Abstract
Among over 30 000 species of ray-finned fishes, seahorses and pipefishes have a unique feeding mechanism whereby the elastic recoil of tendons allows them to rotate their long snouts extremely rapidly in order to capture small elusive prey. To understand the evolutionary origins of this feeding mechanism, its phylogenetic distribution among closely related lineages must be assessed. We present evidence for elastic recoil-powered feeding in snipefish (Macroramphosus scolopax) from kinematics, dynamics and morphology. High-speed videos of strikes show they achieve extremely fast head and hyoid rotational velocities, resulting in rapid prey capture in as short a duration as 2 ms. The maximum instantaneous muscle-mass-specific power requirement for head rotation in snipefish was above the known vertebrate maximum, which is evidence that strikes are not the result of direct muscle power. Finally, we show that the over-centre conformation of the four-bar linkage mechanism coupling head elevation to hyoid rotation in snipefish can function as a torque reversal latch, preventing the head from rotating and providing the opportunity for elastic energy storage. The presence of elastic recoil feeding in snipefish means that this high-performance mechanism is not restricted to the Syngnathidae (seahorses and pipefish) and may have evolved in parallel.
Keywords: over-centre latch, torque reversal, Syngnathiformes, suction feeding, trigger mechanism
1. Introduction
Power, or work performed in a given amount of time, is bounded in both biological and engineered systems by a fundamental trade-off between force and velocity. To increase the power output of a mechanism with a given muscle or motor, energy can be temporarily stored in an elastic structure, then rapidly released, thus decoupling the rate of energy delivery from the rate of energy generation [1]. Such elastic recoil mechanisms have evolved across the tree of life and allow organisms to increase performance during a diverse array of behaviours directly related to survival and fitness, such as prey capture, defence, reproduction and escape from predators [1–3].
Biological elastic recoil mechanisms are composed of a similar suite of features: an ‘engine’ to produce energy, a ‘spring’ or deformable structure to store energy and a ‘tool’ to which that stored energy is imparted (e.g. jaws, tongue, smashing appendage). Many also incorporate a ‘latch’ that keeps energy stored before the strike [4]. Latches can be physical structures that block motion until they are removed, or they can be so-called ‘geometric latches’ that prevent or delay motion while a series of linkages are in a particular conformation. From an evolutionary standpoint, it is unknown how these suites of structures and functions become aligned, although research on a number of clades indicates that the evolutionary history of these specialized mechanisms varies in complexity. For instance, there appear to have been multiple independent origins of elastic recoil mechanisms within specific clades, including ballistic tongue protrusion within plethodontid salamanders [5,6], trap-jaw mandible closing within clades of ants [7] and spiders [8], and snapping claws within caridean shrimp [9]. However, in mantis shrimp, power-amplified rapid raptorial strikes are widespread across lineages, presumably as the result of a single origin at the base of this clade [10]. Circumscribing the extent of amplified power output in the relatives of lineages with known elastic recoil mechanisms will yield important insights into how such complex functional systems are assembled during evolution and the selective pressures under which they arise.
Seahorses and pipefish, the family Syngnathidae, have been recently shown to have extremely rapid head rotation and hyoid depression during feeding strikes due to elastic recoil [11,12]. Seahorses and pipefishes belong to a larger clade, Syngnathiformes, which are characterized by elongated snouts. Syngnathiforms use their snout in an unusual form of prey capture, called pivot feeding, in which rapid dorsal rotation of the entire head and snout brings the small mouth very close to prey, which are then captured via suction [13–15]. In seahorses and pipefish, this rapid dorsal head rotation is powered by elastic recoil of large tendons in the epaxial muscles, resulting in strikes that are much faster than if the muscles directly powered head rotation [11,12,16]. While pivot feeding has been noted in all syngnathiforms studied to date and has been shown to be an important feature of the syngnathiform prey capture strategy [17], power amplification by elastic recoil has not been explicitly tested in live fish apart from seahorses and pipefish. A more detailed understanding of the distribution of enhanced power outputs among syngnathiform lineages and morphological features associated with an elastic recoil mechanism will help us understand the evolution of this mechanism and the evolution of complex, high-performance functional morphologies in general.
In this study, we ask whether there is evidence for an elastic recoil mechanism powering head rotation in the feeding strikes of snipefish (Macroramphosus scolopax). Snipefish are in the family Centriscidae and are among the earliest branching lineages of Syngnathiformes [18], and therefore they can provide information about the presence or absence of this trait deep in the syngnathiform clade. Previous work on snipefish has noted fast times to prey capture and morphological features indicating that their strikes could be powered by elastic recoil [19–22]. Snipefish appear to possess a ‘trigger mechanism’ formed by the arrangement of a four-bar linkage coupling head elevation to hyoid depression, which could allow the linkage to become locked and prestressed by contraction of the trunk musculature [20–22]. We find evidence for elastic recoil during feeding in snipefish using multiple approaches: (1) analysis of feeding kinematics, (2) estimation of the muscle-mass-specific power requirement of head rotation, (3) description of snipefish functional morphology from micro-CT scans and (4) quantitative re-evaluation of the proposed four-bar linkage mechanism using morphology and kinematics. Finally, we discuss the implications of these findings for the evolution of elastic recoil-actuated head rotation in the Syngnathiformes.
2. Material and methods
(a). Feeding kinematics
Six snipefish (Macroramphosus scolopax) were filmed feeding on live zebrafish at 2000 frames s−1. At least 10 high-quality videos were collected for each individual. Then we selected the five strikes for each individual with the shortest time to prey capture (time between onset of hyoid depression and prey capture) to focus on high-performance strikes. We digitized nine landmarks on each frame of the video (electronic supplementary material, figure S1) to calculate displacements, angles, timings and velocities of the mouth, head and hyoid (see the electronic supplementary material, supplementary methods).
(b). Power requirement
To estimate the muscle power required for dorsal head rotation during feeding in snipefish, we replicated the approach used for pipefish by Van Wassenbergh et al. [11]. Briefly, an inverse dynamic model was used to estimate the peak instantaneous muscle-mass-specific power requirement of head rotation, including calculation of the power for dorsal head rotation and for body recoil during head rotation. The head and body were each modelled as a series of 20 elliptical cylinders using morphological measurements taken from lateral and dorsal photographs of euthanized specimens. Motions of the head and body were parametrized with kinematics from the videos, including the calculation of the centre of rotation of the head relative to the earthbound frame of reference. Countermovement of the body was modelled as a decreasing wave travelling down the body from the back of the head to a stationary point on the body. The maximum instantaneous power required for the rotation of the head and body recoil was divided by the mass of the muscles involved in powering head rotation and hyoid depression (sternohyoideus muscle and the anterior portions of the epaxial and hypaxial muscles; electronic supplementary material, figure S2). Details about the kinematics and adaptation of the model to snipefish can be found in the electronic supplementary material.
(c). Four-bar linkage analysis
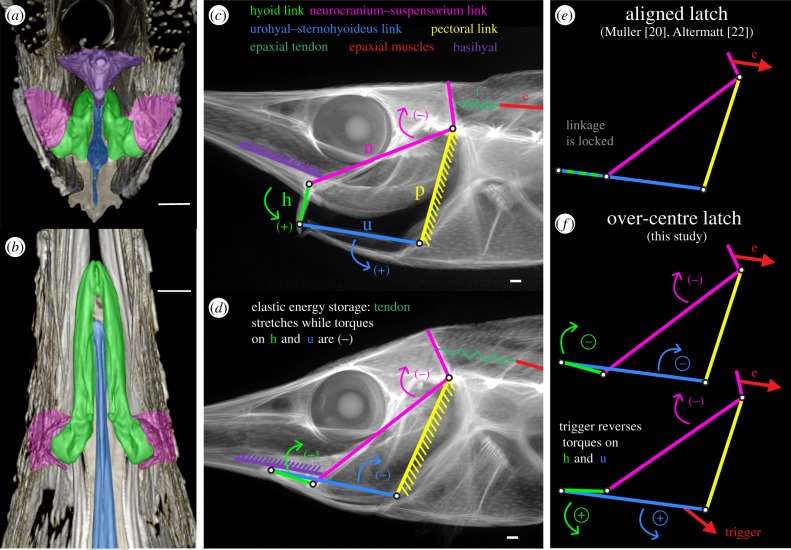
Preliminary observations of snipefish morphology and manipulation of fresh specimens indicated that the four-bar linkage proposed by Muller [20] and Altermatt [21,22] to couple head elevation to hyoid motion was appropriate: a suspensorium–neurocranium link (n), which runs from the joint of the interhyal with the suspensorium to the occipital–vertebral joint; a pectoral girdle link (p), which runs from the occipital–vertebral joint to the origin of the sternohyoideus muscle on the pectoral girdle (as inferred from the orientation of the urohyal); a link composed of the urohyal bone and sternohyoideus muscle (u), which runs from the origin of this muscle on the pectoral girdle to the insertion on the distal tip of the hyoid apparatus; and a hyoid link (h), composed of the hyoid apparatus including the ceratohyal, epihyal and interhyal bones.
We used micro-CT and digital radiography to describe morphology and measure relative lengths and angles of the proposed four-bar linkage mechanism. We also validated the four-bar linkage mechanism by digitizing the locations of all four rotation points in videos and testing whether the model allows accurate predictions of movement. Further details are provided in the electronic supplementary material.
3. Results
(a). Kinematics
Snipefish feeding strikes were similar to those described for other pivot-feeding syngnathiform fishes [11,12,14,15,23]. The kinematic profile for a typical strike can be seen in figure 1 (see also electronic supplementary material, figure S3). Snipefish readily approached prey, but slowed before performing a strike. During the strike, the entire head and snout was rapidly rotated dorsally, bringing the mouth extremely close to the prey, which was captured via rapid suction. Time to prey capture ranged from 2.0 to 7.5 ms across the 30 videos we digitized (electronic supplementary material, table S1). Snipefish approach prey with an open mouth, and change in gape during the strike was extremely small (mean 1.3 mm ± 0.3 mm). Hyoid movement was rapid and began relatively early. On average, hyoid onset was 1.0 ms (±0.6) after gape onset and 0.5 ms (±0.2) before the onset of head rotation. Onset of gape and head rotation began on the same frame as the hyoid in some strikes, but head rotation was never observed before hyoid onset. In general, peak gape occurred before prey capture, while peak hyoid rotation and head rotation were reached after prey capture. Average hyoid rotation was 114° (±5.6°) and reached 30 200° s−1 (±5300° s−1). Average head rotation relative to the body was 20° (±1.9°) and reached 6 090° s−1 (±890° s−1). For further kinematic details see electronic supplementary material, table S1.
Figure 1.
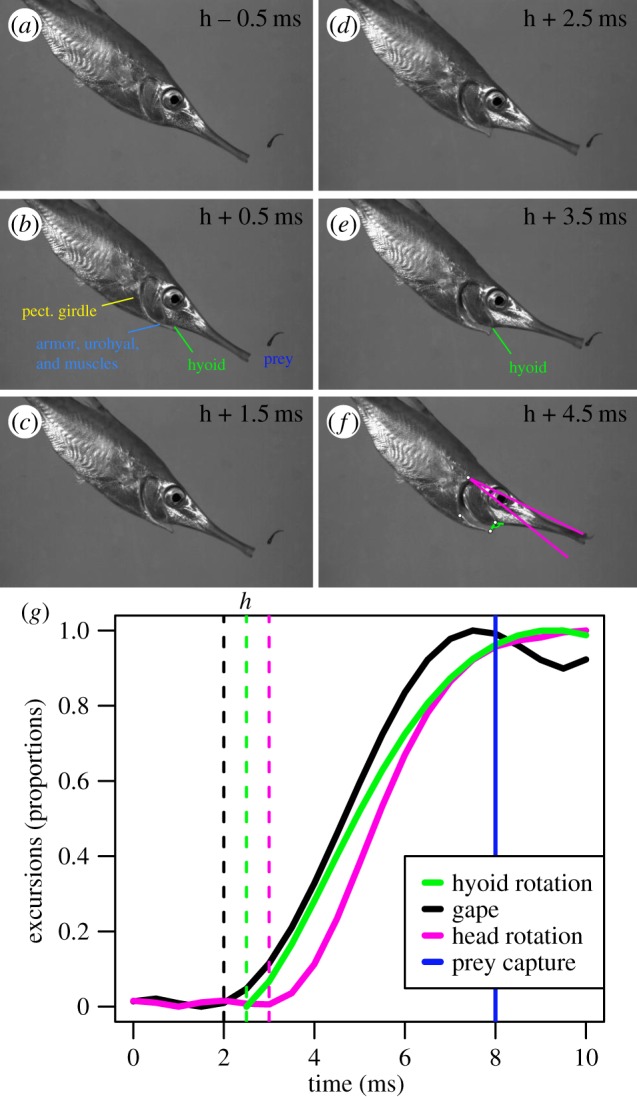
A representative snipefish strike. (a–f) Select frames from a feeding strike, beginning one frame before hyoid onset (time h in g) and ending at prey capture. Frame (f) illustrates total head rotation (pink angle) and hyoid rotation (green angle). Dots correspond to linkage joints similar to figure 3c. (g) Kinematic profile for a different strike, showing excursions relative to their maximum values (for magnitudes, see electronic supplementary material, figure S3). Dashed lines indicate onset times and the solid blue line is prey capture.
(b). Power calculations
Peak instantaneous muscle-mass-specific power requirement was reached soon after the onset of hyoid rotation, between 2.0 and 4.0 ms across all strikes (electronic supplementary material, table S2). The mean peak instantaneous power requirement for head rotation (including body recoil) was 2800 W kg−1 (±560), and the range across all strikes was between 1090 and 5500 W kg−1 (figure 2). Power required to rotate the head (as opposed to body recoil) was the main contribution to the total power requirement (electronic supplementary material, figure S4), and power required to overcome inertial forces on the head was the greatest proportion of power to rotate the head at the time of maximum power output.
Figure 2.
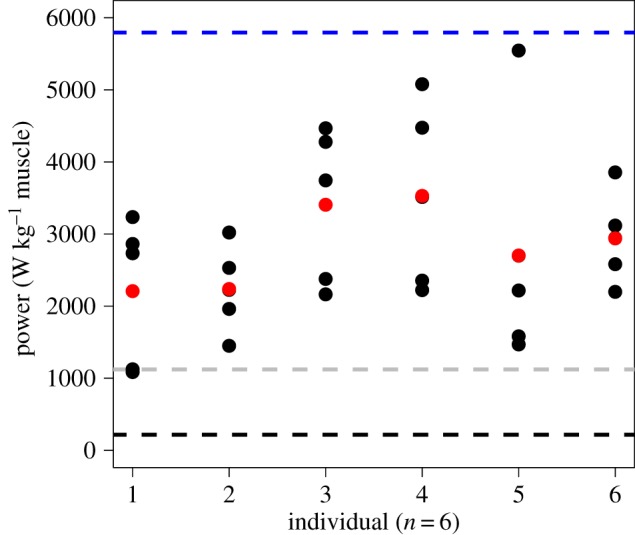
Peak instantaneous muscle-mass-specific requirements for each strike in our dataset by individual (means in red). Most values are above the in vitro peak instantaneous muscle power output recorded for a vertebrate (middle grey-dashed line) [24] and well above that for fish (bottom black-dashed line) [25]. This is evidence for elastic recoil, because no vertebrate muscle is known to possess such high power output. For comparison, the top blue dashed line indicates the pipefish peak instantaneous power requirement [11].
(c). Morphology
The morphology of snipefish has been previously described [21]. Here, we briefly mention a few key features relevant to our study. The anterior region of the vertebral column is highly reinforced. Anterior vertebrae are elongate and fused together and make connections with the dermal armour, encasing the anterior epaxial musculature. Two large epaxial tendons insert on the back of the skull, running between the dorsal and ventral epaxial muscle bundles. These tendons are not ossified (electronic supplementary material, figure S2). The pectoral girdle appears to be immobile relative to the vertebral column, being fused in part to the ventral armour and plates encasing the epaxial muscles. The head rotates upon a hinge created by the occipital–vertebral joint and laterally with the supracleithrum. Although the pectoral girdle (cleithrum) does attach directly to the body wall laterally, it separates from the body wall as it tapers towards the ventral midline. Thin extensions of the hypaxial muscles run through this gap; they appear to insert on the urohyal more ventrally than the sternohyoideus. As in seahorses and pipefishes, the interhyal is not of the typical rod shape observed in other ray-finned fishes, but is instead reduced to a knob and rotates with the hyoid bar in a socket-like joint on the inside of the preoperculum [14,21,22,26]. Movement of the hyoids when adducted is extremely restricted, each hyoid being nestled between the basihyal dorsally, the urohyal ventrally and medially, and the preoperculum laterally (figure 3a,b).
Figure 3.
The snipefish elastic recoil mechanism. (a) Posterior view of the hyoid region from a cross section made just posterior to the hyoid–preopercular joint. Each hyoid (green) fits into a joint with the preoperculum (pink highlight) and is further bounded by the basihyal (purple) and urohyal (blue). Ventral armour is coloured tan for contrast. (b) Dorsal view of the hyoid region from a transverse section (the basihyal has been digitally removed for visibility). The hyoid is adducted in A and B. (c) Digital radiograph with the hyoid depressed in the orientation seen near the end of the strike. The four-bar linkage coupling head elevation and hyoid depression is overlaid. (d) Four-bar linkage when the hyoid is adducted. (e) Muller's hypothesis was that the hyoid and urohyal–sternohyoideus linkages are aligned and lock the system in place, compared with our finding (f) that the linkages are partially overlapped. Note how the circled torques for the hyoid and urohyal–sternohyoideus linkages change sign depending on their arrangement. (+) and (−) refer to the direction of torque on specific linkages. The trigger arrow in (f) is a schematic of the proposed hypaxial trigger. Pink, neurocranium–suspensorium linkage (n); yellow, pectoral linkage (p); blue, urohyal–sternohyoideus linkage (u); lime green, hyoid linkage (h); green, epaxial tendon (t); red, epaxial muscle (e); purple, basihyal. Scale bar, 1 mm.
(d). Four-bar linkage mechanism
Manipulation of freshly euthanized specimens supported the existence of a four-bar linkage mechanism coupling head elevation and hyoid depression, as has been proposed for snipefish [20–22]. The head could not be rotated dorsally while the suspensorium and hyoid apparatus was adducted. Instead, upward force applied to the head or snout was observed to more firmly adduct the suspensorium and hyoid. The planar arrangement of the four-bar linkage mechanism when the hyoid was adducted in micro-CT scans, and digital X-rays (figure 3c,d) revealed that the linkage is folded, such that the hyoid (h, green) sits just ventral to the urohyal–sternohyoideus (u, blue), which in turn overlaps the neurocranium–suspensorium (pink) linkage. This over-centre conformation deviates from the arrangement shown previously by Altermatt [21,22] and Muller [20], in which these linkages were perfectly aligned (figure 3e,f).
The laterally compressed craniofacial morphology of snipefish and measurements from kinematics support the interpretation of the four-bar linkage as a planar mechanism at and near the time of hyoid rotation onset; link lengths do not significantly change during the first few frames after hyoid rotation. Relative lengths of links measured from videos satisfied the inequality h + n < p + u (Grashoff's Criterion) for all individuals in all frames (not shown), where h and n are the shortest and longest links, respectively. The pectoral girdle (p) is anatomically the grounded link; it is largely immobilized relative to the vertebral column. This indicates that the four-bar linkage mechanism in snipefish is bistable and capable of reversing torque depending on the arrangement of the linkages [27,28].
We also validated the mechanical model using kinematics digitized from strikes. Given the head angle and the initial linkage lengths, we were able to accurately predict the hyoid angle in most frames (electronic supplementary material, figure S5). Occasionally, predicted and measured output angles differed (Holm–Bonferroni-corrected p-values < 0.05, asterisks in electronic supplementary material, figure S5), but there was not a consistent pattern across individuals. The average predicted output angle tended to be greater than the average observed angle (electronic supplementary material, figure S5) early in the sequence, but less than the average observed angle later in the sequence. These small but consistent deviations may result from an inability to perfectly digitize the intersection of the sternohyoideus on the pectoral girdle or from changes in length of this muscle.
4. Discussion
Multiple forms of evidence indicate that the feeding mechanism of snipefish is powered by elastic recoil. Kinematics and calculations of muscular power requirements show that snipefish exhibit dorsal head rotational velocities during pivot feeding that are not achievable by direct muscle activation. We also demonstrate that an over-centre (torque reversal) mechanism is present in the snipefish morphology, which would allow the system to become latched and elastic energy to be stored in enlarged tendons. After a trigger unlatches it, the quick-release nature of the linkage mechanism would allow for rapid conversion of stored elastic energy into kinetic energy, resulting in elevated power output. Finally, possession of elastic recoil in snipefish indicates that this complex, high-performance feeding mechanism has a wider distribution and potentially more complex evolutionary history than previously thought.
(a). Evidence for elastic recoil
Snipefish feeding strikes are characterized by extremely rapid dorsal rotation of the head and snout towards prey. Prey capture occurred in as little as 2 ms, placing snipefish feeding performance among the fastest values recorded for fishes [29]. Short times to prey capture are achieved through rapid head elevation, hyoid depression and lateral expansion of the head, which move the mouth to the prey and produce suction to pull the prey into the mouth. Maximum head rotational velocities relative to the body reached approximately 8700° s−1. For comparison, peak head rotational velocity in largemouth bass, which do not use elastic recoil to power head rotation, is under 1000° s−1 [30]. Other kinematic features of snipefish feeding also depart from typical suction feeding fish but resemble seahorses and pipefish, including the early, high-velocity onset of hyoid rotation nearly coincident with rapid head elevation [11,12,14,15,23].
We calculated the peak instantaneous muscle-mass-specific power requirement for strikes to determine if the high rotational velocities observed during head rotation exceed possible muscular power output. Estimates of the maximum power requirement ranged from 1090 to 5500 W kg−1 across multiple individuals and strikes (figure 2). Our power calculations assume that all power production is used for head rotation and body recoil, but does not include the power for concurrent motions such as hyoid rotation and lateral expansion of the mouth, snout and opercular chamber during the production of suction. Therefore, the actual power required for snipefish feeding is probably still greater than our estimate. Furthermore, we included the sternohyoideus and hypaxial muscles in our calculations, even though they (or portions of them) probably function as the trigger rather than in energy storage (see below). Nevertheless, the power requirements we estimated for all but one strike were above the maximum in vitro vertebrate power requirement, 1121 W kg−1 muscle [24], and all were well above the maximum in vitro instantaneous power outputs measured for fish epaxial muscle: approximately 216 W kg−1 in a largemouth bass [25]. In addition, power estimates for snipefish fall within the ranges reported for other mechanisms with tendon-based energy storage (e.g. 3000 W kg−1 for chameleon tongues [31]). Power requirements above those measured for fish and other vertebrates provide strong support for power amplification during head rotation in snipefish feeding.
(b). Snipefish possess an over-centre hyoid latch
In many cases, kinematic evidence for elastic recoil precedes an understanding of the morphology that might account for this enhanced performance. However, previous work by Muller [20] and Altermatt [22] has already described how the relative dimensions and arrangement of the four-bar linkage system coupling head elevation to hyoid depression in snipefish acts as a ‘quick release’ or ‘trigger mechanism’ (figure 3). When the hyoid and urohyal linkages are aligned, the system becomes immobilized and energy can be stored in the enlarged epaxial tendons. A trigger that perturbs the system slightly would then result in extremely rapid hyoid rotation and head elevation as the elastic energy recoils. This mechanism neatly accounts for the unusual kinematic features of snipefish strikes, including that the head and the hyoid begin rotating extremely rapidly and nearly simultaneously. We corroborated the presence of the linkages and joints proposed by Muller [20] and Altermatt [22], and micro-CT scans show in new detail how modifications to the snipefish anatomy allow this simplified model to work so well, compared to more typical suction feeding fish. For instance, the interhyal has been reduced to a knob that rotates with the rest of hyoid apparatus in a socket-like joint on the inside of the preoperculum (figure 3a,b). This allows the hyoid apparatus to preform much like the idealized rigid rod rotating in a pin joint. We also show that the linkage model allows for fairly accurate prediction of observed snipefish kinematics (electronic supplementary material, figure S5).
A key feature of the snipefish four-bar linkage mechanisms is its ability to function as a latch or trigger mechanism, and it is here that we amend the model for snipefish. While it has been previously proposed that the hyoid is aligned with the urohyal–sternohyoideus linkage [20,22], our use of high-resolution imaging technologies revealed that the neurocranium–suspensorium (pink) and urohyal–sternohyoideus (blue) linkages are in fact overlapping (figure 3d). This is referred to as a ‘folded’ or ‘over-centre’ arrangement of the linkages in a mechanism with more than one stable state or centre point. In this arrangement, torque exerted by the trunk musculature to elevate the head causes the hyoid link to rotate in the ‘wrong’ direction (elevating further into the head instead of depressing); it cannot rotate far, because a robust basihyal and characteristics of the interhyal-suspensorium joint prevent further elevation (figure 3). The system therefore becomes firmly locked or latched shut, and can remain so against forces from the epaxial muscles. We verified this behaviour of the hyoid apparatus through manipulation of fresh specimens. When we manually attempted to elevate the head with the hyoid and suspensorium adducted, the hyoid was observed to strain medially (inwards). If the same force is applied with the hyoid initially depressed slightly, the head easily elevates while the hyoid depresses further (compare figure 3c,d). This is typical of torque reversal mechanisms, which are characteristically bistable such that the direction of output movement depends on the specific arrangement of the linkages. Switching from one state to the other can result in explosive movement when the sign of the torque changes, especially when stored elastic energy is released, becoming kinetic energy. Relative lengths of links measured in micro-CT scans and on videos indicate that the snipefish four-bar is consistent with such a bistable mechanism, where the hyoid (the smallest link) can rotate in opposite directions depending on the linkage geometry (figure 3c–f). To be clear, the aligned or centred arrangement shown by Muller and Altermatt also allows the system to become latched and exhibits bistable quick-release behaviour, but theirs is a more tenuous instability, where slight deviations in hyoid angle could cause it to either depress or elevate. Our work therefore shows that snipefish possess a more ‘fool-proof’ latching arrangement of the four-bar linkage mechanism. We refer to this as a ‘torque reversal’ or ‘over-centre latching mechanism’, rather than a ‘trigger mechanism’, because this arrangement prevents movement while the system is loaded. The trigger, on the other hand, allows the system to unlatch and must in fact disrupt the four-bar linkage model.
(c). Components of the elastic recoil mechanism
Elastic recoil mechanisms across the tree of life tend to have four components. In snipefish, we have made the case that the anterior epaxial and hypaxial muscles are the ‘motor’, the enlarged epaxial tendons function as the ‘spring’ (electronic supplementary material, figure S2) and a torque reversal four-bar linkage forms the ‘latch’. We haven't mentioned yet the fourth component, the ‘trigger’, that quickly unlatches the system. Given the over-centre arrangement of the linkage, it seems most likely that an active trigger muscle is needed to alter the conformation of the linkages. Even a seemingly small change that allows the hyoid link to shift dorsally relative to the urohyal–sternohyoideus link would result in a rapid rotation of the head and hyoid as the stored elastic energy recoils. Muscles that could change the orientation of the hyoid or urohyal linkages include the protractor hyoidei or the sternohyoideus and hypaxial muscles attached to the urohyal [20]. The latter hypothesis was favoured by Altermatt [22], and we agree it is the most probable candidate given the relative size and orientation of this muscle. In particular, the slips of hypaxial musculature that insert on the ventral ridge of the urohyal could pull it ventrally, which would unlatch the mechanism and result in rapid hyoid depression. Compared with the rest of the hypaxial musculature posterior to the pectoral girdle, these slips of hypaxial connecting to the urohyal are thin and small in cross section, and as such are reminiscent of fast-contracting trigger muscles in other elastic recoil mechanisms (e.g. [9,32]).
The latch and triggering mechanism we have described is different from that in seahorses and pipefishes (syngnathids). First of all, the sternohyoideus and hypaxial muscles of syngnathids form a single complex, which does not insert on the urohyal in a way that would cause a conformation change of the four-bar linkage [33]. Instead, it has been shown that active adduction of the sides of the head (suspensorium) by a muscle (adductor arcus palatini) is needed to prevent the hyoid from rotating ventrally during loading in seahorses. Relaxation of this muscle then triggers hyoid release and rapid head elevation [33]. The four-bar linkage coupling head elevation to hyoid depression in syngnathids has never been described as over-centre, and as such does not seem to be sufficient to lock the hyoid in place during loading. In contrast to snipefish, the hyoid of a pipefish will often depress when upward forces are applied to the head of a fresh specimen (S.J.L. 2018, personal observation). Because of the specific arrangement and behaviour of the snipefish four-bar linkage mechanism, we do not think that active suspensorium adduction is necessary to latch or trigger the mechanism, but it could still help the mechanism behave in a highly precise and coordinated way, and cannot be ruled out without direct evidence from approaches like EMG. In any case, the observed differences between snipefish and syngnathid four-bar behaviour and associated musculature indicates that this is an important area for further study across Syngnathiformes.
(d). Evolution of elastic recoil
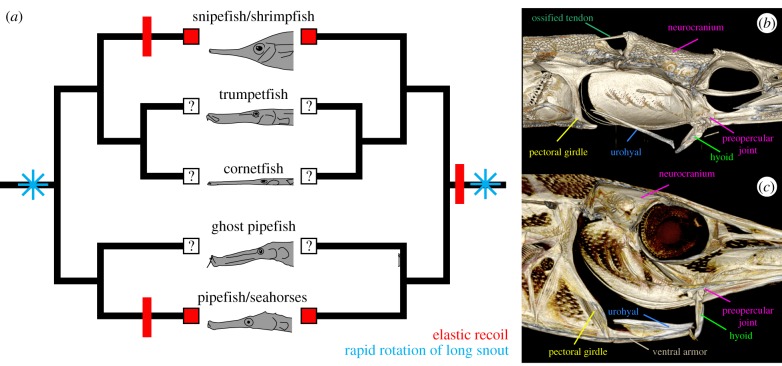
The possession of rapid head rotation by elastic recoil in snipefish reveals that this high-performance mechanism is more broadly distributed among syngnathiforms than previously thought, which in turn has important evolutionary implications by ruling out a single origin at the base of Syngnathidae (seahorses and pipefishes). Our finding indicates that either there was a single origin of this mechanism in a common ancestor of snipefish and syngnathids, or the system evolved independently at least twice (figure 4). The Syngnathidae are well known for many interesting functional, morphological and behaviour traits that are apomorphic, including pelvic fin loss, reduction of the anal fin, male-brooding and segmented body armour. Our work indicates that the elastic recoil feeding mechanism did not arise concomitantly with these features, arguing against any role for them in the origin of elastic recoil feeding.
Figure 4.
The two possible scenarios for the evolution of power-amplified head rotation by elastic recoil in Syngnathiformes. (a) This mechanism may have evolved multiple times (one possible scenario shown on the left), or it may have evolved once at the base of the clade (right). Pivot feeding, or rapid rotation of the long snout (*), seems to characterize syngnathiform feeding [17] and may have been an important preadaptation facilitating the origin of elastic recoil. The feeding functional morphology of (b) pipefish and (c) snipefish are similar, but also notably different. These differences could hint at parallel evolution of elastic recoil among lineages or simply be a consequence of evolutionary divergence.
All syngnathiforms possess a long snout, and dorsal head rotation appears to be the primary contribution to their prey capture [17]. Morphological and functional modifications for head rotation may have served as preadaptations facilitating the independent, parallel origin of elastic recoil in multiple lineages, especially if there were similar selective pressures for fast times to prey capture. Many features of the snipefish four-bar linkage and the hyoid–preopercular joint are similar to that described for seahorses and pipefishes (figure 4) [20,26,11], yet snipefish and syngnathids still appear to latch their hyoid in place in different ways, and the architecture of the epaxial, hypaxial, and sternohyoideus muscles and their associated tendons also differ. These key differences in all components of the elastic recoil mechanism (motor, spring, latch and trigger) strongly suggest that parallel evolution could have given rise to the mechanism in each clade, and minimally indicates divergence in major components of the system if there was a single origin of elastic recoil feeding. At present, it is not possible to perform a robust ancestral state reconstruction for elastic recoil-powered feeding across Syngnathiformes without information from other lineages, including trumpetfish (Aulostomidae), cornetfish (Fistulariidae), ghost pipefish (Solenostomidae) and shrimpfish (sister to snipefish within Centriscidae). Further studies on the biomechanics of feeding in other syngnathiforms are necessary to flesh out our understanding of this unique mechanism, including the circumstances under which it may have arisen.
This study contributes to a growing trend in the literature of elastic recoil mechanisms, whereby broad comparative studies reveal that these complex mechanisms are often not restricted to the most specialized clades but instead are widespread [5–9]. Given the evidence in other groups for repeated, independent origins of elastic recoil mechanisms and the differences between the details of latching and triggering between syngnathids and snipefish, it would not be surprising if elastic recoil feeding did originate more than once within Syngnathiformes.
Finally, over-centre or torque reversal latching mechanisms such as that found in snipefish may be widespread in nature. Such mechanisms have been proposed for a variety of other movements powered by elastic recoil, including snapping shrimp strikes, and flea and locust jumps [9,34–36]. A key feature of torque reversal mechanisms is their explosive behaviour as the torque changes sign. Recent work modelling a spring-driven motion showed the latch dynamics play an important role in performance [37]. Latches mediate the delivery of energy between recoiling springs and output structures, and the construction of latches that can rapidly release stored energy therefore pose extremely important constraints on the evolution of elastic recoil mechanisms. By relying on a subtle geometric change rather than the removal of a physical block, torque reversal mechanisms are probably a highly effective latching strategy, especially at the size scales of vertebrates such as snipefish.
Supplementary Material
Acknowledgements
We are grateful to S. Van Wassenbergh for providing example files and helpful feedback on power calculations. We thank Blue Corner, A. Kuwamoto, M. Foley and J. Harris for their help in acquiring fish, C. Oufiero and J. Patrocinio for help with aquaria, B. Draper for providing live prey, and C. Baldwin, S. Raredon and the Smithsonian Museum of Natural History for assistance with digital radiography. We thank U. Müller, our anonymous reviewers, S. Patek and members of the Patek laboratory for constructive feedback on drafts of the manuscript.
Ethics
All animal care and experimental procedures used in this research followed a protocol reviewed by the University of California, Davis Institutional Animal Care and Use Committee.
Data accessibility
Videos, R scripts and datasets supporting this article have been uploaded as part of the electronic supplementary material or deposited to Dryad (http://dx.doi.org/10.5061/dryad.hb6b0) [38].
Authors' contributions
S.J.L. and P.C.W. designed the study. S.J.L. and T.G. collected high-speed videos, and digitized and analysed kinematics. S.J.L. performed biomechanical analyses, radiography, and micro-CT scans and reconstructions. S.J.L. and P.C.W. described the morphology. S.J.L wrote the manuscript, with comments from P.C.W. and T.G. All the authors reviewed the final version of the text.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the National Science Foundation (NSF) Graduate Research Fellowship Program under grant no. 1148897, NSF Doctoral Dissertation Improvement Grant DEB-1500800, the Center for Population Biology at the University of California, Davis, and the Society for Integrative and Comparative Biology.
References
- 1.Patek SN, Dudek DM, Rosario MV. 2011. From bouncy legs to poisoned arrows: elastic movements in invertebrates. J. Exp. Biol. 214, 1973–1980. ( 10.1242/jeb.038596) [DOI] [PubMed] [Google Scholar]
- 2.Sakes A, van der Wiel M, Henselmans PWJ, van Leeuwen JL, Dodou D, Breedveld P. 2016. Shooting mechanisms in nature: a systematic review. PLoS ONE 11, e0158277 ( 10.1371/journal.pone.0158277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronenberg W. 1996. Fast actions in small animals: springs and click mechanisms. J. Comp. Physiol. A 178, 727–734. ( 10.1007/BF00225821) [DOI] [Google Scholar]
- 4.Claverie T, Chan E, Patek SN. 2011. Modularity and scaling in fast movements: power amplification in mantis shrimp. Evolution 65, 443–461. ( 10.1111/j.1558-5646.2010.01133.x) [DOI] [PubMed] [Google Scholar]
- 5.Deban SM, O'Reilly JC, Dicke U, van Leeuwen JL. 2007. Extremely high-power tongue projection in plethodontid salamanders. J. Exp. Biol. 210, 655–667. ( 10.1242/jeb.02664) [DOI] [PubMed] [Google Scholar]
- 6.Mueller RL, Macey JR, Jaekel M, Wake DB, Boore JL. 2004. Morphological homoplasy, life history evolution, and historical biogeography of plethodontid salamanders inferred from complete mitochondrial genomes. Proc. Natl Acad. Sci. USA 101, 13 820–13 825. ( 10.1073/pnas.0405785101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patek SN, Baio JE, Fisher BL, Suarez AV. 2006. Multifunctionality and mechanical origins: ballistic jaw propulsion in trap-jaw ants. Proc. Natl Acad. Sci. USA 103, 12 787–12 792. ( 10.1073/pnas.0604290103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood HM, Parkinson DY, Griswold CE, Gillespie RG, Elias DO. 2015. Repeated evolution of power-amplified predatory strikes in trap-jaw spiders. Curr. Biol. 26, 1057–1061. ( 10.1016/j.cub.2016.02.029) [DOI] [PubMed] [Google Scholar]
- 9.Kaji T, Anker A, Wirkner CS, Palmer AR. 2018. Parallel saltational evolution of ultrafast movements in snapping shrimp claws. Curr. Biol. 28, 106–113.e4. ( 10.1016/j.cub.2017.11.044) [DOI] [PubMed] [Google Scholar]
- 10.Patek SN, Rosario MV, Taylor JRA. 2013. Comparative spring mechanics in mantis shrimp. J. Exp. Biol. 216, 1317–1329. ( 10.1242/jeb.078998) [DOI] [PubMed] [Google Scholar]
- 11.Van Wassenbergh S, Strother JA, Flammang BE, Ferry-Graham LA, Aerts P. 2008. Extremely fast prey capture in pipefish is powered by elastic recoil. J. R. Soc. Interface 5, 285–296. ( 10.1098/rsif.2007.1124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Wassenbergh S, Roos G, Genbrugge A, Leysen H, Aerts P, Herrel A. 2009. Suction is kid's play: extremely fast suction in newborn seahorses. Biol. Lett. 5, 200–203. ( 10.1098/rsbl.2008.0765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lussanet MHE, Muller M. 2007. The smaller your mouth, the longer your snout: predicting the snout length of Syngnathus acus, Centriscus scutatus and other pipette feeders. J. R. Soc. Interface 4, 561–573. ( 10.1098/rsif.2006.0201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flammang BE, Ferry-Graham LA, Rinewalt C, Ardizzone D, Davis C, Trejo T. 2009. Prey capture kinematics and four-bar linkages in the bay pipefish, Syngnathus leptorhynchus. Zoology 112, 86–96. ( 10.1016/j.zool.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 15.Roos G, Van Wassenbergh S, Herrel A, Aerts P. 2009. Kinematics of suction feeding in the seahorse Hippocampus reidi. J. Exp. Biol. 212, 3490–3498. ( 10.1242/jeb.033050) [DOI] [PubMed] [Google Scholar]
- 16.Van Wassenbergh S, Aerts P. 2008. Rapid pivot feeding in pipefish: flow effects on prey and evaluation of simple dynamic modelling via computational fluid dynamics. J. R. Soc. Interface 5, 1291–1301. ( 10.1098/rsif.2008.0101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longo SJ, McGee MD, Oufiero CE, Waltzek TB, Wainwright PC. 2016. Body ram, not suction, is the primary axis of suction-feeding diversity in spiny-rayed fishes. J. Exp. Biol. 219, 119–128. ( 10.1242/jeb.129015) [DOI] [PubMed] [Google Scholar]
- 18.Longo SJ, Faircloth BC, Meyer A, Westneat MW, Alfaro ME, Wainwright PC. 2017. Phylogenomic analysis of a rapid radiation of misfit fishes (Syngnathiformes) using ultraconserved elements. Mol. Phylogenet. Evol. 113, 33–48. ( 10.1016/j.ympev.2017.05.002) [DOI] [PubMed] [Google Scholar]
- 19.Waterworth GD. 1979. Functional morphology of the feeding mechanism of the slender snipefish Macrorhamposus gracilis. Unpublished MA thesis, California State University, Long Beach, CA. [Google Scholar]
- 20.Muller M. 1987. Optimization principles applied to the mechanism of neurocranium levation and mouth bottom depression in bony fishes (Halecostomi). J. Theor. Biol. 126, 343–368. ( 10.1016/S0022-5193(87)80241-2) [DOI] [Google Scholar]
- 21.Altermatt RU. 1991. Zur Kopfanatomie des Schnepfenfisches Macroramphosus scolopax (Linnaeus, 1758) (Teleostei, Syngnathiformes). Eine beschreibend-morphologische Studie unter Berucksichtigung funktioneller Aspekte. Unpublished dissertation, Universität Basel, Switzerland. [Google Scholar]
- 22.Altermatt RU. 1993. Die Nahrungsaufnahme des Schnepfenfisches Macrorhamphosus scolopax (L., 1758) (Teleostei, Syngnathiformes). Verhandlungen der Naturforschenden Gesellschaft in Basel 103, 59–65. [Google Scholar]
- 23.Bergert BA, Wainwright PC. 1997. Morphology and kinematics of prey capture in the syngnathid fishes Hippocampus erectus and Syngnathus floridae. Mar. Biol. 127, 563–570. ( 10.1007/s002270050046) [DOI] [Google Scholar]
- 24.Askew GN, Marsh RL. 2001. The mechanical power output of the pectoralis muscle of blue-breasted quail (Coturnix chinensis): the in vivo length cycle and its implications for muscle performance. J. Exp. Biol. 204, 3587–3600. [DOI] [PubMed] [Google Scholar]
- 25.Coughlin DJ, Carroll AM. 2006. In vitro estimates of power output by epaxial muscle during feeding in largemouth bass. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 145, 533–539. ( 10.1016/j.cbpa.2006.08.026) [DOI] [PubMed] [Google Scholar]
- 26.Roos G, Leysen H, Van Wassenbergh S, Herrel A, Jacobs P, Dierick M, Aerts P, Adriaens D. 2009a. Linking morphology and motion: a test of a four-bar mechanism in seahorses. Physiol. Biochem. Zool. 82, 7–19. ( 10.1086/589838) [DOI] [PubMed] [Google Scholar]
- 27.Barker C. 1985. A complete classificaiton of planar four-bar linkages. Mech. Mach. 20, 535–554. ( 10.1016/0094-114X(85)90071-0) [DOI] [Google Scholar]
- 28.Hartenberg RS, Denavit J. 1964. Kinematic synthesis of linkages, 435 p New York, NY: McGraw-Hill. [Google Scholar]
- 29.Grobecker DB, Pietsch TW. 1979. High-speed cinematographic evidence for ultrafast feeding in antennariid anglerfishes. Science 205, 1161–1162. ( 10.1126/science.205.4411.1161) [DOI] [PubMed] [Google Scholar]
- 30.Svanbäck R, Wainwright PC, Ferry-Graham LA. 1997. Linking cranial kinematics, buccal pressure, and suction feeding performance in largemouth bass. Physiol. Biochem. Zool. 75, 532–543. ( 10.1086/344495) [DOI] [PubMed] [Google Scholar]
- 31.de Groot JH, Van Leeuwen JL. 2004. Evidence for an elastic projection mechanism in the chameleon tongue. Proc. R. Soc. Lond. B 271, 761–770. ( 10.1098/rspb.2003.2637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gronenberg W. 1996b. The trap-jaw mechanism in the dacetine ants Daceton armigerum and Strumigenys sp. J. Exp. Biol. 199, 2021–2033. [DOI] [PubMed] [Google Scholar]
- 33.Van Wassenbergh S, Dries B, Herrel A. 2014. New insights into muscle function during pivot feeding in seahorses. PLoS ONE 9, e109068 ( 10.1371/journal.pone.0109068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cofer D, Cymbalyuk G, Heitler J, Edwards DH. 2010. Neuromechanical simulation of the locust jump. J. Exp. Biol. 213, 1060–1068. ( 10.1242/jeb.034678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritzmann R. 1974. Mechanisms for the snapping behavior of two alpheid shrimp, Alpheus californiensis and Alpheus heterochelis. J. Comp. Physiol. 95, 217–236. ( 10.1007/BF00625445) [DOI] [Google Scholar]
- 36.Bennet-Clark HC, Lucey ECA. 1967. The jump of the flea: a study of the energetics and a model of the mechanism. J. Exp. Biol. 47, 59–76. [DOI] [PubMed] [Google Scholar]
- 37.Ilton M, et al. 2018. The principles of cascading power limits in small, fast biological and engineered systems. Science 360, eaao1082 ( 10.1126/science.aao1082) [DOI] [PubMed] [Google Scholar]
- 38.Longo SJ, Goodearly T, Wainwright PC. 2018. Data from: Extremely fast feeding strikes are powered by elastic recoil in a seahorse relative, the snipefish, Macroramphosus scolopax Dryad Digital Repository. ( 10.5061/dryad.hb6b0) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Longo SJ, Goodearly T, Wainwright PC. 2018. Data from: Extremely fast feeding strikes are powered by elastic recoil in a seahorse relative, the snipefish, Macroramphosus scolopax Dryad Digital Repository. ( 10.5061/dryad.hb6b0) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Videos, R scripts and datasets supporting this article have been uploaded as part of the electronic supplementary material or deposited to Dryad (http://dx.doi.org/10.5061/dryad.hb6b0) [38].