Abstract
Background
Synovitis is an important disease that cause intractable pain in temporomandibular joint (TMJ), and the inflammation process played a crucial role in the initiation and development of temporomandibular joint disorder. A series of investigations suggested that the increasing expression of interleukin-(IL) 1β secreted by synovial lining cells plays an important role in synovial inflammation and cartilage destruction in TMJ. In this present study, we investigated the signaling pathways which regulate the expression of IL-1β.
Material/Methods
The occlusal interference animal model was created to induce synovial injury. Forty-eight rats were divided into 4 groups: 1) control group, 2) occlusal interference group, 3) TAK-242 (a specific inhibitor targeting the Toll-like receptor (TLR)-4) group, and 4) SB203580 (a specific inhibitor targeting the p38) group. The inflammation changes were observed, and the expression of p38 and IL-1β in the synovial membranes were assayed.
Results
The results showed that downstream p38 MAPK (mitogen-activated protein kinase) signaling was triggered following the activation of TLR4. Moreover, the injection of SB203580 could inhibit the inflammatory reactions and the increased expression of IL-1β at both mRNA and protein levels.
Conclusions
The results prompted us that TLR4 may stimulates synovial inflammatory reactions and increased expression of IL-1β in rats through the activation of p38 MAPK signaling pathway, p38 was an important mediator in the mechanisms of the initiation and development of synovial injury by regulating the expression of IL-1β in synovial membranes.
MeSH Keywords: Interleukin-1 Receptor-Associated Kinases, MAP Kinase Signaling System, Synovitis, Temporomandibular Joint Disorders, Toll-Like Receptor 4
Background
Temporomandibular joint disorder (TMD) is a common oral disease that refers to several clinical symptoms, including headache, pain in the masticatory muscles and temporomandibular joint (TMJ), disturbances in jaw movements, and sounds in the joints while opening and closing the mouth [1–3]. The initiation and development of TMD is considered to be related to many risk factors, such as occlusal interferences, psychological factors, and biomechanical and neuromuscular factors [4–6]. Nevertheless, there are no definitive conclusions. Synovial membrane is located in the inner side of the joint capsule, it secretes synovial fluid, and it is sensitive tissue that feels stress in the articular cavity. Many studies have demonstrated [7,8] the occurrence of inflammation in the synovial membrane, and the inflammation process played a crucial role in the initiation and development of TMD.
Toll-like receptor 4 (TLR4) has been widely known as a key regulator, which is involved in both initiation of innate resistance and the development of adaptive immune responses against infectious pathogens and damage-associated molecule patterns (DAMPs) from endogenous proteins that are elevated due to stress, inflammation, and cell death [9–11]. Engagement of ligands to TLR4 initiates a series of intracellular signaling events, such as mitogen-activated protein kinase (MAPK) cascades, ultimately culminating in the activation of nuclear factor-κB (NF-κB) or nuclear transcription factor activation protein-1 (AP-1) [12]. These factors are, in turn, responsible for the transcriptional activation of a set of genes that mediate inflammation, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) [13, 14]. Among these inflammatory cytokines, IL-1β is particularly important. Abnormal expression of IL-1β has been associated with the pathogenesis of a variety of human diseases, including synovial inflammation and cartilage destruction in TMJ [15,16]. Therefore, it is critical to understand how to control the expression of IL-1β, from synthesis to degradation.
In our previous research [17], we created an occlusal interference animal model to induce synovial injury by bonding crowns with a thickness of 0.6 mm to the right mandibular first molars of rats, and then observed obvious inflammation changes and increased expression of TLR4 and IL-1β at both mRNA and protein levels in the synovial membranes. In addition, these changes could be restrained by treatment with TAK-242, a blocker of TLR4 signaling. The results suggested that the activation of TLR4 may be involved in the inflammatory reactions and increased expression of IL-1β. While a proinflammatory effect of TLR4 has been implicated in these diseases, the adaptor molecules that participate in intracellular signaling and the pathways of intracellular signaling transduction triggered by TLR4 and that induce production of inflammatory mediators like IL-1β were not studied.
In this present study, we created an occlusal interference animal model to induce synovial injury by the method aforementioned to learn more about signaling pathways. The results showed that downstream p38MAPK signaling was triggered following the activation of TLR4. Moreover, we evaluated that p38 MAPK may be responsible for the inflammatory responses and the expression of IL-1β in rats induced by occlusal interference by treatment with SB203580, a specific inhibitor targeting p38. The aim of this study was to provide valuable information that will improve our understanding of the pathologic mechanism of synovial inflammation in TMJ.
Material and Methods
Animals
Forty-eight male Wistar rats (6-weeks old, obtained from the Shandong University Center of Laboratory Animals, China) were housed under a 12-h light/dark cycle with food and water available ad libitum. This study was approved by the Animal Care and Use Committee at the Shandong University.
Animal model of occlusal interference
Rats were anesthetized with intraperitoneal injection of pentobarbital sodium (0.5%, 40 mg/kg). A metallic crown (0.6 mm, uniform thickness) was bonded to the right mandibular first molar using resin cement (Super-Bond C&B, Osaka, Japan). Crowns were fabricated using cobalt chromium casting alloys and designed to cover the occlusal, buccal, lingual, and medial surfaces of the molars. Sham-treated rats in the control group were anesthetized and their mouths were forced opened for approximately 5 min using a protocol similar to the occlusal interference groups, however, no crowns were cemented.
Forty-eight rats were randomly divided into 4 groups (12 rats in each group) and treated as follows: 1) the control group, these rats were anesthetized and their mouths were forced open for about 5 min and received saline injections (10 uL, twice a week) into the upper joint cavities of both sides of TMJs; 2) the occlusal interference group, these rats were treated to create an occlusal interference animal model according to the aforementioned methods and these rats received saline injections (10 uL, twice a week) into the upper joint cavities of both sides of TMJs; 3) the TAK-242 group, these rats were treated to create an occlusal interference animal model according to the aforementioned methods and these rats received TAK-242 injections (3 mg/kg, diluted in 10 uL DMSO (Invitrogen, San Diego, CA, USA), twice a week, into the upper joint cavities of both sides of TMJs; and 4) the SB203580 group, these rats were treated to create an occlusal interference animal model according to the aforementioned methods and received SB203580 injections (2 mg/kg, diluted in 10uL DMSO (Invitrogen, San Diego, CA, USA), twice a week, into the upper joint cavities of both sides of TMJs.
Tissue preparation
After 2 weeks, 6 rats in each group were randomly selected and were euthanized by an overdose of pentobarbital sodium. Then, the rats were perfused with heparinized saline followed by a cold fixative containing 4% paraformaldehyde in 0.01 M phosphate buffer saline (PBS, pH 7.2). The right TMJs were removed, fixed in 4% paraformaldehyde, and then demineralized in 15% EDTA. After decalcification in 10% EDTA, the TMJs were dehydrated, embedded in paraffin, and sectioned on the sagittal plane at a thickness of 4 μm.
The other 6 rats in each group were also anesthetized with an overdose of pentobarbital sodium. The synovial tissues were harvested from the right TMJs, rinsed with cold sterile saline solution and stored at –80°C for real-time quantitative polymerase chain reaction (RT-PCR) assay.
Histopathologic examination
The sagittal sections of the central portion of the rat TMJ were selected from each TMJ in all rats, and then stained with hematoxylin and eosin. The histopathological findings were evaluated as previously described [18,19]. Briefly, 1) synovial lining hyperplasia was graded on a scale from 0 to 2: grade 0, staining of 1–3 layers; grade 1, staining of 4–6 layers; and grade 2, staining of 7 or more layers. 2) Dilated vasculature was graded on a scale from 0 to 3: grade 0, not present; grade 1, involving less than one-third of the synovial membrane length; grade 2, involving one-third to two-thirds of the synovial membrane length; grade 3, involving more than two-thirds of the synovial membrane length. 3) Fibrin deposits were graded on a scale from 0 to 3 (as described for the vasculature). 4) Vascularity was graded on a scale from 0 to 2: grade 0, a limited number (less than 5) of blood vessel profiles/mm2; grade 1, focal occurrence of 5–10 small blood vessel profiles/mm2; grade 2, focal occurrence of a large number (more than 10) of small blood vessel profiles/mm2.
Immunohistochemistry
After routine deparaffinization and rehydration, the sections underwent antigen retrieval in 0.125% trypsin-EDTA (Solarbio, Beijing, China) for 20 min at 37°C. Histostain™-Plus kits (ZSGB-Bio, Beijing, China) were used according to the manufacturer’s recommendations. After incubation in goat serum, sections were incubated with the primary antibodies against P-p38, IL-1β (1: 1000, Cell Signaling, Beverly, MA, USA), respectively, overnight at 4°C. After rinsing with 0.01 M PBS, the sections were exposed to goat anti-rabbit secondary antibody (ZSGB-Bio, Beijing, China) for 30 min at 37°C, then were exposed to a solution of horseradish peroxidase-conjugated avidin-biotin complex (ZSGB-Bio, Beijing, China) for 20 min at 37°C. Then, sections were visualized with 0.1% DAB (3, 30-diaminobenzidine dihydrochloride) (ZSGB-Bio, Beijing, China), and the sections were counterstained with hematoxylin. The digital images were captured using a microscopy digital camera system (Olympus, Tokyo, Japan. The results were evaluated semi-quantitatively using the Image-Pro Plus 6.0 software. Five sections per rat were assayed in high power, and the mean optical density (MOD) was measured respectively. The mean of MOD of 5 sections was seen as relative protein expression of this rat.
Real-time quantitative PCR
Total RNA was extracted from synovial tissues using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The first strand complementary DNA (cDNA) was synthesized by reverse transcription using SYBR Prime Script TM RT reagent Kit (Takara, Dalian, China). The levels of target mRNA in synovial tissues were analyzed by quantitative real-time PCR using SYBR Green I dye (Takara, Dalian, China). The primer pairs used for PCR were as follows: forward 5′-ACAAGGAGAGACAAGCAACGA-3′ and reverse 5′-TCTGCTTGAGAGGTGCTGATG-3′ for IL-1β, and forward 5′-GAAGGTGAAGGTCGGAGTCG-3′ and reverse 5′-GAAGATGG TGATGGGATTTC-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
The amplifications were performed in triplicate on a LightCycler 480 QPCR System (Roche Diagnostics Ltd., Bern, Switzerland). Each gene was normalized against the corresponding GAPDH levels and relative gene expression of each sample was fold change (2−ΔΔCt) using the control group as calibrator.
Statistical analysis
Normally distributed variables were expressed as means ±SD. Unpaired Student’s t-test was used to compare differences between groups. Differences in data values were defined significant at a P<0.05 using SPSS statistical software package Version 17.0.
Results
Effect of TLR4 on the expression of P-p38 in the synovial membranes
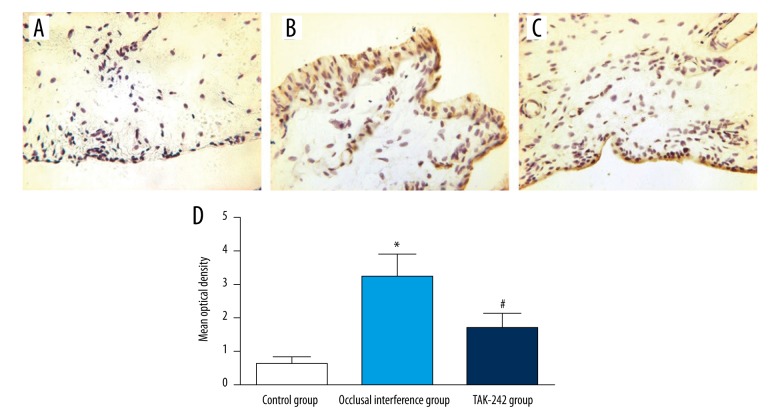
As shown in Figure 1A, the immunohistochemistry revealed that there were few synovial membranes that could be stained, and the synovial membranes in the control group hardly expressed P-p38. Compared with the control group, the area of synovial membranes stained was increased by the experiment of occlusal interference (Figure 1B), and the expression of P-p38 (Figure 1D) in the occlusal interference group was improved (P<0.05). In the TAK-242 group, treatment with TAK-242 could reduce the area of synovial membranes stained (Figure 1C). As shown in Figure 1D, the occlusal interference induced increased expression of P-p38 was significantly reduced by the injection of TAK-242 compared with the occlusal interference group (P<0.05).
Figure 1.
Effect of TLR4 on the expression of P-p38 in the synovial membranes. Immunohistochemical staining for P-p38 in the synovial membranes of (A) the control group, (B) the occlusal interference group, and (C) the TAK-242 group. (D) The mean optical density of each groups. As the results show, in comparison with the control group, the expression of P-p38 was significantly increased in the occlusal interference group. However, this effect could be inhibited significantly after treatment with the TAK-242. Data shows all the values from independent samples of n=6, * P<0.05 vs. control group, # P<0.05 vs. occlusal interference group.
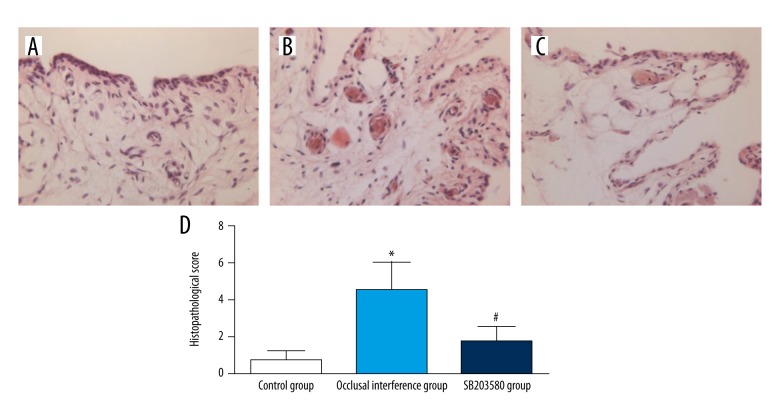
Effect of P-p38 on the histopathological findings in the synovial membranes
In the control group (Figure 2A), the synovial membranes of the TMJs did not show inflammatory changes. In the occlusal interference group (Figure 2B), obvious inflammation changes were observed in the synovial membranes, such as apparent hyperplasia of synovial lining cells, dilated blood vessels, proliferation of blood vessels and fibrin deposition. As shown in Figure 2D, in comparison with that in the controls, the histopathological score was significantly increased in the occlusal interference group (P<0.05). In the SB203580 group (Figure 2C), the treatment of SB203580 markedly inhibited the inflammatory reactions, although slight hyperplasia of synovial lining cells and dilated blood vessels were still present. As shown in Figure 2D, the histopathological score became significantly lower after treatment with the SB203580 when compared with the occlusal interference group (P<0.05).
Figure 2.
Histological examination of synovial membranes: (A) the control group, (B) the occlusal interference group, and (C) the SB203580 group. (D) The histopathological score of each group. As the results show, in comparison with the control group, the histopathological score was significantly increased in the occlusal interference group. However, this effect could be inhibited significantly after treatment with the SB203580. Data shows all the values from independent samples of n=6, * P<0.05 vs. control group, # P<0.05 vs. occlusal interference group.
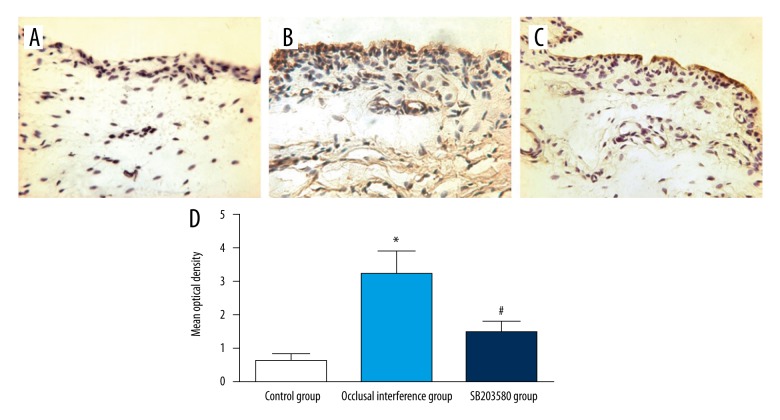
The expression of P-p38 in the synovial membranes
The result of immunohistochemistry staining for P-p38 in the synovial membranes of the TMJ in each group are shown in Figure 3A–3C. Compared with the control group, the expression of P-p38 of synovial membranes in the occlusal interference group was improved (Figures 3D, P<0.05). In the SB203580 group, the treatment with SB203580 significantly reduced occlusal interference-enhanced P-p38 expression (Figure 3D) compared with the occlusal interference group (P<0.05).
Figure 3.
The expression of P-P38 in the synovial membranes. Immunohistochemical staining for P-p38 in the synovial membranes of (A) the control group, (B) the occlusal interference group, and (C) the SB203580 group. (D) The mean optical density of each group. As the results show, in comparison with the control group, the expression of P-p38 was significantly increased in the occlusal interference group. However, this effect could be inhibited significantly after treatment with the SB203580. Data shows all the values from independent samples of n=6, * P<0.05 vs. control group, # P<0.05 vs. occlusal interference group.
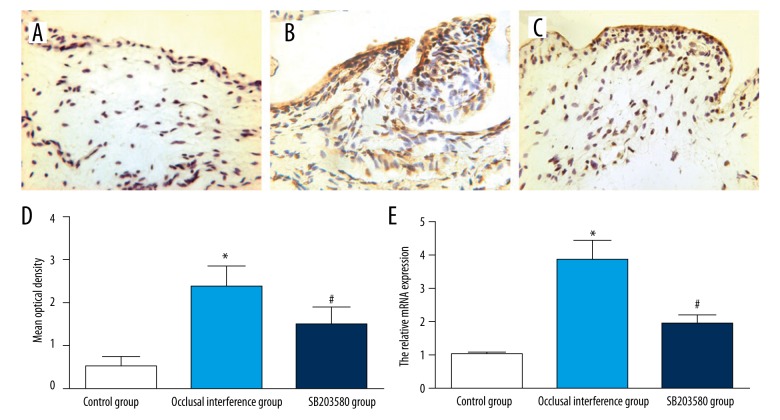
Effect of P-p38 on the expression of IL-1β
The result of immunohistochemistry staining for IL-1β in the synovial membranes of the TMJ in each group are shown in Figure 4A–4C. Compared with the control group, the expression of IL-1β of synovial membranes in the occlusal interference group was improved at both protein (Figure 4D) and mRNA (Figure 4E) levels (P<0.05). In the SB203580 group, the treatment with SB203580 significantly reduced occlusal interference-enhanced IL-1β expression at both protein (Figure 4D) and mRNA (Figure 4E) levels compared with the occlusal interference group (P<0.05).
Figure 4.
Effect of P-p38 on the expression of IL-1β in the synovial membranes. Immunohistochemical staining for IL-1β in the synovial membranes of (A) the control group, (B) the occlusal interference group, and (C) the SB203580 group. (D) The mean optical density of each groups. (E) The relative mRNA expression of IL-1β of each group. As the results show, in comparison with the control group, the expression of IL-1β was significantly increased in the occlusal interference group at both protein and mRNA levels. However, this effect could be inhibited significantly after treatment with the SB203580. Data shows all the values from independent samples of n=6, * P<0.05 vs. control group, # P<0.05 vs. occlusal interference group.
Discussion
Synovitis is an inflammation mainly occurs in synovial membrane and joint capsule of TMJ. The patients often suffered from pain in TMJ. The initiation of synovitis is considered to involve many risk factors, nevertheless, there are no definitive conclusions about the mechanisms of the initiation and development of this disease. Therefore, investigations into the detailed mechanisms of the pathophysiological processes that occur during synovitis are of great clinical significance to optimize therapeutic measures. In our previous investigation, we created an occlusal interference animal model by bonding crowns with a thickness of 0.6 mm to right mandibular first molar, and observed obvious inflammation changes in the synovial membranes, such as apparent hyperplasia of synovial lining cells, dilated blood vessels, proliferation of blood vessels and fibrin deposition. In this study, we established an occlusal interference rat model with the method described to investigate the pathophysiological mechanisms.
TLR4 is a member of the TLR (Toll-like receptor) family of transmembrane proteins. TAK-242 is a specific inhibitor of TLR4, which could selectively suppress TLR4-mediated myeloid differentiation factor 88 (MyD88)-dependent pathway as well as TIR domain-containing adapter-inducing IFN-β (TRIF) dependent pathway by binding to Cys747 in the intracellular domain of TLR4 and its inhibitory effect is largely unaffected by LPS concentration and types of TLR4 ligands, and finally inhibit the expression of NO, TNF-α, IL-6, and IL-1β [20,21]. In a previous study, we found TAK-242 could restrain the inflammation responses and increased expression of IL-1β at both mRNA and protein levels in the synovial membranes, and the inflammatory reactions and expression of IL-1β in synovitis of rats induced by occlusal interference maybe regulated by TLR4. In this study, we explored its signaling and downstream processing, and found that the expression of phosphorylated p38 (P-p38) was improved in the occlusal interference group. In addition, the increased P-p38 could be restrained by treatment with TAK-242. These results suggest that the phosphorylation of p38 MAPK may be involved in the TLR4 triggered immune responses induced by occlusal interference.
The MAPK signaling pathway, which includes p38, c-Jun N-terminal protein kinase (JNK), and extracellular signal-regulated kinase 1/2(ERK1/2) MAPK signaling, has been shown to be key in the transduction of extracellular signals to cell responses. Once activated, the phosphorylation of pathways could relay, amplify, and integrate signals from a wide range of stimuli, and are crucial for regulating cell growth, proliferation, differentiation, apoptosis, and the expression levels of inflammatory cytokines [22–27]. SB203580, a 2,4,5-triarylimidazole, is a potent p38 MAPK inhibitor that is highly selective relative to other kinases, including other closely related MAP kinases [28]. As a classical pyridinyl imidazole p38 inhibitor, SB203580 binds to the ATP-binding site of p38 [29], so that p38 cannot activate the substrates like ATF2 without ATP binding.
A series of studies have demonstrated that the p38MAPK signaling pathway plays an important role in the progression of many diseases. As shown in studies [30,31], high levels of non-esterified fatty acid (NEFA) treatment significantly increased levels of phosphorylated p38MAPK and induced apoptosis in bovine hepatocytes in vitro. Serum retinol-binding protein 4 (RBP4) induces phospho-activation of p38, and inhibitors of p38 activation can partially inhibit RBP4-induced inflammation in human retinal microvascular endothelial cells (HRECs) [32]. In our study, we investigated whether p38MAPK participated in the inflammatory responses and the expression of IL-1β in rats induced by occlusal interference. As the results showed in the occlusal interference group, obvious inflammation changes were observed in the synovial membranes, the expression of P-p38 was increased, and IL-1β in the synovial membranes was significantly increased at both protein and mRNA levels. However, the effect of occlusal interference was significantly decreased by the use of SB203580. In the SB203580 group, the histologic severity score of synovial membranes became significantly lower after treatment with the SB203580 when compared with the occlusal interference group. Consistent with the inflammatory reactions, the increased expression of IL-1β was obviously reduced at both mRNA and protein levels. This may suggest the important role of p38MAPK in increased expression of proinflammatory cytokines, like IL-1β, and the inflammatory reactions of synovial membranes in rats treated with occlusal interference.
Conclusions
In the current study, we demonstrated that downstream p38MAPK signaling was triggered following the activation of TLR4 in synovitis of rats, and the activation of p38 MAPK may be involved in the TLR4 triggered immune responses induced by occlusal interference in rats. Additionally, the injection of SB203580 could inhibit the inflammatory reactions and the increased expression of IL-1β at both mRNA and protein levels. The study results suggested that TLR4 may stimulate synovial inflammatory reactions and increased expression of IL-1β in rats through the activation of p38 MAPK signaling pathway; and that p38 may be an important mediator in the mechanisms of the initiation and development of synovial injury by regulating the expression of inflammatory mediators, like IL-1β, in synovial membranes. Our research results provided new valuable information that will improve our understanding of the pathologic mechanism of synovial inflammation in TMJ.
Footnotes
Conflict of interests
None.
Source of support: This research was supported by science and technology development plans of Shandong province (Grant No.2014GSF118027) and the Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20120131110074)
References
- 1.Stohler CS. Craniofacial pain and motor function: pathogenesis, clinical correlates, and implications. Crit Rev Oral Biol Med. 1999;10(4):504–18. doi: 10.1177/10454411990100040601. [DOI] [PubMed] [Google Scholar]
- 2.Stegenga B. Nomenclature and classification of temporomandibular joint disorders. J Oral Rehabil. 2010;37(10):760–65. doi: 10.1111/j.1365-2842.2010.02146.x. [DOI] [PubMed] [Google Scholar]
- 3.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol. 2011;97:179–206. doi: 10.1016/B978-0-12-385198-7.00007-2. [DOI] [PubMed] [Google Scholar]
- 4.Seligman DA, Pullinger AG. Analysis of occlusal variables, dental attrition, and age for distinguishing healthy controls from female patients with intracapsular temporomandibular disorders. J Prosthet Dent. 2000;83(1):76–82. doi: 10.1016/s0022-3913(00)70091-6. [DOI] [PubMed] [Google Scholar]
- 5.Celić R, Braut V, Petricević N. Influence of depression and somatization on acute and chronic orofacial pain in patients with single or multiple TMD diagnoses. Coll Antropol. 2011;35(3):709–13. [PubMed] [Google Scholar]
- 6.Suvinen TI, Reade PC, Hanes KR, et al. Temporomandibular disorder subtypes according to self-reported physical and psychosocial variables in female patients: a re-evaluation. J Oral Rehabil. 2005;32(3):166–73. doi: 10.1111/j.1365-2842.2004.01432.x. [DOI] [PubMed] [Google Scholar]
- 7.Sato H, Fujii T, Kitamori H. The clinical significance of the horizontal condylar angle in patients with temporomandibular disorders. Cranio. 1997;15(3):229–35. doi: 10.1080/08869634.1997.11746016. [DOI] [PubMed] [Google Scholar]
- 8.Gynther GW, Holmlund AB, Reinholt FP. Synovitis in internal derangement of the temporomandibular joint: correlation between arthroscopic and histologic findings. J Oral Maxillofac Sur. 1994;52(9):913–17. doi: 10.1016/s0278-2391(10)80066-7. [DOI] [PubMed] [Google Scholar]
- 9.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1(2):135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Sohn KH, Rhee SH, et al. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through toll-like receptor 4. J Biol Chemi. 2001;276(20):16683–89. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 11.Heinola T, Kouri V-P, Clarijs P, et al. High mobility group box-1 (HMGB-1) in osteoarthritic cartilage. Clin Exp Rheumatol. 2010;28(4):511–18. [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Akira A. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Investigation. 2006;86(1):9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- 15.Vincenti MP, Brinckerhoff CE. Early response genes induced in chondrocytes stimulated with the inflammatory cytokine interleukin-1. Arthritis Res. 2001;3(6):381–88. doi: 10.1186/ar331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn DK, Chae JM, Choi HS, et al. Central cyclooxygenase inhibitors reduced IL-1b-induced hyperalgesia in temporomandibular joint of freely moving rats. Pain. 2005;117(1):204–13. doi: 10.1016/j.pain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Kong J, Yang Y, Sun S, et al. Effect of toll-Like receptor 4 on synovial injury of temporomandibular joint in rats caused by occlusal interference. Mediators Inflamm. 2016;2015:7694921. doi: 10.1155/2016/7694921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gynther GW, Dijkgraaf LC, Reinhoh FP, et al. Synovial inflammation in arthroscopieally obtained biopsy specimens from the temporomandibular joint: A review of the literature and a proposed histologie grading system. Oral Maxillofac Surg. 1998;56(11):1281–86. doi: 10.1016/s0278-2391(98)90609-7. [DOI] [PubMed] [Google Scholar]
- 19.Ozaki M, Kaneko S, Soma K. Masseter muscular weakness affects temporomandibular synovitis induced by jaw opening in growing rats. Angle Orthod. 2008;78(5):819–25. doi: 10.2319/072407-342.1. [DOI] [PubMed] [Google Scholar]
- 20.Takashima K, Matsunaga N, Yoshimatsu M, et al. Analysis of binding site for the novel small-molecule TLR4 signal transduction inhibitor TAK-242 and its therapeutic effect on mouse sepsis model. Bri J Pharmacol. 2009;157(7):1250–62. doi: 10.1111/j.1476-5381.2009.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsunaga N, Tsuchimori N, Matsumoto T, et al. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011;79(1):34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Zhang X, Liu Y, Liu Z. Morphine postconditioning protects against reperfusion injury via inhibiting jnk/p38 mapk and mitochondrial permeability transition pores signaling pathways. Cell Physiol Biochem. 2016;39(1):61–70. doi: 10.1159/000445605. [DOI] [PubMed] [Google Scholar]
- 23.Odaka H, Numakawa T, Yoshimura A, et al. Chronic glucocorticoid exposure suppressed the differentiation and survival of embryonic neural stem/progenitor cells: Possible involvement of erk and pi3k/akt signaling in the neuronal differentiation. Neuro Sci Res. 2016;113:28–36. doi: 10.1016/j.neures.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Yao J, Weng Y, Yan S, et al. Nov inhibits proliferation while promoting apoptosis and migration in osteosarcoma cell lines through p38/mapk and jnk/mapk pathways. Oncol Rep. 2015;34(4):2011–21. doi: 10.3892/or.2015.4153. [DOI] [PubMed] [Google Scholar]
- 25.Chi H, Barry SP, Roth RJ, et al. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) ininnate immune responses. Proc Natl Acad Sci USA. 2006;103(7):2274–79. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases regulating the immune response. Nat Rev Immunol. 2007;7(2):202–12. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 27.Ma FY, Sachchithananthan M, Flanc RS, et al. Mitogen activated protein kinases in renal fibrosis. Front Biosci. 2009;1(1):171–87. doi: 10.2741/s17. [DOI] [PubMed] [Google Scholar]
- 28.Cuenda A, Rouse J, Doza YN, et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364(2):229–33. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 29.Lisnock J, Tebben A, Frantz B, et al. Molecular basis for p38 protein kinase inhibitor specificity. Biochemistry. 1998;37(47):16573–81. doi: 10.1021/bi981591x. [DOI] [PubMed] [Google Scholar]
- 30.Song Y, Li X, Li Y, et al. Non-esterified fatty acids activate the ros-p38-p5 3/n rf2 signaling pathway to induce bovine hepatocyte apoptosis in vitro. Apoptosis. 2014;19(6):984–97. doi: 10.1007/s10495-014-0982-3. [DOI] [PubMed] [Google Scholar]
- 31.Song Y, Li N, Gu J, et al. β-hydroxybutyrate induces bovine hepatocyte apoptosis via an ros-p38 signaling pathway. J Dairy Sci. 2016;99(11):9184–98. doi: 10.3168/jds.2016-11219. [DOI] [PubMed] [Google Scholar]
- 32.Du M, Martin A, Hays F, et al. Serum retinol-binding protein-induced endothelial inflammation is mediated through the activation of toll-like receptor 4. Mol Vis. 2017;23:185–97. [PMC free article] [PubMed] [Google Scholar]