Abstract
Males are known to adjust their expenditure on testes growth and sperm production in response to sperm competition risk. Genital morphology can also contribute to competitive fertilization success but whether male genital morphology can respond plastically to the sperm competition environment has received little attention. Here, we exposed male house mice to two different sperm competition environments during their sexual development and quantified phenotypic plasticity in baculum morphology. The sperm competition environment generated plasticity in body growth. Males maturing under sperm competition risk were larger and heavier than males maturing under no sperm competition risk. We used a landmark-based geometric morphometric approach to measure baculum size and shape. Independent of variation in body size, males maintained under risk of sperm competition had a relatively thicker and more distally extended baculum bulb compared with males maintained under no sperm competition risk. Plasticity in baculum shape paralleled evolutionary responses to selection from sperm competition reported in previous studies of house mice. Our findings provide experimental evidence of socially mediated phenotypic plasticity in male genitalia.
Keywords: genital morphology, competitive growth, sperm competition, phenotypic plasticity
1. Introduction
Phenotypic plasticity is defined as the capacity for a single genotype to produce multiple phenotypes in response to variation in the environment [1]. Phenotypic plasticity allows individuals to adapt to new conditions, or anticipate future conditions, by accessing environmental or social cues that predict environmental change [2]. Many different types of traits can exhibit plasticity, which may lead to transient or permanent changes in morphological, physiological, and/or behavioural phenotypes [1]. Phenotypic plasticity is a developmental process occurring within the lifetime of an individual. Nevertheless, it can be a major avenue of evolutionary divergence among populations [3]. Adaptive phenotypic plasticity has been reported in a number of important life-history traits including predator defences, immune responses, and sexual traits important in reproductive competition [4–7].
Phenotypic plasticity in male allocation to reproductive competition has been described in a range of taxa [7]. Theoretical models predict that males should strategically allocate resources to increase ejaculate expenditure when faced with an increased risk of sperm competition [8]. Consistent with this prediction, empirical studies have reported phenotypic plasticity in ejaculate expenditure by males, cued by their social environment [9]. For example, males make adaptive changes in ejaculatory frequency [10,11], the size of the ejaculate [12], the number of sperm within the ejaculate [13–15], and the composition of seminal fluid [16,17] depending on their perceived risk of sperm competition. In house mice, exposure of males to potential rivals induces increased levels of sperm production [18,19], an increase in the production of key seminal fluid proteins [20], and changes in mating behaviour, including a decrease in the number of thrusts, intromissions, and duration of copulation, and an increase in the probability of repeated ejaculation [21]. These studies of house mice illustrate how the sperm competition environment can induce phenotypic plasticity in a variety of male reproductive traits and that phenotypic plasticity need not be limited to sperm production.
There is now considerable evidence that male genital morphology is subject to sexual selection, and variation in genital shape can impact mating, insemination, fertilization, and overall male reproductive success [22–24]. Preliminary evidence from ducks suggests that males may have the capacity to adjust the morphology of their penis in response to reproductive competition [25]. The ossified penis or baculum is a feature of several mammalian orders [26,27]. In house mice, the shape of the baculum affects competitive male reproductive success [28] and covaries with the strength of selection from sperm competition among natural and experimentally evolving populations [29]. The house mouse baculum is comprised of two structures, a proximal ossified and a distal cartilaginous structure [30,31]. The growth of these structures occurs primarily during the first 60 days post-birth [30] and is known to depend on androgens and endogenous oestrogen expression during pre- and post-natal development [30,32,33].
In this study, we assessed whether house mouse baculum morphology exhibits phenotypic plasticity in response to sperm competition risk during pre-adult development. Given the wide-ranging effects of sperm competition risk on male reproductive physiology and behaviour [18–21,34], and the importance of baculum shape for male reproductive success [28], we might expect baculum morphology to show similar plasticity in response to cues in the social environment [25]. We thus raised males in two different social environments, either with or without cues to sperm competition risk and quantified variation in the shape of the baculum as well as a naturally selected bone, the hind femur. We expected males to show phenotypic plasticity in baculum morphology in response to the sperm competition environment, but not in the morphology of the hind femur.
2. Material and methods
(a). Experimental animals
Wild house mice (Mus musculus domesticus) were sourced from an isolated population on Rat Island (28°42′ S, 113°47′ E) off the coast of Western Australia (n = 100) and maintained in the laboratory at the University of Western Australia. The animals were held in constant temperature rooms (CTRs; 24°C) on a reverse 10 D : 14 L cycle and provided with water and food ad libitum. Mice were outbred under common-garden conditions for two generations. Male and female pairs were housed together for a maximum of 14 days. When noted to be pregnant, females were housed alone. At weaning age (21 days) female and male offspring were separated; female offspring were housed in groups and male offspring were housed individually. A total of 48 males and eight females were used in this study.
(b). Social manipulation
We raised males in one of two social environments throughout their sexual development by manipulating their exposure to rival males and their odours. Two sibling males from 24 families were weighed and then randomly assigned to either a ‘Risk’ or ‘No Risk’ environment. Details of the experimental manipulation are described elsewhere [19]. Briefly, males in both environments were housed individually in cages (16 × 33 × 12 cm). The individual cages in the ‘No Risk’ environment were placed alone in a large plastic tub (49 × 74 × 41 cm), while those in the ‘Risk’ environment were co-housed in a large plastic tub with those of two unrelated males. Males were housed under these conditions from weaning (21 days of age) until sexual maturity (90 days of age). The tubs were spread across two large (9 × 4 m) CTR, such that each room contained four ‘Risk’ tubs (total N males = 12) and 12 ‘No Risk’ tubs (total N males = 12). Tubs were spaced 60 cm apart. Sexually mature females (N = 4) were housed in their own cages within each CTR. Thus, females were placed in the centre of the room in large cages (28 × 46 × 13 cm) approximately equidistant to tubs containing males.
Each week, males in the ‘Risk’ treatment were exposed to soiled chaff (15 g) of the two rival males within their tub. For this, chaff was taken from the cage of each male and placed at the front of their rival male's cages. In the ‘No Risk’ treatment, males were ‘exposed’ to their own soiled chaff, i.e. it was moved from the back to the front of their cage. Once a fortnight ‘encounters’ were conducted whereby males were released into the tub and allowed to roam freely for 30 min. In the ‘Risk’ treatment, males were released one at a time and allowed to interact with their neighbouring males through the bars of their cages. ‘No Risk’ treatment males were released inside their tub but did not experience any interaction with other individuals. The same general procedures were performed using females; thus, once a fortnight, males of both treatments were exposed to female soiled chaff (15 g) and interacted with a female through the bars of their cages.
(c). Baculum and femur morphometric analyses
At 90 days of age, sexually mature males were sacrificed, and the baculum and right femur were dissected for analysis. At the time of dissection, the majority of the tissue surrounding the baculum and the femur was removed. However, to ensure complete tissue removal, both structures were stored overnight in 1 ml of 5% KOH. Following this, the specimens were stored in 1 ml of Dietrich's Fixative solution.
Digital images of the baculum (ventral view) and femur (external lateral view) were taken using a binocular microscope at ×20 and ×10 magnification, respectively. Geometric morphometric analysis of bone shape was conducted using the software developed by Rohlf (2006), blind to the treatment group from which images were obtained. Landmarks were placed around the periphery of the baculum (36 sliding, 4 fixed) and the femur (71 sliding, 8 fixed) using the software tpsDig2 v. 2.29 (for details, see electronic supplementary material, figure S1). The software Tpsrelw v. 1.65 was then used to extract relative warps (RWs) and centroid size. RWs represent the variation in shape relative to the consensus configuration across all samples [35]. In this study, we focused on those RWs that individually explained greater than 10% of the variance for both the baculum and femur. Centroid size (square root of the summed distances of each landmark to the centroid in x and y distances) provided a multivariate measure of the size of each bone [36]. The repeatability of landmark placement was assessed by calculating the Euclidean distances between fixed landmarks for 12 individuals (six from each of the risk and non-risk treatment) on two separate occasions. We also assessed the repeatability of centroid size and shape scores derived from our geometric morphometric analyses of these landmarks (for details, see electronic supplementary material, figure S2).
(d). Statistical analysis
Examination of the residuals identified outliers from the femur RWs analysis (for details, see electronic supplementary material, table S1). Shapiro–Wilks tests confirmed that data residuals were normally distributed, and parametric tests were applied in all cases. We used linear mixed models fitted by maximum-likelihood estimation using the lmer procedure in the lme4 R package [37]. Significance values were extrapolated from Type II Wald χ2 tests using the ANOVA function in the car package of R [38]. All morphological traits, except the RW scores, were log transformed prior to analysis. Male family identity and replicate tub identity were included in the model as random factors; log body length was entered as a covariate. Non-significant interaction terms were removed from statistical models.
We conducted bivariate line-fitting methods for estimating the relationships between baculum and femur centroid size and body length. We used SMATR version 2.0 freeware which allowed us to fit bivariate lines to the data and make inferences about such relationships [39]. Following previous studies [40–42], we opted to conduct standard major axis (SMA) regressions.
3. Results
Descriptive statistics for body size, genital and non-genital traits can be found in the online electronic supplementary material, table S1. Individuals did not differ in body weight when first assigned to their social environment (weight at 21 days of age: ‘Risk’ males: 8.14 ± 0.28 g; ‘No Risk’ males: 8.17 ± 0.28 g; t46 = −0.079, p = 0.937). However, at sexual maturity (90 days of age) males reared in the risk environment were on average larger and heavier than males reared under no risk of sperm competition (length: ‘Risk’ males: 8.59 ± 0.062 cm; ‘No Risk’ males: 8.16 ± 0.073 cm, t45 = −4.47, p < 0.001. weight: ‘Risk’ males: 18.19 ± 0.383 g; ‘No Risk’ males: 16.14 ± 0.323 g; t45 = −4.47, p < 0.001).
Baculum centroid size increased with body length (figure 1a), but there was no significant interaction between body length and social environment (χ2 = 0.945, p = 0.331), indicating that baculum allometry did not vary between the social environments. The common allometric slope (SMA slope = 0.9125 ± 0.131) did not differ statistically from 1.0 (χ2 = 0.608, p = 0.436). Baculum centroid size did not differ significantly between social environments (table 1). The same patterns of allometry were evident in a trait expected to be under natural selection (figure 1b). Thus, femur centroid size increased with body length (figure 1b), but there was no evidence for a significant interaction between social environment and body length (χ2 = 0.037, p = 0.859), yielding a common slope (SMA slope = 1.122 ± 0.144) that did not differ from 1.0 (χ2 = 0.851, p = 0.356). Femur centroid size did not differ between social environments (table 2).
Figure 1.
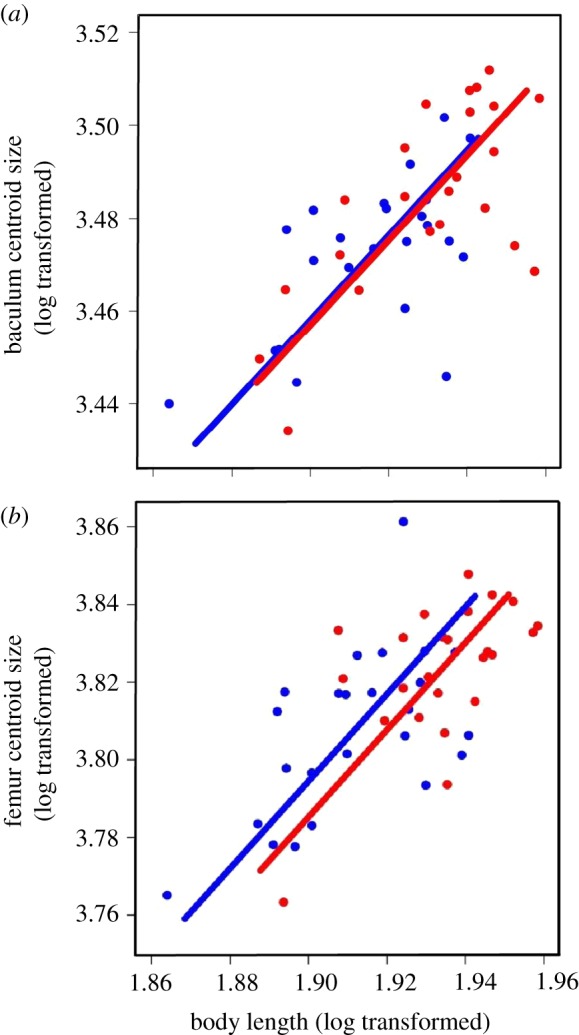
Allometric scaling of baculum centroid size (a) and femur centroid size (b) in male house mice. Blue symbols (greyscale—black) represent the ‘No Risk’ social environment and red symbols (greyscale—grey) the ‘Risk’ social environment. One regression line is fitted per treatment based on standardized major axis regression. (Online version in colour.)
Table 1.
Linear mixed model (LMM) of the effect of social environment on the morphology of the baculum of male house mice. Values in italics are significant at p < 0.05.
| fixed effects | estimate | ±s.e. | type II, Wald χ2 | p | random effects | variance | ±s.d. |
|---|---|---|---|---|---|---|---|
| centroid size | |||||||
| intercept | 5.7536 | 0.4747 | tub | 0.00010 | 0.01220 | ||
| social environment | 0.0153 | 0.0104 | 2.178 | 0.1400 | family | 0.00020 | 0.01340 |
| body length | 0.5042 | 0.1091 | 21.358 | <0.0001 | |||
| RW1 | |||||||
| intercept | −0.0531 | 0.2656 | tub | 0.00002 | 0.00470 | ||
| social environment | −0.0305 | 0.0059 | 26.1630 | <0.0001 | family | 0.00000 | 0.00190 |
| body length | 0.0226 | 0.0610 | 0.0028 | 0.7133 | |||
| RW2 | |||||||
| intercept | −0.1718 | 0.1747 | tub | 0.00000 | 0.00000 | ||
| social environment | 0.0026 | 0.0032 | 0.6299 | 0.4274 | family | 0.00006 | 0.00780 |
| body length | 0.0376 | 0.0402 | 0.8777 | 0.3488 | |||
| RW3 | |||||||
| intercept | −0.3938 | 0.1308 | tub | 0.00002 | 0.00464 | ||
| social environment | 0.0051 | 0.0033 | 2.4461 | 0.1178 | family | 0.00000 | 0.00000 |
| body length | 0.0872 | 0.0301 | 8.4015 | 0.0037 | |||
Table 2.
LMM of the effect of social environment on the morphology of the hind femur of male house mice. Values in italics are significant at p < 0.05.
| fixed effects | estimate | ±s.e. | type II, Wald χ2 | p | random effects | variance | ±s.d. |
|---|---|---|---|---|---|---|---|
| centroid size | |||||||
| intercept | 5.9378 | 0.5642 | tub | 0.00020 | 0.01260 | ||
| social environment | 0.0036 | 0.0126 | 0.113 | 0.7725 | family | 0.00010 | 0.01020 |
| body length | 0.6419 | 0.1297 | 23.471 | <0.0001 | |||
| RW1 | |||||||
| intercept | 0.1696 | 0.2237 | tub | 0.00008 | 0.00850 | ||
| social environment | −0.0040 | 0.0055 | 0.5510 | 0.4579 | family | 0.00002 | 0.00470 |
| body length | −0.0373 | 0.0514 | 0.5273 | 0.4677 | |||
| RW2 | |||||||
| intercept | 0.0167 | 0.1451 | tub | 0.00000 | 0.00000 | ||
| social environment | 0.0032 | 0.0029 | 1.1878 | 0.2758 | family | 0.00001 | 0.00380 |
| body length | −0.0051 | 0.0333 | 0.0238 | 0.8773 | |||
| RW3 | |||||||
| intercept | 0.1455 | 0.1082 | tub | 0.00002 | 0.00000 | ||
| social environment | −0.0025 | 0.0023 | 1.1624 | 0.2810 | family | 0.00000 | 0.00120 |
| body length | −0.0313 | 0.0248 | 1.5861 | 0.2079 | |||
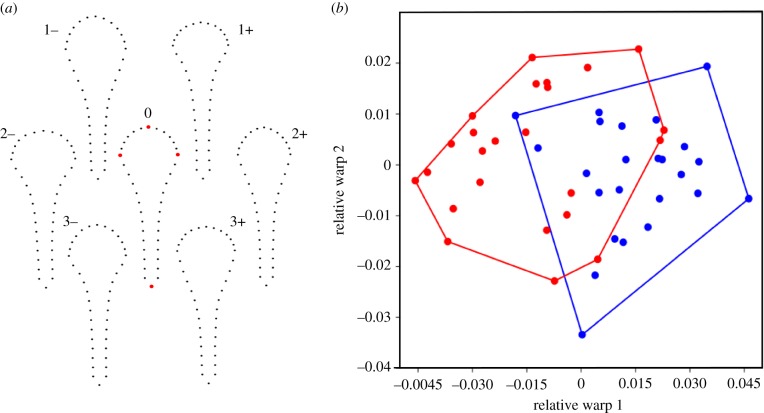
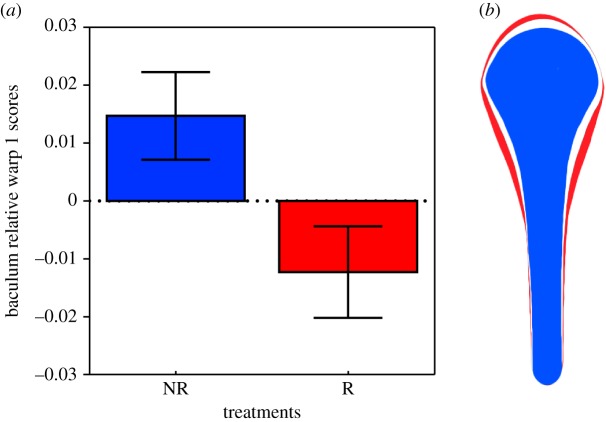
Geometric morphometric analysis of the baculum returned three relative warps (BRW) that each explained more than 10% of the variation in baculum shape (BRW1: 54.19%, BRW2: 15.92%, BRW3: 12.23%). BRW1 described variation in the relative thickness and extension of the baculum bulb along the shaft, and the thickness of the shaft itself (figure 2). BRW2 described similar variation in the relative thickness of the baculum bulb (figure 2), while BRW3 described variation in the rate of transition between bulb and shaft along the length of the baculum (figure 2). Our analyses revealed a significant effect of social environment on BRW1 (table 1; figure 3a) but no effect on BRW2 and BRW3. Males from the ‘Risk’ environment had a relatively thicker and more distally extended baculum bulb, and a relatively thicker baculum shaft compared to males from the ‘No Risk’ environment (figure 3b). The interaction terms between social environment and body length were not significant (BRW1: χ2 = 2.718, p = 0.09; BRW2: χ2 = 1.346, p = 0.246; BRW3: χ2 = 0.210, p = 0.647) and were, therefore, removed from all analyses. Male body length had no effect on BRW1 or BRW2 but did explain some of the variation in BRW3 (table 1).
Figure 2.
(a) Variation in baculum shape described by the extremes of relative warp 1 (1+, 1–), relative warp 2 (2+, 2−), and relative warp 3 (3+, 3−). The positions of the 40 landmarks around the periphery of the baculum for both positive and negative extremes are shown. The consensus shape (0) is displayed in the centre, with the four fixed landmarks identified as red symbols (greyscale—grey). (b) Morphospace plot of relative warp 1 against relative warp 2 with polygons encompassing individuals in each treatment. Blue symbols (greyscale—black) represent the ‘No Risk’ social environment and red symbols (greyscale—grey) represent the ‘Risk’ social environment. (Online version in colour.)
Figure 3.
Variation in baculum shape of male house mice from different sperm competition environments. (a) Mean (±95% CL) score on the first relative warp. (b) Superimposition of relative warp 1 thin-plate splines; blue (greyscale—black) represents the ‘No Risk’ social environment, red shape (greyscale—grey) represents the ‘Risk’ social environment, and white represents the consensus shape across all samples. (Online version in colour.)
Geometric morphometric analysis of the hind femur returned three relative warps (FRW) that each explained more than 10% of the variation in femur shape (FRW1: 24.50%, FRW2: 17.34%, FRW3: 11.09%). FRW1 explained variation in the shape of the femur head, variation in the shape of the lesser trochanter, the position of the third trochanter, and thickness of the femur shaft (electronic supplementary material, figure S3). FRW2 explained variation in the position of the third trochanter, and the thickness of the femur shaft (electronic supplementary material, figure S3). FRW3 explained variation in the relative length and thickness of the femur (electronic supplementary material, figure S3). Femur shape was not influenced by social environment or male body length (table 2). The interaction terms between social environment and body length were not significant (FRW1: χ2 = 0.514, p = 0.474: FRW2: χ2 = 0.970, p = 0.325; FRW3: χ2 = 0.665, p = 0.415) and were removed from the statistical models.
4. Discussion
Our study has revealed that male house mice exposed to rivals during sexual development grew a relatively thicker baculum with a distally extended bulb, compared with males that did not experience rivals during their development. While phenotypic plasticity in male genitalia has been reported previously in response to temperature and diet in Drosophila [43], and population density and wave-exposure in barnacles [44,45], there has been little evidence of socially mediated plasticity in genital morphology. A recent study of waterfowl reported how the penis of Lesser Scaup (Aythya affinis) grew longer in males housed together compared with those housed with a single female [25]. The authors considered this evidence preliminary because groups were not replicated and other environmental differences between groups could have explained their results. We provide replicated experimental evidence of phenotypic plasticity in male genital morphology. Socially induced plasticity was not apparent in a naturally selected bone, the hind femur, providing further support that the mammalian baculum is subject to sexual selection.
Remarkably, the variation in baculum shape that we observed between our sperm competition risk treatments reflects the genetic variation in baculum shape reported previously among populations of house mice [29]. Among natural populations, males under high risk of sperm competition have evolved thicker bacula than males from populations with a relatively lower risk of sperm competition [29]. Further, males from experimental populations subjected to multiple generations of post-copulatory sexual selection were found to evolve thicker bacula compared to males from populations evolving under enforced monogamy [29]. Consistent with these previous studies, we found no effect of sperm competition risk on baculum size [29,40]. Collectively, these studies support the hypothesis that it is the shape of male genitalia that is under sexual selection, and not genital size [23]. Considering that baculum thickness predicts male reproductive success under competitive conditions [28], our data provide strong evidence that the mammalian baculum is a trait subject to selection via sperm competition, and shows that males can prepare for future sperm competition via adjustments in their genital morphology.
Variation in bone morphogenesis offers a potential mechanism for phenotypic plasticity in baculum shape. Sex steroids play a fundamental role in osteogenesis [46–49], being actively responsible for longitudinal and radial bone growth during sexual maturation [46,50]. Artificial manipulation of the expression or activation of androgen, oestrogen, oestrogen receptor alpha (ERα), and oestrogen aromatase enzyme, all lead to abnormal osteogenesis [30,32,33,47,49]. The proximal segment of the mouse baculum is an ossified structure, and previous studies have shown that anti-androgen treatments arrest baculum development [30]. Studies conducted on rats and house mice have shown that during sexual maturation, androgen involvement in baculum growth is partly dependent on the aromatization of oestrogen [32,33]. ERα and oestrogen aromatase activation/expression has also been shown to be responsible for radial bone growth [48,49]. Taken together, these findings suggest the differential expression of ERα and/or differential local oestrogen aromatase enzyme expression as candidate mechanisms for the observed phenotypic plasticity in baculum thickness [48,49].
Our results also revealed socially mediated differences in adult size at sexual maturity. Androgen expression during sexual development is known to have a direct impact on spermatogenesis [51] and indirect impact on body growth via growth hormone secretion [50,52]. A previous study of this same population of mice found that sperm competition risk induced higher sperm production [19] and differential body growth may be associated with higher expression of testosterone during the pre-adult developmental stages. Overall, the perception of rival males may influence the regulation and expression of sex steroids that affect a range of sexual traits preparing males for sperm competition.
Increased male body size in response to sexual competition may be important in establishing dominance over rivals, increasing a male's ability to gain access to receptive females, or be the first male to copulate. In house mice, dominance and fighting ability are both positively correlated with body mass [53,54], and mating position is a strong predictor of paternity success, with the first male to mate siring the majority of offspring [55]. Larger male house mice deliver greater copulatory stimulation [21], and those with an evolutionary history of sperm competition have longer copulations and paternity success compared to males evolving under enforced monogamy [56], so it is conceivable that phenotypic plasticity in body size might be driven by pre-copulatory male–male competition over access to females. Indeed, socially mediated plasticity in traits that contribute to pre-mating male contest competition has been demonstrated in studies of invertebrates and vertebrates [57–60]. When exposed to same-sex rivals, Kalahari meerkat individuals have been shown to adjust their growth according to that of their rivals, so-called competitive growth that prepares them for future sexual competition [60]. Phenotypic plasticity in pre-and post-copulatory competitive traits is expected when male investment in these traits is costly. For house mice, one cost of competitive traits may relate to sex steroid expression. In general, testosterone is known to suppress vertebrate immune function [61], which has the potential to impact lifespan, a well-documented cost of male investment in pre- and post-copulatory traits [62–64].
The mechanism(s) by which post-copulatory sexual selection acts on baculum shape remains unknown. Of those proposed [65–67], vaginal stimulation represents a plausible candidate. The baculum is an integral part of the glans penis [31] and is likely to impart mechanical stimulation during copulation. House mouse sexual behaviour is characterized by multiple intromissions per mount and multiple mounts before the male reaches ejaculation [68]. The baculum within the glans penis will impart rigidity to facilitate penetration as well as promote friction between the penis and the female reproductive tract [65]. Differences in baculum thickness may lead to variation in the degree of stimulation that a female receives during copulation. In particular, a more distal thickening of the basal bulb might promote greater levels of stimulation throughout a longer section of the vaginal tract, increasing neuroendocrine responses that affect sperm migration, embryo implantation rate, and embryo viability [69–71]. Greater stimulation delivered by a thicker baculum may also influence female sexual behaviour by dampening their propensity to re-mate with a rival male [72]. Genital stimulation is important in directing sexual behaviour, activating the brain reward system and reinforcing and facilitating sexual behaviour [73]. Indeed, manipulation of neurochemical states of sexual reward has been shown to affect female preference [73].
In conclusion, we provide evidence of socially mediated phenotypic plasticity in male genital morphology. Our study adds to growing evidence that supports a role of sexual selection in the evolution of the mammalian baculum and re-enforces its role in male reproductive fitness [27–29]. Consistent with earlier studies of house mice, variation in baculum shape, but not size, was found to respond to socially mediated cues of sperm competition risk. Further investigation of the mechanism(s) underlying plasticity in baculum development, as well as those responsible for selection acting on the baculum, are required. In particular, we suggest that research on the role of sex steroids and their receptor distribution in the baculum will offer insight into the mechanisms that mediate plasticity in baculum shape.
Supplementary Material
Acknowledgement
We thank J. Moran for help with animal maintenance.
Ethics
The work reported in this article followed all the guidelines for ethical treatment of animals in research under UWA Ethics Committee approval (03/100/1456).
Data accessibility
Data are available at the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.57v6627) [74].
Authors' contributions
G.I.A., R.C.F., and L.W.S. designed the study. R.C.F. collected the source animals and assisted with establishing the experimental treatments. G.I.A collected and analysed the data. G.I.A. wrote the first draft of the manuscript and all authors contributed to the final version.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by the Australian Research Council (LWS DP 170101315).
References
- 1.Fordyce JA. 2006. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. J. Exp. Biol. 209, 2377–2383. ( 10.1242/jeb.02271) [DOI] [PubMed] [Google Scholar]
- 2.Whitman DW, Agrawal AA. 2009. What is Phenotypic Plasticity and Why is it Important? In Phenotypic Plasticity of Insects (eds Whitman DW.), pp. 1–63. New Hampshire, USA: Science Publishers; ( 10.1201/b10201-1) [DOI] [Google Scholar]
- 3.Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP. 2010. Phenotypic plasticity's impacts on diversification and speciation. Trends Ecol. Evol. 25, 459–467. ( 10.1016/j.tree.2010.05.006) [DOI] [PubMed] [Google Scholar]
- 4.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 5.Lyytinen A, Brakefield PM, Lindstrom L, Mappes J. 2004. Does predation maintain eyespot plasticity in Bicyclus anynana? Proc. R. Soc. Lond. B 271, 279–283. ( 10.1098/rspb.2003.2571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50, 529–551. ( 10.1146/annurev.ento.50.071803.130420) [DOI] [PubMed] [Google Scholar]
- 7.Bretman A, Gage MJG, Chapman T. 2011. Quick-change artists: male plastic behavioural responses to rivals. Trends Ecol. Evol. 26, 467–473. ( 10.1016/j.tree.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 8.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 9.Kelly CD, Jennions MD. 2011. Sexual selection and sperm quantity: meta-analyses of strategic ejaculation. Biol. Rev. 86, 863–884. ( 10.1111/j.1469-185X.2011.00175.x) [DOI] [PubMed] [Google Scholar]
- 10.Candolin U, Reynolds JD. 2002. Adjustments of ejaculation rates in response to risk of sperm competition in a fish, the bitterling (Rhodeus sericeus). Proc. R. Soc. Lond. B 269, 1549–1553. ( 10.1098/rspb.2002.2055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DelBarco-Trillo J, Ferkin MH. 2006. Male meadow voles respond differently to risk and intensity of sperm competition. Behav. Ecol. 17, 581–585. ( 10.1093/beheco/ark001) [DOI] [Google Scholar]
- 12.Garcia-Gonzalez F, Gomendio M. 2004. Adjustment of copula duration and ejaculate size according to the risk of sperm competition in the golden egg bug (Phyllomorpha laciniata). Behav. Ecol. 15, 23–30. ( 10.1093/beheco/arg095) [DOI] [Google Scholar]
- 13.Evans JP, Pierotti M, Pilastro A. 2003. Male mating behavior and ejaculate expenditure under sperm competition risk in the eastern mosquitofish. Behav. Ecol. 14, 268–273. ( 10.1093/beheco/14.2.268) [DOI] [Google Scholar]
- 14.del Barco-Trillo J, Ferkin MH. 2004. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature 431, 446–449. ( 10.1038/nature02845) [DOI] [PubMed] [Google Scholar]
- 15.Smith C, Pateman-Jones C, Zięba G, Przybylski M, Reichard M. 2009. Sperm depletion as a consequence of increased sperm competition risk in the European bitterling, Rhodeus amarus. Anim. Behav. 77, 1227–1233. ( 10.1016/j.anbehav.2009.01.027) [DOI] [Google Scholar]
- 16.Simmons LW, Lovegrove M. 2017. Socially cued seminal fluid gene expression mediates responses in ejaculate quality to sperm competition risk. Proc. R. Soc. B 284, 20171486 ( 10.1098/rspb.2017.1486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sloan NS, Lovegrove M, Simmons LW. 2018. Social manipulation of sperm competition intensity reduces seminal fluid gene expression. Biol. Lett. 14, 20170659 ( 10.1098/rsbl.2017.0659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramm SA, Stockley P. 2009. Adaptive plasticity of mammalian sperm production in response to social experience. Proc. R. Soc. B 276, 745–751. ( 10.1098/rspb.2008.1296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Firman RC, Klemme I, Simmons LW. 2013. Strategic adjustments in sperm production within and between two island populations of house mice. Evolution 67, 3061–3070. ( 10.1111/evo.12164) [DOI] [PubMed] [Google Scholar]
- 20.Ramm SA, Edward DA, Claydon AJ, Hammond DE, Brownridge P, Hurst JL, Beynon RJ, Stockley P. 2015. Sperm competition risk drives plasticity in seminal fluid composition. BMC Biol. 13, 87 ( 10.1186/s12915-015-0197-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston BT, Stockley P. 2006. The prospect of sexual competition stimulates premature and repeated ejaculation in a mammal. Curr. Biol. 16, 239–241. ( 10.1016/j.cub.2006.03.018) [DOI] [PubMed] [Google Scholar]
- 22.Hosken DJ, Stockley P. 2004. Sexual selection and genital evolution. Trends Ecol. Evol. 19, 87–93. ( 10.1016/j.tree.2003.11.012) [DOI] [PubMed] [Google Scholar]
- 23.Simmons LW. 2014. Sexual selection and genital evolution. Austral. Entomol. 53, 1–17. ( 10.1111/aen.12053) [DOI] [Google Scholar]
- 24.Eberhard WG. 1985. Sexual selection and animal genitalia. Cambridge, MA: Harvard University Press. [Google Scholar]
- 25.Brennan PLR, Gereg I, Goodman M, Feng D, Prum RO. 2017. Evidence of phenotypic plasticity of penis morphology and delayed reproductive maturation in response to male competition in waterfowl. Auk 134, 882–893. ( 10.1642/AUK-17-114.1) [DOI] [Google Scholar]
- 26.Ramm SA. 2007. Sexual selection and genital evolution in mammals: a phylogenetic analysis of baculum length. Am. Nat. 169, 360–369. ( 10.1086/510688) [DOI] [PubMed] [Google Scholar]
- 27.Brindle M, Opie C. 2016. Postcopulatory sexual selection influences baculum evolution in primates and carnivores. Proc. R. Soc. B 283, 20161736 ( 10.1098/rspb.2016.1736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockley P, Ramm SA, Sherborne AL, Thom MDF, Paterson S, Hurst JL. 2013. Baculum morphology predicts reproductive success of male house mice under sexual selection. BMC Biol. 11, 66 ( 10.1186/1741-7007-11-66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simmons LW, Firman RC. 2014. Experimental evidence for the evolution of the mammalian baculum by sexual selection. Evolution 68, 276–283. ( 10.1111/evo.12229) [DOI] [PubMed] [Google Scholar]
- 30.Glucksmann A, Ooka-Souda S, Miura-Yasugi E, Mizuno T. 1976. The effect of neonatal treatment of male mice with antiandrogens and of females with androgens on the development of the os penis and os clitoridis. J. Anat. 121, 363–370. [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez E, et al. 2011. New insights on the morphology of adult mouse penis. Biol. Reprod. 85, 1216–1221. ( 10.1095/biolreprod.111.091504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez E, Weiss DA, Ferretti M, Wang H, Menshenia J, Risbridger G, Handelsman D, Cunha G, Baskin L. 2012. Specific morphogenetic events in mouse external genitalia sex differentiation are responsive/dependent upon androgens and/or estrogens. Differentiation 84, 269–279. ( 10.1016/j.diff.2012.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yonezawa T, Higashi M, Yoshioka K, Mutoh K. 2011. Distribution of aromatase and sex steroid receptors in the baculum during the rat life cycle: effects of estrogen during the early development of the baculum. Biol. Reprod. 85, 105–112. ( 10.1095/biolreprod.110.089508) [DOI] [PubMed] [Google Scholar]
- 34.Ramm SA, Stockley P. 2007. Ejaculate allocation under varying sperm competition risk in the house mouse, Mus musculus domesticus. Behav. Ecol. 18, 491–495. ( 10.1093/beheco/arm003) [DOI] [Google Scholar]
- 35.Zelditch ML, Swiderski DL, Sheets HD. 2012. Chapter 3 - Simple Size and Shape Variables: Shape Coordinates. In Geometric Morphometrics for Biologists (Second Edition) (eds Zelditch ML, Swiderski DL, Sheets HD), pp. 51–74. San Diego: Academic Press; ( 10.1016/B978-0-12-386903-6.00003-4) [DOI] [Google Scholar]
- 36.Zelditch ML, Swiderski DL, Sheets HD. 2012. Chapter 4 - Theory of Shape. In Geometric Morphometrics for Biologists (Second Edition) (eds Zelditch ML, Swiderski DL, Sheets HD), pp. 75–102. San Diego: Academic Press; ( 10.1016/B978-0-12-386903-6.00004-6) [DOI] [Google Scholar]
- 37.Bates D, Maechler M, Bolker B, Walker S.. 2017. Linear Mixed-Effects Models using ‘Eigen’ and S4 [R package lme4 version 1.1-13]. See https://cran.r-project.org/web/packages/lme4/index.html (accessed 1 June 2017).
- 38.Fox J, et al. 2016. Package ‘car’. CRAN Repos., 171 See https://cran.r-project.org/web/packages/car/car.pdf. [Google Scholar]
- 39.Warton DI, Wright IJ, Falster DS, Westoby M. 2006. Bivariate line-fitting methods for allometry. Biol. Rev. 81, 259 ( 10.1017/S1464793106007007) [DOI] [PubMed] [Google Scholar]
- 40.Ramm SA, Khoo L, Stockley P. 2009. Sexual selection and the rodent baculum: an intraspecific study in the house mouse (Mus musculus domesticus). Genetica 138, 129–137. ( 10.1007/s10709-009-9385-8) [DOI] [PubMed] [Google Scholar]
- 41.Tasikas DE, Fairn ER, Laurence S, Schulte-Hostedde AI. 2009. Baculum variation and allometry in the muskrat (Ondatra zibethicus): a case for sexual selection. Evol. Ecol. 23, 223–232. ( 10.1007/s10682-007-9216-2) [DOI] [Google Scholar]
- 42.Lϋpold S, Mcelligott AG, Hosken DJ. 2004. Bat genitalia: allometry, variation and good genes. Biol. J. Linn. Soc. 83, 497–507. ( 10.1111/j.1095-8312.2004.00407.x) [DOI] [Google Scholar]
- 43.Andrade CAC, Hatadani LM, Klaczko LB. 2005. Phenotypic plasticity of the aedeagus of Drosophila mediopunctata: effect of the temperature. J. Therm. Biol. 30, 518–523. ( 10.1016/j.jtherbio.2005.05.011) [DOI] [Google Scholar]
- 44.Hoch JM. 2009. Adaptive plasticity of the penis in a simultaneous hermaphrodite. Evolution 63, 1946–1953. ( 10.1111/j.1558-5646.2009.00668.x) [DOI] [PubMed] [Google Scholar]
- 45.Neufeld CJ, Palmer AR. 2008. Precisely proportioned: intertidal barnacles alter penis form to suit coastal wave action. Proc. R. Soc. B 275, 1081–1087. ( 10.1098/rspb.2007.1760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. 2004. Androgens and bone. Endocr. Rev. 25, 389–425. ( 10.1210/er.2003-0003) [DOI] [PubMed] [Google Scholar]
- 47.Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C. 2000. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc. Natl Acad. Sci. USA 97, 5474–5479. ( 10.1073/pnas.97.10.5474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanderschueren D, Venken K, Ophoff J, Bouillon R, Boonen S. 2006. Clinical review: sex steroids and the periosteum - reconsidering the roles of androgens and estrogens in periosteal expansion. J. Clin. Endocrinol. Metab. 91, 378–382. ( 10.1210/jc.2005-1766) [DOI] [PubMed] [Google Scholar]
- 49.Bouillon R, Bex M, Vanderschueren D, Boonen S. 2004. Estrogens are essential for male pubertal periosteal bone expansion. J. Clin. Endocrinol. Metab. 89, 6025–6029. ( 10.1210/jc.2004-0602) [DOI] [PubMed] [Google Scholar]
- 50.Venken K, Movérare-Skrtic S, Kopchick JJ, Coschigano KT, Ohlsson C, Boonen S, Bouillon R, Vanderschueren D. 2006. Impact of androgens, growth hormone, and IGF-I on bone and muscle in male mice during puberty. J. Bone Miner. Res. 22, 72–82. ( 10.1359/jbmr.060911) [DOI] [PubMed] [Google Scholar]
- 51.O'Shaughnessy PJ. 2014. Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol. 29, 55–65. ( 10.1016/j.semcdb.2014.02.010) [DOI] [PubMed] [Google Scholar]
- 52.Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A. 2001. Roles of growth hormone and insulin-like growth factor 1 in mouse postnatal growth. Dev. Biol. 229, 141–162. ( 10.1006/dbio.2000.9975) [DOI] [PubMed] [Google Scholar]
- 53.DeFries JC, McClearn GE. 1970. Social dominance and Darwinian fitness in the laboratory mouse. Am. Nat. 104, 408–411. ( 10.1086/282675) [DOI] [Google Scholar]
- 54.Cunningham CB, Ruff JS, Chase K, Potts WK, Carrier DR. 2013. Competitive ability in male house mice (Mus musculus): genetic influences. Behav. Genet. 43, 151–160. ( 10.1007/s10519-012-9577-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Firman RC, Simmons LW. 2008. Polyandry, sperm competition, and reproductive success in mice. Behav. Ecol. 19, 695–702. ( 10.1093/beheco/arm158) [DOI] [Google Scholar]
- 56.Klemme I, Firman RC. 2013. Male house mice that have evolved with sperm competition have increased mating duration and paternity success. Anim. Behav. 85, 751–758. ( 10.1016/j.anbehav.2013.01.016) [DOI] [Google Scholar]
- 57.Simmons LW, Buzatto BA. 2014. Contrasting responses of pre- and post-copulatory traits to variation in mating competition. Funct. Ecol. 28, 494–499. ( 10.1111/1365-2435.12211) [DOI] [Google Scholar]
- 58.Kasumovic MM, Hall MD, Try H, Brooks RC. 2011. The importance of listening: juvenile allocation shifts in response to acoustic cues of the social environment. J. Evol. Biol. 24, 1325–1334. ( 10.1111/j.1420-9101.2011.02267.x) [DOI] [PubMed] [Google Scholar]
- 59.Allen LE, Barry KL, Holwell GI, Herberstein ME. 2011. Perceived risk of sperm competition affects juvenile development and ejaculate expenditure in male praying mantids. Anim. Behav. 82, 1201–1206. ( 10.1016/j.anbehav.2011.09.009) [DOI] [Google Scholar]
- 60.Huchard E, English S, Bell MBV, Thavarajah NK, Clutton-Brock TH. 2016. Competitive growth in a cooperative mammal. Nature 533, 532–534. ( 10.1038/nature17986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foo YZ, Nakagawa S, Rhodes G, Simmons LW. 2017. The effects of sex hormones on immune function: a meta-analysis. Biol. Rev. 92, 551–571. ( 10.1111/brv.12243) [DOI] [PubMed] [Google Scholar]
- 62.Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussière LF. 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432, 1024–1027. ( 10.1038/nature03084) [DOI] [PubMed] [Google Scholar]
- 63.Robinson MR, Pilkington JG, Clutton-Brock TH, Pemberton JM, Kruuk LEB. 2006. Live fast, die young: trade-offs between components and sexually antagonistic selection on weaponry in Soay Sheep. Evolution 60, 2168–2181. ( 10.1111/j.0014-3820.2006.tb01854.x) [DOI] [PubMed] [Google Scholar]
- 64.Bretman A, Westmancoat JD, Gage MJG, Chapman T. 2013. Costs and benefits of lifetime exposure to mating rivals in male Drosophila Melanogaster. Evolution 67, 2413–2422. ( 10.1111/evo.12125) [DOI] [PubMed] [Google Scholar]
- 65.Greenwald GS. 1956. The reproductive cycle of the field mouse, Microtus californicus. J. Mammal. 37, 213 ( 10.2307/1376680) [DOI] [Google Scholar]
- 66.Ewer RF. 1973. The carnivores. Ithaca, NY: Cornell University Press [Google Scholar]
- 67.Dixson AF. 1987. Observations on the evolution of the genitalia and copulatory behaviour in male primates. J. Zool. 213, 423–443. ( 10.1111/j.1469-7998.1987.tb03718.x) [DOI] [Google Scholar]
- 68.Bronson FH. 1979. The reproductive ecology of the house mouse. Q. Rev. Biol. 54, 265–299. ( 10.1086/411295) [DOI] [PubMed] [Google Scholar]
- 69.Yang JJ, Larsen CM, Grattan DR, Erskine MS. 2009. Mating-induced neuroendocrine responses during pseudopregnancy in the female mouse. J. Neuroendocrinol. 21, 30–39. ( 10.1111/j.1365-2826.2008.01803.x) [DOI] [PubMed] [Google Scholar]
- 70.Firman RC, Simmons LW. 2012. Male house mice evolving with post-copulatory sexual selection sire embryos with increased viability. Ecol. Lett. 15, 42–46. ( 10.1111/j.1461-0248.2011.01706.x) [DOI] [PubMed] [Google Scholar]
- 71.Miki K, Clapham DE. 2013. Rheotaxis guides mammalian sperm. Curr. Biol. 23, 443–452. ( 10.1016/j.cub.2013.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eberhard WG. 1996. Female control: sexual selection by cryptic female choice. Princeton, NJ: Princeton University Press. [Google Scholar]
- 73.Pfaus JG, Kippin TE, Coria-Avila GA, Gelez H, Afonso VM, Ismail N, Parada M. 2012. Who, what, where, when (and maybe even why)? How the experience of sexual reward connects sexual desire, preference, and performance. Arch. Sex. Behav. 41, 31–62. ( 10.1007/s10508-012-9935-5) [DOI] [PubMed] [Google Scholar]
- 74.André GI, Firman RC, Simmons LW. 2018. Data from: Phenotypic plasticity in genitalia: baculum shape responds to sperm competition risk in house mice Dryad Digital Repository. ( 10.5061/dryad.57v6627) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- André GI, Firman RC, Simmons LW. 2018. Data from: Phenotypic plasticity in genitalia: baculum shape responds to sperm competition risk in house mice Dryad Digital Repository. ( 10.5061/dryad.57v6627) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available at the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.57v6627) [74].