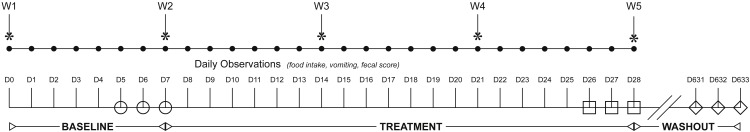
Figure 1. Flowchart illustrating study design, duration, observations, and sampling.
The study spanned 633 days (D1-633) and was broken into three study periods: baseline (D1–D7), treatment (D8–D28), and washout. Cats were randomized to receive either a placebo or synbiotic with 150 mg clindamycin PO once daily during treatment. Food intake, vomiting, and fecal score were recorded daily (•) and weight (W) weekly (*) by an individual blinded to treatment group. Two grams were collected from the center portion of each first morning naturally-voided fecal samples for each cat once daily for the last three days of each study period: baseline (open circles), treatment (open squares), and recovery (open diamonds). Each sample was subdivided into two aliquots, with each aliquot placed into a 2 mL cryovial and immediately frozen at −80 °C pending completion of data collection.