Abstract
Some aquatic mammals appear to care for their dead, whereas others abandon their live offspring when conditions are unfavourable. This incredible variety in behaviours suggests the importance of comparing and contrasting mechanisms driving death-related behaviours among these species. We reviewed 106 cases of aquatic mammals (81 cetaceans and 25 non-cetaceans) reacting to a death event, and extrapolated ‘participant’ (age class, sex, relationship and decomposition) and ‘social’ characteristics (escorting, calf dependence, alloparental care, herding and dispersal patterns) from published and unpublished literature. A multiple correspondence analysis (MCA) was performed to explore the relationships between these characteristics and death-related behaviours, with species clustered based on MCA scores. Results showed that both cetaceans and non-cetaceans react to death but in different ways. Non-cetaceans, characterized by a short maternal investment, were observed to protect the dead (defending it from external attacks), while cetaceans spent much longer with their offspring and display carrying (hauling, spinning, mouthing with the carcass and diving with it) and breathing-related (lifting and sinking the carcass) activities with the dead generally in association with other conspecifics. Our work emphasizes the need of increased documentation of death-related cases around the world to improve our understanding of aquatic mammals and their responses to death.
This article is part of the theme issue ‘Evolutionary thanatology: impacts of the dead on the living in humans and other animals’.
Keywords: death, behaviour, sociality, aquatic mammals, multiple correspondence analyses
1. Background
‘Death-related behaviour’ [1], including grieving and other complex responses to dying or to distressed conspecifics, was long considered an exclusive prerogative of our species [2]. Darwin was one of the earliest to suggest that other animal species, like humans, are capable of pleasure, pain, happiness and misery [3]. Death-related behaviour is described as a subcategory of epimeletic or nurturant behaviours (i.e. a healthy individual gives attention to an injured or dead one, as summarized in [4]) and is usually seen as a consequence of the cooperative, succouring and protective nature of social mammals [4–8]. Considering that the individual receiving this attention is often an offspring, some authors suggested that this behaviour could be a consequence of the strong mother–offspring bond [9–12], or a revival attempt through violent manipulation of the bodies [13,14]. In certain cases where the dead or dying individuals were adults, a sexual component and/or a dominance display is involved as observers recorded erections, mounting attempts and other dominance display behaviours [15,16]. Generally, social structure features, anatomical constraints and ecological conditions could influence death-related behaviours, while an evolutionary or direct benefit is still far from being inferred [8].
According to the available literature, epimeletic death responses among land and marine mammals include stereotyped behaviours such as the carrying of dead offspring (primates using hands, cetaceans against their dorsal fin or similarly to Canis in their mouth) and having the mother–dead infant pair (or simply the dead infant) protected or escorted by other members of their groups, as observed in elephants, cetaceans and primates [4,7]. In cases where individuals were unable to carry the dead (e.g. lemurs, giraffes and elephants), these species are known to stay near a dead conspecific for extended periods and move back and forth between their groups and their distressed or dead offsprings [6–8]. Many aquatic mammal species react to the death of a conspecific, most often a calf, and adults can be observed staying close to, maintaining physical contact with, lifting, keeping at the surface or carrying the dead one, even in an advanced state of decomposition. While carrying the carcass adults may stop eating, focusing all their attention on it. They can also display defensive and aggressive behaviours if predators or conspecifics (e.g. pinnipeds) cross their paths, and have escorts accompany and defend them from intruders (e.g. cetaceans, [4,7]). There are also records of species (e.g. sea otters, [17–19], Antarctic fur seals [20]) that have been observed to routinely abandon their live pups, owing to environmental changes, illness or nutritional stress.
While death-related behaviours of dolphins are known to be highly variable [4,7], there has not been a comprehensive review of the available information on this topic including all cetaceans. The only exception is a recent encyclopaedia chapter focusing on epimeletic behaviour among cetaceans [4]. Regarding pinnipeds, sea otters and manatees, the current available information is lacking, and the few studies are often descriptive and include sporadic observations. However, in the majority of reported cases, a change in behaviour occurred after the death of a conspecific, suggesting that such event may have caused disruption/distress in the species displaying death-related behaviours. In this study, the association between aquatic mammal species' social characteristics and death-related behaviours was explored by using a multiple correspondence analysis (MCA) of the literature and available unpublished material. The aim was to answer the following questions: (i) Can behaviours displayed during death events be linked to certain species' social characteristics?, (ii) If so, which social characteristics can be used to categorize the type of behaviour displayed? and (iii) How do these behaviours differ among cetaceans and non-cetacean species? Sightings published in the literature and in the field were collected and were critically assessed, highlighting inconsistencies and identifying key areas for further work and future analysis.
2. Systematic and analytical literature review
We used the List of marine mammal species and subspecies [21] to create an updated list of aquatic mammals, distinguishing cetacean (odontocetes and mysticetes) from non-cetaceans (pinnipeds, sea otters and manatees) for this study. To find published cases, we used a combination of search words (see electronic supplementary material, table S1A for how these words were combined) including calf, pup, adult, mortality, died, dead, death, mother and behaviour, with the Latin name of each species, in the search engine Google Scholar. We also searched the reference section of online published papers to find additional articles not located in the online searches. Lastly, we contacted authors who had published several papers focusing on sociality, death and mother–calf bond among non-cetacean species. We added new field sightings from other researches to the literature review, and a complete list of all the cases including reference, species and participants characteristics is reported in electronic supplementary material, table S1B. Video and photographs available on the web and collected by a non-scientific audience were excluded owing to potential bias caused by cinematographic editing in videos (such as the loss of the correct temporal sequence of events owing to efforts to increase the dramatic nature of the images) and to the lack of detailed information about death events for photographs. A total of nine ‘characteristics’ (adapted from [22]) were gathered and were categorized as follows. Four of these were used to describe the ‘participant characteristics': age class (adult, juvenile, subadult and calf), sex (male and female), relationship (between the alive ‘giver’ and the dead ‘receiver’: mother, inferred mother and unrelated) and decomposition (fresh, moderate and advanced; following [23]). Five provided information about the sociality, hereafter ‘social characteristics' (see electronic supplementary material, table S2): alloparental care (presence and absence), calf dependence (defined as when a calf relies on its mother for food, protection, spending the majority of its time with her: 6–11 months, 1–1.5 years, 2–5 years, 4 years, 5.5 years, 6 years, 6–10 years), herding (mother–calf pair living in female groups, living in mixed-sex groups, living in mother–calf pair groups only and solitary), dispersal patterns (intended as the choice of offspring to stay, to leave their natal group once they reached sexual maturity or to return after a period of separation) and escorting (defined by the presence or absence of other conspecifics involved: helper, group and none). We chose these social characteristics because the death of a conspecific can affect group composition and survivability, with group composition potentially influenced by age class, sex, reproductive condition and kinship [24–26], and the social characteristics by group cohesion, parental care, social structure and reproductive success [27]. Dependence, alloparental care, herding and dispersal patterns categories were inferred and generalized from population studies found in the literature (see electronic supplementary material, table S2). The category unknown was used when the information was not certain, not applicable when the receiver was an object, another species or the receiver was severely wounded and close to death (alive-then-dead) and not reported when a parameter was not described in the literature.
An ethogram of death-related behaviours for cetacean and non-cetacean species was created consisting of a total of 23 behavioural types using terms that were found in the literature review we conducted. Potential sources of bias in our dataset are linked to (i) low frequencies of some behavioural components, and (ii) the species-specificity of some behaviours. In order to prevent the low frequency of some behaviours from biasing our results, we created behavioural categories and grouped multiple behaviours within them. To avoid creating categories that include behaviours displayed solely by one species, we included behavioural components displayed by both cetaceans and non-cetaceans. The only exception is the category ‘protection’, (see electronic supplementary material, table S3), which is only displayed by non-cetaceans. In some species of pinnipeds females display a protecting behaviour towards their young ones in response to aggressive juvenile male competitors wanting to separate them from their calves during the mating season. Given the uniqueness and importance of this category we decided to retain it. We therefore classified all behavioural types into the following six behavioural categories: (i) carriage: carrying, hauling, spinning, mouthing and diving; (ii) breathing: lifting and sinking; (iii) contacts: striking, licking, body contact, nosing, arousing, suckling and grooming; (iv) protection: protecting; (v) other: vocalizing, kidnapping, searching, unknown, sniffing and sexual; (vi) resting: laying beside the carcass and stationing. See electronic supplementary material, table S3 for the full list of types and categories.
An exploratory analysis of the potential relationships between aquatic mammal species and their death-related behaviours and social characteristics was performed using an MCA [28,29]. MCA allows the analysis of multivariate categorical data and visualization of the results in a graphical manner. For each species, each behavioural and social parameter was marked as a ‘1’ if present and ‘0’ if absent (see electronic supplementary material, table S4).
The matrix data, comprising 23 behavioural types for 28 aquatic species (see details below in ‘Participant characteristics and death-related behaviour’), were then converted into dimensions that were structured from the most explicative to the least. To permit visualization, the scores from the two dimensions that account for the most variance are projected to create a factor plane. The scores on the factor plane can be used to explore the relationship between species where the distances between points reflect the similarities in type of social and behavioural characteristics, with the shorter the distance, the greater the similarity. We clustered species into groups by using the scores of the first n axes where n is defined by finding the cut-off where an increase in the axes does not provide significant discriminative properties (inertial gain). A hierarchical clustering is performed with the scores from these n axes using the Euclidian distance and Ward's clustering method. All analyses were performed using the R programming environment (R Core Team 2017) using FactoMineR [30] and associated packages for the MCA and clustering analysis.
3. Participant characteristics and death-related behaviour
A total of 106 cases were found (81 of cetacean and 25 of non-cetacean species), with 28 species involved (20 were cetaceans and eight non-cetaceans; see electronic supplementary material, table S1). For cetaceans, Tursiops sp., Globicephala macrorhynchus and Sousa chinensis were the most recorded species displaying death-related behaviours (see electronic supplementary material, histogram S5a), and Otaria flavescens and Phoca vitulina for non-cetacean species (see electronic supplementary material, histogram S5b). However, it must be acknowledged that the results presented in this study refer to the number of death cases found through search engines and do not indicate the total number of existing cases. The results in this study could also be biased downwards owing to a possible omission of pertinent papers, although care was taken by the authors to provide the most comprehensive systematic review of death-related cases across all aquatic mammals. For both cetaceans and non-cetaceans, ‘givers’ were adults, females and usually inferred mothers, while ‘receivers’ were most often dead calves in a fresh state of decomposition. ‘Receivers’ were calves in 84 cases, adults in 11, subadults in one and juveniles in seven, while an amniotic sac was targeted in one case (see electronic supplementary material, histograms S5f,g).
4. Social characteristics and death-related behaviour
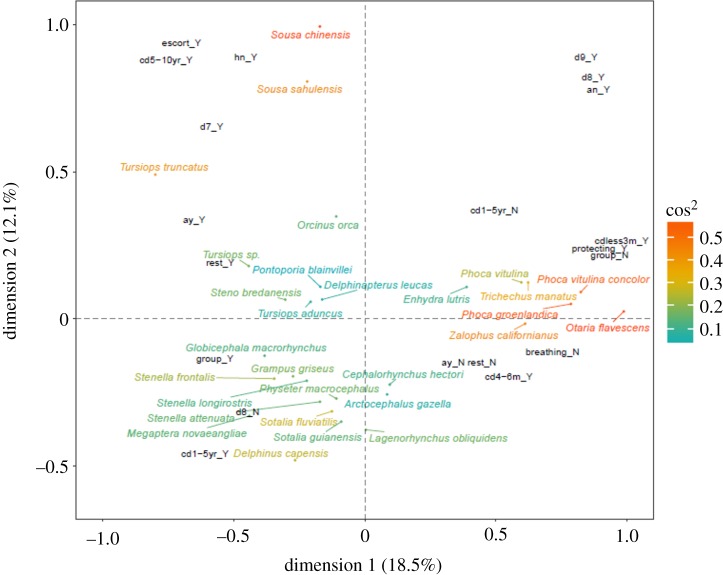
Both cetaceans and non-cetaceans react to death but display different behaviours. MCA (figure 1) results highlight that parameters such as calf dependence and the presence of alloparental care are related to the behavioural type that is displayed and to the participation of other individuals during death events (i.e. escorting). Cetaceans with more dynamic moving patterns and with a longer time spent with their offspring are found to display carrying- (hauling, spinning, mouthing the carcass and diving with it) and breathing-related (lifting and sinking the carcass) activities, generally in association with other conspecifics. More than one individual commonly interacted with the mother–calf pair in cetaceans, either approaching the couple or contributing (see electronic supplementary material, histograms S5h,i). Conversely, non-cetacean species, with a shorter maternal investment, react to the death of a conspecific by displaying ‘protecting’ as a behavioural type. Both cetaceans and seals live in fission–fusion societies [31], so the different shades of gregariousness typical of these groups could explain the frequent involvement of other members of the same species during death events.
Figure 1.
The first factor plane (dimension 1 and dimension 2) of the MCA which explains 30.6% of the total inertia within the dataset. Species are coloured by their cosine squared (cos2) value with larger values, suggesting a stronger association with each axis. The categories that represent the greatest contribution to both the dimension 1 and dimension 2 axes are shown in black (see table 1 and electronic supplementary material, tables S4 and S3 for a description of each category).
5. Cetacean versus non-cetacean species
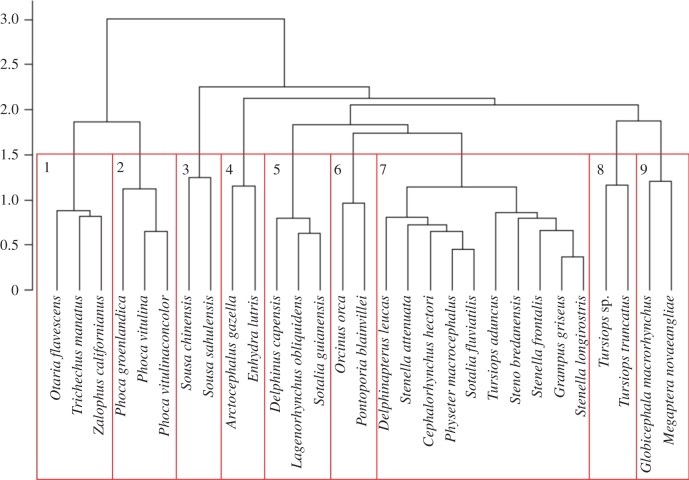
For non-cetaceans, the dendrogram shows that Phocidae (Phoca groenlandica, P. vitulina and P. vitulina concolor), Trichechidae (Trichechus manatus) and the majority of Otariidae (O. flavescens and Zalophus californianus) are clustered separately from cetaceans (figure 2 and table 1). The grouping between cetacean and non-cetacean species mirrors the behavioural and social differences existing between these two animal groups (see §4 ‘Social characteristics and death-related behaviour’). Our data also show that females of P. vitulina, P. vitulina concolor and O. flavescens display protective behaviours towards their dead young. This could relate to the protective behaviour that mothers display towards their offspring after birth to defend them against danger. For example, females in O. flavescens protect their calves from juvenile male competitors who want to reproduce with them, separating mothers from their calves [32,33]. The remaining Otariidae, Arctocephalus gazella, clusters with Enhydra lutris (Mustelidae) as these species both display grooming during death events and, although abandonment was not considered in the analysis, both species are also reported to abandon their alive pups due to changes in environmental or body conditions [19,20]).
Figure 2.
The results of the hierarchical clustering dendrogram using scores derived from the first eight axes of the MCA. Clusters were determined by finding the optimum level of inertia gain (p < 0.05).
Table 1.
The cosine squared (cos2) scores for the most important variables used to characterize the first two axes (dimension 1 and dimension 2) of the MCA with variables close to one are best represented by the two dimensions. Also shown is the percentage (%) contribution of the yes/no assignments for each category.
| % contribution |
||||||
|---|---|---|---|---|---|---|
| category | ID | dimension 1 cos2 | dimension 2 cos2 | total | yes | no |
| dispersal | d8 | 0.52 | 0.26 | 0.78 | 16.9 | 6.16 |
| calf dependence | cd5–10yr | 0.18 | 0.44 | 0.62 | 16.44 | 5.23 |
| herding (no) | hn | 0.02 | 0.54 | 0.56 | 20.01 | 2.39 |
| escorting | group | 0.53 | 0.03 | 0.56 | 5.16 | 8.44 |
| calf dependence | cd1–5yr | 0.3 | 0.17 | 0.47 | 7.56 | 6.14 |
| alloparental (no) | an | 0.31 | 0.13 | 0.44 | 10.05 | 2.49 |
| death-related behaviour | protecting | 0.4 | 0.01 | 0.41 | 8.63 | 2.21 |
| escorting | helper | 0.13 | 0.23 | 0.36 | 9.49 | 2.42 |
| calf dependence | cdless3 m | 0.34 | 0.01 | 0.35 | 7.35 | 1.9 |
| calf dependence | cd4–6 m | 0.34 | 0 | 0.34 | 7.22 | 2.64 |
| death-related behaviour | breathing | 0.31 | 0.01 | 0.32 | 2.9 | 4.74 |
| death-related behaviour | rest | 0.25 | 0.04 | 0.29 | 3.97 | 3.22 |
| alloparental (yes) | ay | 0.22 | 0.06 | 0.28 | 4.94 | 1.92 |
| dispersal | d9 | 0.15 | 0.07 | 0.22 | 6.07 | 2.87 |
For cetaceans, the dendrogram (figure 2) shows S. chinensis and S. sahulensis clustering together, which is likely due to their display of carrying, lifting and stationing behaviours, and having a long period of calf dependence (5–10 years). In the death-event cases analysed for these two species, mothers initially stayed alone with the dead, but were later assisted by escorts in the carrying of the carcass. Another cluster was composed of Delphinus capensis, Sotalia guianensis and Lagenorhynchus obliquidens, which share a short calf dependence (less than 1 year), the tendency of mother–calf pairs to live in groups with other conspecifics [34–36] and the presence of escorts intervening and carrying the dead. Another group was made by Orcinus orca and Pontoporia blainvillei, which were clustered together owing to the fact that juveniles remain with the maternal groups for the duration of their lives [37,38], they both show alloparental care, and during death events they always had at least one escort present with the mother. The largest cluster consisted of Cephalorhynchus hectori, Delphinapterus leucas, Grampus griseus, Physeter macrocephalus, Sotalia fluviatilis, Stenella attenuata, S. frontalis, S. longirostris, Steno bredanensis and Tursiops truncatus, which all displayed carrying, diving, mouthing and lifting as death-related behaviours. Lastly, Globicephala macrorhynchus and Megaptera novaeangliae were clustered together, sharing lifting and sexual behaviours directed towards dead adults (erections and intromission of a male towards dead female for G. macrorhynchus; erection and genital slit opened for M. novaeangliae). They also displayed their death-related behaviours in the presence of other individuals, a calf dependence period lasting 1–5 years and a tendency of mothers and calves to group together [39,40].
6. Conclusion and future recommendations
An important step when summarizing the findings of this work is to address the three aims we outlined at the beginning of this study that relate to the investigation of the association between aquatic mammals' species' social characteristics and death-related behaviours. (i) Can behaviours displayed during death events be linked to certain species' social characteristics? A high number of species show death-related behaviours that can occur due to a mix of ecological, taxonomical, cultural and abiotic factors. Here, we have shown that the behaviours displayed during death events in marine mammals can be linked to certain social characteristics. (ii) Which social characteristics can categorize the type of behaviour displayed? Death events represent for highly social species the definitive breaking of a strong social bond. Outcomes of this work highlight that for marine mammals, some social characteristics, such as calf dependence and the presence of alloparental care, can categorize death-related behavioural patterns. Lastly, (iii) How do these behaviours differ among cetaceans and non-cetacean species? Social characteristics, like alloparental care and calf dependence, differ among cetacean and non-cetacean species and consequently their behavioural patterns are influenced by this variation. Our results highlight that the differences in social characteristics shown by these two groups exert a strong influence on the variation of the observed death-related behaviours.
In the context of the new interdisciplinary area of comparative thanatology [41], which incorporates animal cognition, social behaviour, inter-individual relatedness and emotion, this study provides scientific advances in understanding how aquatic mammals face death through a systematic and analytical approach to link behaviour and social characteristics. However, fully understanding how aquatic mammals perceive and react to death will require more time. As a future consideration, a larger number of death-related events is needed to improve our understanding of grieving, abandonment and neglect towards the dead. We therefore hope to encourage an increasing number of researchers to report sightings of similar events, collecting acoustic recordings alongside photographs and videos with scientific rigour, and strictly accompanied by an accurate description of all behaviours displayed in chronological order. Future analysis could also include the use of mortality rate and predation risk as parameters investigated, as they are known to affect group cohesion and composition, which might ultimately influence how mammals relate to death.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to James Anderson and the editors of this issue for the invitation to contribute. We are also thankful to Giovanni Bearzi for his constructive suggestions and his help on designing this study. Thanks also go to Claudio Campagna, Fritz Trillmich and Burney LeBoeuf for sharing their knowledge and publications on pinnipeds with us. Thanks to all field assistants who collected sightings presented in this paper. The authors would like to dedicate this work to the memory of Carla Magliocco for her encouragement and support.
Data accessibility
Data are available in the electronic supplementary material.
Authors' contributions
M.A.L.V.R. and C.G.B. conceived the work, performed the systematic literature review, participated in data analysis and drafted the manuscript. L.E. contributed to most of the cetacean literature review. E.P. contributed to a field case, participated in data analysis and drafted the manuscript. G.A.deL. contributed with E.P. to a case described in the paper. N.McG. analysed the data, prepared all figures present in the paper, drafted the manuscript and helped revise the use of English. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
No funding was used to produce this paper.
References
- 1.Pierce J. 2013. The dying animal. Bioeth. Inq. 10, 469–478. ( 10.1007/s11673-013-9480-5) [DOI] [PubMed] [Google Scholar]
- 2.Fashing P, et al. 2011. Death among geladas (Theropitechus gelada): a broader perspective on mummified infants and primate thanatology. Am. J. Primatol. 73, 405–409. ( 10.1002/ajp.20902) [DOI] [PubMed] [Google Scholar]
- 3.Darwin C. 1871. The descent of man and selection in relation to sex. Princeton, NJ: Princeton University Press; (reprinted 1981). [Google Scholar]
- 4.Bearzi G, Eddy L, Piwetz S, Reggente MAL, Cozzi B. 2017. Cetacean behavior toward the dead and dying. In Encyclopedia of animal cognition and behavior (eds Vonk J, Shackelford TK), pp. 1–8. Berlin, Germany: Springer International Publishing. [Google Scholar]
- 5.de Waal FMB, Preston SD. 2017. Mammalian empathy: manifestation and neural basis. Nat. Rev. Neurosci. 18, 498–509. ( 10.1038/nrn.2017.72) [DOI] [PubMed] [Google Scholar]
- 6.Perez-Manrique A, Gomila A. 2017. The comparative study of empathy: sympathetic concern and empathic perspective-taking in non-human animals. Biol. Rev. 93, 248–269. ( 10.1111/brv.12342) [DOI] [PubMed] [Google Scholar]
- 7.Reggente MA, Alves F, Nicolau C, Freitas L, Cagnazzi D, Baird RW, Galli P. 2016. Nurturant behaviour toward dead conspecifics in free-ranging mammals: new records for odontocetes and a general review. J. Mammal. 97, 1428–1434. ( 10.1093/jmammal/gyw089) [DOI] [Google Scholar]
- 8.Douglas-Hamilton I, Bhalla S, Wittemyer G, Vollrath F. 2006. Behavioural reactions of elephants towards a dying and deceased matriarch. Appl. Anim. Behav. Sci. 100, 87–102. ( 10.1016/j.applanim.2006.04.014) [DOI] [Google Scholar]
- 9.Smith TG, Sleno GA. 1986. Do white whales, Delphinapterus leucas, carry surrogates in response to early loss of their young? Can. J. Zool. 64, 1581–1582. ( 10.1139/z86-237) [DOI] [Google Scholar]
- 10.Wells RS. 1991. Bringing up baby. Nat. Hist. 8, 56–62. [Google Scholar]
- 11.Fertl D, Schiro A. 1994. Carrying of dead calves by free-ranging Texas bottlenose dolphins (Tursiops truncatus). Aquat. Mamm. 20, 53–56. [Google Scholar]
- 12.Krasnova VV, Chernetsky AD, Zheludkova AI, Bel'kovich VM. 2014. Parental behaviour of the beluga whale (Delphinapterus leucas) in natural environment. Biol. Bull. Russ. Acad. Sci. 41, 349–356. ( 10.1134/S1062359014040062) [DOI] [PubMed] [Google Scholar]
- 13.Mann J, Barnett H. 1999. Lethal tiger shark (Galeocerdo cuvieri) attack on bottlenose dolphin (Tursiops sp.) calf: defense and reactions by the mother. Mar. Mamm. Sci. 15, 568–575. ( 10.1111/j.1748-7692.1999.tb00823.x) [DOI] [Google Scholar]
- 14.Rickards SH, Vanderlip C, Oliver G. 2001. Spinner dolphins (Stenella longirostris) of Midway Atoll, northwest Hawaiian archipelago: February–November 2001. Report for National Marine Fisheries Service and U.S. Fish and Wildlife Service Midway Atoll National Wildlife Refuge, p. 41.
- 15.Dudzinski KM, Sakai M, Masaki K, Kogi K, Hishii T, Kurimoto M. et al. 2003. Behavioural observations of bottlenose dolphins towards two dead conspecifics. Aquat. Mamm. 29, 108–116. ( 10.1578/016754203101023951) [DOI] [Google Scholar]
- 16.Pack AA, Salden DR, Ferrari MJ, Glockner-Ferrari DA, Herman LM, Stubbs HA, Straley JM. 1998. Male humpback whale dies in competitive group. Mar. Mamm. Sci. 14, 861–873. ( 10.1111/j.1748-7692.1998.tb00771.x) [DOI] [Google Scholar]
- 17.Garshelis DL, Garshelis JA. 2006. Atypical pup rearing strategies by sea otters. Mar. Mamm. Sci. 3, 263–270. ( 10.1111/j.1748-7692.1987.tb00167.x) [DOI] [Google Scholar]
- 18.Shapiro LLM, Murdock C, Jacobs GR, Thomas MB. 2016. Dual congenital transmission of Toxoplasma gondii and Sarcocystis neurona in a late-term aborted pup from a chronologically infected southern sea otter (Enhydra lutris nereis). Parasitology 143, 276–288. ( 10.1017/S0031182015001377) [DOI] [PubMed] [Google Scholar]
- 19.Thometz NM, Tinker MT, Staedler MM, Mayer KA, Williams TM. 2014. Energetic demands of immature sea otters from birth to weaning: implications for maternal costs, reproductive behaviour and population-level trends. J. Exp. Biol. 217, 2053–2061. ( 10.1242/jeb.099739) [DOI] [PubMed] [Google Scholar]
- 20.Lunn NJ. 1992. Fostering behaviour and milk stealing in Antarctic fur seals. Can. J. Zool. 70, 837–839. ( 10.1139/z92-119) [DOI] [Google Scholar]
- 21.Committee on Taxonomy. 2014. List of marine mammal species and subspecies. Society for Marine Mammalogy. See https://www.marinemammalscience.org/species-information/list-marine-mammal-species-subspecies/.
- 22.Holt-Lunstad J, Smith TB, Layton JB. 2010. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7, e1000316 ( 10.1371/journal.pmed.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geraci IR, Lounsbury VJ. 1993. Marine mammals ashore. A field guide for strandings. Galveston, TX: Texas A&M Sea Grant Publication. [Google Scholar]
- 24.Shane SH, Wells RS, Würsig B. 1986. Ecology, behaviour and social organization of the bottlenose dolphin: a review. Mar. Mamm. Sci. 2, 34–63. ( 10.1111/j.1748-7692.1986.tb00026.x) [DOI] [Google Scholar]
- 25.Wells RS, Scott MD, Irvine AB. 1987. The social structure of free-ranging bottlenose dolphins. In Current mammalogy (ed. Genoways HH.), pp. 247–305. New York, NY: Plenum Press. [Google Scholar]
- 26.Smolker RA, Richards AF, Connor RC, Pepper JW. 1992. Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour 123, 38–69. ( 10.1163/156853992X00101) [DOI] [Google Scholar]
- 27.Clutton-Brock T. 2002. Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72. ( 10.1126/science.296.5565.69) [DOI] [PubMed] [Google Scholar]
- 28.Greenacre M. 1984. Theory and applications of correspondence analysis. London, UK: Academic Press. [Google Scholar]
- 29.Benzécri JP. 1992. Handbook of correspondence analysis. New York, NY: Dekker. [Google Scholar]
- 30.Lê S, Josse J, Husson F. 2008. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1–18. ( 10.18637/jss.v025.i01) [DOI] [Google Scholar]
- 31.Couzin ID, Laidre ME. 2009. Fission–fusion populations. Curr. Biol. 19, 633–635. ( 10.1016/j.cub.2009.05.034) [DOI] [PubMed] [Google Scholar]
- 32.Riedman M. 1990. The pinnipeds: seals, sea lions, and walruses, p. 12 Berkeley, CA: University of California Press. [Google Scholar]
- 33.Campagna C, Le Boeuf BJ, Cappozzo HL. 1988. Pup abduction and infanticide in southern sea lions. Behaviour 107, 44–60. ( 10.1163/156853988X00188) [DOI] [Google Scholar]
- 34.Chivers SJ, Perryman WL, Lynn MS, Gerrodette T, Archer FI, Danil K, Berman-Kowalewski M, Dines JP. 2016. Comparison of reproductive parameters for populations of eastern North Pacific common dolphins: Delphinus capensis and D. delphis. Mar. Mam. Sci. 32, 57–85. ( 10.1111/mms.12244) [DOI] [Google Scholar]
- 35.Santos MDO, Rosso S, Siciliano S, Zerbini AN, Zampirolli E, Vicente A, Alvarenga F. 2000. Behavioral observations of the marine tucuxi dolphin (Sotalia fluviatilis) in São Paulo estuarine waters, southeastern Brazil. Aquat. Mamm. 26, 260–267. [Google Scholar]
- 36.Kasuya T, Miyazaki N. 1976. An observation of epimeletic behaviour of Lagenorhynchus obliquidens. Sci. Rep. Whales Res. Inst. 28, 141–143. [Google Scholar]
- 37.Baird RW. 1994. Foraging behaviour and ecology of transient killer whales (Orcinus orca). Doctoral dissertation, Simon Fraser University, VA, Canada. [Google Scholar]
- 38.Costa-Urrutia P, Abud C, Secchi ER, Lessa EP. 2011. Population genetic structure and social kin associations of franciscana dolphin Pontoporia blainvillei. J. Hered. 103, 92–102. ( 10.1093/jhered/esr103) [DOI] [PubMed] [Google Scholar]
- 39.Heimlich-Boran JR. 1993. Social organisation of the short-finned pilot whale, Globicephala macrorhynchus, with special reference to the comparative social ecology of delphinids. PhD thesis, University of Cambridge, UK. [Google Scholar]
- 40.Cartwright R, Sullivan M. 2009. Associations with multiple male groups increase the energy expenditure of humpback whale (Megaptera novaeangliae) female and calf pairs on the breeding grounds. Behaviour 146, 1573–1600. ( 10.1163/156853909X458377) [DOI] [Google Scholar]
- 41.Anderson JR. 2016. Comparative thanatology. Curr. Biol. 26, R553 ( 10.1016/j.cub.2015.11.010) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the electronic supplementary material.