Abstract
Artificial light at night (ALAN) affects over 20% of the earth's surface and is estimated to increase 6% per year. Most studies of ALAN have focused on a single mechanism or life stage. We tested for indirect and direct ALAN effects that occurred by altering American toads' (Anaxyrus americanus) ecological interactions or by altering toad development and growth, respectively. We conducted an experiment over two life stages using outdoor mesocosms and indoor terraria. In the first phase, the presence of ALAN reduced metamorphic duration and periphyton biomass. The effects of ALAN appeared to be mediated through direct effects on toad development, and we found no evidence for indirect effects of ALAN acting through altered ecological interactions or colonization. In the second phase, post-metamorphic toad growth was reduced by 15% in the ALAN treatment. Juvenile-stage ALAN also affected toad activity: in natural light, toads retreated into leaf litter at night whereas ALAN toads did not change behaviour. Carry-over effects of ALAN were also present; juvenile toads that had been exposed to larval ALAN exhibited marginally increased activity. In this time frame and system, our experiments suggested ALAN's effects act primarily through direct effects, rather than indirect effects, and can persist across life stages.
Keywords: ecological light pollution, night lighting, direct and indirect effects, legacy effects, American toad, Bufo americanus
1. Background
Artificial light at night (ALAN) is one of the most significant human-induced environmental changes [1,2], yet many of its effects are still poorly understood [3]. Determining ALAN's effects on organisms' growth and survival is complicated by the fact that these effects emerge from both how ALAN changes an organism's interactions with other species (‘indirect effects’) and how ALAN alters an individual's physiology (‘direct effects’) [4]. Further adding to the challenge of studying ALAN is the fact that it may have different effects at different life stages.
ALAN may indirectly affect individuals through two distinct ecological mechanisms: changing dispersal into and out of an ecological community, or altering the outcome of interspecific interactions within a community [4]. Examples of ALAN altering community composition include changes in the colonization behaviour of marine invertebrates [5] or inhibition of drifting behaviour in freshwater invertebrates [6]. Regardless of whether ALAN changes community composition, ALAN can also indirectly affect individuals by altering the outcome of species interactions [4]. For example, nocturnal illumination similar to that of a full moon facilitates owls' ability to hunt mice [7]. As a result, nocturnal mice exposed to ALAN may alter the time when they forage, and consequently increase competition with diurnal congeners [8]. ALAN can also cause asymmetric interspecific competition, such that competition is stronger in lit habitats (e.g. urban) compared with darker habitats (e.g. forest) [9].
ALAN also directly affects individuals by altering their physiology [10–12]. For example, exposure to ALAN can alter the amount or timing of feeding [13,14], accelerate reproductive organ development [15] and slow larval development [16,17]. Altered behaviour has been one of the most studied direct effects of ALAN, and includes improper orientation [18,19], and altered anti-predator behaviours [20,21] or activity periods [22]. Although there are many examples of direct effects of ALAN, the majority of studies are done in the laboratory, which limits our ability to relate ALAN's effects on survival and growth in natural populations [3].
Further limiting our inferences is the fact that ALAN studies typically focus on a single life stage. Early exposure to a wide range of anthropogenic and natural stressors [23–26] can affect individuals throughout their lives. To our knowledge, only one study has tested for carry-over effects of early exposure to ALAN: 67% of newborn mice exposed to constant light had disrupted circadian rhythms [27]. Subsequent exposure to normal photoperiods, however, restored regular circadian rhythms within 3–5 months [27], demonstrating the potential to reverse carry-over effects. Carry-over effects of ALAN may be particularly important for wild animals that migrate or use different environments at different life stages. These animals may be carrying the effects of early ALAN exposure, even if their subsequent habitats do not experience ALAN. As a consequence, studies that are constrained to a single life stage may under- or over-estimate the effects of ALAN.
Due to the many mechanisms and life stages through which ALAN may affect an organism, it is important to assess these multiple mechanisms and stages. Here, we describe a study in which we investigated direct and indirect effects of ALAN across two life stages using a widespread amphibian. Amphibians may be vulnerable to ALAN for three main reasons: (i) amphibians use natural light as a cue for behaviours across both larval and post-metamorphic life stages (e.g. activity [28]); (ii) ALAN may indirectly affect amphibians by influencing other community members that have strong interactions with amphibians; and (iii) ALAN may have different effects in each stage, and these effects may carry over.
2. Material and methods
(a). Study system
We used the American toad (Anaxyrus americanus) because they are geographically widespread and a habitat generalist. They breed in water bodies ranging from permanent lakes to roadside ditches [29], including environments that are exposed to ALAN. Larval toads feed on periphyton, and the growth of periphyton can be affected by ALAN [30]. Larval toads are also preyed upon by a wide range of aquatic predatory invertebrates [29], and thus if ALAN changes the colonization rate of aquatic invertebrates, it can change predation risk for larval toads.
(b). Experiment 1: testing for direct and indirect effects of artificial light at night in larvae
We conducted an outdoor experiment in 2 m diameter mesocosms to investigate indirect and direct effects of artificial light on American toad larval growth, development and survival. We used a 2 × 2 × 2 experimental design in which we crossed the presence or absence of ALAN with tightly fitting lids (to control colonization) and toad larvae (electronic supplementary material, figures S1 and S2). Mesocosms were placed into 10 groups of four mesocosms (40 total mesocosms split into 10 groups). Five groups of four mesocosms (20 mesocosms) were assigned to the ALAN light treatment, and the other five groups were assigned to the natural light treatment. Within each group of mesocosms in the light treatments, half of the mesocosms (2 mesocosms per group) were assigned to a limited-colonization treatment (i.e. lids made of shade cloth are present to reduce colonization) and the other half were assigned to be a free-colonization treatment (i.e. the other two mesocosms did not receive shade cloth lids). Finally, toad presence and absence was manipulated such that half of the mesocosms in each lid group were stocked with 50 larvae (2 mesocosms per group, 1 of each colonization treatment), and the other half of the mesocosms in each lid group did not receive toad larvae. Thus, all eight combinations of light, colonization and toad presence were initially replicated five times. Early in the experiment, we removed one group of four mesocosms in the ALAN treatment because heavy shading and leaf input from a nearby tree caused temperature and dissolved oxygen to be very different from the other mesocosms.
We used a single 20-watt LED outdoor floodlight (Zitrades, China) mounted in the middle of each ALAN mesocosm group approximately 1.5 m above ground and 0.75 m above the top of the mesocosms (electronic supplementary material, figure S2). These floodlights measured 835 lux directly underneath with peak wavelengths in the blue-green spectrums (electronic supplementary material, figure S4). Average light levels reaching the water surface were 15.07 lux ± 7.42 s.e. without lids and 3.12 lux ± 1.84 s.e. with lids. Our light intensities were within the range (4.14–349.2 lux) of ALAN measured near wetlands around greater Cleveland, Ohio (electronic supplementary material, figure S3). We chose LED lights because their use in outdoor lighting is becoming increasingly widespread and they have the potential to become the predominant outdoor light source [31]. All ALAN floodlights were connected to a timer set to turn on at 20.00 and turn off at 07.00. Data loggers were placed approximately 10 cm below the surface in each mesocosm to monitor temperature hourly from 29 May to 2 July 2015.
All mesocosms (2 m diameter polyethylene cattle tanks) were filled with approximately 700 l of tap water between 14 and 15 April 2015. Between 17 April and 9 May 2015, we added approximately 11 l of mixed hardwood leaves, 3.8 l of pond water, 1.45 l of pond mud, 0.38 l of zooplankton, one water scorpion (Nepidae), five snails (Planorbidae) and eight dragonfly larvae (Libellulidae) into each mesocosm. These organisms and resources were added to make our mesocosms as realistic as possible, thus pond mud, leaves and water were not sterilized before adding it to our mesocosms. We did sift through the mud and water to ensure no large predatory larvae were added to any mesocosm. However, it is likely that other small invertebrates found in the pond mud or water were added to our mesocosms. On 7 May 2015, we collected toad egg strings from five areas of one pond at the Case Western Reserve University's Squire Valleevue and Valley Ridge Farm in Hunting Valley, Ohio. By selecting different areas of the pond to collect egg strings from, we expect this resulted in collecting at least five maternal lines of toads. On 8 May 2015, 50 toad larvae were added to each of the 20 toad treatment mesocosms. These 50 toad larvae consisted of 10 toads from each of the five collection areas to increase genetic diversity. On 8 May 2015, we also began the colonization-limited treatment by adding the tightly fitting lids onto the colonization-limited mesocosms. Lids were constructed of 60% shade cloth and were weighed down by sand-filled tubing around the edges. All lids were approximately 2.5 m in diameter and completely covered the mesocosm. ALAN lights were maintained on the timer from 11 May 2015 until all toads metamorphosed.
To test for treatment effects on toad growth, development and survival, we counted and weighed all metamorphosing toads. Once metamorphs completely resorbed their tails, the first eight metamorphs in each free-colonization mesocosm were transported to the laboratory and used in the second experiment (described below). We weighed, euthanized (using tricaine methanesulfonate; MS-222) and preserved all other metamorphs in 70% ethanol as they emerged.
To test for treatment effects on the relative abundance of periphyton (main food resource for toad larvae) and phytoplankton, we measured relative chlorophyll-a using an Aquafluor fluorometer (Turner Designs, Sunnyvale, CA) [32] following methods in [23]. We used mean leaf fluorescence values for each mesocosm in statistical analyses. We measured relative phytoplankton and periphyton biomass on 10 June and 2 July 2015.
To test for treatment effects on invertebrate and amphibian colonization, we performed pipe sampling using plastic garbage cans with the bottoms removed (25 cm long × 16 cm wide). The authors stood on opposite sides of the mesocosm and each pushed the can down into the water column until it hit the bottom. We swept dipnets from the bottom to the top and collected all invertebrates and amphibians until we had 10 empty nets in a row. All invertebrates (except for Nepidae) were immediately preserved in 70% ethanol, and amphibians were euthanized using MS-222 and then preserved in 70% ethanol. Pipe samples were performed on 3 June and 19 June 2015 but combined for statistical analyses.
(i). Statistical analyses
To distinguish between direct and indirect effects, we tested for effects of the ALAN and colonization treatments on mean toad survival, mass at metamorphosis, larval duration and metamorphic duration using a general linear mixed model with a random effect of mesocosm nested within light treatment to account for the groups of four mesocosms. Our experimental unit was the mesocosm. Of particular interest were interactions between the colonization and ALAN treatments, because such an interaction would indicate an indirect effect of ALAN acting through colonization. Survival was calculated as the number of metamorphosed toads divided by the initial stocking density, then cube transformed to meet assumptions of normality. Mean mass at metamorphosis was calculated as the average mass of all metamorphosed toads from each mesocosm. Larval duration was calculated as the average number of days from hatch (8 May) to metamorphosis. Metamorphic duration was the number of days between the first and the last toad to metamorphosis in each mesocosm. Metamorphic duration was reciprocally transformed (1/metamorphic duration) to meet the assumption of normality.
We tested for treatment effects on relative algal biomass using repeated measures ANOVA with a random effect of light treatment nested within group. For relative periphyton and phytoplankton biomass, we used mean relative biomass from both sampling days as response variables. No interactions between main effects were significant and therefore we eliminated them from our analyses.
We tested for treatment effects on invertebrate abundance and diversity and larval amphibian abundance. Within each mesocosm, pipe samples from both sampling days were combined. All invertebrates were identified to order and categorized as whether or not they were a predator of tadpoles. We tested for treatment effects on total invertebrate abundance, diversity of invertebrate taxonomic orders, and log-transformed invertebrate predator abundance using ANOVA. We also tested for treatment effects on the abundance of non-predatory invertebrates using a general linear model with a Poisson distribution. Aside from the intentionally stocked toads, we also found tadpoles from the treefrog family Hylidae, which colonized the mesocosms. We pooled hylid counts from both sampling days and tested for treatment effects on total hylid abundance. To account for the grouping of mesocosms, we used a general linear mixed model with a random effect of light treatment nested within group with a Poisson distribution. We performed all analyses in R using packages car, Hmisc and MASS [33].
(c). Experiment 2: prior and subsequent effects of light on post-metamorphic toads
Using toads from experiment 1, we collected the first eight metamorphosing toads from the free colonization treatments (five natural and four ALAN mesocosms) and transported them back to the laboratory (electronic supplementary material, figure S1) to test whether ALAN affected post-metamorphic growth and survival. All toads for this experiment metamorphosed by 6 June 2015. While in the laboratory, toads were randomly assigned to either the natural or ALAN light treatment, for a total of 18 experimental units (9 per juvenile light treatment), each holding 4 toads.
Toads were kept in plastic terraria (42.5 × 30.2 × 17.8 cm) with lids. We placed terraria at a slant; the upper portion of the terraria contained dried leaves and the lower portion held 0.25 l of dechlorinated tap water. All juvenile toads were housed in the same room at approximately 23°C with the room lights on a 14 : 10 light:dark photoperiod. ALAN treatment lights used in the laboratory were 24-watt LED string lights (LE, China; 61 lux at the top of the terraria) placed 50.8 cm above the terraria. The average of four measurements at the cardinal directions taken at the terraria surface was 16.9 ± 3.04 lux. Similar to the outdoor LED lights used previously, laboratory LED lights had peak wavelengths in the blue-green spectrums (electronic supplementary material, figure S4). On 17 June 2015, our experiment started and the ALAN lights were set on a timer to turn on at 21.00 and turn off at 06.00, coinciding with the times in which the main laboratory lights turn off and on, respectively. We fed toads fruit flies or crickets ad libitum daily. Coinciding with weekly water and leaf replacement, we weighed each toad to determine growth. We kept toads in their treatments until 2 September 2015, at which time we weighed and euthanized all toads.
On 16 July 2015, we measured toad activity during the day and night. During each observation period, we observed toads for 30 s, after a 10 s acclimation period. We approached the terraria, waited 10 s, and then counted how many toads were visible for a 30 s period. Two observations were made during the day (all terraria lit) and three observations were made at night (only ALAN terraria lit). We made all night observations using a dimly lit red headlamp. While it was easier to see into the terraria with ALAN in the absence of the red headlamp, the red headlamp sufficiently lit the natural light treatment terraria such that we could clearly see into the terraria in order to count visible toads.
(i). Statistical analyses
We tested for effects of larval and juvenile light on toad survival, mass and activity. To determine if there were effects of ALAN on survival, we used ANOVA with larval light, juvenile light and their interaction as explanatory variables, and mean survival as the response variable.
We used repeated-measures ANOVA to test for treatment effects on toad growth on the following dates: 24 June, 9 July, 24 July, 8 August, 20 August and 2 September. This statistical model included larval and juvenile light treatments and initial mass (17 June) as between-subject test variables, and time, the interaction between time and juvenile light, larval light and initial mass as within-subject test variables.
For activity, we used repeated-measures ANOVA using the proportion of visible toads (number of toad visible divided by total number of toads present) as the response variable and larval light, juvenile light, observation time and their interactions as explanatory variables. We performed all analyses in R using packages car, ggplot2, Hmisc, MASS and Rmisc [33].
3. Results
(a). Experiment 1: testing for direct and indirect effects of artificial light at night in larvae
Mean temperature was affected by the presence of lids (F1,30 = 15.47, p < 0.001) but not light (F1,30 = 0.86, p = 0.36) or their interaction (F1,30 = 2.67, p = 0.11). Free-colonization mesocosms had mean temperatures of 22.55°C ± 0.09 standard error (s.e.) whereas colonization-limited mesocosms were 21.62°C ± 1.22 s.e. Mean survival (78.67% ± 0.03 s.e.) and mass at metamorphosis (0.16 g ± 0.01 s.e.) were not affected by any treatment or interaction (electronic supplementary material, table S1). Larval duration was marginally reduced by 1 day in ALAN treatments (F1,7 = 5.04, p = 0.06) and reduced by 8 days in the free-colonization treatments (F1,7 = 179.99, p < 0.001). Metamorphic duration was reduced by approximately 30% in the ALAN treatments (F1,7 = 6.39, p = 0.04).
Relative periphyton biomass was reduced by ALAN (F1,32 = 7.43, p = 0.01; electronic supplementary material, table S2). Mean periphyton abundance was 79.8 ± 10.62 s.e. in mesocosms under natural light conditions and 47.02 ± 6.26 s.e. for mesocosms in ALAN treatments. Relative phytoplankton biomass was increased in colonization-limited mesocosms (F1,32 = 4.17, p = 0.05; electronic supplementary material, table S2). Mean phytoplankton biomass was 23.85 ± 3.59 s.e. for mesocosms with lids present and 19.7 ± 1.77 s.e. for mesocosms with lids absent.
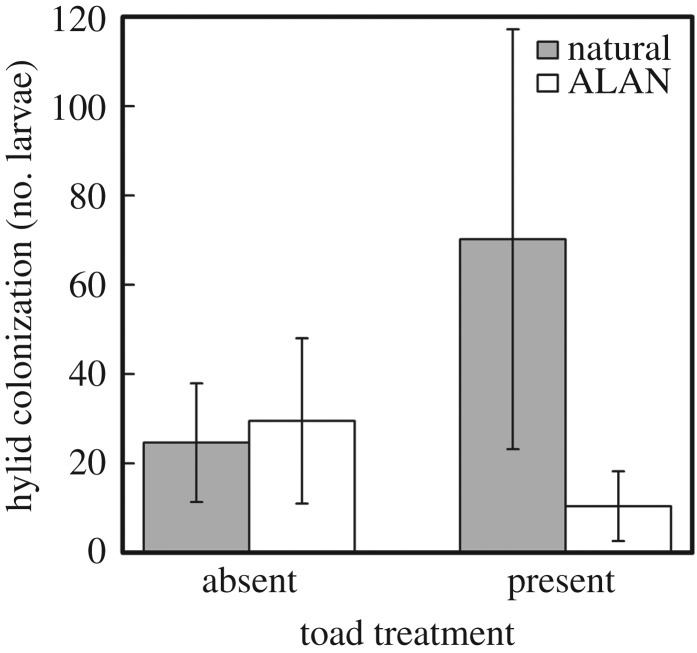
While the total number of invertebrates was similar across treatments, the colonization-limited treatment reduced the diversity of invertebrates in the mesocosms (F1,28 = 6.94, p = 0.01; electronic supplementary material, table S3). Mesocosms with free colonization had on average 2.1 ± 0.2 s.e. taxonomic orders of invertebrates, while colonization-limited mesocosms had 1.3 ± 0.2 s.e. orders of invertebrates. Hylid frogs (spring peepers Pseudacris crucifer; grey treefrogs Hyla versicolor) readily colonized 24 out of 36 mesocosms (range: 4–481 total individuals collected within all pipe samples, median: 43 individuals across all pipe samples). Many Hylidae tadpoles captured during dipnet sampling had recently hatched and were too small for us to distinguish between species. However, we found breeding H. versicolor adults and mostly larger H. versicolor tadpoles later in the season, suggesting the majority of the hylid tadpoles were H. versicolor. Hylid abundance was significantly affected by the interaction between ALAN and toad presence (F1,28 = −3.24, p = 0.004; figure 1). Tukey post hoc tests (p-value < 0.05) indicate that hylid abundance was significantly higher when toads were present under natural light conditions compared with the other treatments.
Figure 1.
Effects of light and toad treatments on hylid colonization. Hylid abundance was significantly affected by the interaction between ALAN and toad presence. Tukey post hoc tests indicated that hylid abundance was significantly higher when toads were present under natural light conditions compared to all other treatments. Letters represent significant (p < 0.05) differences between treatments. Error bars represent standard error.
(b). Experiment 2: prior and subsequent effects of light on post-metamorphic toads
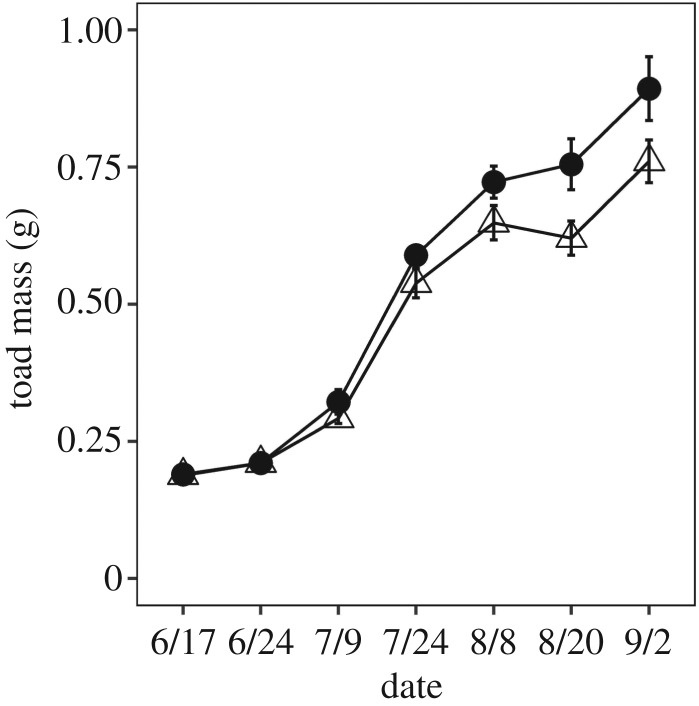
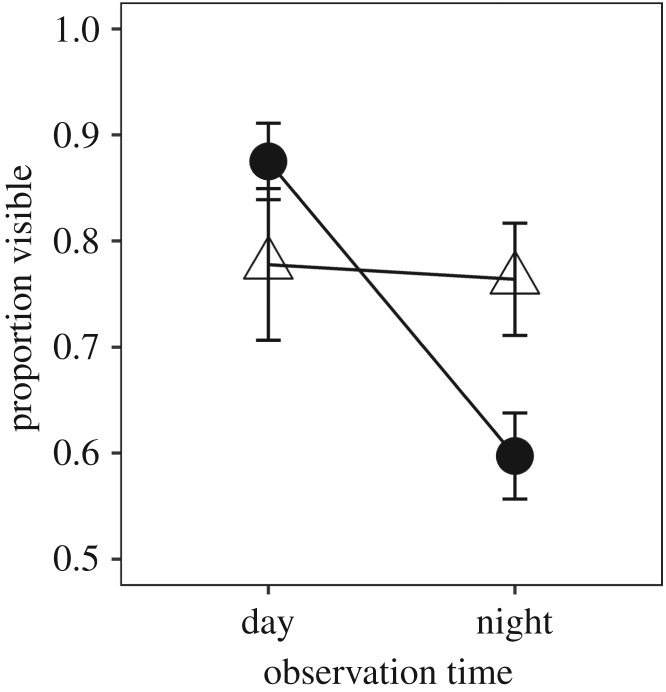
While juvenile toad survival was high across all treatments (83% ± 5 s.e.), juvenile toad growth was reduced by exposure to juvenile-stage ALAN (F1,14 = 6.46, p = 0.02; figure 2; electronic supplementary material, table S4). Juvenile toads in the laboratory were marginally more active if they had been raised with ALAN as tadpoles (F1,14 = 4.17, p = 0.06; electronic supplementary material, figure S5). Toad activity was also significantly affected by the interaction of juvenile light treatment and observation time (F1,14 = 14.56, p = 0.002; electronic supplementary material, table S5). Tukey post hoc test indicated juvenile toads in the natural light treatment were visible during the day but went under the leaves at night (p = 0.002); juvenile toads in the ALAN light treatment, however, were equally visible in the day and night (p = 0.99) (figure 3).
Figure 2.
Effects of juvenile-stage light exposure on juvenile toad mass. Juvenile toad growth was reduced by exposure to juvenile-stage ALAN. Triangles represent ALAN light treatments, circles represent natural light treatments. Error bars represent standard error.
Figure 3.
The proportion of visible toads in the ALAN treatment did not differ between day and night observations, whereas toads in natural light treatments were more active during the day than at night. Triangles represent ALAN treatments and circles represent natural light treatments. Error bars represent standard error.
4. Discussion
We were surprised that we did not find evidence for indirect effects of ALAN in our study, despite these effects occurring in other systems (e.g. [5,34,35]). There were two ways through which we expected indirect effects. First, ALAN might have increased periphyton growth [30], which would in turn affect food availability for toad larvae. However, while ALAN actually reduced periphyton growth, this did not cause food limitation for the toads: there was no effect of toad presence on periphyton growth, which we would have expected if periphyton was a limiting resource for toads. The second way we could have detected an indirect effect was if ALAN affected colonization of invertebrates, particularly if ALAN increased the abundance of predators on larval toads. We predicted that ALAN would alter invertebrate immigration and thus community composition through differential attraction of invertebrates to ALAN [5,6,36]. We found no interaction between the light and colonization treatments, which indicated that ALAN did not affect colonization by invertebrates in a way that affected larval toads.
Although there was no effect of ALAN-mediated colonization on toad larvae, hylid treefrogs preferentially oviposited in mesocosms under natural light treatments when toads were present. Previous studies found female hylids avoided ovipositing in wetlands with certain types of predators and conspecifics but did not respond to heterospecifics or nutrients [37–40]. The presence of toad larvae may signal suitable habitat quality for breeding hylids, although mesocosm experiments have found that toad larvae can have a strong competitive effect on hylids [41]. Regardless of the mechanism that caused hylid colonization to be biased towards mesocosms without ALAN and with toads, we found no evidence that hylid colonization affected the toads.
The primary direct effect of ALAN in the larval stage was increased metamorphic synchrony (i.e. reduced metamorphic duration). In ALAN treatments, toads from a single mesocosm typically metamorphosed over six days, compared with nine days in natural light. Increased synchrony of life-history events has previously been shown to occur as an anti-predator response [42–44]. Perceived predation risk associated with light, natural or artificial, can alter predation rates and thus prey behaviour in mammals [7,45], fish [46], amphibians [47] and birds [48]. Therefore, the perceived risk associated with light may have induced the metamorphic synchrony in toads under ALAN even in the absence of a predator.
We did not find evidence for strong carry-over effects of ALAN exposure, despite carry-over effects being widespread for other stressors [23–26]. Our experiment found ALAN had a marginally significant carry-over effect: larval-stage ALAN exposure led to marginally more active juvenile toads. We also did not find larval-stage exposure effects on juvenile growth. There were direct effects of ALAN exposure during the juvenile stage; ALAN reduced juvenile growth by 15% and eliminated the transition from diurnal to nocturnal behaviour. The increased nocturnal activity in ALAN treatments may have resulted in greater energy expenditure and therefore significantly slower juvenile growth despite being fed ad libitum. In contrast, laboratory mice raised in ALAN altered behaviour such that the mice ate during normal times of inactivity which increased weight gain even when the quantity of food consumed between groups remained constant [14]. There are many negative consequences of reduced growth, including delayed reproductive maturity, lower fecundity and reduced survival [49–52]. Thus, toads inhabiting terrestrial habitats with ALAN may ultimately have lower fitness, which could scale up to affect survival, fecundity or population growth rates.
5. Conclusion
Natural light is a critical part of circadian and circannual rhythms, which affect ecological systems and individual physiology [53]. As a result, ALAN can have wide-reaching effects across taxa and habitats [3]. Amphibians in particular are rapidly declining, and this decline has been linked to multiple anthropogenic factors. Despite the many ecological and physiological effects of ALAN, ALAN has not been investigated as a potential contributor to amphibian decline. As a result of this research focusing on multiple mechanisms and life stages, we detected a particularly troubling finding: reduced juvenile toad growth in response to ALAN. This reduced growth may suggest toads exposed to ALAN may incur reduced fitness later in life, especially if additional stressors (e.g. predators, competitors, desiccation risk) are present and alter the effect of ALAN. Thus, our work extends our understanding of the scope of ALAN's effects across ecological contexts and life stages.
Supplementary Material
Acknowledgements
We thank Ana Locci, Shane Brown, Joe Miller, and Alan Aldridge of the CWRU Squire Valleevue and Valley Ridge Farms for logistical support. Hilary Rollins, Colin Cope, Mimi Guo, Addie Klimek, Erin Conway, Tim Nicholas, Alex Grossman and Catherine Chervenek provided valuable assistance in conducting this experiment. We benefitted from helpful feedback on this manuscript from members of the Benard Lab and the CWRU Biology Department's Ecology and Evolution journal club.
Ethics
All experimental research was done under Institutional Animal Care and Use Committee approved protocol 2015-0054. Amphibians were collected under Ohio Department of Natural Resources Wild Animal Permit 18-69 to M.F.B.
Data accessibility
Data are available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.pq54gh5) [54].
Authors' contributions
K.L.D. and M.F.B. conceived of the study, performed fieldwork, analysed data and wrote the manuscript. Both authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
We received no funding for this study.
References
- 1.Gaston KJ, Bennie J. 2014. Demographic effects of artificial nighttime lighting on animal populations. Environ. Rev. 22, 1–8. ( 10.1139/er-2014-0005) [DOI] [Google Scholar]
- 2.Navara KJ, Nelson RJ. 2007. The dark side of light at night: physiological, epidemiological, and ecological consequences. J. Pineal Res. 43, 215–224. ( 10.1111/j.1600-079X.2007.00473.x) [DOI] [PubMed] [Google Scholar]
- 3.Gaston KJ, Visser ME, Hölker F. 2015. The biological impacts of artificial light at night: the research challenge. Phil. Trans. R. Soc. B 370, 20140133 ( 10.1098/rstb.2014.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaston KJ, Duffy JP, Gaston S, Bennie J, Davies TW. 2014. Human alteration of natural light cycles: causes and ecological consequences. Oecologia 176, 917–931. ( 10.1007/s00442-014-3088-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies TW, Coleman M, Griffith KM, Jenkins SR. 2015. Night-time lighting alters the composition of marine epifaunal communities. Biol. Lett. 11, 20150080 ( 10.1098/rsbl.2015.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perkin EK, Hölker F, Tockner K, Richardson JS. 2014. Artificial light as a disturbance to light-naïve streams. Freshw. Biol. 59, 2235–2244. ( 10.1111/fwb.12426) [DOI] [Google Scholar]
- 7.Clarke JA. 1983. Moonlight's influence on predator/prey interactions between short-eared owls (Asio flammeus) and deermice (Peromyscus maniculatus). Behav. Ecol. Sociobiol. 13, 205–209. ( 10.1007/BF00299924) [DOI] [Google Scholar]
- 8.Rotics S, Dayan T, Kronfeld-Schor N. 2011. Effect of artificial night lighting on temporally partitioned spiny mice. J. Mammal. 92, 159–168. ( 10.1644/10-MAMM-A-112.1) [DOI] [Google Scholar]
- 9.Petren K, Bolger DT, Case TJ. 1993. Mechanisms in the competitive success of an invading sexual gecko over an asexual native. Science 259, 354–358. ( 10.1126/science.259.5093.354) [DOI] [PubMed] [Google Scholar]
- 10.Beier P. 2006. Effects of artificial night lighting on terrestrial mammals. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T), pp. 19–42. Washington, DC: Island Press. [Google Scholar]
- 11.Inger R, Bennie J, Davies TW, Gaston KJ. 2014. Potential biological and ecological effects of flickering artificial light. PLoS ONE 9, e98631 ( 10.1371/journal.pone.0098631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry G, Buchanan BW, Fisher RN, Salmon M, Wise SE. 2008. Effects of artificial night lighting on amphibians and reptiles in urban environments. Urban Herpetol. 3, 239–256. [Google Scholar]
- 13.Pan J, Yang Y, Yang B, Yu Y. 2014. Artificial polychromatic light affects growth and physiology in chicks. PLoS ONE 9, 113595 ( 10.1371/journal.pone.0113595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. 2010. Light at night increases body mass by shifting the time of food intake. Proc. Natl Acad. Sci. USA 107, 18 664–18 669. ( 10.1073/pnas.1008734107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominoni D, Quetting M, Partecke J. 2013. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280, 20123017 ( 10.1098/rspb.2012.3017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez P, Delgado MJ, Alonso-Bedate M. 1984. Influence of photoperiod and melatonin administration on growth and metamorphosis in Discoglossus pictus larvae. Comp. Biochem. Physiol. 79A, 255–260. ( 10.1016/0300-9629(84)90425-0) [DOI] [Google Scholar]
- 17.Edwards MLO, Pivorun EB. 1991. The effects of photoperiod and different dosages of melatonin on metamorphic rate and weight gain in Xenopus laevis tadpoles. Gen. Comp. Endocrinol. 81, 28–38. ( 10.1016/0016-6480(91)90122-M) [DOI] [PubMed] [Google Scholar]
- 18.Salmon M. 2006. Protecting sea turtles from artificial night lighting at Florida's oceanic beaches. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T), pp. 141–168. Washington, DC: Island Press. [Google Scholar]
- 19.Gauthreaux SA, Belser CG. 2006. Effects of artificial night lighting on migrating birds. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T), pp. 67–93. Washington, DC: Island Press. [Google Scholar]
- 20.Frank KD. 2006. Effects of artificial night lighting on moths. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T), pp. 305–344. Washington, DC: Island Press. [Google Scholar]
- 21.Yorzinski JL, Chisholm S, Byerley SD, Coy JR, Aziz A, Wolf JA, Gnerlich AC. 2015. Artificial light pollution increases nocturnal vigilance in peahens. PeerJ 3, e1174 ( 10.7717/peerj.1174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Da Silva A, Valcu M, Kempenaers B. 2015. Light pollution alters the phenology of dawn and dusk singing in common European songbirds. Phil. Trans. R. Soc. B 370, 20140126 ( 10.1098/rstb.2014.0126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dananay KL, Krynak KL, Krynak TJ, Benard MF. 2015. Legacy of road salt: apparent positive larval effects counteracted by negative post-metamorphic effects in wood frogs. Environ. Toxicol. Chem. 34, 2417–2424. ( 10.1002/etc.3082) [DOI] [PubMed] [Google Scholar]
- 24.Rohr JR, Raffel TR, Halstead NT, McMahon TA, Johnson SA, Boughton RK, Martin LB. 2013. Early-life exposure to a herbicide has enduring effects on pathogen-induced mortality. Proc. R. Soc. B 280, 20131502 ( 10.1098/rspb.2013.1502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck CW, Congdon JD. 2000. Effects of age and size at metamorphosis on performance and metabolic rates of Southern Toad, Bufo terrestris, metamorphs. Funct. Ecol. 14, 32–38. ( 10.1046/j.1365-2435.2000.00386.x) [DOI] [Google Scholar]
- 26.Benard MF, Fordyce JA. 2003. Are induced defenses costly? Consequences of predator-induced defenses in Western toads, Bufo boreas. Ecology 84, 68–78. ( 10.1890/0012-9658(2003)084%5B0068:AIDCCO%5D2.0.CO;2) [DOI] [Google Scholar]
- 27.Ohta H, Mitchell AC, McMahon DG. 2006. Constant light disrupts the developing mouse biological clock. Pediatr. Res. 60, 304–308. ( 10.1203/01.pdr.0000233114.18403.66) [DOI] [PubMed] [Google Scholar]
- 28.Beiswenger RE. 1977. Diel patterns of aggregative behavior in tadpoles of Bufo americanus, in relation to light and temperature. Ecology 58, 98–108. ( 10.2307/1935111) [DOI] [Google Scholar]
- 29.Brune CR. 2013. Eastern American toad. In Amphibians of Ohio (eds Pfingsten RA, Davis JG, Matson TO, Lipps GJ, Wynn D, Armitage BJ), pp. 467–482. Columbus, OH: Ohio Biological Survey. [Google Scholar]
- 30.Schulze PSC, Barreira LA, Pereira HGC, Perales JA, Varela JCS. 2014. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 32, 422–430. ( 10.1016/j.tibtech.2014.06.001) [DOI] [PubMed] [Google Scholar]
- 31.Schubert EF, Kim JK. 2005. Solid-state light sources getting smart. Science 308, 1274–1278. ( 10.1126/science.1108712) [DOI] [PubMed] [Google Scholar]
- 32.Chase JM, Burgett AA, Biro EG. 2010. Habitat isolation moderates the strength of top-down control in experimental pond food webs. Ecology 91, 637–643. ( 10.1890/09-0262.1) [DOI] [PubMed] [Google Scholar]
- 33.R Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 34.Gaston KJ, Bennie J, Davies TW, Hopkins J. 2013. The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol. Rev. 88, 912–927. ( 10.1111/brv.12036) [DOI] [PubMed] [Google Scholar]
- 35.Gaston KJ, Gaston S, Bennie J, Hopkins J. 2015. Benefits and costs of artificial nighttime lighting of the environment. Environ. Rev. 23, 14–23. ( 10.1139/er-2014-0041) [DOI] [Google Scholar]
- 36.Davies TW, Bennie J, Gaston KJ. 2012. Street lighting changes the composition of invertebrate communities. Biol. Lett. 8, 764–767. ( 10.1098/rsbl.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binckley CA, Resetarits WJ Jr. 2003. Functional equivalence of non-lethal effects: Generalized fish avoidance determines distribution of gray treefrog, Hyla chrysoscelis, larvae. Oikos 102, 623–629. ( 10.1034/j.1600-0706.2003.12483.x) [DOI] [Google Scholar]
- 38.Resetarits WJ Jr, Wilbur HM. 1989. Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology 70, 220–228. ( 10.2307/1938428) [DOI] [Google Scholar]
- 39.Binckley CA, Resetarits WJ Jr. 2008. Oviposition behavior partitions aquatic landscapes along predation and nutrient gradients. Behav. Ecol. 19, 552–557. ( 10.1093/beheco/arm164) [DOI] [Google Scholar]
- 40.Resetarits WJ., Jr 2005. Habitat selection behaviour links local and regional scales in aquatic systems. Ecol. Lett. 8, 480–486. ( 10.1111/j.1461-0248.2005.00747.x) [DOI] [PubMed] [Google Scholar]
- 41.Lawler SP, Morin PJ. 1993. Temporal overlap, competition, and priority effects in larval anurans. Ecology 74, 174–182. ( 10.2307/1939512) [DOI] [Google Scholar]
- 42.Sweeney BW, Vannote RL. 1982. Population synchrony in mayflies: a predator satiation hypothesis. Evolution 36, 810–821. ( 10.1111/j.1558-5646.1982.tb05447.x) [DOI] [PubMed] [Google Scholar]
- 43.Ims RA. 1990. The ecology and evolution of reproductive synchrony. Trends Ecol. Evol. 5, 135–140. ( 10.1016/0169-5347(90)90218-3) [DOI] [PubMed] [Google Scholar]
- 44.DeVito J. 2003. Metamorphic synchrony and aggregation as antipredator responses in American toads. Oikos 103, 75–80. ( 10.1034/j.1600-0706.2003.12527.x) [DOI] [Google Scholar]
- 45.Bird BL, Branch LC, Miller DL. 2004. Effects of coastal lighting on foraging behavior of beach mice. Conserv. Biol. 18, 1435–1439. ( 10.1111/j.1523-1739.2004.00349.x) [DOI] [Google Scholar]
- 46.Scheuerell MD, Schindler DE. 2003. Diel vertical migration by juvenile sockeye salmon: empirical evidence for the antipredation window. Ecology 84, 1713–1720. ( 10.1890/0012-9658(2003)084%5B1713:DVMBJS%5D2.0.CO;2) [DOI] [Google Scholar]
- 47.Buchanan BW. 2006. Observed and potential effects of artificial night lighting on anuran amphibians. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T), pp. 192–220. Washington, DC: Island Press. [Google Scholar]
- 48.Mougeot F, Bretagnolle V. 2000. Predation risk and moonlight avoidance in nocturnal seabirds. J. Avian Biol. 31, 376–386. ( 10.1034/j.1600-048X.2000.310314.x) [DOI] [Google Scholar]
- 49.Cabrera-Guzmán E, Crossland MR, Brown GP, Shine R. 2013. Larger body size at metamorphosis enhances survival, growth and performance of young Cane Toads (Rhinella marina). PLoS ONE 8, e70121 ( 10.1371/journal.pone.0070121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarvin RD, Silva Bermúdez C, Briggs VS, Warkentin KM. 2015. Carry-over effects of size at metamorphosis in red-eyed treefrogs: higher survival but slower growth of larger metamorphs. Biotropica 47, 218–226. ( 10.1111/btp.12198) [DOI] [Google Scholar]
- 51.Berven KA. 1990. Factors affecting population fluctuations in larval and adult stages of the Wood Frog (Rana sylvatica). Ecology 71, 1599–1608. ( 10.2307/1938295) [DOI] [Google Scholar]
- 52.Taylor BW, Anderson CR, Peckarsky BL. 1998. Effects of size at metamorphosis on stonefly fecundity, longevity, and reproductive success. Oecologia 114, 494–502. ( 10.1007/s004420050473) [DOI] [PubMed] [Google Scholar]
- 53.Bradshaw WE, Holzapfel CM. 2010. What season is it anyway? Circadian tracking vs. photoperiodic anticipation in insects. J. Biol. Rhythms 25, 155–165. ( 10.1177/0748730410365656) [DOI] [PubMed] [Google Scholar]
- 54.Dananay KL, Benard MF. 2018. Data from: Artificial light at night decreases metamorphic duration and juvenile growth in a widespread amphibian Dryad Digital Repository. ( 10.5061/dryad.pq54gh5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dananay KL, Benard MF. 2018. Data from: Artificial light at night decreases metamorphic duration and juvenile growth in a widespread amphibian Dryad Digital Repository. ( 10.5061/dryad.pq54gh5) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.pq54gh5) [54].