Abstract
Objective
Hwa-byung is one of the cultural concept of distress in Korea resulted from chronic accumulated anger. It is characterized by various symptoms like stuffy in the chest, hot or heat sensation, something pushing up in the chest, feeling of mortification, and a flush of anger. This protocol aims to explore the efficacy and safety of Huanglian-jie-du decoction on various somatic symptoms and insomnia in patients with Hwa-byung.
Methods
This is study protocol for a randomized, double-blind, placebo-controlled trial. A total of 44 patients will be randomly assigned to the experimental group or the placebo group in a 1:1 ratio. All medications will be taken orally 3 times per day for 7 consecutive days. The primary outcomes are the mean changes in Patient Health Questionnaire of physical symptoms (PHQ-15) and Insomnia Severity Index (ISI) after the 7 days of administration. The secondary outcomes include the scales to assess stress response, symptoms of Hwa-byung, and state anger.
Conclusion
The results of this study will provide high quality and explorative evidence to investigate the effect of Huanglian-jie-du decoction on Hwa-byung.
Keywords: Huanglian-jie-du decoction, Oren gedoku to, Hwa-byung, medically unexplained symptoms, insomnia, randomized controlled trial
1. Introduction
Hwa-byung, which literally means “fire disease” in Korean, is one of the cultural concepts of distress that results from chronic accumulated anger [1, 2]. Resulting from interpersonal conflicts or injustices, the symptoms that reflect oppressed anger are often manifested in the cultural context of Korean society’s internalization of their emotions [3, 4]. As part of the Confucian culture, Koreans traditionally have been discouraged to display or acknowledge conflict or negative emotions and forced to maintain personal temperament and harmonious interpersonal relationships [5]. Unrelieved anger accumulated inside of the body develops into Hwa-byung, which is characterized by physical symptoms like chest congestion, hot or heat sensation, and chest pressure; and psychological symptoms like feelings of mortification and a flush of anger [6, 7]. The prevalence of Hwa-byung has been reported to be 5.4% in the local Korean community population and is relatively high in middle-aged or older housewives of the lower socioeconomic class [8].
The clinical course of Hwa-byung includes four stages: anger, conflict, resignation (giving-up), and symptom stages [9]. In the early stage of Hwa-byung, expression of anger is predominant; while anger suppression is predominant in the late stage of Hwa-byung [10]. Though the proper intervention in the early stage is especially important to prevent the progression of Hwa-byung, previous studies were not focused on the stages of Hwa-byung. Various treatment modalities including herbal medicines like Bunsimgi-eum [11], Sihogayonggolmoryeo-tang [12]; acupuncture [13–15]; emotional freedom techniques [16]; and Qigong-based stress reduction programs [17] have been suggested to manage the symptoms of Hwa-byung. However, none of the previous studies addressed the stage of Hwa-byung in patients or examined how the various modalities impact patients based upon the stage of the disease. In this study, we will recruit patients who are in the early (anger) stage of Hwa-byung and the onset of Hwa-byung symptoms are within the past 4 weeks.
A huanglian-jie-du decoction (HJD decoction; Hwangryunhaedok tang in Korean; and Oren-gedoku-to in Japanese) has been commonly used in patients with Hwa-byung. It is composed of four medicinal herbs that traditionally clears heat and detoxifies: the roots of Coptis chinensis Franch, Scutellaria baicalensis Georgi, and Phellodendron chinense Schneid, and the fruit of Gardenia jasminoides Ellis. Its main active components are berberine, baicalin, wogonoside, and gardenoside [18]. In the animal model of chronic unpredictable stress, an HJD extract was reported to ameliorate the depressive-like behaviors in rats through the BDNF-TrkB-CREB pathway [19]. Given the clinical experience with HJD decoctions as the first line therapy in Hwa-byung with predominant heat symptoms and insomnia [9], we hypothesize that a HJD decoction will relieve somatic symptoms and insomnia in patients with early stage Hwa-byung.
2. Materials and Methods
2.1. Study design
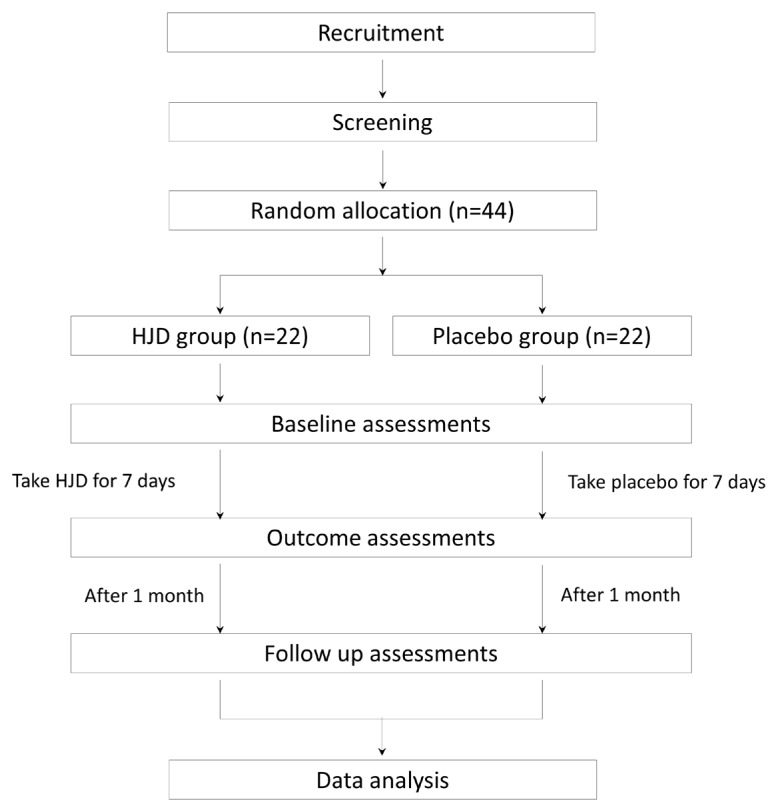
This study is designed as a randomized, double-blind, prospective, placebo-controlled trial with two parallel groups to explore the efficacy and safety of a HJD decoction on Hwa-byung. It will be conducted by a single center, Kyung Hee University Medical Center, in Seoul, Korea. Forty-four patients will be recruited and randomly allocated to either the HJD group or placebo group in a 1:1 ratio. All medications will be taken orally 3 times per day for 7 consecutive days. The efficacy and safety outcomes will be collected after the 7 days of the treatment period and at a 1-month follow-up. The flow chart in Figure 1 illustrates the proposed structure of the study.
Figure 1.
Study flow chart
2.2. Ethics
This trial has been approved by the Kyung Hee University Korean Medicine Hospital Institutional Review Board (Approval Number KOMCIRB-170217-HR-004) and registered in the Clinical Research Information Service, Republic of Korea (KCT0002379). The study will be conducted in accordance with the Declaration of Helsinki and the Korean good clinical practice (KGCP) guidelines. Prior to participation, participants will be fully informed about the study by a qualified Korean medicine doctor and voluntarily sign an informed consent form.
2.3. Participants and recruitment
Forty-four eligible participants will be recruited through advertising at the hospital notice boards, apartments near hospital, and subways.
Inclusion criteria
Participants included in this study will possess all of the following: (1) diagnosis of Hwa-byung; (2) onset of Hwa-byung symptoms occurring within the prior 4 weeks; (3) no problems with communication; and (4) voluntarily signed informed consent form.
Exclusion criteria
Participants with any of the following will be excluded from this study: (1) high risk of suicide; (2) current or lifetime bipolar disorder, schizophrenia; (3) current or previous intake of psychotropic drugs within 30 days; non-psychotropic drugs having psychiatric adverse effect within 14 days; psychotherapy, electroshock treatment, or transcranial magnetic therapy within 30 days; or oriental medical treatment within 14 days; (4) serious unstable medical condition; (5) diabetic patients not controlled by hypoglycemic agents or insulin; (6) systolic blood pressure greater than 160 mmHg or diastolic blood pressure greater than 95 mmHg; (7) alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels 1.5 times as high as or higher than the normal upper limit; (8) blood urea nitrogen (BUN) and creatinine (Cr) levels 1.5 times as high as or higher than the normal upper limit; (9) hyperthyroidism or hypothyroidism; (10) pregnancy or likelihood of pregnancy due to lack of proper contraception, or lactation.
Diagnostic criteria
The diagnosis of Hwa-byung will be established by using a structured clinical interview for Hwa-byung by a qualified resident or specialist that majored in neuropsychiatry of Korean medicine. The structured clinical interview was developed based on the diagnostic criteria for Hwa-byung as displayed in Table 1. The validity and reliability of the diagnostic criteria for Hwa-byung have been reported to be high in Korean populations [20].
Table 1.
Diagnostic criteria for Hwa-byung
| Items | Descriptions |
|---|---|
| A. Hwa-byung major symptoms (presence of four or more of the following) |
|
| B. Hwa-byung-related somatic symptoms (presence of two or more of the following) |
|
| C. Hwa-byung-related psychological symptoms (presence of two or more of the following) |
|
| D. Related stress | The symptoms are significantly related to the anger-inducing events or situations, exposing the individual to unfairness in relationships (ex. spouse, parents-in-law) |
| E. Decline of psychological and social skills | The symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| F. Medical disorders | The symptoms are not attributable to the physiological effects of a substance or to another medical condition. |
mixed feeling of missing someone, sorrow, regret, sadness and depression, along with some feelings of hatred and revenge
2.4. Randomization and blinding
Forty-four eligible participants will be randomly assigned to the HJD group or the placebo group in a 1:1 ratio. The randomization sequences will be generated by an independent investigator that is not involved in the recruitment and enrollment of participants. Block randomization will be done with a block size of four, using the R (version 3.3.3) statistical software. The generated numbers will be concealed in lightproof, sealed envelopes and delivered to the Kyungjin pharmaceutical Co., Ltd (Icheon, Korea) which will be responsible for packaging the HJD and placebo. This pharmaceutical company will make the numbered drug containers based on the random allocation sequence and group information. Each drug container will be labeled with random allocation numbers. The participants and investigators who will be involved in the enrollment, assignment, treatment, and outcome assessment will be blinded to the treatment allocation. Until completion of the statistical analysis, the group information (whether HJD or placebo group) will be replaced with “A” or “B” as blind codes.
2.5. Intervention
All participants will orally ingest 2.5 grams of granules with 150 ml of warm water, three times a day. The treatment duration will be 7 days with a 1-month follow-up. The HJD extract granule (TSUMURA Orengedokuto, TJ-15) was manufactured by the Tsumura & Co, Japan, and the placebo granule, which is nearly identical to the experimental granules in shape, color, scent, and taste and containing no effective ingredients, was manufactured by the Kyungjin Pharmaceutical Co., Ltd (Icheon, Korea). The manufacturing number is UGR901 for HJD decoction and 17001 for placebo. Both of these drugs will be packaged in identical drug containers.
2.6. Efficacy assessments
The primary outcomes of this study are the changes in the means scores of the Patient Health Questionnaire of Physical Symptoms (PHQ-15) [21] and Insomnia Severity Index (ISI) [22] after 7 days of treatment.
The secondary outcomes include the scores of the Stress Response Inventory (SRI) [23], Visual Analogue Scale for Hwa-byung Symptoms (VAS-HS) [9], State Trait Anger Expression Inventory-State Anger (STAXI-S) [24], EuroQol 5 Dimensions Questionnaire (EQ-5D), EuroQol-Visual Analogue Scales (EQ-VAS), 36-Item Short Form Survey (SF-36), and heart rate variability [25]. Additionally, the surface temperature of the chest, abdominal, and upper back regions will be measured using digital infrared thermal imaging (DITI) and a resting electroencephalogram will be measured on 32-channel system for the brain according to 10–20 systems. The schedule for collecting outcomes is provided in Table 2.
Table 2.
Schedule for enrollment, interventions, and assessment
| STUDY PERIOD | |||||
|---|---|---|---|---|---|
| Enrollment | Allocation | Post-allocation | Close-out | ||
| Time point | −t1 | 0 | day 1 | day 8 (±1d) | day 36 (±7d) |
| ENROLMENT: | |||||
| Informed consent | x | ||||
| Eligibility screen | x | ||||
| Random allocation | x | ||||
| INTERVENTIONS: | |||||
| Take medications |

|
||||
| ASSESSMENTS: | |||||
| Demographic data | x | ||||
| Medical history | x | ||||
| Vital signs | x | ||||
| Laboratory test | x | ||||
| Hwa-byung diagnosis | x | ||||
| PHQ-15 | x | x | x | ||
| ISI | x | x | x | ||
| SRI | x | x | x | ||
| VAS-HS | x | x | x | ||
| STAXI-S | x | x | x | ||
| EQ-5D | x | x | x | ||
| EQ-VAS | x | x | x | ||
| SF-36 | x | x | x | ||
| HRV | x | x | x | ||
| DITI | x | x | x | ||
| EEG | x | x | |||
| Adverse events | x | x | x | ||
PHQ-15: Patient health questionnaire-physical symptoms; ISI: Insomnia severity index; SRI: Stress response inventory; VAS-HS: Visual analogue scale for Hwa-byung symptoms; STAXI-S: State trait anger expression inventory-state anger; EQ-5D: EuroQol 5 dimensions questionnaire; EQ-VAS: EuroQol-visual analogue scales; SF-36: 36-item short form survey; HRV: Heart rate variability; DITI: Digital infrared thermal imaging; EEG: Electroencephalogram
2.7. Safety assessment
Every adverse event that occurs during the treatment and follow-up phases will be recorded on the case report form. The symptoms, severity, duration and treatment for the adverse events will be carefully documented. If the adverse symptoms are severe, the participant will stop taking the medication and be given appropriate medical treatment.
2.8. Sample size calculation
According to a previous report, a clinically relevant reduction in the ISI score was determined to be 5 [26]. The results from a recent clinical trial of Korean medicine treatment for Hwa-byung demonstrated that the standard deviation of the change in ISI scores were assumed to be 5.73 [13]. With a 5% significance level and 21 participants per group, we have 80% power to detect a difference of 5. A total of 44 participants will be recruited to account for an expected 5% dropout rate.
2.9. Statistical analysis
Independent sample t-tests will be performed to determine the change in mean scores between before and after the treatment. Additionally, repeated measure analyses of variance will be conducted to assess the mean change between experiments, including the 1-month follow-up. Adjustments for imbalanced baseline values will be performed as supplementary analyses. For all analyses, a α-value of 5% and power of 80% will be considered. The analysis will be conducted using the SPSS 22.0 software (IBM Inc., Armonk, NY, USA).
3.Results
We propose a randomized, double-blind, placebo-controlled trial to explore the efficacy and safety of a HJD decoction on Hwa-byung. Hwa-byung is the cultural concept of distress in Korea that results from chronic accumulated anger. Though Korean medicine is commonly used to manage symptoms of Hwa-byung in the clinic, there have been few studies that suggest treatment options for Hwa-byung. Meta-analyses with well-designed randomized controlled studies examining Hwa-byung treatment are needed [27]. A HJD decoction has been used for patients with Hwa-byung in Korean clinical practices. The results of this study will facilitate a better understanding of the efficacy and safety of a HJD decoction on Hwa-byung. A HJD decoction is composed of four medicinal herbs that have the traditional effects of clearing heat and detoxifying. Hwa-byung, which literally means “fire disease” in Korean, is characterized by various fire-like symptoms that results from accumulated anger [2]. Taking this into consideration, a HJD decoction is one of the most appropriate herbal medicine therapy for patients with Hwa-byung. An antidepressant-like effect of the HJD decoction has been reported [19], and a similar effect on the stress response is expected in this study also.
Various aspects, including somatic symptoms and insomnia, will be measured to investigate the effect of the HJD decoction on patients with Hwa-byung. Somatic distress like chest discomfort and flushing occur in patients with Hwa-byung. The PHQ-15 was selected to measure the somatic symptoms of Hwa-byung. Insomnia is a typical symptom that indicates an alarmed response in acute stressed circumstances. Thus, the PHQ-15 and ISI are the primary outcomes and representative scales for early stage Hwa-byung.
The state of anger, which is the core emotion of Hwa-byung, will be identified via the State Trait Anger Expression Inventory (STAXI). Since Hwa-byung is caused by interpersonal conflicts or injustices, the Stress Response Inventory (SRI) is also selected. Hwa-byung is associated with difficulties in household affairs, jobs and personal relationships; so a scale for measuring quality of life was adopted. Additionally, a bio-signal measurement including heart rate variability, body surface temperature, and resting electroencephalography will explore the effects of a HJD decoction on physiological signals.
In summary, Hwa-byung is cultural-bound distress in Korea, but effective treatment approaches are limited. A HJD decoction has been commonly used in clinics for patients with Hwa-byung. This randomized controlled trial will provide high-quality, explorative evidence to investigate the effects of a HJD decoction on Hwa-byung.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HB16C0068)
Abbreviations
- DITI
Digital infrared thermal imaging
- EEG
Electroencephalogram
- EQ-5D
EuroQol 5 dimensions questionnaire
- EQ-VAS
EuroQol-visual analogue scales
- HJD
Huanglian-jie-du
- HRV
Heart rate variability
- ISI
Insomnia severity index
- PHQ-15
Patient health questionnaire- physical symptoms
- SF-36
36-item short form survey
- SRI
Stress response inventory
- STAXI-S
State trait anger expression inventory- state anger
- VAS-HS
Visual analogue scale for Hwa-byung symptoms
Footnotes
This paper meets the requirements of KS X ISO 9706, ISO 9706-1994 and ANSI/NISO Z39.48-1992 (Permanence of Paper).
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This study has been approved by the Kyung Hee University Korean Medicine Hospital Institutional Review Board (Approval Number KOMCIRB-170217-HR-004). Written informed consent will be obtained from all participants by the investigator.
References
- 1.Kohrt BA, Rasmussen A, Kaiser BN, Haroz EE, Maharjan SM, Mutamba BB, et al. Cultural concepts of distress and psychiatric disorders: Literature review and research recommendations for global mental health epidemiology. Int J Epidemiol. 2014;43(2):365–406. doi: 10.1093/ije/dyt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Min SK. Clinical correlates of hwa-byung and a proposal for a new anger disorder. Psychiatry Investiq. 2008;5(3):125–41. doi: 10.4306/pi.2008.5.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin KM, Lau JK, Yamamoto J, Zheng YP, Kim HS, Cho KH, et al. Hwa-byung: A community study of Korean Americans. J Nerv Ment Dis. 1992;180(6):386–91. doi: 10.1097/00005053-199206000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Wachholtz A, Choi KH. A Review of the Korean Cultural Syndrome Hwa-Byung: Suggestions for Theory and Intervention. Asia Taepyongyang Sangdam Yongu. 2014;4(1):49. doi: 10.18401/2014.4.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ninnemann K. Hwa-Byung. In: Loue S, Sajatovic M, editors. Encyclopedia of Immigrant Health. New York, NY: Springer New York; 2012. pp. 862–3. [DOI] [Google Scholar]
- 6.Park YJ, Kim HS, Kang HC, Kim JW. A survey of Hwa-Byung in middle-age Korean women. J Transcult Nurs. 2001;12(2):115–22. doi: 10.1177/104365960101200205. [DOI] [PubMed] [Google Scholar]
- 7.Min SK, Suh SY, Song KJ. Symptoms to use for diagnostic criteria of hwa-byung, an anger syndrome. Psychiatry Investiq. 2009;6(1):7–12. doi: 10.4306/pi.2009.6.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JG, Lee JH. Study on the Prevalence of Hwa-Byung Diagnosed by HBDIS in General Population in Kang-won Province. J Orient Neuropsychol. 2008;19(2):133. [Google Scholar]
- 9.Kim J, Jung I, Kang H, Lee S, Jung S. Clinical guideline for Hwa-Byung. Clinical Research Center for Hwa-Byung Seoul; Korea: 2013. [Google Scholar]
- 10.Chon K, Whang W, Kim J, Park H. Relationship between hwabyung and emotional stress. Korean J Health Psychology. 1997;2:170–80. [Google Scholar]
- 11.Kim SH, Park YC, Hong KE, Kang W, Lee SR, Jung IC. The effect of Bunsimgi-eum on Hwa-byung: randomized, double blind, placebo controlled trial. J Ethnopharmacol. 2012;144(2):402–7. doi: 10.1016/j.jep.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Choi WC, Park DM, Kang WC, Lee WC, Jung IC. Interim Report about The Effect of Sihogayonggolmoryeo-tang on the Anxiety of Hwa-byung. J Orient Neuropsychol. 2012;23(4):133–52. doi: 10.7231/jon.2012.23.4.133. [DOI] [Google Scholar]
- 13.Lee GE, Kim NK, Kim HY, Kang HW. The Effects of Acupuncture Treatment on Hwa-byung patient’s Insomnia: Patient-assessor blind, Randomized, Placebo-controlled Clinical trial. J Orient Neuropsychol. 2012;23(1):31–48. doi: 10.7231/JON.2012.23.1.031. [DOI] [Google Scholar]
- 14.Jeong IC, Lee SR, Park YC, Hong KE, Lee YK, Kang HC, et al. The Effect of Sa-am Acupuncture Simjeongkyeok Treatment for Major Symptom of Hwa-byung. J Orient Neuropsychol. 2008;19(1):1–18. [Google Scholar]
- 15.Jung DJ, Lee JH. The clinical trial for the significant effects of acupuncture on decreasing anxiety symptom of Hwa-Byung in a single institute- single-arm with Hwa-Byung, open lable. J Orient Neuropsychol. 2012;23(1):49–58. doi: 10.7231/JON.2012.23.1.049. [DOI] [Google Scholar]
- 16.Suh JW, Chung SY, Kim SY, Lee JH, Kim JW. Anxiety and Anger Symptoms in Hwabyung Patients Improved More following 4 Weeks of the Emotional Freedom Technique Program Compared to the Progressive Muscle Relaxation Program: A Randomized Controlled Trial. Evidence-based complementary and alternative medicine: eCAM. 2015;2015:203612. doi: 10.1155/2015/203612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang EY, Chung SY, Cho JH, Song MY, Kim S, Kim JW. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC complementary and alternative medicine. 2013;13:113. doi: 10.1186/1472-6882-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Y, Huang C, Jiang L, Shen X, Zhu S, Rao Y, et al. Huanglian-Jie-Du-Tang extract protects against chronic brain injury after focal cerebral ischemia via hypoxia-inducible-factor-1alpha-regulated vascular endothelial growth factor signaling in mice. Biological & pharmaceutical bulletin. 2012;35(3):355–61. doi: 10.1248/bpb.35.355. [DOI] [PubMed] [Google Scholar]
- 19.Ye YL, Zhong K, Liu DD, Xu J, Pan BB, Li X, et al. Huanglian-Jie-Du-Tang Extract Ameliorates Depression-Like Behaviors through BDNF-TrkB-CREB Pathway in Rats with Chronic Unpredictable Stress. Evidence-based complementary and alternative medicine: eCAM. 2017;2017:7903918. doi: 10.1155/2017/7903918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Kwon J, Lee M, Park D. Development of hwa-byung diagnostic interview schedule (HBDIS) and its validity test. The Korean Journal of Health Psychology. 2004;9(2):321–31. [Google Scholar]
- 21.Han C, Pae CU, Patkar AA, Masand PS, Kim KW, Joe SH, et al. Psychometric properties of the Patient Health Questionnaire-15 (PHQ-15) for measuring the somatic symptoms of psychiatric outpatients. Psychosomatics. 2009;50(6):580–5. doi: 10.1176/appi.psy.50.6.580. [DOI] [PubMed] [Google Scholar]
- 22.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 23.Koh KB, Park JK, Kim CH, Cho S. Development of the stress response inventory and its application in clinical practice. Psychosomatic medicine. 2001;63(4):668–78. doi: 10.1097/00006842-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. 1970 [Google Scholar]
- 25.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Annals of noninvasive electrocardiology: the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc. 2005;10(1):88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong MK, Kim W, Lee WB, Cho SH. Korean medicine for Hwa-byung: a systematic and meta-analysis. Orient Pharm Exp Med. 2016;16(4):251–7. doi: 10.1007/s13596-016-0233-y. [DOI] [Google Scholar]