Abstract
Patient: Male, 87
Final Diagnosis: Lung cancer
Symptoms: Abnormal shadow on Chest X-ray
Medication: —
Clinical Procedure: Transbronchial lung biopsy
Specialty: Pulmonology
Objective:
Rare disease
Background:
Whereas non-tuberculous mycobacterium (NTM) pulmonary disease can mimic lung cancer as a solitary pulmonary nodule or mass, the coexistence of lung cancer and NTM pulmonary disease in a single nodule or mass is rare. We report such a rare case, highlighting that during a bronchoscopes examination which comprises taking a transbronchial lung biopsy (TBLB), bronchial brushing, and bronchial lavage, a positive mycobacterium culture result for sputum or bronchial lavage fluid does not exclude the possibility of a concomitant lung cancer.
Case Report:
An 87-year-old male was referred to our institution for evaluation of an abnormal shadow on a chest x-ray scan. He had been previously healthy with no symptoms and an unremarkable medical history. A contrast-enhanced CT scan showed a cavitating mass measuring 20×40 mm with a thick ring-enhancing irregular wall in the left lower lobe. Although the TBLB of the lesion showed no malignant cells, sputum acid-fast bacilli smear and culture of the bronchial lavage fluid yielded positive results. An NTM infection, instead of lung cancer was suspected to have caused the mass because a Mycobacterium tuberculosis polymerase chain reaction showed negative results. However, we performed the surgery because NTM pulmonary disease and lung cancer cannot be differentiated. The results of a pathological examination of the mass showed an adenocarcinoma, and M. avium complex was detected in the cancer tissue culture.
Conclusions:
Physicians should suspect the co-existent lung cancer and NTM infection in patients with solitary lung masses that yield a positive mycobacterium culture result for sputum or bronchial lavage fluid.
MeSH Keywords: Biopsy, Lung Neoplasms, Mycobacterium Infections, Nontuberculous
Background
Non-tuberculous mycobacterium (NTM) pulmonary disease has been found in 2.0–8.5% of patients with lung cancer [1–4]. However, the coexistence of lung cancer and NTM pulmonary disease in a solitary nodule or mass is very rare [2,4,5]. On the other hand, the computed tomography (CT) findings of NTM pulmonary disease can mimic lung cancer as a solitary pulmonary nodule or mass [5]. Therefore, during a bronchoscope examination, which comprises a transbronchial lung biopsy (TBLB) as well as a bronchial brushing and lavage, is very important to distinguish one from the other; a positive culture result for sputum or bronchial lavage fluid does not exclude the possibility of concomitant lung cancer [5]. We herein report such a rare case.
Case Report
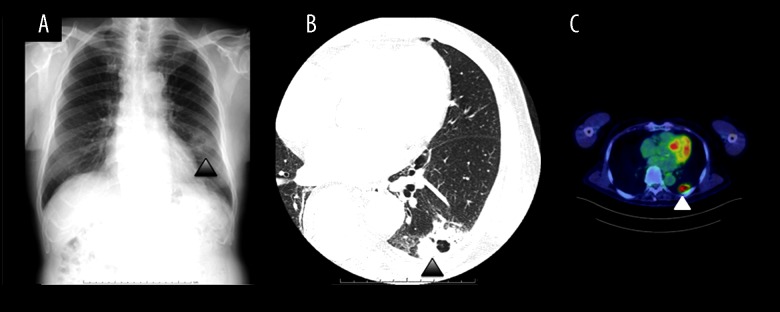
An 87-year-old male was referred to our institution for evaluation of an abnormal shadow on a chest x-ray scan for lung cancer screening in December 2016. He had been previously healthy with no symptoms, had an unremarkable medical and family history, and was a non-smoker. The chest x-ray showed an abnormal shadow in the left lower lobe (Figure 1A). A physical examination revealed that lymphadenopathy was absent in the cervical, axillary, and inguinal regions. On auscultation of the chest, his breath sounds were normal. The laboratory data revealed a total leukocyte count of 4610 cells/mm3 and C-reactive protein level of 0.07 mg/doll (normal range: 0–0.3 mg/dell). The levels of serum tumor markers, such as CEA, CYFLA, and Props, were within the normal ranges. A contrast-enhanced CT scan showed a cavitating mass with a thick ring-enhancing irregular wall in the left lower lobe, and there was no evidence of bronchiectasis or consolidation in the other lobes. The mass measured 20×40 mm, had poorly defined margins, and no gas collection or calcification was detected. There were no enlarged lymph nodes in the mediastinum (Figure 1B). A PET/CT scan demonstrated a high up-take in the lesion, with a maximum standardized uptake value (SUV) of 10.1 (Figure 1C). Lung cancer was initially suspected based on the imaging findings; however, there were no signs of distant metastasis.
Figure 1.
(A) An x-ray scan showing an abnormal shadow in the left lower lobe (arrowhead). (B) Chest CT scan with contrast enhancement showing a cavitating mass measuring 20×40 mm in size in the left lower lobe (arrowhead). (C) A PET/CT scan revealing a high uptake in the mass, with a maximum SUV of 10.1 (arrowhead).
Whereas a TBLB of the lesion showed no malignant cells or sputum, his bronchial lavage fluid examinations for acid-fast bacilli (AFB) were positive. Thus, NTM infection instead of lung cancer was suspected as the cause of the mass because a Mycobacterium tuberculosis polymerase chain reaction (PCR) test showed negative results. However, we planned surgery for his lesion because there were no other lung lesions suggestive of NTM pulmonary disease in the pulmonary parenchyma and it is difficult to differentiate one from the other. A pathological diagnosis of lung cancer was confirmed based on the results of a frozen section biopsy of the mass. Consequently, we performed a left lower lobectomy. There were no intraoperative complications, and the patient had an uneventful recovery. The final results of the pathological examination of the mass showed a stage 1B adenocarcinoma (pT2aN0M0) (Figure 2), and Mycobacterium avium complex was detected in the cancer tissue culture.
Figure 2.
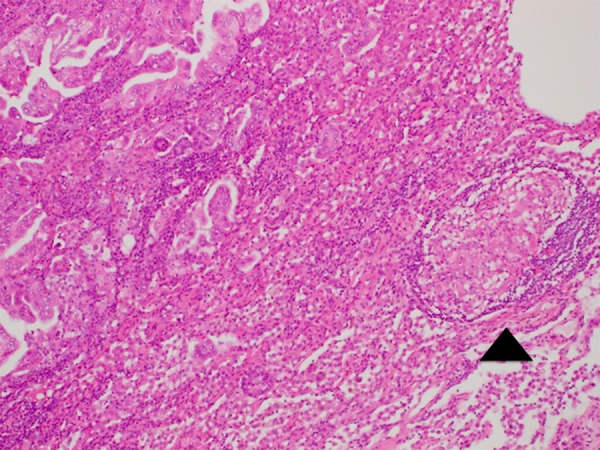
A lung pathology specimen of the present case depicting adenocarcinoma adjacent to a region of granulomatous inflammation typical of NTM infection (arrowhead) (hematoxylin and eosin stain 40×).
Discussion
Pulmonary NTM infections often occur in the context of preexisting lung disease, especially chronic obstructive pulmonary disease (COPD), bronchiectasis, and previous tuberculosis [4,5]. NTM pulmonary diseases are classified into fibrocavitary disease and nodular bronchiectatic disease depending on radiologic patterns [6]. The characteristic radiologic features of fibrocavitary disease include heterogeneous nodular and cavitary opacities, whereas those of nodular bronchiectatic disease are bronchiectasis and branching centrilobular nodules [5,6].
NTM pulmonary disease can manifest as a solitary pulmonary nodule or mass mimicking lung cancer [5]. Hong et al. [5] studied patients with NTM pulmonary disease presenting as solitary mass mimicking lung cancer. They found that the lesions typically showed poor contrast-enhancement (75%) and internal calcification (43%), which are more frequently observed in benign lesions including NTM pulmonary disease; however, they also observed the CT features of solitary lesions, such as a lobulated border (71%) or pleural retraction (28%), overlapping with those of primary lung cancer. Furthermore, the lesions showed strong fluorodeoxyglucose uptake, simulating malignant lesions on PET/CT imaging.
In general, an SUV cutoff of 2.5 or greater has been traditionally associated with malignant pulmonary nodules [7]. However, PET/CT may not be able to provide additional information to help differentiation of benign tumors from lung cancer [5]. In a solitary mass, it can be difficult to accurately distinguish lung cancer from NTM pulmonary disease based on image findings.
Therefore, in general, microbiologic procedures, such as TBLB and brushing, or lavage during bronchoscopy, are important for the diagnosis. We suspected the solitary mass to be a malignancy because CT image findings demonstrated no other lung lesions suggestive of NTM pulmonary disease in the pulmonary parenchyma, such as consolidation or bronchiectasis, despite the patient’s positive microbiological findings suggestive of NTM infection. This case report, therefore, suggests that a positive culture result for sputum or bronchial lavage fluid does not exclude the possibility of concomitant lung cancer [5], even if no malignant cells are seen in a TBLB, as was the case for this patient.
Although the association between NTM pulmonary disease and lung cancer has not been well-recognized, chronic pulmonary inflammation caused by NTM is linked to the development of lung cancer [4,8]. Although the subtype of cancer in this patient was an adenocarcinoma, previous studies [2,4] have reported a higher proportion of the squamous cell carcinoma subtype. The subtype of adenocarcinoma in our patient might be related to the recent increase in the proportions of adenocarcinoma in lung cancer patients [3,9]. More thorough scientific documentation is needed to discuss the association between this histological subtype and NTM infection as there have previously been few published cases.
In general, the need for medical therapy after a surgical resection for NTM pulmonary disease may be dependent upon the extent of remaining disease or the pathogenicity of the organism [10]. The role of postoperative medical therapy in patients who have had resection of an isolated solitary mass for NTM pulmonary disease is still unclear [10]. A study with a large study sample or a prospective trial is needed to assess this.
Conclusions
In conclusion, this case report suggests that physicians should suspect the coexistence of lung cancer and NTM infection in patients with a solitary lung mass and a positive culture result for sputum or bronchial lavage fluid.
Footnotes
Conflict of interest
None.
References:
- 1.Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features. Am J Respir Crit Care Med. 2010;182:977–82. doi: 10.1164/rccm.201003-0503OC. [DOI] [PubMed] [Google Scholar]
- 2.Tamura A, Hebisawa A, Sagara Y, et al. Pulmonary nontuberculous mycobacteriosis in patients with lung cancer. Kekkaku. 2004;79(6):367–73. [PubMed] [Google Scholar]
- 3.Tamura A, Hebisawa A, Kusaka K, et al. Relationship between lung cancer and Mycobacterium avium complex isolated using bronchoscopy. Open Respir Med J. 2016;10:20–28. doi: 10.2174/1874306401610010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lande L, Peterson DD, Gogoi R, et al. Association between pulmonary Mycobacterium avium complex infection and lung cancer. J Thorac Oncol. 2012;7(9):1345–51. doi: 10.1097/JTO.0b013e31825abd49. [DOI] [PubMed] [Google Scholar]
- 5.Hong SJ, Kim TJ, Lee JH, Park JS. Nontuberculous mycobacterial pulmonary disease mimicking lung cancer. Medicine (Baltimore) 2016;95(26):e3978. doi: 10.1097/MD.0000000000003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 7.Lowe VJ, Hoffman JM, DeLong DM, et al. Semiquantitative and visual analysis of FDG-PET images in pulmonary abnormalities. J Nucl Med. 1994;35:1771–76. [PubMed] [Google Scholar]
- 8.Daley CL, Iseman M. Mycobacterium avium complex and lung cancer: Chicken or egg? Both? J Thorac Oncol. 2012;7(9):1329–30. doi: 10.1097/JTO.0b013e318265a7ef. [DOI] [PubMed] [Google Scholar]
- 9.Hosoda C, Hagiwara E, Shinohara T, et al. Clinical characteristics of pulmonary Mycobacterium avium complex infection complicated with lung cancer. Kekkaku. 2014;89(8):691–95. [PubMed] [Google Scholar]
- 10.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6(3):210–20. doi: 10.3978/j.issn.2072-1439.2013.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]