Abstract
The question of parallel evolution—what causes it, and how common it is—has long captured the interest of evolutionary biologists. Widespread urban development over the last century has driven rapid evolutionary responses on contemporary time scales, presenting a unique opportunity to test the predictability and parallelism of evolutionary change. Here we examine urban evolution in an acorn-dwelling ant species, focusing on the urban heat island signal and the ant's tolerance of these altered urban temperature regimes. Using a common-garden experimental design with acorn ant colonies collected from urban and rural populations in three cities and reared under five temperature treatments in the laboratory, we assessed plastic and evolutionary shifts in the heat and cold tolerance of F1 offspring worker ants. In two of three cities, we found evolved losses of cold tolerance, and compression of thermal tolerance breadth. Results for heat tolerance were more complex: in one city, we found evidence of simple evolved shifts in heat tolerance in urban populations, though in another, the difference in urban and rural population heat tolerance depended on laboratory rearing temperature, and only became weakly apparent at the warmest rearing temperatures. The shifts in tolerance appeared to be adaptive, as our analysis of the fitness consequences of warming revealed that while urban populations produced more sexual reproductives under warmer laboratory rearing temperatures, rural populations produced fewer. Patterns of natural selection on thermal tolerances supported our findings of fitness trade-offs and local adaptation across urban and rural acorn ant populations, as selection on thermal tolerance acted in opposite directions between the warmest and coldest rearing temperatures. Our study provides mixed support for parallel evolution of thermal tolerance under urban temperature rise, and, importantly, suggests the promising use of cities to examine parallel and non-parallel evolution on contemporary time scales.
Keywords: adaptation, phenotypic plasticity, thermal physiology, global change, natural selection
1. Introduction
Do the same selective pressures yield parallel evolution of phenotypes across populations? There is considerable evidence for parallel evolution—the consistent evolution of similar traits in similar environments—from morphology in three-spine stickleback [1] and anoles [2], to life-history evolution in poeciliid fishes [3,4], and host-use traits in walking sticks [5] and bacteria [6]. But there are also many exceptions to parallel evolution under a common agent of natural selection (reviewed in [7]). Deviations from parallel evolutionary responses could be generated from a variety of sources. The influence of other evolutionary mechanisms—including genetic drift, mutation and migration [8], the action of other independent or interacting agents of selection [9], or convergence upon the same functional outcome via different phenotypic optima [10]—can all contribute to deviations from parallelism. In theory, determining why adaptive evolution produces parallel responses is not a difficult task, though in practice, there can be significant challenges. This difficulty arises from the need to evaluate and distinguish among the myriad potential causes of evolution in complex natural environments.
Experimental evolution provides a means to manipulate the agents of selection potentially driving parallel evolutionary responses while controlling for other factors. But these types of studies are often performed in the laboratory [11] due to the difficulty of manipulating agents of natural selection and measuring phenotypic responses in the field (but see [12,13]). Cities provide an alternative solution. Urban environments are ready-made, highly replicated mesocosms of altered selective landscapes distributed at a global scale, often with well-documented histories. As such, cities represent a massive experiment played out against the background of the natural environment [13,14]. Because urban areas often share some common features (e.g. artificial light, impervious surfaces), but differ considerably in others (e.g. city age, structure and background climate), cities provide an excellent opportunity to examine the causes of parallel and non-parallel evolution [14–16].
A consistent signature of urbanization among different cities, and a likely shared selective pressure, is in the thermal landscape [15]. Through urban heat island effects, cities are often warmer (by several °C) than nearby rural habitats [17]. An open question is whether urban heat islands lead to parallel evolution of organismal thermal physiologies among different cities [15]. For urbanization gradients, there is increasing evidence of evolved differences in urban and rural thermal performance and tolerance. Both water fleas [18] and acorn ants [19] exhibit evolved increases in heat tolerance in cities compared with nearby undeveloped habitats, and there appears to be a genetic differentiation in thermal performance curves for growth and development of urban and rural populations of damselflies [20] and chitinolytic fungus [21]. But how repeatable are these patterns across multiple cities? Parallel evolution in response to cities has been demonstrated (to varying degrees) for other types of traits, including limb morphology of anolis lizards [22] and cyanogenesis in white clover plants (notably, a correlate of cold tolerance) [23]. Yet, with the field of urban evolution still nascent, the patterns of parallel evolution in urban environments remain unresolved both broadly and specifically for realized thermal tolerance in response to urban heat islands [14,15,24].
Cities also provide an opportunity to explore the causes of non-parallel evolutionary responses to shared selective agents. Although urban heat islands are a consistent feature of most cities, variation between cities in the background climates they occur in could drive unique, but predictable, aspects of the evolutionary response in thermal tolerance. The thermal tolerance breadths of ectothermic species are generally narrower in warmer locations (e.g. at lower latitude) compared with colder locations (e.g. higher latitude) [25]. Thermal tolerance breadths across the combined warming effects of urban heat islands and decreasing latitude should also follow this macrophysiological pattern, such that thermal tolerance breadths are predicted to be broadest within higher latitude undeveloped areas and most narrow within cities at lower latitudes. As a consequence the magnitude of the evolutionary response in thermal tolerance may also decrease with decreasing latitude, as ectotherms at lower latitudes inhabit environments at or near their thermal optimum and thermal maximum [26,27].
Although examining the evidence for evolved trait differences in response to shared environmental change (e.g. urban warming) is an important first step for understanding urban evolution, it is likewise important to assess the adaptive nature of these changes. Despite the fact that other mechanisms, such as drift, could drive evolved trait differences between urban and rural environments, only fitness trade-offs could result in adaptive evolutionary divergence. However, surprisingly few studies have directly tested the prediction that fitness trade-offs should exist between divergent urban and rural populations due to local adaptation [14]. Moreover, comparing the magnitude of fitness trade-offs among urbanization gradients may help explain patterns of parallel and non-parallel evolution. For example, weak fitness trade-offs between environments may result in non-parallel evolution due to the greater influence of other shared selective pressures, migration or drift.
Here we explore the potential for parallel (and non-parallel) evolution of thermal tolerance across three cities using acorn ants as a model system. Acorn ants are highly sensitive to temperature, including in their development rate [28], running speed [29] and thermal tolerance [30]. Previously, we found evidence for evolved differences in heat and cold tolerance across a single urbanization gradient in Cleveland, Ohio, such that urban populations exhibited increases in heat tolerance and losses in cold tolerance relative to rural populations [19]. To assess the repeatability of this pattern, in this study, we collected acorn ants from urban and rural populations across three cities in the eastern USA (Cleveland, Ohio; Cincinnati, Ohio; and Knoxville, Tennessee), and reared the acorn ant colonies under five common-garden temperature treatments in the laboratory to assess plastic and evolved divergence in thermal tolerances. We predicted that across each urbanization gradient, urban populations would show evolved increases in heat tolerance and losses in cold tolerance when compared to rural populations in response to the shared selective pressure of the urban heat island. We further predicted that the magnitude of these evolutionary responses in thermal tolerance would decrease with decreasing latitude, as populations at lower latitudes would be closer to their thermal maximum, constraining evolutionary responses in urban environments. Finally, we predicted that evolutionary divergence in thermal tolerance would be associated with fitness trade-offs (as assessed by the number of reproductive ants or alates) across warmer and cooler rearing temperatures (i.e. providing evidence of local adaptation in typically urban and rural thermal environments). To investigate the targets of these fitness trade-offs, we additionally asked if selection was acting on thermal tolerance traits in our common-garden experiment.
2. Material and methods
(a). Ant colony collections
We collected acorn ant (Temnothorax curvispinosus; see electronic supplementary information for the natural history of the system) colonies from urban and rural sites across three cities: Cleveland, Ohio (42° N latitude), Cincinnati, Ohio (39° N latitude) and Knoxville, Tennessee (36° N latitude). To identify urban and rural sites, we used percent developed impervious surface area (ISA) [17], such that urban sites were categorized as approximately 40–60% ISA, and rural sites were categorized as 0% ISA (electronic supplementary material, table S1). Although ISA is strongly correlated (r > 0.9) with environmental temperature in our study region [17], we ground-truthed the temperature difference between our urban and rural sites with temperature loggers placed at two urban and two rural sites for each of our three focal cities (electronic supplementary material, figure S1). Because of the latitudinal signal in the onset of the growing season across the three cities, our collections from Knoxville occurred earliest in the year (27 April–7 June 2016), followed by Cleveland (2 June–6 July 2016) and Cincinnati (5 June–8 July 2016).
(b). Common-garden experiment
After collection from the field, ant colonies were allowed to acclimate to laboratory conditions (approx. 25°C, an intermediate temperature among our five temperature treatments) for approximately 48 h prior to being randomly assigned to one of five temperature treatments. We used a relatively simple diurnal fluctuation in temperature for all treatments, where night-time temperatures (10 h span) increased by 5°C to a non-peak daytime temperature for 7 h, followed by an increase of 5°C to a peak daytime temperature for 2 h before returning to the non-peak daytime temperature for 5 h (electronic supplementary material, figure S2). We refer to the temperature treatments by their non-peak daytime temperatures: 21, 23, 25, 27 and 29°C. Photoperiod (14 L : 10 D) was synchronized with the temperature changes between night-time and daytime temperatures. The temperature treatments were developed based on nest temperature data during the peak growing season (June–July) across our three focal cities and across urban and rural sites (electronic supplementary material, figure S3); the range of temperatures across all treatments represents a non-stressful range for this species [19,30].
To generate F1 offspring for thermal tolerance testing, we reared colonies for a minimum of 10 weeks (mean duration ± 1 s.d. = 79.4 ± 4.35 days). The acclimation period allowed sufficient time for turnover of workers within the colonies, i.e. the original workers died and the queens produced a new generation of workers from eggs laid entirely within the common-garden treatments, prior to assessment of thermal tolerance. This aspect of the experimental design allowed us to eliminate developmental acclimation effects and some, but not all, potential maternal effects [31]. Each colony was maintained individually in a 120 ml plastic cup. Resource tubes with sugar water (25% solution) and plain tap water were provided to colonies along with a continuous supply of dead mealworms.
We used a dynamic temperature ramping protocol to assess the critical thermal maximum and minimum (CTmax and CTmin), each defined as the loss of muscular coordination, which yield ecologically relevant limits on performance and serve as our measures of heat and cold tolerance [32]. We tested workers and queens individually for either CTmax or CTmin, as the assessment of thermal tolerance is a semi-destructive process that precludes assessment of both CTmax and CTmin on the same individual (electronic supplementary material, text). Ants were placed individually into 1.5 ml Eppendorf tubes with a cotton plug in the lid. Temperatures were manipulated using a dry block incubator (Boekel Scientific Tropicooler), and increased or decreased at a rate of 1°C min−1. Initial temperature for the estimation of CTmax was 34°C, and was 16°C for CTmin. These starting temperatures lie outside the range needed to induce loss of muscular coordination or death. We assessed the thermal tolerance of 260 T. curvispinosus colonies across the three cities (nKnoxville = 81; nCincinnati = 87; nCleveland = 92), two source environments (nurban = 125, and nrural = 135) and five rearing temperatures (n21 = 53; n23 = 53; n25 = 50; n27 = 52; n29 = 52) (see electronic supplementary material, tables S2, S3 for breakdowns of colony-level and site-level replication for each combination of city, source environment and rearing temperature). The mean ±1 s.d. of the number of workers tested per colony for CTmax was 9.62 ± 1.14, and for CTmin was 9.67 ± 1.08.
(c). Statistical analyses
(i). Thermal tolerance models
To evaluate the evidence for (1) evolved differences between urban and rural populations, (2) phenotypic plasticity between rearing temperatures and (3) evolutionary divergence in plasticity between populations and rearing temperatures, we constructed models with (in order of their above interpretation) (1) source environment (urban or rural), (2) rearing temperature (treated as a continuous variable) and (3) the interaction of source environment and rearing temperature as predictors of worker ant CTmax and CTmin (electronic supplementary material, table S4). Separate models were performed for CTmax and CTmin, and for the three focal cities (Knoxville, Cincinnati and Cleveland). Initially, we developed a global model that included up to the three-way interaction between source environment, rearing temperature and city, though many of the interaction terms involving city (specifically, city × source population and city × rearing temperature) were significant (electronic supplementary material, tables S4 and S5), which justified the separate treatment of each city. In each model, we included colony identity as a random effect to account for non-independence among individuals from the same colony. All model assumptions were verified for this and all subsequent models (see electronic supplementary material, text for details of diagnostic tests). Significance of the fixed effects was assessed using likelihood ratio tests.
For thermal tolerance breadth, or the difference between mean colony-level worker ant CTmax and CTmin, we constructed linear models with source environment, rearing temperature, and the interaction of source environment and rearing temperature as the predictor variables. We performed separate models for each of the three focal cities. Because our analysis of tolerance breadth uses colony-level means, we did not include a random effect structure in this model. Significance of the predictor variables was assessed using likelihood ratio tests. Our primary models focus on worker thermal tolerances, as these were F1 offspring; however, we performed complementary analyses for queens (field-caught and reared for 10 weeks under the common-garden temperature treatments). The analytical approaches for queens were similar to those for workers except that for CTmax and CTmin, we did not need to include a random effects term for colony identity. For analyses of tolerance breadth of queens, we were limited to those cases where we had multiple queens present in the same colony.
(ii). Fitness models
We used generalized linear models to examine colony-level fitness responses (the number of reproductive individuals, or alates, produced) to rearing temperature for urban and rural populations. Source environment, rearing temperature, and the interaction of source environment and rearing temperature were included as the predictor variables. We performed separate analyses for each of our three focal cities. Because the number of alates represent count data, but contained many zeroes (colonies that failed to produce alates), we used a zero-inflated Poisson model (electronic supplementary material, table S4). We used likelihood ratio tests to assess the significance of each of the predictors.
With the fitness data and thermal tolerance trait data, we were also able to estimate selection on thermal tolerance. We focused on estimating selection on CTmax and CTmin in the warmest temperature treatment (29°C) and the coolest (21°C). To estimate selection, we used generalized additive models, with the number of alates as the response and CTmax and CTmin as predictors (electronic supplementary material, table S4). We performed selection analyses separately for each city and the two extreme temperature treatments, but pooled across source population. Because alate production is a function of the colony unit, but thermal tolerance data were recorded at the level of individual ants, we computed colony mean CTmax and CTmin of worker ants for this analysis.
(iii). Tests of parallelism among cities
To test for general patterns of parallelism in plastic and evolved responses to urbanization among our three focal cities, we compared the magnitude and direction of shifts in thermal tolerance trait values from rural to urban populations across each city. We specifically evaluated the evidence for our expectations that warmer rearing temperatures will lead to increased heat tolerance and diminished cold tolerance, and that urban populations will have increased heat tolerance and diminished cold tolerance regardless of rearing temperature as we found previously in this system [19]. We also explored the potential for systematic variation in plastic and evolutionary responses to urbanization resulting from the latitudinal position of each city. Specifically, we tested the hypotheses that thermal tolerance breadths would be most compressed within lower latitude city populations owing to the combined effects of warmer background climates and urban warming, and that the differentiation among urban and rural populations would be weakest at lower latitudes owing to the already narrow separation between environmental temperatures and the thermal optimum and thermal maximum in these locations.
3. Results and discussion
How common is parallel evolution? The evidence so far is mixed as to how frequently evolutionary responses to a shared agent of natural selection lead to similar phenotypic outcomes [3,7]. Although urbanization entails many changes to the environment, one largely consistent feature among cities is the increase in environmental temperature compared with nearby undeveloped areas [17]. Here we used a laboratory common-garden experiment to test for the parallel evolution of thermal tolerance in the acorn ant (an abundant species in both rural and urban habitats) across three different cities. Two out of the three cities showed remarkably similar patterns of evolution in urban environments, characterized by increases in heat tolerance (though for one city, the response was weak and depended on laboratory rearing temperature), substantial losses in cold tolerance, and compression of thermal tolerance breadth. We also found evidence of local adaptation of urban and rural populations in these two cities: acorn ants exhibited the highest fitness at the laboratory rearing temperature that more closely matched with their source environment. Patterns of phenotypic selection also link these fitness trade-offs with thermal tolerance. Selection favoured increased cold and decreased heat tolerance in the coldest rearing environments, but favoured increased heat and decreased cold tolerance in the warmest. Our data provide mixed support for parallel adaptive evolution of thermal tolerance under urban temperature rise.
(a). Urban evolution in acorn ants: the case for and against parallelism
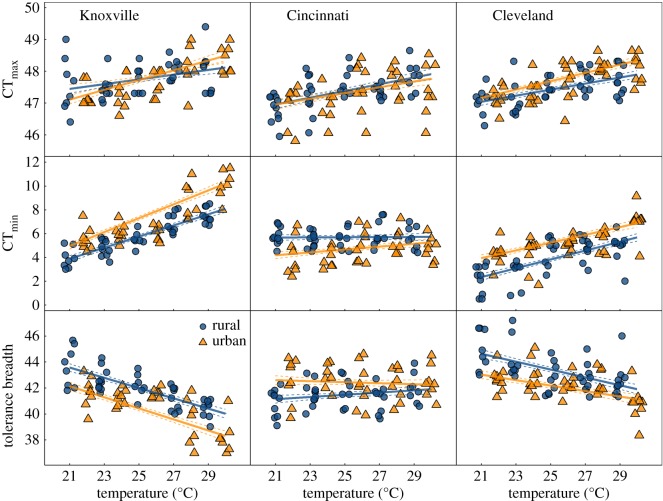
For two out of the three focal cities, Cleveland and Knoxville (which lie at the northern and southern extremes of the latitudinal extent of our study), we found parallel responses of worker ant thermal tolerances to urbanization. Heat tolerance increased with increasing laboratory rearing temperature in urban and rural populations from both cities, indicating phenotypic plasticity in heat tolerance. Consistent with the findings of our earlier study [19], urban populations exhibited overall greater heat tolerance compared with rural populations in Cleveland, indicating evolved differences between urban and rural population heat tolerance (figure 1 and table 1). This response was somewhat more complex in Knoxville, where we found a borderline significant interaction between population (urban versus rural) and laboratory rearing temperature such that urban populations exhibited greater heat tolerances at warmer rearing temperatures but were more comparable with rural populations at lower rearing temperatures (table 1). Although we did find weak evidence of evolved plasticity in heat tolerance for Knoxville (i.e. where the slope of the tolerance response to different rearing temperatures differed among urban and rural populations), we found no evidence of evolved plasticity in the other two cities, Cleveland and Cincinnati. Further, we found no significant difference in mean heat tolerance among urban and rural populations in Cincinnati (figure 1 and table 1). At least for Cleveland and Knoxville, the patterns we observed for heat tolerance appear to be consistent with responses from other taxa where similar common-garden approaches to disentangle plastic and evolved responses to urban warming were employed. Daphnia show an increase in heat tolerance [18] and chitinolytic fungi show an increase in the thermal optimum (often correlated with heat tolerance [33]) for growth rate in urban compared with rural populations [21].
Figure 1.
Thermal tolerance responses (CTmax, CTmin and tolerance breadth) of urban and rural acorn ant workers to rearing temperature. Responses from three cities are shown in separate panels. Lines represent predicted values ±1 s.e. from linear mixed effects models (performed separately for each city). See table 1 for model coefficients. Points indicate colony mean thermal tolerance, and are jittered within each combination of source population and temperature treatment.
Table 1.
Model estimates (with standard errors) and likelihood ratio tests for the significance of source environment, rearing temperature and their interaction on worker ant thermal tolerances, including CTmax, CTmin and tolerance breadth. Significant p-values at the 0.05 level are indicated in italics.
| response | city | term | estimate | s.e. | χ2 | p |
|---|---|---|---|---|---|---|
| CTmax | Cleveland | rearing temperature | 0.0847 | 0.0195 | 55.3 | 1.03 × 10−13 |
| source environment | −0.562 | 0.688 | 9.59 | 0.00195 | ||
| temperature × source | 0.0319 | 0.0272 | 1.38 | 0.241 | ||
| Cincinnati | rearing temperature | 0.0943 | 0.0255 | 22.1 | 2.59 × 10−6 | |
| source environment | 0.292 | 0.942 | 0.279 | 0.597 | ||
| temperature × source | −0.014 | 0.0376 | 0.139 | 0.709 | ||
| Knoxville | rearing temperature | 0.0747 | 0.0273 | 32.3 | 1.31 × 10−8 | |
| source environment | −2 | 0.996 | 0.0389 | 0.844 | ||
| temperature × source | 0.079 | 0.0396 | 3.99 | 0.0456 | ||
| CTmin | Cleveland | rearing temperature | 0.371 | 0.0556 | 77.5 | 1.32 × 10−18 |
| source environment | 2.55 | 2 | 38.4 | 5.78 × 10−10 | ||
| temperature × source | −0.0456 | 0.0795 | 0.331 | 0.565 | ||
| Cincinnati | rearing temperature | 0.00606 | 0.0533 | 2.33 | 0.127 | |
| source environment | −3.91 | 1.96 | 22.5 | 2.14 × 10−6 | ||
| temperature × source | 0.115 | 0.0782 | 2.17 | 0.141 | ||
| Knoxville | rearing temperature | 0.467 | 0.0516 | 191 | 1.92 × 10−43 | |
| source environment | −1.38 | 1.9 | 50.9 | 9.76 × 10−13 | ||
| temperature × source | 0.117 | 0.0758 | 2.38 | 0.123 | ||
| tolerance breadth | Cleveland | rearing temperature | −0.303 | 0.0649 | 31.7 | 1.81 × 10−8 |
| source environment | −3.54 | 2.29 | 22.5 | 2.15 × 10−6 | ||
| temperature × source | 0.0923 | 0.0909 | 1.03 | 0.31 | ||
| Cincinnati | rearing temperature | 0.0855 | 0.0636 | 0.348 | 0.555 | |
| source environment | 4.1 | 2.34 | 13.9 | 0.000191 | ||
| temperature × source | −0.125 | 0.0934 | 1.8 | 0.18 | ||
| Knoxville | rearing temperature | −0.395 | 0.0598 | 87.1 | 1.03 × 10−20 | |
| source environment | −0.796 | 2.21 | 38.8 | 4.74 × 10−10 | ||
| temperature × source | −0.0306 | 0.0879 | 0.121 | 0.728 |
We found remarkably similar patterns for cold tolerance, where decreasing rearing temperature led to a greater ability to tolerate cold temperatures (i.e. cold tolerance values were lower) in urban and rural populations from both Cleveland and Knoxville, indicating phenotypic plasticity in cold tolerance. In addition, urban populations from these two cities exhibited losses in their ability to tolerate cold temperatures (i.e. cold tolerance values were higher) compared with rural populations, indicating evolved differences between urban and rural population cold tolerance (figure 1 and table 1). By comparison, Cincinnati once again diverged from the patterns of cold tolerance for Cleveland and Knoxville, exhibiting the opposite pattern, with a greater ability of urban populations to tolerate cold temperatures compared with rural populations. We did not find evidence of evolved plasticity in cold tolerance for any of the three cities (figure 1 and table 1). The evolved increase in heat tolerance in Cleveland urban populations, and the same, at least for Knoxville urban populations reared under warm temperature treatments, seems perfectly reasonable given the increased environmental temperature in cities. However, the evolution of diminished cold tolerance in urban areas is less clear. Trade-offs between cold and heat tolerance and/or underlying genetic correlations between them could potentially explain the loss of cold tolerance in these urban populations (electronic supplementary material, text).
Because we estimated both heat and cold tolerance, we were able to explore how urbanization impacted overall tolerance breadth. The greater magnitude of evolved differences in cold tolerance relative to heat tolerance yielded substantially narrower tolerance breadths of urban populations compared with rural populations for both Knoxville and Cleveland. Owing to the unexpected shift in cold tolerance in Cincinnati with urban populations being more tolerant of low temperatures, thermal tolerance breadth was greater in urban populations compared with rural populations (figure 1 and table 1).
The foregoing analyses focused on worker ant thermal tolerances. Queens exhibited quite similar patterns to workers, particularly for cold tolerance and tolerance breadth (electronic supplementary material, figure S4 and table S6). The congruence between queen and worker responses is important, as queens are the reproductive units of the colony. However, we note that queens were field-caught and thus carry-over effects from the field cannot be ruled out in these analyses.
The shifts we observed in thermal tolerance traits were consistent with the shifts in environmental temperature in acorn ant nest sites across urban and rural sites in two cities. Knoxville exhibited a shift of 3.64°C (the difference between the maximum values of the kernel density distributions of environmental temperature; electronic supplementary material, figure S1) and Cleveland exhibited a shift of 4.45°C. By linking increases in environmental temperature within cities to evolved increases in heat tolerance and losses in cold tolerance, we are able to establish a strong case for temperature rise as the agent of selection in two of our focal cities (Cleveland and Knoxville). Yet why the third city in our study (Cincinnati) showed not only no differentiation among urban and rural populations for heat tolerance, but also the opposite patterns to our expectations and the observed patterns for Cleveland and Knoxville for cold tolerance and consequently tolerance breadth, is unclear. In one respect, the temperature difference between urban and rural environments was more modest (1.61°C between the peaks of the kernel density distributions of environmental temperature) compared with the other two urbanization gradients (electronic supplementary material, figure S1). This weaker temperature difference may explain the lack of differentiation in heat tolerance, but does not explain the reversal of expected patterns, specifically, the greater cold tolerance of urban populations and consequently their broader tolerance breadth. The reversal of expected patterns of traits related to thermal tolerance has been observed in white clover, and was driven by the insulating snow layer being removed in cities, but being kept intact in rural habitats, leading to decreased cyanogenesis (a proxy of cold tolerance) in urban clover populations [23]. It is possible a similar phenomenon, whereby the insulating effects of snow supersede the effects of air temperature, is responsible for the patterns of thermal tolerance responses of acorn ants in Cincinnati. However, more data are needed on snow coverage and removal at our urban Cincinnati sites to evaluate this hypothesis. It is also possible that body size differences could play a role in the Cincinnati patterns as heat and cold tolerance generally improve with greater body size for terrestrial insects, including ants [34]. However, body sizes of acorn ants exhibit no differentiation based on rearing temperature or source population across the Cleveland urbanization gradient where body size has been measured (electronic supplementary material, table S7).
(b). Macrophysiology: the influence of background climate on urban evolution
The patterns of thermal adaptation we observed in Cleveland and Knoxville mirror observed macrophysiological patterns for ectotherms. For many ectothermic species, heat tolerance increases and the ability to tolerate cold temperatures decreases with increasing environmental temperature across latitude, though with a bias towards shifts in cold tolerance such that thermal tolerance breadth decreases with increasing environmental temperature [25]. Indeed, recent syntheses show that cold tolerance appears to be more strongly related to environmental temperature variation than heat tolerance [35], including specifically in ants [36]. We found similar patterns across the temperature clines between rural and urban environments in Cleveland and Knoxville, where heat tolerance increased modestly and the ability to tolerate cold temperatures decreased substantially under urbanization (figure 1 and table 1; electronic supplementary material, figure S4 and table S6). As a consequence of this asymmetry, in both cities, thermal tolerance breadth was narrower in urban populations. Because our rural-to-urban warming clines were positioned against a latitudinal cline in environmental temperature, with Cleveland positioned at a relatively high latitude with a cooler environmental temperature regime and Knoxville positioned at a relatively low latitude with a warmer environmental regime, we found the narrowest tolerance breadth in the Knoxville urban population and the broadest in the Cleveland rural population (figure 1 and table 1).
As we further expected, the differentiation in heat tolerance was more modest for the lower latitude city of Knoxville compared with Cleveland. For example, the difference in predicted mean heat tolerance of urban versus rural populations at the warmest rearing temperature, 29°C, was 0.295°C for Knoxville compared with 0.363°C for Cleveland, and this difference was even greater at cooler temperatures (figure 1 and table 1). Because Temnothorax is already a very heat-tolerant species compared with other ants [27], it is possible that low-latitude populations are beginning to push the evolutionary limits of heat tolerance, leading to a diminished evolved response with increased warming in cities. Importantly, these results taken together indicate that rapid evolutionary change in thermal tolerances of acorn ants across rural-to-urban gradients can recapitulate evolved differences among species in thermal tolerance across latitude. Of course more work needs to be done in this area, particularly involving greater sampling of urban and rural populations along latitudinal clines, but our results provide an initial exploration of how macrophysiology can be united with urban evolutionary ecology.
(c). Fitness consequences of warming among urban and rural populations
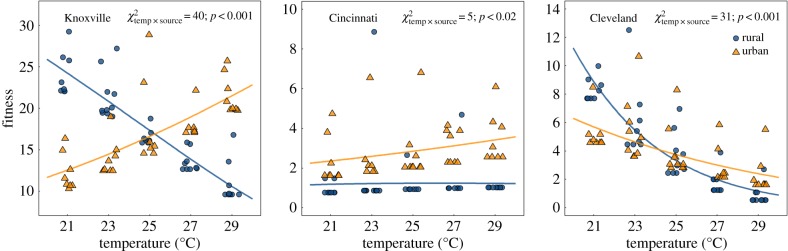
There is now a considerable amount of evidence for phenotypic shifts under urbanization, but few studies link these trait changes to their fitness consequences [14,37]. As a result, it is difficult to assess whether these urban-driven changes are adaptive. The classic pattern of local adaptation is seen when populations achieve higher fitness in their source environment than foreign populations, and lower fitness when reared in foreign environments, demonstrating fitness trade-offs [38]. The results of our study suggest the phenotypic changes in thermal tolerance that we observed in acorn ants from urban populations are adaptive, at least for Cleveland and Knoxville where the observed patterns of thermal adaptation match the expectations for local adaptation. We found that for these two cities, reproductive success defined as the number of alates (i.e. winged, sexual ants) produced over the course of the experiment, either increased with increasing temperature (Knoxville) or remained high across all rearing temperatures (Cleveland), whereas rural populations exhibited reduced alate production with increasing temperature in both Knoxville and Cleveland, resulting in fitness trade-offs indicated by significant temperature by source population interactions (figure 2; electronic supplementary material, table S8). These results take into account relative differences in the number of alates produced per colony as well as the differences between whether or not colonies produced alates at all [39].
Figure 2.
Fitness (number of alates or reproductive ants) as a function of rearing temperature for urban and rural populations from three cities. Lines represent predicted values from zero-inflated Poisson models (performed separately for each city) and points (jittered) represent partial residuals. See electronic supplementary material, table S8 for model coefficients.
Because Cleveland acorn ants appear to be more cool-adapted compared with Knoxville (figure 1), the lack of marked increase in alate production for urban populations under the warmest temperature regimes is unsurprising. The patterns of alate production for Cincinnati are less surprising than thermal tolerance responses: urban populations have higher overall alate production compared with rural populations, and while urban alate production increases with rearing temperature, rural alate production decreases with rearing temperature (figure 2; electronic supplementary material, table S8). Critically, the alate production results negate a simple temperature dependence of development, in which case we would expect all populations to have greater alate production in warmer rearing temperatures. Because alates were F1 offspring produced entirely within the laboratory treatments, and because urban colonies (Cleveland and Knoxville especially) exhibit higher fitness under warmer conditions while rural colonies exhibit higher fitness under cooler conditions, the phenotypic shifts in thermal tolerance we observed are likely to be adaptive. Moreover, for the Cleveland and Knoxville populations, when detected, the direction of selection acting on temperature tolerance in the warmest and coldest rearing temperatures was in the direction expected if these shifts are adaptive (electronic supplementary material, table S9). Specifically, when reared at 21°C, colonies with greater cold tolerance tended to produce the most alates in both the Cleveland and Knoxville populations (CTmin: βCleveland = −0.918; βKnoxville = −0.609), and conversely, when reared at 29°C, greater heat tolerance and decreased cold tolerance were both associated with increased fitness in the Knoxville populations (CTmin: βKnoxville = 0.492; CTmax: βKnoxville = 0.691). Therefore, we have evidence to link variation in thermal tolerance to fitness in contrasting rearing environments.
Of course, these data on alate production provide a snapshot of fitness over the growing season. Ideally, we would quantify fitness over the entire year or even life cycle of the queen [40], particularly given the diminished cold tolerance of urban populations and the above-ground nesting habit that exerts strong viability selection in acorn ants [41]. Nonetheless, the presence of fitness trade-offs among urban and rural acorn ant populations across rearing temperatures provides critical information on the adaptive nature of phenotypic shifts in thermal tolerance.
(d). Study limitations
There are two important limitations to our study. First, our sampling of multiple urban and rural sites within each city is relatively minimal at 2–3 sites and in some cases, unbalanced with respect to the number of colonies per site (electronic supplementary material, table S1). Ideally, the number of sites would be increased and the colony-level replication more evenly represented among sites to maximize the generality of our results. Second, with F1 offspring ant workers, we are unable to completely eliminate maternal effects with our experimental design. Ideally, F2 offspring would be tested to rule out potential confounding maternal effects. However, such maternal effects are often small and most important early in ontogeny [31], and our F1 offspring tested for thermal tolerance were mature adult workers.
(e). Implications and future directions for parallel urban evolution
In this study, we were able to link phenotypic shifts in temperature tolerance with environmental changes in temperature, and show that these phenotypic shifts are likely to be adaptive. For two out of three cities, we found that patterns of evolved differences in thermal tolerance under urbanization were remarkably similar, particularly for cold tolerance and tolerance breadth, and to a lesser extent for heat tolerance, indicating a fairly consistent pattern of parallel evolution. Future research would benefit from the exploration of additional urbanization gradients to evaluate the generality of parallel evolution of thermal tolerance traits and their fitness consequences in acorn ants and other species.
Supplementary Material
Acknowledgements
We are grateful to Marc Johnson, James Santangelo and two anonymous reviewers for helpful comments, and to Crystal Zhao and Bethany Lutter for laboratory assistance. The Squire Valleevue and Valley Ridge Farm, the Holden Forests and Gardens, Mt. Airy Forest, Cincinnati Park Board, the University of Tennessee, and Great Smoky Mountains National Park (permit no.: GRSM-2016-SCI-1265) provided access to field sites.
Data accessibility
Data are available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.4bk56qr) [42].
Authors' contributions
S.E.D. and R.A.M. designed the study. L.D.C., A.P. and S.A.S. collected data. S.E.D. and R.A.M. analysed the data and wrote the first draft of the manuscript. All authors contributed to revisions.
Competing interests
We have no competing interests.
Funding
An Oglebay Fund grant provided partial financial support.
References
- 1.Colosimo PF, et al. 2005. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science 307, 1928–1933. ( 10.1126/science.1107239) [DOI] [PubMed] [Google Scholar]
- 2.Mahler DL, Ingram T, Revell LJ, Losos JB. 2013. Exceptional convergence on the macroevolutionary landscape in island lizard radiations. Science 341, 292–295. ( 10.1126/science.1232392) [DOI] [PubMed] [Google Scholar]
- 3.Moore MP, Riesch R, Martin RA. 2016. The predictability and magnitude of life-history divergence to ecological agents of selection: a meta-analysis in livebearing fishes. Ecol. Lett. 19, 435–442. ( 10.1111/ele.12576) [DOI] [PubMed] [Google Scholar]
- 4.Langerhans RB. 2017. Predictability and parallelism of multitrait adaptation. J. Hered. 109, 59–70. ( 10.1093/jhered/esx043) [DOI] [PubMed] [Google Scholar]
- 5.Nosil P, Crespi BJ, Sandoval CP. 2002. Host-plant adaptation drives the parallel evolution of reproductive isolation. Nature 417, 440–443. ( 10.1038/417440a) [DOI] [PubMed] [Google Scholar]
- 6.Reid SD, Herbelin CJ, Bumbaugh AC, Selander RK, Whittam TS. 2000. Parallel evolution of virulence in pathogenic Escherichia coli. Nat. Lond. 406, 64–67. ( 10.1038/35017546) [DOI] [PubMed] [Google Scholar]
- 7.Oke KB, Rolshausen G, LeBlond C, Hendry AP. 2017. How parallel is parallel evolution? A comparative analysis in fishes. Am. Nat. 190, 1–16. ( 10.1086/691989) [DOI] [PubMed] [Google Scholar]
- 8.Olson-Manning CF, Wagner MR, Mitchell-Olds T. 2012. Adaptive evolution: evaluating empirical support for theoretical predictions. Nat. Rev. Genet. 13, 867–877. ( 10.1038/nrg3322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart YE, et al. 2017. Contrasting effects of environment and genetics generate a continuum of parallel evolution. Nat. Ecol. Evol. 1, 0158 ( 10.1038/s41559-017-0158) [DOI] [PubMed] [Google Scholar]
- 10.Wainwright PC, Alfaro ME, Bolnick DI, Hulsey CD. 2005. Many-to-one mapping of form to function: a general principle in organismal design? Integr. Comp. Biol. 45, 256–262. ( 10.1093/icb/45.2.256) [DOI] [PubMed] [Google Scholar]
- 11.Kawecki TJ, Lenski RE, Ebert D, Hollis B, Olivieri I, Whitlock MC. 2012. Experimental evolution. Trends Ecol. Evol. 27, 547–560. ( 10.1016/j.tree.2012.06.001) [DOI] [PubMed] [Google Scholar]
- 12.Calsbeek R, Cox RM. 2010. Experimentally assessing the relative importance of predation and competition as agents of selection. Nature 465, 613–616. ( 10.1038/nature09020) [DOI] [PubMed] [Google Scholar]
- 13.Logan ML, Cox RM, Calsbeek R. 2014. Natural selection on thermal performance in a novel thermal environment. Proc. Natl Acad. Sci. USA 111, 14 165–14 169. ( 10.1073/pnas.1404885111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson MTJ, Munshi-South J. 2017. Evolution of life in urban environments. Science 358, eaam8327 ( 10.1126/science.aam8327) [DOI] [PubMed] [Google Scholar]
- 15.Diamond SE, Dunn RR, Frank SD, Haddad NM, Martin RA. 2015. Shared and unique responses of insects to the interaction of urbanization and background climate. Curr. Opin. Insect Sci. 11, 71–77. ( 10.1016/j.cois.2015.10.001) [DOI] [PubMed] [Google Scholar]
- 16.Donihue CM, Lambert MR. 2015. Adaptive evolution in urban ecosystems. Ambio 44, 194–203. ( 10.1007/s13280-014-0547-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imhoff ML, Zhang P, Wolfe RE, Bounoua L. 2010. Remote sensing of the urban heat island effect across biomes in the continental USA. Remote Sens. Environ. 114, 504–513. ( 10.1016/j.rse.2009.10.008) [DOI] [Google Scholar]
- 18.Brans KI, Jansen M, Vanoverbeke J, Tüzün N, Stoks R, De Meester L. 2017. The heat is on: genetic adaptation to urbanization mediated by thermal tolerance and body size. Glob. Change Biol. 23, 5218–5227. ( 10.1111/gcb.13784) [DOI] [PubMed] [Google Scholar]
- 19.Diamond SE, Chick L, Perez A, Strickler SA, Martin RA. 2017. Rapid evolution of ant thermal tolerance across an urban–rural temperature cline. Biol. J. Linn. Soc. 121, 248–257. ( 10.1093/biolinnean/blw047) [DOI] [Google Scholar]
- 20.Tüzün N, de Beeck L Op, Brans KI, Janssens L, Stoks R. 2017. Microgeographic differentiation in thermal performance curves between rural and urban populations of an aquatic insect. Evol. Appl. 10, 1067–1075. ( 10.1111/eva.12512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLean MA, Angilletta MJ, Williams KS. 2005. If you can't stand the heat, stay out of the city: thermal reaction norms of chitinolytic fungi in an urban heat island. J. Therm. Biol. 30, 384–391. ( 10.1016/j.jtherbio.2005.03.002) [DOI] [Google Scholar]
- 22.Winchell KM, Reynolds RG, Prado-Irwin SR, Puente-Rolón AR, Revell LJ. 2016. Phenotypic shifts in urban areas in the tropical lizard Anolis cristatellus. Evolution 70, 1009–1022. ( 10.1111/evo.12925) [DOI] [PubMed] [Google Scholar]
- 23.Thompson KA, Renaudin M, Johnson MTJ. 2016. Urbanization drives the evolution of parallel clines in plant populations. Proc. R. Soc. B 283, 20162180 ( 10.1098/rspb.2016.2180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond SE, Martin RA. 2016. The interplay between plasticity and evolution in response to human-induced environmental change. F1000Research 5, 2835 ( 10.12688/f1000research.9731.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Addo-Bediako A, Chown SL, Gaston KJ. 2000. Thermal tolerance, climatic variability and latitude. Proc. R. Soc. Lond. B 267, 739–745. ( 10.1098/rspb.2000.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diamond SE, Sorger DM, Hulcr J, Pelini SL, Toro ID, Hirsch C, Oberg E, Dunn RR. 2012. Who likes it hot? A global analysis of the climatic, ecological, and evolutionary determinants of warming tolerance in ants. Glob. Change Biol. 18, 448–456. ( 10.1111/j.1365-2486.2011.02542.x) [DOI] [Google Scholar]
- 28.Penick CA, Diamond SE, Sanders NJ, Dunn RR. 2017. Beyond thermal limits: comprehensive metrics of performance identify key axes of thermal adaptation in ants. Funct. Ecol. 31, 1091–1100. ( 10.1111/1365-2435.12818) [DOI] [Google Scholar]
- 29.Maclean HJ, Penick CA, Dunn RR, Diamond SE. 2017. Experimental winter warming modifies thermal performance and primes acorn ants for warm weather. J. Insect Physiol. 100, 77–81. ( 10.1016/j.jinsphys.2017.05.010) [DOI] [PubMed] [Google Scholar]
- 30.Diamond SE, Penick CA, Pelini SL, Ellison AM, Gotelli NJ, Sanders NJ, Dunn RR. 2013. Using physiology to predict the responses of ants to climatic warming. Integr. Comp. Biol. 53, 965–974. ( 10.1093/icb/ict085) [DOI] [PubMed] [Google Scholar]
- 31.Massamba-N'Siala G, Prevedelli D, Simonini R. 2014. Trans-generational plasticity in physiological thermal tolerance is modulated by maternal pre-reproductive environment in the polychaete Ophryotrocha labronica. J. Exp. Biol. 217, 2004–2012. ( 10.1242/jeb.094474) [DOI] [PubMed] [Google Scholar]
- 32.Terblanche JS, Hoffmann AA, Mitchell KA, Rako L, le Roux PC, Chown SL. 2011. Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 214, 3713–3725. ( 10.1242/jeb.061283) [DOI] [PubMed] [Google Scholar]
- 33.Huey RB, Kingsolver JG. 1993. Evolution of resistance to high temperature in ectotherms. Am. Nat. 142, S21–S46. ( 10.1086/285521) [DOI] [Google Scholar]
- 34.Baudier KM, Mudd AE, Erickson SC, O'Donnell S. 2015. Microhabitat and body size effects on heat tolerance: implications for responses to climate change (army ants: Formicidae, Ecitoninae). J. Anim. Ecol. 84, 1322–1330. ( 10.1111/1365-2656.12388) [DOI] [PubMed] [Google Scholar]
- 35.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond SE, Chick L. 2018. The Janus of macrophysiology: stronger effects of evolutionary history, but weaker effects of climate on upper thermal limits are reversed for lower thermal limits in ants. Curr. Zool 64, 223–230. ( 10.1093/cz/zox072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alberti M, Correa C, Marzluff JM, Hendry AP, Palkovacs EP, Gotanda KM, Hunt VM, Apgar TM, Zhou Y. 2017. Global urban signatures of phenotypic change in animal and plant populations. Proc. Natl Acad. Sci. USA 114, 8951–8956. ( 10.1073/pnas.1606034114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hereford J. 2009. A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 173, 579–588. ( 10.1086/597611) [DOI] [PubMed] [Google Scholar]
- 39.Wagner D, Gordon DM. 1999. Colony age, neighborhood density and reproductive potential in harvester ants. Oecologia 119, 175–182. ( 10.1007/s004420050774) [DOI] [PubMed] [Google Scholar]
- 40.Ingram KK, Pilko A, Heer J, Gordon DM. 2013. Colony life history and lifetime reproductive success of red harvester ant colonies. J. Anim. Ecol. 82, 540–550. ( 10.1111/1365-2656.12036) [DOI] [PubMed] [Google Scholar]
- 41.Mitrus S. 2013. Cost to the cavity-nest ant Temnothorax crassispinus (Hymenoptera: Formicidae) of overwintering aboveground. Euro. J. Entomol. 110, 177–179. ( 10.14411/eje.2013.026) [DOI] [Google Scholar]
- 42.Diamond SE, Chick LD, Perez A, Strickler SA, Martin RA Data from: Evolution of thermal tolerance and its fitness consequences: parallel and non-parallel responses to urban heat islands across three cities. Dryad Digital Respository. ( 10.5061/dryad.4bk56qr) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Diamond SE, Chick LD, Perez A, Strickler SA, Martin RA Data from: Evolution of thermal tolerance and its fitness consequences: parallel and non-parallel responses to urban heat islands across three cities. Dryad Digital Respository. ( 10.5061/dryad.4bk56qr) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.4bk56qr) [42].