination decreases bee visit duration at sunflowers. Urban Ecosyst. 20, 1221–1228. ( 10.1007/s11252-017-0674-1) [DOI] [Google Scholar]
- 115.Steinberg DA, Pouyat RV, Parmlee RW, Groffman PM. 1997. Earthworm abundance and nitrogen mineralization rates along an urban–rural land use gradient. Soil Biol. Biochem. 29, 427–430. ( 10.1016/S0038-0717(96)00043-0) [DOI] [Google Scholar]
- 116.Pouyat RV, McDonnell MJ, Pickett STA. 1997. Litter decomposition and nitrogen mineralization in oak stands along an urban–rural land use gradient. Urban Ecosyst. 1, 117–131. ( 10.1023/A:1018567326093) [DOI] [Google Scholar]
- 117.McDonnell MJ, Pickett STA, Groffman P, Bohlen P, Pouyat RV, Zipperer WC, Parmlee RW, Carreiro MM, Medley K. 1997. Ecosystem processes along an urban–rural gradient. Urban Ecosyst. 1, 21–36. ( 10.1023/A:1014359024275) [DOI] [Google Scholar]
- 118.Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S. 2008. Adaptation, migration, or extirpation: climate change outcomes for tree populations. Evol. Appl. 1, 95–111. ( 10.1111/j.1752-4571.2007.00013.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. 2012. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377. ( 10.1111/j.1461-0248.2011.01736.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Franks SJ, Weber JJ, Aitken SN. 2013. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol. Appl. 7, 123–139. ( 10.1111/eva.12112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Munshi-South J, Zolnik CP, Harris SE. 2016. Population genomics of the Anthropocene: urbanization is negatively associated with genome-wide variation in white-footed mouse populations. Evol. Appl. 9, 546–564. ( 10.1111/eva.12357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dieleman WIJ, et al. 2012. Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob. Change Biol. 18, 2681–2693. ( 10.1111/j.1365-2486.2012.02745.x) [DOI] [PubMed] [Google Scholar]
- 123.Leuzinger S, Luo Y, Beier C, Dieleman W, Vicca S, Ch K. 2011. Do global change experiments overestimate impacts on terrestrial ecosystems? Trends Ecol. Evol. 26, 236–241. ( 10.1016/j.tree.2011.02.011) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.