Cellular signaling processes rely on the presence of intrinsically disordered proteins (IDPs) or regions of proteins (IDRs), employing unique attributes of disorder that include pathway cross-talk, brokered by interactions of the same polypeptide with multiple partners, and control of signaling by combinatorial post-translational modifications.1 Disordered proteins such as the cyclin-dependent kinase inhibitor p21, retinoblastoma tumor suppressor protein pRb, and the tumor suppressor p53 play central roles in regulation of the cell cycle. Viruses also employ disordered proteins, which serve to disrupt and subvert the cell cycle and normal cellular signaling processes. Viruses are an established cause of cancer in humans and other species. Disordered viral oncoproteins are central to the cancer phenotype; they compete with cellular proteins for binding to central control systems.
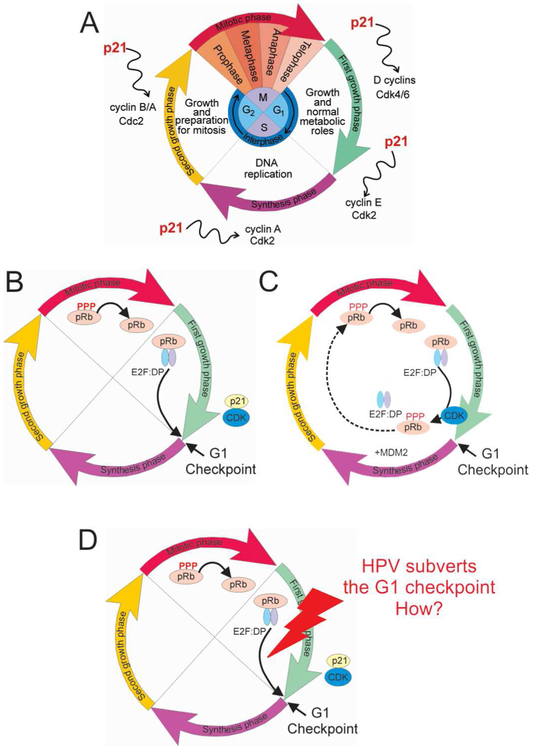
One of the proximate causes of virus-induced cancer is the interaction of disordered viral oncoproteins with the retinoblastoma protein pRb.2 In the normal cell cycle, (Figure 1A) the E2F and DP transcription factors, which allow the cell to progress beyond the G1 checkpoint at the end of the first growth phase and into S phase, are sequestered in complex with pRb (Figure 1B). The normal signal for progression beyond the G1 checkpoint is the phosphorylation of pRb, which releases E2F and DP (Figure 1C). However, many viral oncoproteins subvert this process, releasing E2F from its pRb complex and leading to aberrant cell cycle progression and uncontrolled cell proliferation and cancer (Figure 1D). One intriguing puzzle is that even for very similar viral strains, the tendency for infection to lead to cancer differs markedly. For example, some strains of the human papilloma virus HPV have an extremely high cancer risk, while others cause only benign papillomas (warts).3 Nearly all cases of cervical cancer are related to infection with high-risk strains of HPV – this devastating disease can now be prevented by vaccination against HPV.
Figure 1.
Schematic diagram of the cell cycle and its control mechanism.
A. General cell cycle showing phases where growth, DNA replication and cell division occur. B. Role of pRb in the cell cycle. pRb dephosphorylated during the mitotic phase binds transcription factors E2F and DP, preventing the cell from proceeding through the G1 checkpoint. C. The normal signal to proceed through the G1 checkpoint is given by the degradation of p21 in the Cdk2-cyclin E complex, releasing Cdk2, which phosphorylates pRb, leading to the release of E2F and DP. D. HPV subverts the normal cell cycle by releasing the G1 checkpoint without the normal signal from the Cdk2-cyclin E complex.
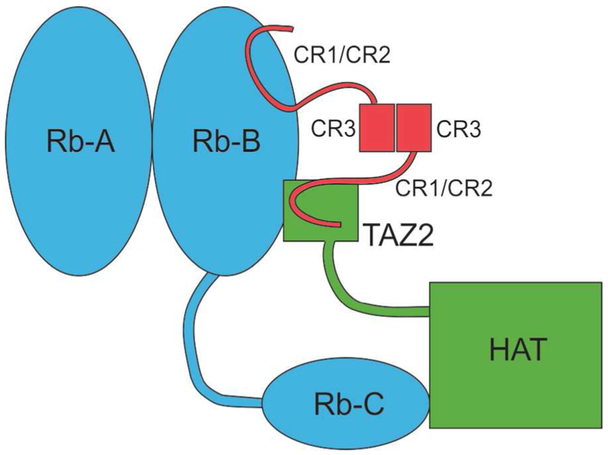
What makes some strains of HPV high-risk, while closely-related strains are low risk? Comparative studies of the interactions of the E7 protein of HPV derived from two strains, one high-risk (HPV16) and one low-risk (HPV6b)4 showed that the difference in oncogenic potential is correlated with a difference in the E7 affinity for the transcriptional activator CREB-binding protein (CBP). The E7 protein is a dimer of two 98-residue monomers, with a folded C-terminal dimerization domain and an N-terminal disordered domain that binds to the A-B pocket domain of pRb and to the TAZ2 domain of CBP. The affinity of E7 from HPV16 for TAZ2 is much higher than that of the HPV6b E7, and the high-risk E7 forms a stable ternary complex with TAZ2 and pRb. This ternary complex likely explains the high oncogenic potential of high-risk E7: in the absence of E7, there is no interaction between pRb and CBP. However, in the ternary complex, the pRb is placed in close proximity to the catalytic acetyltransferase domain of CBP (Figure 2), promoting acetylation and degradation of Rb, which blocks normal Rb phosphorylation and stabilizes the E7:Rb complex.4, 5 In addition, E7 directly competes with E2F for binding to Rb, freeing the E2F transcription factors and precipitating the aberrant release of the G1 cell cycle checkpoint.
Figure 2.
Model for pRb:CBP:E7 ternary complex formation.4 E7 dimerization through the CR3 domain facilitates recruitment of CBP to Rb. The disordered CR1/CR2 domain of one monomer interacts with the pRb B domain, while the other interact with TAZ2 in a ternary complex that brings the catalytic HAT domain of CBP close to the pRb C domain, enabling acetylation at pRb lysines 873 and 874 and promoting pRb degradation. (Reproduced with permission from ref. 4).
These observations provide one possible mechanism for the oncogenesis of high-risk HPV E7, intimately related to the disorder of the constituent domains and their ability to broker cross-talk between cellular components that would otherwise not interact.
Acknowledgments
Funding: CA96865 (PEW)
References
- [1].Wright PE, and Dyson HJ (2015) Intrinsically disordered proteins in cellular signalling and regulation, Nat. Rev. Mol. Cell Biol 16, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bellacchio E, and Paggi MG (2013) Understanding the targeting of the RB family proteins by viral oncoproteins to defeat their oncogenic machinery, J. Cell. Physiol 228, 285–291. [DOI] [PubMed] [Google Scholar]
- [3].zur Hausen H (2009) Papillomaviruses in the causation of human cancers -- a brief historical account, Virology 384, 260–265. [DOI] [PubMed] [Google Scholar]
- [4].Jansma AL, Martinez-Yamout MA, Liao R, Sun P, Dyson HJ, and Wright PE (2014) The high-risk HPV16 E7 oncoprotein mediates interaction between the transcriptional coactivator CBP and the retinoblastoma protein pRb, J. Mol. Biol 426, 4030–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oh KJ, Kalinina A, and Bagchi S (2010) Destabilization of Rb by human papillomavirus E7 is cell cycle dependent: E2-25K is involved in the proteolysis, Virology 396, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]