Abstract
Clinical trials in the past decade have established the antitumor effects of immune checkpoint inhibition as a revolutionary treatment for cancer. Namely, blocking antibodies to cytotoxic T-lymphocyte antigen 4 and programmed death 1 or its ligand have reached routine clinical use. Manipulation of the immune system is not without side effects, and autoimmune toxicities often known as immune-related adverse events (IRAEs) are observed. Endocrine IRAEs, such as hypophysitis, thyroid dysfunction, and insulin-dependent diabetes mellitus, can present with unique profiles that are not seen with the use of traditional chemotherapeutics. In this Review, we discuss the current hypotheses regarding the mechanism of these endocrinopathies and their clinical presentations. Further, we suggest guidelines and algorithms for patient management and future clinical trials to optimize the detection and treatment of immune checkpoint–related endocrinopathies.
Immune checkpoint proteins are T-cell surface receptors that deliver inhibitory signals when they bind to their ligands, limiting immune responses when no longer needed. Tumors can co-opt these inhibitory mechanisms to evade rejection. At least two checkpoint proteins have been demonstrated as operational in cancer, cytotoxic T-lymphocyte antigen 4 (CTLA4), which inhibits activation and T-cell proliferation within the lymphoid compartment, and programmed death 1 (PD1), which inhibits T-cell function within the tumor microenvironment. Monoclonal antibodies that inhibit CTLA4 or PD1 have clinical efficacy in melanoma as single agents or in combination (1–6), including ipilimumab approved to treat unresectable or metastatic melanoma (Table 1). PD1 antibodies have also been approved to treat non–small cell lung cancer, renal cell cancer, bladder cancer, head and neck cancer, Hodgkin lymphoma, hepatocellular carcinoma, gastric cancer, Merkel cell carcinoma, and unresectable or metastatic, microsatellite instability–high (MSI-H), or mismatch repair–deficient (dMMR) solid tumors that have progressed after prior treatment (7). Programmed death 1 ligand (PDL1) antibodies are approved to treat non–small cell lung cancer, urothelial cancer, and Merkel cell carcinoma. Additional agents are under development, and the indications for immune checkpoint inhibition (ICI) use continue to expand.
Table 1.
Checkpoint blockade drug classes and FDA-approved indications*
| Drug class | Name | Disease sites approved |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanoma | NSCLC | RCC | HL | Urothelial | HNSCC | Merkel | MSI-H CRC | Gastric | HCC | MSI-H | ||
| CTLA4 blockade | Ipilimumab | x | ||||||||||
| Tremelimumab | ||||||||||||
| PD1 blockade | Nivolumab | x | x | x | x | x | x | x | x | |||
| Pembrolizumab | x | x | x | x | x | x | x | x | ||||
| PDL1 blockade | Atezolizumab | x | x | |||||||||
| Durvalumab | x | x | ||||||||||
| Avelumab | x | x | ||||||||||
CTLA4 = cytotoxic T-lymphocyte antigen 4; FDA = US Food and Drug Administration; HCC = hepatocellular carcinoma; HL = Hodgkin lymphoma; HNSCC = head and neck squamous cell carcinoma; MSI-H = microsatellite instability–high cancer, unresectable or metastatic; MSI-H CRC = microsatellite instability–high colorectal cancer NSCLC = non–small cell lung cancer; PD1 = programmed death 1; PDL1 = programmed death 1 ligand; RCC = renal cell carcinoma.
Clinical responses to ICI occur rapidly and are often durable with long-term survival. Furthermore, some responses persist if the agents are stopped due to toxicity (principally immune-related). While infrequent, profound adverse events reported with ICI therapies involve endocrine autoimmunity. Anti-CTLA4 therapy has primarily been associated with hypophysitis, or inflammation of the pituitary gland, and thyroid dysfunction. Rarely, adrenalitis has been observed. Anti-PD1/PDL1 therapy has been predominantly associated with thyroid dysfunction and, less commonly, insulin-dependent diabetes. Immune-related toxicity due to induced autoimmunity can involve multiple organs, including the skin, colon, lung, liver, kidney, nervous system, and endocrine organs. Low-grade toxicities can be managed with symptomatic care and potentially a delay in therapy. High-grade toxicities may necessitate holding therapy to administer high-dose corticosteroids until improvement and slow tapering of oral corticosteroids over a four- to six-week period. In this review, we discuss the manifestations and management of autoimmune endocrinopathies and provide recommendations for assessment and management.
Possible Mechanisms of Immunotherapy-Related Endocrinopathy
Endocrinopathies can develop during cancer immunotherapy with monoclonal antibodies (mAb) targeting immune checkpoints CTLA4 and PD1/PDL1 and with cytokines such as interleukin-2 (IL-2) or interferon alpha. The association of autoimmune endocrine dysfunction and treatment response with cytokine-based cancer immunotherapy presaged the findings observed with immune checkpoint inhibitors and suggested that the mechanism of antitumor effects of immunotherapy was autoimmune in nature.
The capacity of self-reactive T cells to cause autoimmune disease is normally offset by negative selection in the thymus and peripheral tolerance mechanisms. These mechanisms include CTLA4 protein, which is a negative regulator of T-cell activation, and PD1/PDL1, which act as a physiologic brake on unrestrained cytotoxic T effector function. Cancer immunotherapies may result in endocrinopathies by disrupting self-reactive quiescent T cells. CTLA4 mutations in humans result in CTLA4 haploinsufficiency or impaired ligand binding in various clinical autoimmune syndromes, including autoimmune thyroiditis (8). Genotypic alterations have been linked with multiple autoimmune endocrinopathies. Polymorphisms in the CLTA4 gene region of chromosome 2q33 and the relative expression levels of CTLA4 are associated with Graves’ disease and Hashimoto’s thyroiditis (9). These alleles may result in impaired function of CTLA4, permitting unchecked T-cell self-reactivity (10). In CTLA4 mAb-associated hypophysitis (CTLA4-H), binding of CTLA4 autoantibodies or ipilimumab to native CTLA4 proteins ectopically expressed on normal pituitary cells has been suggested to lead to IgG1-mediated activation of the classical complement pathway (11,12). Additionally, the IgG1 subclass has more potent activation of the classic complement pathway than IgG2, supporting the observation of an increased incidence of hypophysitis with ipilimumab IgG1 (9.1%) (1,2,6,13–22) compared with IgG2b-based tremelimumab (1.3%) (3,23–26). In a cadaveric study of patients after anti-CTLA4 therapy, the subject who presented with severe hypophysitis during therapy had the highest ectopic expression of CTLA4 on the pituitary gland (27). The presence of CTLA4 in normal pituitary cells could explain the rarity of hypophysitis with anti-PD1/PDL1 treatment. In addition, in seven patients with ipilimumab-induced hypopituitarism, antibodies against the pituitary hormones thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and adrenocorticotropic hormone (ACTH) were found (11).
Disruption of immune tolerance via PD1/PDL1 has been identified in the pathogenesis of autoimmune endocrinopathy. For example, genetic polymorphisms in PD1 and PDL1 have been associated with Addison’s and autoimmune thyroid disease (28). Authors have hypothesized that polymorphisms in the PD1 gene may confer an increased risk of thyroid dysfunction in some patients (29). Loss of circulatory PD1+ CD4+ and CD8+ T cells, an increase in peripheral CD56+CD16+ NK cells, and an increase in activated monocytes have been found in patients with pembrolizumab-induced thyroiditis (30). Some studies have also found an increase in thyroid autoantibodies in patients with thyroid dysfunction following anti-PD1 treatment (31). It is unclear whether thyroid autoantibodies cause thyroid dysfunction or if they are a result of a humoral response to thyroid antigens released during destructive thyroiditis (30). PD1 or PDL1 blockade has also been shown to rapidly precipitate diabetes in prediabetic female nonobese diabetic mice via a T-cell-mediated pathway (32). Evidence of both cellular and humoral autoimmunity have been observed in a case series of insulin-dependent diabetes following anti-PD1 therapy. Both increased diabetes antigen-specific CD8+ T cells (two of four patients) and autoantibodies positive to diabetes autoantigens (three of five patients) were identified using flow cytometry in these subjects (33). ICI exposure may interact with factors such as genetic susceptibility and environmental factors that lead to the development of endocrinopathy. Active research is ongoing to identify patients who are most susceptible and the mechanisms that drive these adverse events, as well as to quantify the effect of ICI on existent autoimmune conditions.
Incidence and Description of Endocrinopathies Induced by ICI
Hypopituitarism
Reported incidence of hypophysitis related to anti-CTLA4 therapy has varied widely, from 0.4% (one of 251 patients) to 17% (eight of 46 patients) (34). As above, tremelimumab has a reported lower incidence of hypophysitis compared with ipilimumab (0.4%–2.6% vs 0.7–18.1%) (1–3,6,13–26,34). Improving awareness revealed elevated incidence rates in two ipilimumab studies, with a reported 7.4% (19 of 256 patients) to 11% (17 of 154 patients) incidence of hypophysitis (15,17), and in a phase III trial of ipilimumab, treatment-induced hypophysitis was reported as 16.3% or 77 of 471 melanoma patients (35). Hypophysitis induced by anti-PD1/PDL1 monotherapy is rare (≤1%) (34,36–40).
Nonspecific symptoms of headache, fatigue, and weakness are the most commonly reported presenting symptoms of hypophysitis related to anti-CTLA4 therapy (15,41). Other less common symptoms include nausea, decreased appetite, weight loss, vision changes, mental status changes, temperature intolerance, and arthralgias (15,17,41). CTLA4-H may be more common in men (15). This phenomenon has been suggested to be the result of the increased prevalence of men with melanoma in these trials. Age may also be a risk factor for CTLA-H (15).
Damage to the pituitary gland can result in a variety of hormonal abnormalities, among the most dangerous being ACTH and TSH deficiency, which results in secondary adrenal insufficiency and secondary hypothyroidism, respectively. ACTH and TSH deficiency are the most common pituitary endocrine abnormality reported with ICI-associated hypophysitis; rarely, polyuria and polydipsia secondary to diabetes insipidus or posterior pituitary compromise have been reported (15,17,34,41). CTLA4-H has been associated with clinically significant morbidity thought to be largely related to secondary adrenal insufficiency, with a reported incidence of approximately 6% across studies (37/608 patients) (34). Adrenal insufficiency can be life-threatening if untreated. Symptoms have been shown to rapidly improve following initiation of steroids with or without thyroid hormone replacement (15,17). Hyponatremia can also occur in up to 50% of patients and improves after hormonal replacement (15,41). Secondary hypogonadism can also occur. Insulin growth factor–1 (IGF-1) levels may be low but are less often assessed, as treatment with growth hormone replacement would be contraindicated in active malignancy (15,34). Prolactin has been reported to be both elevated and decreased in patients following anti-CTLA4 therapy (15,38,41,42).
Secondary adrenal insufficiency is rarely reversible in patients with CTLA4-H, and these patients typically require long-term glucocorticoid replacement (38,41). Reported frequency of recovery of pituitary-thyrotroph function varies from 6% (one of 17 patients) to 64% (14 of 22 patients) (15,38), and similarly, reports for gonadal axis recovery vary from 12% (two of 17 patients) to 57% (67 of 118 patients) (15,42,43). Assessment of initial thyroid and gonadotropin function in sick patients is complicated because thyroid and gonadal lab values during illness may be similar to those seen in hypophysitis-related pituitary insufficiency (ie, sick euthyroid syndrome/sickness-induced hypogonadism). Therefore, it may be difficult to assess recovery after illness from true recovery from hypophysitis-induced thyroid or gonadal hormone deficiency. In contrast, for pituitary-adrenal function, cortisol levels typically rise during illness.
Although there are no direct serum or CSF-specific markers to identify autoimmune hypophysitis, dedicated pituitary magnetic resonance imaging (MRI) can show mild to moderate diffuse enlargement of the pituitary with either homogenous or heterogeneous appearance after contrast administration in up to 75%–100% of patients (15,34). The pituitary stalk can be thickened and infrequently causes optic nerve compression. However, the timing of the MRI in relation to the clinical diagnosis of CTLA4-H and the familiarity of the radiologist with this diagnosis may result in a lower likelihood of positive MRI findings. From a retrospective review of MRIs, relative pituitary enlargement has been documented to precede the clinical diagnosis of CTLA4-H, with the median time to onset of pituitary enlargement occurring one week earlier than biochemical evidence of pituitary hormone deficiency (15,38). During follow-up, the enlarged pituitary from CTLA4-H has been shown to decrease in size over four to 12 weeks (15,34,43,44). Comparatively, the pituitary does not appear enlarged in patients receiving ipilimumab without clinical hypophysitis (15). Incidence of CTLA4-H has also been described as being dose-related, although there have been conflicting reports (15,17,38). The average time to onset of symptoms of CTLA4-H has ranged from six to 14 weeks after treatment initiation, usually occurring after the third dose (15,41).
Although high-dose steroids have been used to attempt to reduce inflammation associated with hypophysitis and to preserve or reverse pituitary damage and hormonal insufficiency, they do not appear to improve the course of hormonal recovery (17,38,41). In addition, there is a theoretical concern that the immunosuppressant effect of high-dose steroids could negatively affect the antitumor efficacy of immune checkpoint inhibition. Accordingly, high-dose steroids should be reserved for those with clinically significant illness, hyponatremia, severe headache, or marked pituitary enlargement that approaches the optic apparatus (15,34).
Primary Thyroid Dysfunction
Thyroid dysfunction can be primary (related to thyroid gland abnormality) or secondary to hypophysitis/pituitary dysfunction. The majority of cases of primary thyroid dysfunction are related to thyroiditis and can be seen as diffuse uptake on a positron emission tomography scan (45). Thyroiditis can present initially as thyrotoxicosis due to the release of thyroid hormone from inflamed thyroid tissue. This can subsequently result in hypothyroidism from inflammatory damage to the thyroid gland. Graves’ disease is less commonly seen after ICI therapy. In contrast to hypophysitis from CTLA4 blockade, primary thyroid dysfunction can result from both anti-CTLA4 and anti-PD1/PDL1 therapy, although it may be more common with PD1/PDL1 blockade (17,30). Two retrospective ipilimumab studies looking specifically for primary hypothyroidism reported rates of 5.2% (eight of 154 patients) and 5.9% (15 of 256 patients) (15,17). In a retrospective review of clinical trials using ipiluimumab without PD1 blockade, primary hypothyroidism presented from five months to three years after treatment (17).
Assessing rates of thyroid dysfunction with PD1 inhibition, the overall rate in initial studies appeared to be approximately 5%–8% for hypothyroidism and approximately 3% for hyperthyroidism (34). As above, in thyroiditis, thyrotoxicosis can proceed hypothyroidism in the same patient. More recent studies looking specifically for primary thyroid dysfunction after PD1 inhibition note that the rates could be as high as 14%–20%, especially following combination ICI therapy (30,31). The onset of hypothyroidism has been reported as early as three weeks after treatment and up to 10 months following therapy, but most cases appear to occur within the first one to three months of therapy (30,31,45). It has been reported that up to 50% of the cases of thyrotoxicosis can be transient, with patients returning to the euthyroid state (30). However, if hypothyroidism develops after anti-PD1 therapy, it is more likely to be permanent (30,31,45). A high titer of thyroid peroxidase antibodies at baseline or history of hypothyroidism may predict an increased risk of worsening/recurrent hypothyroidism following treatment with anti-PD1 therapy (30). With respect to anti-PDL1 therapy, an atezolizumab trial reported an incidence of hypothyroidism of 8.6% (46). Further clinical evaluation will be needed to determine whether autoimmune effects of anti-PDL1 antibodies will be similar to those observed with other ICI therapies.
Primary Adrenal Insufficiency
Only a few cases of adrenalitis have been reported, and association to immune checkpoint blockade is unclear (47,48). Two studies have described adrenal gland enlargement three to four months after ipilimumab treatment that subsequently resolved. One patient developed primary adrenal insufficiency with an elevated ACTH and low cortisol levels and an abnormal response to cosyntropin (47), whereas the other was noted to have initially high cortisol levels that normalized (48).
Hyperglycemia
In a phase II/III trial of pembrolizumab, three of 682 patients (<1%) acquired new-onset insulin-dependent diabetes during the trial (49). In an atezolizumab multicenter phase II trial in locally advanced or metastatic urothelial tumors, hyperglycemia was reported in 7% (eight of 119) of the patients (50).
Several cases of insulin-dependent diabetes (IDDM) have been reported after anti-PD1/PDL1 treatment (33,49,51–54). The majority initially presented with diabetic ketoacidosis as early as one week and up to 12 months after initiation of treatment with a median of 8.5 weeks, a median glucose of 530 mg/dL, and low C-peptide levels (54). Positive autoantibodies to diabetic autoantigens can be seen with PD1 inhibitor-associated IDDM as well as upregulation of CD8+ T-cell activity (33,54). Insulin-dependent diabetes has not yet been reported with clinical use of anti-CTLA4 treatment.
Combination Therapy
In the hope of yielding an additive benefit by combining CTLA4 and PD1 blockade, several combination trials have been undertaken (2,6,55). The concurrent administration of nivolumab and ipilimumab showed superior rates of objective response at 61% (44 of 72 patients) compared with 11% (four of 37 patients) of the ipilimumab monotherapy arm (2). It did, however, elevate the rate of grade III–IV adverse events from 24% (11 of 46 patients) in the ipilimumab monotherapy arm to 54% (51 to 94 patients) in the combination arm. In terms of specific endocrinopathies observed in the combination arm, 15 cases of hypothyroidism (16% vs 15.2% in ipilimumab arm), 11 cases of hypophysitis (11.7% vs 6.5% ipilimumab arm), four cases of hyperthyroidism (4.3% vs 0% ipilimumab arm), and six cases of adrenal insufficiency (6.4% vs 4.3% ipilimumab arm) were observed in a cohort of 94 patients (46 patients, ipilimumab arm). Of note, no statistical analysis of differences in endocrinopathy rates was performed between the combination and monotherapy treatment arms. Such additive benefits and more severe or prevalent side effects with combination therapy were corroborated by the CheckMate 067 phase III trial and CheckMate 069 phase II/III trial of nivolumab/ipilimumab combination vs monotherapy. Approximately 59% of the combination arm presented with grade III–IV toxicities, whereas the monotherapy arm (nivolumab or ipilimumab) presented with lower rates of adverse events (21% and 28%, respectively) (6). Similar to specific endocrinopathy incidence in the previously mentioned study, the CheckMate 067 trial reported 53 cases of hypothyroidism (17%; nivolumab only 11%, ipilimumab only 5%), 35 cases of hyperthyroidism (11%; nivolumab 4%, ipilimumab 1.0%), and 23 cases of hypophysitis (7%; nivolumab 1%, ipilimumab 4%) in the combination arm (n = 313; nivolumab arm = 313, ipilimumab arm = 311); a statistical comparison of differences observed between treatment arms was not presented. In addition, a retrospective analysis by Morganstein et al. revealed that the rate of melanoma patients presenting with any type of thyroid abnormalities could be as high as 50% in CTLA4/PD1 combined therapy (56). A review of the PD1/CTLA4 combination therapy literature revealed an overall incidence of hypothyroidism of 15.2% (70 cases of 461 patients), hyperthyroidism of 8.9% (41 of 461), and hypophysitis of 7.8% (36 of 461) (2,6,57).
Adverse Events and Treatment Response
Association of clinical response and immune-related adverse events (IRAEs) from ICI therapy has been observed in several clinical trials (58). In a retrospective analysis of multiple ipilimumab phase II trials assessing 343 cases of stage III–IV melanoma, a trend toward superior disease control was reported in those with at least grade II autoimmunity when compared to those with grade I autoimmunity or less (58). Further, in a subanalysis of patients with ipilimumab-related hypophysitis, a prolonged median survival time (19.4 months vs 8.8 months in patients without hypophysitis) was observed (P = .05) (15). In another recent retrospective study of 27 patients with CTLA4-H, 89% of all subjects with stage III (n = 4) and stage IV malignancy (n = 17) were alive at three years, and 100% of those patients with advanced melanoma (n = 21) were alive at three years (41). In contrast, in a recent separate study looking at patients with advanced melanoma receiving nivolumab plus ipilimumab combination, the three-year overall survival rate was 63% in all patients regardless of IRAEs, which is the highest observed for this patient population (59). In a retrospective study, adverse events were described as possible prognostic factors in anti-PD1 therapy when 35 of 83 patients receiving pembrolizumab reported cutaneous adverse effects and had clinically significantly longer progression-free survival compared with those without cutaneous IRAEs (37). An extended survival has also been described with anti-PD1 therapy and the development of thyroid dysfunction, although data have been conflicting (29,31). In a study of patients with non–small cell lung cancer receiving pembrolizumab, the median overall survival in patients who developed thyroid dysfunction was significantly longer statistically than in those without thyroid dysfunction (median = 40 vs 14 months, P = .029) (31). A possible lead time bias may also occur when evaluating these outcomes as only those benefiting from prolonged survival from ICI therapy may be followed long enough to develop IRAEs.
Recommendations for Clinical Trials
Patient Selection
Selection of appropriate patients for enrollment in clinical trials or treatment with already approved ICI should consider a patient’s history of autoimmune endocrinopathies. Although the US Food and Drug Administration labels for both ipilimumab and pembrolizumab warn of autoimmune endocrinopathies, it is notable that the labels have no contraindications for these conditions (60,61). Table 3 lists potential eligibility criteria for clinical trial participation in patients with autoimmune endocrinopathies. These suggestions are guided by the principle that safety must be prioritized in human trials. Lingering endocrine toxicities from previous ICI treatment should not preclude enrollment in future ICI studies, provided the condition is controlled with hormone replacement, did not necessitate removal of the patient from study, and no longer requires immune suppression. In the case of adrenal insufficiency, patients requiring replacement levels of steroid hormones should be considered eligible for future ICI study participation.
Table 3.
Potential eligibility criteria as per clinical trial scenario*
| Trial scenario | Eligibility of autoimmune endocrinopathies |
|---|---|
| First in human, first in class ICI phase I | Excluded no exceptions |
| Phase I of single ICI, not first in class | Most endocrinopathies excluded except for a few select low-risk disorders, ie, autoimmune hypothyroidism on stable thyroid hormone replacement |
| Combination phase I of multiple ICI or ICI (not first in class) with MTAs, radiation, chemotherapy, or other agents | Most endocrinopathies excluded except for a few select low-risk disorders, ie, autoimmune hypothyroidism on stable thyroid hormone replacement |
| Phase II/III | Allow autoimmune endocrinopathies except patients with disorders that are active or uncontrolled requiring immunosuppression or supraphysiologic doses of steroids |
ICI = immune checkpoint inhibition; MTA = mutation-derived tumor antigens.
Reporting of Endocrine Adverse Events
Severe adverse events that occur within a specified time period, usually the first cycle of treatment, are termed dose-limiting toxicities (DLTs). Grade 3 or 4 endocrine toxicities (Common Terminology Criteria for Adverse Events, version 5.0) have been reported in early phase clinical trials of ICI, but rarely have these adverse events met DLT criteria (62). The incidence of DLTs at particular dose levels is used to determine the recommended phase II dose (RP2D) of the drug or combination regimen, but delayed adverse events present a challenge as these toxicities may not influence dose escalation or de-escalation decisions (62). Endocrine toxicities can be life threatening if not recognized early and may require long-term hormone replacement. These adverse events should be considered toxicities of special interest that require rigorous reporting so that the timing, management, resolution, or reversibility of the event can be described. These data can be used to inform the design of future studies of the tested agent or drugs in that class and to modify the DLT definitions for adequately controlled grade 3 endocrinopathies.
Monitoring, Diagnosis, and Management
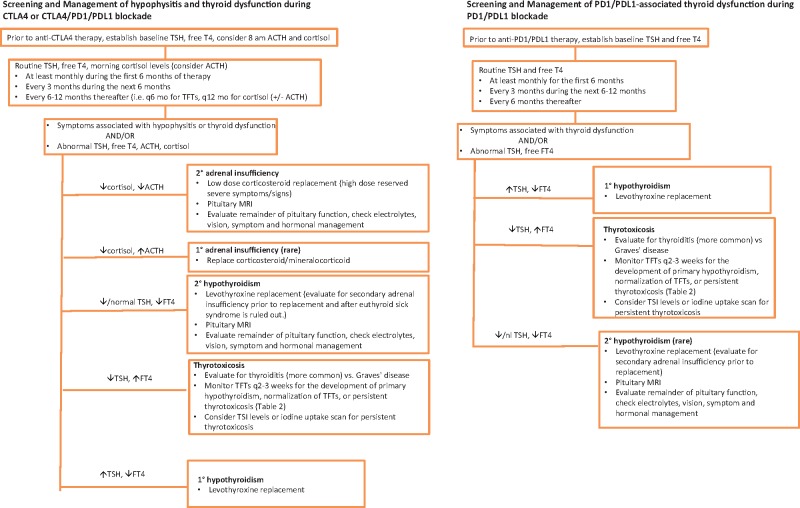
Clinical protocols often stipulate algorithms for the management of endocrine adverse events, but there are no widely accepted standards for routine monitoring. Figure 1 provides recommendations that can be applied to both clinical trials and standard of care treatment with ICI. Endocrine consultation can be very helpful in cases of ICI-induced endocrinopathies.
Figure 1.
Algorithm for hormonal testing. ACTH = adrenocorticotropic hormone; CTLA4 = cytotoxic T-lymphocyte antigen 4; MRI = magnetic resonance imaging; PD1 = programmed death 1; PDL1 = programmed death 1 ligand; TFT = thyroid function test; TSH = thyroid-stimulating hormone; FT4 = free T4; TSI = Thyroid-Stimulating Immunoglobulin.
Hypophysitis
Hypophysitis has been predominantly reported using anti-CTLA4 therapy either alone or in combination with anti-PD1/PDL1 agents and is rare with anti-PD1/PDL1 therapy alone (≤ 1%), as described above. Recommended routine screening for therapy with anti-CTLA4 and anti-PD1/PDL1 agents has included baseline and follow-up thyroid function tests (TFTs) but not adrenal function tests. Given the potentially life-threatening outcome from adrenal insufficiency and its incidence with anti-CTLA4 therapy, we recommend monitoring morning ACTH and cortisol levels during treatment with CTLA4 blockade (Figure 1). While on immunotherapy, patients who report symptoms suggestive of hypophysitis should have a prompt evaluation for hypopituitarism. Initial evaluation includes TSH, free T4, and morning ACTH and cortisol levels (ideally at or before 8 am). Pituitary imaging with MRI, or computed tomography/computed tomography when MRI is contraindicated, is recommended when hypophysitis is suspected or biochemically evident. Formal visual field exam may also be indicated for visual field changes or evidence of optic chiasm compression.
For patients treated with anti-CTLA4 therapies, we recommend baseline levels for TSH, free T4, and considering evaluation of baseline 8 am ACTH and cortisol levels. Repeat evaluation can be considered monthly for the first six months on therapy. If values are normal and a patient is asymptomatic, the monitoring interval can decrease to every three months for the next six months and further to every six to 12 months thereafter (Figure 1). This monitoring schedule is recommended for phase I studies. In circumstances when adrenal function is not tested, TFTs show low or low-normal TSH and low free T4, surveillance brain scans show enlarged pituitary, or symptoms of hypophysitis develop; morning 8 am paired ACTH and cortisol levels should be checked. Often patients with secondary adrenal insufficiency from CTLA4-H have a frankly low 8 am cortisol (<3 mcg/dL) with a low ACTH (<5 pg/mL) (41). Normal subjects have serum cortisol concentrations in the early morning (at or before 8 am), ranging from 10 to 20 mcg/dL (63). If early morning labs are not possible or there is an urgent need for evaluation at a clinical visit, random ACTH and cortisol levels can be checked as these levels can be very low for any time of day in patients with CTLA4-H. It is important to confirm that the patient has not been receiving exogenous steroids (ie, for another nonendocrine IRAE) as this will influence results. For example, patients taking exogenous dexamethasone can have a low ACTH and cortisol because of hypothalamic-pituitary-adrenal (HPA) axis suppression by exogenous steroids. In contrast, a patient actively taking prednisone or hydrocortisone can have a low ACTH but a normal or high cortisol because these medications can be detected in the cortisol assay, whereas dexamethasone is not. Caution should be exercised if a patient is being tapered off steroids after being treated for a non-endocrine-related IRAE. Prolonged steroid use can result in HPA axis suppression, although steroid treatment for another IRAE could also potentially mask the presentation of ICI-induced adrenal insufficiency or hypophysitis. Consultation with an endocrinologist is particularly useful in these settings. An ACTH stimulation test is not as helpful in diagnosing early secondary adrenal insufficiency because in initial pituitary injury, the adrenal glands may respond to ACTH stimulation normally because they have not yet atrophied from the chronic absence of pituitary ACTH stimulation (64). In patients with diagnosed hypophysitis, gonadotropins, testosterone (in males), and estrogen (in premenopausal females) levels should be measured. Prolactin levels can be checked in those with secondary hypogonadism as hyperprolactinemia can result in hypogonadotropic hypogonadism. Serum sodium levels should be measured, as there is also a high incidence of hyponatremia (34). Patients should be evaluated clinically for diabetes insipidus (DI) by assessing for polyuria and polydipsia, with the use of DDAVP as a potential method of management. However, DI is uncommon in hypophysitis related to CTLA4 blockade (15,41). Growth hormone and IGF1 levels do not need to be measured because growth hormone replacement is contraindicated in active cancer patients. If therapy includes both CTLA4 and PD1/PDL1 blockade, either combined or sequentially, we recommend monitoring thyroid function and adrenal function following the recommendation algorithm for anti-CTLA4 monotherapy.
Management
Immunotherapy-related hypophysitis can result in either an isolated hormonal deficiency or multiple pituitary hormone deficiencies (15,41). Goals of therapy are hormone replacement and symptom relief. Headaches may be managed by acetaminophen, nonsteroidal anti-inflammatories, or steroids. Hydrocortisone at approximately 10 mg/m2 (ie, 10–15 mg in the morning and 5–10 mg in the afternoon) or the equivalent dose of daily prednisone is commonly used to replace central/secondary adrenal insufficiency (41). ICI therapy can often proceed uninterrupted after hormonal replacement is initiated. Systemic high-dose glucocorticoids can be reserved for life-threatening or critical illness, clinically significant hyponatremia, severe headache, visual disturbances or other neurologic sequela, or marked pituitary enlargement that approaches or abuts the optic chiasm. In these cases, ICI therapy can be held until the patient is stabilized on physiologic hormonal replacement. Although glucocorticoid treatment may reduce the size of the pituitary for symptomatic improvement, use of high-dose steroids does not appear to reverse hypopituitarism, so secondary hormonal insufficiencies must be appropriately managed (38,41–43). Once adrenal insufficiency is diagnosed, patients should receive instructions for increasing steroid doses during illness or surgical procedures and should wear a medical alert bracelet. Assessment for pituitary-adrenal axis recovery can be performed every three to six months for the first year and then every six to 12 months thereafter, although secondary adrenal insufficiency is typically permanent (41).
After nonthyroidal illness is ruled out, levothyroxine should be initiated to treat central hypothyroidism. In patients with both ACTH and TSH deficiency, glucocorticoid replacement should be initiated before or simultaneously with thyroid hormone replacement in order to prevent precipitating adrenal crisis (65). In patients with hypogonadism, prolactin can be measured as elevated levels can cause secondary hypogonadism. Testosterone replacement may be used for male hypogonadism after ruling out eugonadal sick syndrome and hyperprolactinemia. Testosterone replacement should follow Endocrine Society guidelines (66). Estrogen replacement may be initiated in female premenopausal patients who develop hypogonadotropic hypogonadism if not contraindicated. Assessment for pituitary-thyroid and pituitary-gonadal axis recovery can be performed every three to six months for the first year and then every six to 12 months thereafter, as recovery of secondary hypothyroidism and secondary hypogonadism has been described (15). Hyponatremia is usually transient and resolves after hormonal replacement.
Primary Thyroid Disorders
Hypothyroidism is the most common thyroid abnormality occurring in both anti-CTLA4 and anti-PD1/PDL1 therapies. For both anti-CTLA4 and anti-PD1/PDL1 therapy, baseline TSH and free T4 are recommended before first treatment and then at least monthly for the first six months. If there are no abnormalities and the patient is asymptomatic, these labs can be checked quarterly for months 6 to 12 and approximately every six months thereafter (or until six months post-treatment). Patients should be monitored for signs of hypothyroidism (ie, fatigue, weight gain). If a patient notes any signs of thyroid dysfunction in between visits or at a visit, thyroid function tests should be measured (Table 2). If there is evidence of primary hypothyroidism or thyrotoxicosis, thyroid auto-antibodies can be measured (67).
Table 2.
Possible immunotherapy-induced changes in thyroid function based on laboratory values
| TSH | Free T4 | T3 total | Possible diagnoses |
|---|---|---|---|
| Low | Normal-high | Normal-high | Transient thyrotoxic phase of thyroiditis* |
| Graves’ disease (less common) | |||
| Low or normal | Low | Low | Secondary hypothyroidism due to hypophysitis/pituitary dysfunction† |
| High | Low-normal | Low-normal | Primary hypothyroidism/hypothyroid phase of thyroiditis* |
May evolve from a thyrotoxic phase to permanent hypothyroidism. TSH = thyroid-stimulating hormone.
Thyroid hormone therapy should be instituted together with adrenal steroid replacement, unless adrenal insufficiency has been ruled out.
In those presenting initially with thyrotoxicosis from thyroiditis, clinical and biochemical abnormalities may resolve in two to four weeks, but prolonged thyrotoxicosis has been observed (29). Patients with thyrotoxicosis should be closely monitored for progression to hypothyroidism, with TFTs monitored at least every two to three weeks (Table 2).
Reports of other thyroid disorders, such as Graves’ hyperthyroidism and ophthalmopathy, are rare (68,69). Thyroid-stimulating immunoglobulins, thyroid-stimulating hormone receptor antibodies, or a thyroid uptake scan can be performed in patients with prolonged thyrotoxicosis, goiter, or ophthalmopathy to rule out Graves’ hyperthyroidism. In Graves’ hyperthyroidism, one would expect a high iodine uptake in the thyroid gland, in contrast to thyroiditis, in which one typically sees a low iodine uptake. Of note, the use of radioactive iodine uptake scans in cancer patients may be inaccurate given frequent exposure to imaging modalities with iodinated contrast, which would lower the thyroid’s iodine uptake (70).
As thyrotoxicosis is usually transient from thyroiditis, symptomatic management with a beta-blocker can be used. Systemic glucocorticoid treatment is rarely indicated and could be considered in patients with preexisting cardiac disease and/or severe symptoms that could warrant holding ICI therapy. For Graves’ hyperthyroidism, an antithyroid agent, such as methimazole, can be used. These drugs are not effective in thyrotoxicosis from thyroiditis, which represents the vast majority of cases of ICI-related thyrotoxicosis.
For primary hypothyroidism (high TSH, low free T4) (Table 2), levothyroxine should be started and titrated up every four to six weeks in order to normalize thyroid function tests (71). If clinically asymptomatic with an elevated TSH lower than 10 and normal free T4, the patient could be closely followed without thyroid hormone replacement (72). In patients with secondary hypothyroidism related to pituitary dysfunction (low free T4 with a low or low-normal TSH), secondary adrenal insufficiency should be ruled out before starting levothyroxine to prevent triggering adrenal crisis. In elderly patients or patients with cardiac disease, a low replacement dose of levothyroxine should be started and increased slowly (71). Temporary discontinuation of immunotherapy can be considered in severe symptomatic cases of hypothyroidism; however, this is uncommon.
Primary Adrenal Insufficiency
Given the rare incidence of primary adrenal insufficiency after ICI therapy, systematic screening and monitoring guidelines have not been established. If adrenal enlargement is observed on routine imaging, it is important to assess adrenal function through the measurement of ACTH and cortisol levels, as well as a cosyntropin stimulation test, to rule out primary adrenal insufficiency. Primary adrenal insufficiency should be treated with physiologic doses of corticosteroid and mineralocorticoid.
Hyperglycemia
Although rare, clinicians should be aware of the signs and symptoms of DKA or hyperglycemia (polyuria, polydipsia, blurred vision, malaise) during anti-PD1/PDL1 therapy as a missed diagnosis may be life threatening. Serum glucoses are typically included in standard laboratory care during ICI therapy, and attention should be made to monitoring glucose trends. Tests for autoantibodies (abs) and endogenous insulin secretion (glutamic acid decarboxylase/GAD65 abs, insulin abs, islet cell abs, zinc transporter 8/Zn-T8 abs, C-peptide, and insulin levels) can distinguish between insulin-dependent and non-insulin-dependent diabetes. Management of insulin-dependent diabetes after anti-PD1/PDL1 therapy, which typically presents with marked hyperglycemia, ketoacidosis, and low C-peptide levels, includes aggressive management of ketoacidosis and individualized insulin regimens. No reports of CTLA4 mAb-related insulin-dependent diabetes have been noted in the literature.
Combination Therapy
For patients undergoing combined ICI agent therapy, we recommend that clinicians follow the recommendations for monotherapy above and be aware of the potential for increased incidence of endocrinopathies with combination immunotherapy.
Summary
Adverse effects on the endocrine system by immune checkpoint blockade have been described over the past decade. Although the exact mechanisms are not fully understood, the exaggerated activity of the immune system induced by immunotherapy appears to have resulted in an unleashing of autoimmunity to normal tissue. CTLA4 blockade is associated with an increased incidence of hypophysitis and primary thyroid dysfunction. Anti-PD1/PDL1 agents are predominantly associated with primary thyroid dysfunction from thyroiditis. Thyroiditis may initially present as transient thyrotoxicosis and progress to permanent hypothyroidism. Patients receiving combination ICI therapies appear to be at a greater risk for both thyroid dysfunction and hypophysitis. Less commonly, fulminant insulin-dependent diabetes mellitus has been described following PD1/PDL1 blockade. There are data to suggest that the presentation of certain immune-related adverse events may be associated with a superior clinical response, although further research is needed to fully understand the association. The endocrine side effect profile for different classes of immunotherapy may lie in differences in protein targets, subtypes of monoclonal antibodies used, environmental factors, and heterogeneous immunologic genotypes and phenotypes observed in patients undergoing therapy. Given the possibility that earlier clinical trials failed to screen for endocrine side effects, the overall incidence of endocrine adverse events may not be fully appreciated. It is important for clinicians to be vigilant in screening and diagnosing endocrinopathies. Although there are effective screening modalities and hormonal replacement therapies for ICI-associated endocrinopathies, future clinical trials should develop concrete guidelines to establish baseline hormonal function to better detect and manage subsequent adverse events when they occur. With the increasing use of ICI therapy across all fields of oncology, data from these clinical studies can decrease the morbidity associated with these endocrinopathies and limit the interruption of life-saving ICI treatments.
Funding
We would like to acknowledge the Coordinating Center for Clinical Trials, Office of the Director, National Cancer Institute, and the Immunotherapy Task Force and Investigational Drug Steering Committee for supporting this effort.
Notes
Affiliations of authors: Endocrine Division, Department of Medicine, Weill Cornell Medical College (MG, AF), and Department of Medicine (DJB), Memorial Sloan-Kettering Cancer Center (MC), New York, NY; Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, Toronto, ON, Canada (AH); Department of Neurosurgery, Brigham and Women’s Hospital, Boston, MA (LM); Moffitt Cancer Center, Tampa, FL (BC, SA); Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC (MA); Cancer Therapy Evaluation Program, National Cancer Institute, Rockville, MD (ES); The Emmes Corporation, Rockville, MD (PW); The Emmes Corporation, Rockville, MD (AG).
Conflicts of interest: Monica Girotra has been a consultant for AstraZeneca and Bristol-Myers Squibb. Michael B. Atkins: consultant: BMS, Merck, Pfizer, Genentech-Roche, Astra Zeneca, Novartis; advisory board: Novartis, Merck. Margaret Callahan: family member employee: BMS; research funding: BMS; advisor: AstraZeneca, Merck. Ben Creelan: speakers’ bureau: Hoffman-La Roche AG, E.R. Squibb & Sons LLC, Takeda, Pharmaceutical Co Ltd, AstraZeneca Plc; consultant: Celgene Corporation, E.R. Squibb & Sons LLC, AbbVie Inc.; research funding: Boehringer-Ingelheim, Iovance Biotherapeutics Inc, Prometheus Laboratories Inc, E.R. Squibb & Sons LLC. Le Min: research funding: BMS. Scott Antonia: advisory board: BMS, Novartis, Merck, Boehringer Ingelheim, AstraZeneca, Memgen; research funding: Novartis; Founder: Nilogen. All other authors have disclosed no conflicts.
References
- 1. Hodi FS, O'Day SJ, McDermott DF et al. , . Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;3638:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Postow MA, Chesney J, Pavlick AC et al. , . Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;37221:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ribas A, Kefford R, Marshall MA et al. , . Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;315:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robert C, Ribas A, Wolchok JD et al. , . Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;3849948:1109–1117. [DOI] [PubMed] [Google Scholar]
- 5. Robert C, Schachter J, Long GV et al. , . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;37226:2521–2532. [DOI] [PubMed] [Google Scholar]
- 6. Wolchok JD, Chiarion-Sileni V, Gonzalez R et al. , . Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;37714:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Food and Drug Administiration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm. Accessed March 2, 2018
- 8. Schubert D, Bode C, Kenefeck R et al. , . Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;2012:1410–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kotsa K, Watson PF, Weetman AP.. A CTLA-4 gene polymorphism is associated with both Graves disease and autoimmune hypothyroidism. Clin Endocrinol (Oxf). 1997;465:551–554. [DOI] [PubMed] [Google Scholar]
- 10. Ni J, Qiu LJ, Zhang M et al. , . CTLA-4 CT60 (rs3087243) polymorphism and autoimmune thyroid diseases susceptibility: A comprehensive meta-analysis. Endocr Res. 2014;394:180–188. [DOI] [PubMed] [Google Scholar]
- 11. Iwama S, De Remigis A, Callahan MK et al. , . Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6230:230ra45. [DOI] [PubMed] [Google Scholar]
- 12. Quirk SK, Shure AK, Agrawal DK.. Immune-mediated adverse events of anticytotoxic T lymphocyte-associated antigen 4 antibody therapy in metastatic melanoma. Transl Res. 2015;1665:412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Albarel F, Gaudy C, Castinetti F et al. , . Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;1722:195–204. [DOI] [PubMed] [Google Scholar]
- 14. Ansell SM, Hurvitz SA, Koenig PA et al. , . Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;1520:6446–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faje AT, Sullivan R, Lawrence D et al. , . Ipilimumab-induced hypophysitis: A detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;9911:4078–4085. [DOI] [PubMed] [Google Scholar]
- 16. Royal RE, Levy C, Turner K et al. , . Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;338:828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryder M, Callahan M, Postow MA et al. , . Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;212:371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang JC, Hughes M, Kammula U et al. , . Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;308:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Attia P, Phan GQ, Maker AV et al. , . Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;2325:6043–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Downey SG, Klapper JA, Smith FO et al. , . Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22 Pt 1):6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hersh EM, O'Day SJ, Powderly J et al. , . A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Invest New Drugs. 2011;293:489–498. [DOI] [PubMed] [Google Scholar]
- 22. Ku GY, Yuan J, Page DB et al. , . Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: Lymphocyte count after 2 doses correlates with survival. Cancer. 2010;1167:1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chung KY, Gore I, Fong L et al. , . Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;2821:3485–3490. [DOI] [PubMed] [Google Scholar]
- 24. Kirkwood JM, Lorigan P, Hersey P et al. , . Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;163:1042–1048. [DOI] [PubMed] [Google Scholar]
- 25. Ralph C, Elkord E, Burt DJ et al. , . Modulation of lymphocyte regulation for cancer therapy: A phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res. 2010;165:1662–1672. [DOI] [PubMed] [Google Scholar]
- 26. Ribas A, Camacho LH, Lopez-Berestein G et al. , . Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;2335:8968–8977. [DOI] [PubMed] [Google Scholar]
- 27. Caturegli P, Di Dalmazi G, Lombardi M et al. , . Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: Insights into pathogenesis from an autopsy series. Am J Pathol. 2016;18612:3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mitchell AL, Cordell HJ, Soemedi R et al. , . Programmed death ligand 1 (PD-L1) gene variants contribute to autoimmune Addison's disease and Graves' disease susceptibility. J Clin Endocrinol Metab. 2009;9412:5139–5145. [DOI] [PubMed] [Google Scholar]
- 29. Orlov S, Salari F, Kashat L et al. , . Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. 2015;1005:1738–1741. [DOI] [PubMed] [Google Scholar]
- 30. Delivanis DA, Gustafson MP, Bornschlegl S et al. , . Pembrolizumab-induced thyroiditis: Comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab. 2017;1028:2770–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Osorio JC, Ni A, Chaft JE et al. , . Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;283:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ansari MJ, Salama AD, Chitnis T et al. , . The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;1981:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hughes J, Vudattu N, Sznol M et al. , . Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;384:e55–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Byun DJ, Wolchok JD, Rosenberg LM et al. , . Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;134:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eggermont AM, Chiarion-Sileni V, Grob JJ et al. , . Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;37519:1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brahmer JR, Tykodi SS, Chow LQ et al. , . Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;36626:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herbst RS, Soria JC, Kowanetz M et al. , . Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;5157528:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Min L, Hodi FS, Giobbie-Hurder A et al. , . Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: A retrospective cohort study. Clin Cancer Res. 2015;214:749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Topalian SL, Hodi FS, Brahmer JR et al. , . Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;36626:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barroso-Sousa R, Barry WT, Garrido-Castro AC et al. , . Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol. 2018;42:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sinha AG, Zheng J, Girotra M. Pituitary dysfunction after CTLA-4 blockade: Time course and hypothalamic-pituitary-adrenal (HPA) axis recovery. Abstract and Oral Presentation in: Endocrine Society 100th Annual Meeting. Endocr Rev. 2018;392; Chicago, IL. [Google Scholar]
- 42. Blansfield JA, Beck KE, Tran K et al. , . Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;286:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Min L, Vaidya A, Becker C.. Association of ipilimumab therapy for advanced melanoma with secondary adrenal insufficiency: A case series. Endocr Pract. 2012;183:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carpenter KJ, Murtagh RD, Lilienfeld H et al. , . Ipilimumab-induced hypophysitis: MR imaging findings. Am J Neuroradiol. 2009;309:1751–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Filette J, Jansen Y, Schreuer M et al. , . Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016;10111:4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McDermott DF, Sosman JA, Sznol M et al. , . Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: Long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;348:833–842. [DOI] [PubMed] [Google Scholar]
- 47. Min L, Ibrahim N.. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013;13:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bacanovic S, Burger IA, Stolzmann P et al. , . Ipilimumab-induced adrenalitis: A possible pitfall in 18F-FDG-PET/CT. Clin Nucl Med. 2015;4011:e518–e519. [DOI] [PubMed] [Google Scholar]
- 49. Herbst RS, Baas P, Kim DW et al. , . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;38710027:1540–1550. [DOI] [PubMed] [Google Scholar]
- 50. Balar AV, Galsky MD, Rosenberg JE et al. , . Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet. 2017;38910064:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gaudy C, Clévy C, Monestier S et al. , . Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;3811:e182–e183. [DOI] [PubMed] [Google Scholar]
- 52. Mellati M, Eaton KD, Brooks-Worrell BM et al. , . Anti-PD-1 and anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care. 2015;389:e137–e138. [DOI] [PubMed] [Google Scholar]
- 53. Martin-Liberal J, Furness AJ, Joshi K et al. , . Anti-programmed cell death-1 therapy and insulin-dependent diabetes: A case report. Cancer Immunol Immunother. 2015;646:765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gauci ML, Laly P, Vidal-Trecan T et al. , . Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: A case report and literature review. Cancer Immunol Immunother. 2017;6611:1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hodi FS, Chesney J, Pavlick AC et al. , . Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;1711:1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morganstein DL, Lai Z, Spain L et al. , . Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf). 2017;864:614–620. [DOI] [PubMed] [Google Scholar]
- 57. Wolchok JD, Kluger H, Callahan MK et al. , . Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;3692:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weber JS, Kähler KC, Hauschild A.. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;3021:2691–2697. [DOI] [PubMed] [Google Scholar]
- 59. Callahan MK, Kluger H, Postow MA et al. , . Nivolumab plus ipilimumab in patients with advanced melanoma: Updated survival, response, and safety data in a phase I dose-escalation study. J Clin Oncol. 2018;364:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ipilimumab [FDA label package insert]. Princeton, NJ: BMS; 2011.
- 61.Pembrolizumab [FDA label package insert]. Whitehouse Station, NJ: Merck; 2014.
- 62. Postel-Vinay S, Gomez-Roca C, Molife LR et al. , . Phase I trials of molecularly targeted agents: Should we pay more attention to late toxicities? J Clin Oncol. 2011;2913:1728–1735. [DOI] [PubMed] [Google Scholar]
- 63. Hägg E, Asplund K, Lithner F.. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol (Oxf). 1987;262:221–226. [DOI] [PubMed] [Google Scholar]
- 64. Suliman AM, Smith TP, Labib M et al. , . The low-dose ACTH test does not provide a useful assessment of the hypothalamic-pituitary-adrenal axis in secondary adrenal insufficiency. Clin Endocrinol (Oxf). 2002;564:533–539. [DOI] [PubMed] [Google Scholar]
- 65. Smith JC. Hormone replacement therapy in hypopituitarism. Expert Opin Pharmacother. 2004;55:1023–1031. [DOI] [PubMed] [Google Scholar]
- 66. Seftel AD, Kathrins M, Niederberger C.. Critical update of the 2010 Endocrine Society clinical practice guidelines for male hypogonadism: A systematic analysis. Mayo Clin Proc. 2015;908:1104–1115. [DOI] [PubMed] [Google Scholar]
- 67. Sinclair D. Clinical and laboratory aspects of thyroid autoantibodies. Ann Clin Biochem. 2006;43(Pt 3):173–183. [DOI] [PubMed] [Google Scholar]
- 68. Min L, Vaidya A, Becker C.. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;1642:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Borodic G, Hinkle DM, Cia Y.. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg. 2011;274:e87–e88. [DOI] [PubMed] [Google Scholar]
- 70. Hamnvik OP, Larsen PR, Marqusee E.. Thyroid dysfunction from antineoplastic agents. J Natl Cancer Inst. 2011;10321:1572–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jonklaas J, Bianco AC, Bauer AJ et al. , . Guidelines for the treatment of hypothyroidism: Prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;2412:1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pearce SH, Brabant G, Duntas LH et al. , . 2013 ETA guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013; 24:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]