Abstract
Intercellular adhesive junctions are essential for maintaining the physical integrity of tissues; this is particularly true for the heart that is under constant mechanical load. The correct functionality of the heart is dependent on the electrical and mechanical coordination of its constituent cardiomyocytes. The intercalated disc (ID) structure located at the termini of the rod shaped adult cardiomyocyte contains various junctional proteins responsible for the integration of structural information and cell-cell communication. According to the classical description, the ID consists of three distinct junctional complexes: adherens junction (AJ), desmosome (Des), and gap junction (GJ) that work together to mediate mechanical and electrical coupling of cardiomyocytes. However, recent morphological and molecular studies indicate that AJ and Des components are capable of mixing together resulting in a ‘hybrid adhering junction’ or ‘area composita’. This review summarizes recent progress in understanding the in vivo function(s) of AJ components in cardiac homeostasis and disease.
Keywords: N-cadherin, catenin, adherens junction, desmosome, arrhythmogenic cardiomyopathy
INTRODUCTION
The coordinated contraction of the heart is dependent on the proper mechanical and electrical coupling of cardiomyocytes. To achieve this goal cardiomyocytes are connected end-to-end by a specialized structure called the intercalated disc (ID) that serves as an organizing center for various cell surface proteins including junctional complexes critical for cell-cell attachment and cell-cell communication (Figure 1). The ID contains three different intercellular junctions: adherens junction (AJ), desmosome (Des), and gap junction (GJ) (Forbes and Sperelakis, 1985). AJ and Des provide mechanical attachment between the myocytes by anchoring the actin cytoskeleton and intermediate filaments, respectively, at the ID. GJs are plaques of multiple intercellular channels that connect the cytoplasm of adjacent cells. A major role of GJs in the myocardium is to enable the rapid and coordinated electrical excitation, a prerequisite for normal rhythmic cardiac function. The expression, distribution, and/or function of many of the junctional components are often perturbed in heart disease (Severs et al., 2008, Saffitz, 2009), however their specific contribution to the disease phenotype is less well understood.
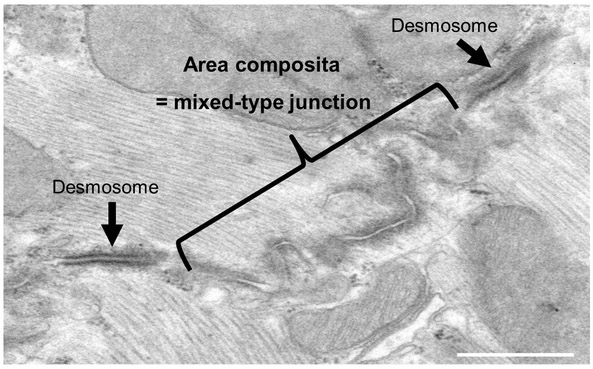
Figure 1: Transmission electron microscopy of a cross section through intercalated disc of a mouse heart.
TEM image showing the intercalated disc with a large mixed-type junction (area composita) surrounded by two dense desmosome structures (black arrows).
The AJ consists of classical cadherins, including E-, P- and N-cadherin, that mediate calcium-dependent cell-cell adhesion. The classical cadherins are single pass transmembrane proteins with five extracellular ‘cadherin domains’, a transmembrane domain, and a cytoplasmic domain (Meng and Takeichi, 2009). Through their homophilic binding and adhesive specificities, cadherins play a critical role in embryonic development and the maintenance of normal tissue architecture in the adult. Cadherin adhesive activity is dependent on cytosolic proteins called catenins that link the cadherin cytoplasmic domain to the actin cytoskeleton. N-cadherin is the sole cadherin expressed in heart muscle. In contrast, different catenin subtypes with overlapping and distinct functions are available for N-cadherin/catenin complex formation. Catenins can be divided into two families: the armadillo (Arm) domain containing catenins that bind directly to the cadherin (β-catenin, γ-catenin, and p120ctn) and the vinculin-homology domain containing catenins (αE-catenin and αT-catenin). β-catenin and γ-catenin (also known as plakoglobin) bind to the carboxy-terminus in a mutually exclusive manner whereas p120ctn binds to the juxtamembrane region of cadherin. The function of p120ctn has not been studied in the heart and will not be discussed further in this review. α-catenin which binds to the cadherin-β-catenin or cadherin-plakoglobin complex, mediates linkage to the actin cytoskeleton.
Desmosomes are present in tissues under constant mechanical stress such as skin and heart, and thus reinforce intercellular adhesion in those tissues (Green et al., 2010). The molecular structure of Des is relatively similar to AJ, however these adhesive junctions are anchored to intermediate filaments (i.e. desmin). The desmosomal cadherins, desmoglein-2 (DSG2) and desmocollin-2 (DSC2), serve as the transmembrane adhesion receptors in the Des. The Arm proteins plakoglobin (PG) and plakophilin-2 (PKP-2) bind directly to the cytoplasmic tail of DSG2 and DSC2, which in turn binds to the plakin protein desmoplakin (DP). DP links the desmomosal cadherins to desmin.
The importance of these adhesion molecules in the heart is highlighted by the fact that human mutations in genes encoding desmosomal proteins cause arrhythmogenic cardiomyopathy (AC, also known as arrhythmogenic right ventricular cardiomyopathy or ARVC) a hereditary heart muscle disease that causes sudden cardiac death (SCD) in young people and athletes (Basso et al., 2012). The pathological features of AC consist of progressive loss of cardiomyocytes, myocardial degeneration, and compensatory replacement with fibro-fatty tissue. AC is considered a disease of the desmosome since almost half of the patients carry a mutation in one of the five genes encoding desmosomal proteins expressed in the heart. A hallmark of AC is incomplete penetrance and variable expressivity of the disease phenotype making it difficult for clinicians to advise patients of their risk of SCD. Adding further to the genetic complexity AC patients were recently identified with more than one mutation in the same or different desmosomal gene, suggesting that AC might require multiple genetic hits in the cell adhesion complex to elicit a cardiac phenotype (den Haan et al., 2009, Bauce et al., 2010, Xu et al., 2010).
Until recently it was thought that AJ and Des represent distinct junctional complexes of the ID. The idea of a “hybrid junction” as part of the normal heart structure was first suggested in 2006 (Franke et al., 2006). In this study, the authors revealed the presence of DP and PKP2 in “desmosome-like” structures as well as “adherens junction-like” structures by immunoelectron microscopy. These hybrid junctions that they called “area composita”, comprise the majority of intercellular junctions in the heart (Franke et al., 2006, Borrmann et al., 2006) (Figure 2). This novel junctional complex is only found in the heart of higher vertebrates raising the intriguing possibility that this reinforced adhesive junction evolved to withstand the increased mechanical load of the four-chamber mammalian heart (Pieperhoff and Franke, 2007, Pieperhoff and Franke, 2008). The concept of a mixed junctional complex containing both AJ and Des components has important ramifications for understanding the molecular mechanisms underlying ID remodeling observed in heart disease. The area composita will be discussed later in the context of specific interactions between the AJ and Des components, αT-catenin and PKP-2.
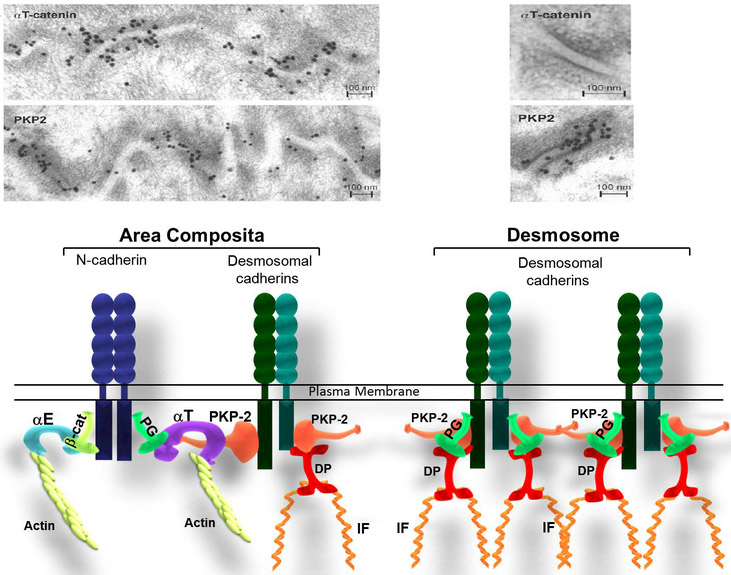
Figure 2: Molecular organization of Area composita vs. desmosome in the heart.
a) Immunoelectron microscopy images of the myocardium of a mouse heart showing the co-localization of PKP2 and αT-catenin at the area composita. Note the absence of αT-catenin in the desmosome. These images were originally published in “J Cell Sci., Goosens et al. 2007”. b) Model for cadherin-based cell-cell adhesion in the heart. This model represents the composition of a hybrid junction compared to a desmosome. αT-catenin recruits desmosomal proteins through its interaction with plakophilin-2 (PKP2), forming a hybrid junction (named area composita), reinforcing the intercalated discs resistance. (αE: αE-catenin; αT: αT-catenin; β-cat: β-catenin; PG: Plakoglobin; PKP2: Plakophillin-2; IF: Intermediate Filaments).
Gap junction channels connect the cytoplasmic compartments of adjacent cells and allow the intercellular flow of ions. In the heart, GJ are critical for the efficient propagation of the action potential throughout the myocardium (Severs et al., 2008). An individual channel is created by stable, noncovalent interactions of two hemichannels, referred to as connexons. Each connexon is composed of six connexin (Cx) proteins. In the mammalian heart, gap junction channels are primarily composed of three different Cx proteins: Cx43, Cx40 and Cx45. Cx43 is the main constituent of cardiac GJ and in rodents is expressed in all atrial and ventricular myocytes. Cx40 is expressed in atrial myocytes, while Cx45 is expressed in the conduction system. In cardiomyopathies, Cx43 is often down-regulated or mislocalized to the lateral membranes instead of being restricted to the ID (Kostin et al., 2003). Experimental animal models demonstrated that altered Cx expression results in slow, heterogeneous conduction and conduction block critical for reentrant arrhythmias (Remo et al., 2012).
In this review, we will focus on AJ components and their essential roles in the organization and function of the cardiac ID. As the ID can only be fully appreciated in the context of the working myocardium, we will focus our discussion on animal models that have enhanced our understanding of the functional interdependence of the different intercellular junctions in the heart. Finally, the new concept of the ‘area composita’ will be discussed and how it may explain the increased risk of arrhythmia in AC patients.
N-cadherin function in the heart
N-cadherin-mediated adhesion plays a critical role in both cardiac morphogenesis and in the adult working myocardium. The importance of N-cadherin in mammalian development was appreciated after germline deletion of N-cadherin in mice (Radice et al., 1997). N-cadherin knockout embryos display multiple embryonic abnormalities that include severe cardiovascular defects. The loss of cardiomyocyte adhesion in the N-cadherin mutant embryos caused malformation of the primitive heart tube and early embryonic lethality. Interestingly, cardiac-specific expression of the epithelial cadherin, E-cadherin, was able to rescue the adhesion defect and restore cardiac looping in the N-cadherin-null embryos demonstrating that these two cadherins are functionally interchangeable during early cardiac development (Luo et al., 2001). In addition to the requirement of N-cadherin in the nascent myocardium, this protein also plays essential roles in other cardiac cell lineages including cardiac neural crest, epicardial, and endocardial cells (Luo et al., 2006, Luo et al., 2005).
Initially, during early cardiac development, the junctional components are distributed all along the cell borders of the polygonal shaped embryonic cardiomyocyte (Hirschy et al., 2006). During the later fetal and early postnatal period the myocyte elongates, myofibrils align, and maturation results in a significantly larger rod shaped cardiomyocyte. During this morphological progression the AJ, Des, and GJ components eventually become restricted to the polarized ends of the cell to form the mature ID (Angst et al., 1997). Pathological remodeling of the ID has been observed in diseased human myocardium (Schaper et al., 1991, Tomaselli et al., 1994) and animal models (Matsushita et al., 1999, Wang and Gerdes, 1999) demonstrating that a heightened risk of arrhythmia and sudden death is associated with abnormal cell-cell coupling.
Mouse models have allowed a detailed characterization of the consequences of interfering with mechanical junctions in the heart (Table I). To overcome the requirement for N-cadherin during embryogenesis, an inducible cardiac-specific N-cadherin (N-cad) conditional knockout (CKO) model was used to investigate N-cadherin function in the adult heart (Kostetskii et al., 2005). Induced deletion of the N-cadherin (Cdh2) gene in the working myocardium results in complete disassembly of the ID as determined by transmission electron microscopy. This study demonstrated the paramount importance of N-cadherin for ID structural integrity. The loss of desmosomes after N-cadherin depletion illustrates the functional hierarchy of these adhesive junctions in the heart (Figure 3). In contrast, mutations in genes encoding desmosomal proteins have minimal, if any effect, on N-cadherin expression in the heart (Kwon et al., 2013, Tavora et al., 2013, Oxford et al., 2007, Cerrone et al., 2012).
Table I.
Genetic manipulation of adherens junction proteins in the adult myocardium
| Mouse model | Stress | Cardiac Phenotype | Reference |
|---|---|---|---|
| N-cadherin LOF | None | Cardiomyopathy, GJ remodeling, arrhythmia, SCD | (Cheng et al., 2011, Franceschini et al., 2013, Kostetskii et al., 2005, Li et al., 2005) |
| N-cadherin HET | PS | Normal cardiac function, GJ remodeling, inducible arrhythmia | (Li et al., 2008) |
| β-catenin LOF | None | Normal cardiac function, increase PG | (Zhou et al., 2007) |
| β-catenin LOF | TAC | Block hypertrophy, decrease Wnt genes | (Chen et al., 2006) |
| β-catenin LOF | AngII | No change in hypertrophy response | (Baurand et al., 2007) |
| β-catenin LOF | MI | Improve LV function, increase cpc | (Zelarayan et al., 2008) |
| β-catenin HET | TAC | Block hypertrophy, increase fetal genes | (Qu et al., 2007) |
| β-cateninΔex3 GOF | None | Cardiomyopathy, no change Wnt genes | (Hirschy et al., 2010) |
| β-cateninΔex3 GOF | MI | No improvement in LV function, decrease cpc differentiation | (Zelarayan et al., 2008) |
| β-cateninΔex3 GOF | AngII | Block hypertrophy, decrease cardiac function | (Baurand et al., 2007) |
| PG LOF | None | AC-like pathology except adipocytes | (Li et al., 2011a, Li et al., 2011b) |
| PG Hypomorph | None | Normal cardiac function, increase β-catenin | (Swope et al., 2012) |
| PG GOF | None | AC-like pathology including adipocytes | (Lombardi et al., 2011) |
| PGNaxos GOF | None | AC-like pathology including adipocytes | (Lombardi et al., 2011) |
| αE-catenin LOF | None | Cardiomyopathy late onset | (Sheikh et al., 2006) |
| αE-catenin LOF | MI | Ventricular wall rupture | (Sheikh et al., 2006) |
| αT-catenin LOF | None | Cardiomyopathy early onset, GJ remodeling | (Li et al., 2012) |
| αT-catenin LOF | I/R | Arrhythmia | (Li et al., 2012) |
LOF, loss-of-function; GOF, gain-of-function; HET, heterozygous; SCD, sudden cardiac death; PS; program stimulation; GJ, gap junction; TAC, transverse aortic constriction; AngII, angiotensinII; MI, myocardial infarction; cpc, cardiac progenitor cells; I/R, ischemia/reperfusion.
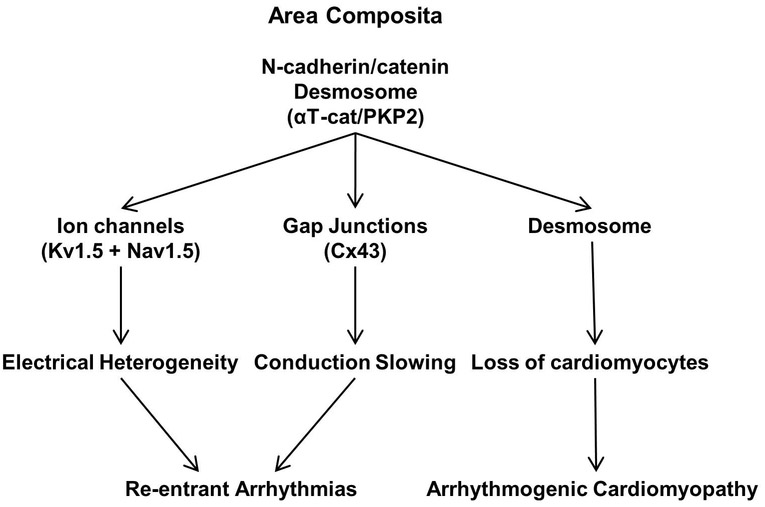
Figure 3: Functional Hierarchy at the Intercalated Disc.
Scheme depicts the relationship between the different junctions and cardiac dysfunction.
It is remarkable that N-cad CKO animals survive even for a limited time without any recognizable ID. The mutant heart retains the ability to contract, albeit weakly, without these mechanical junctions. It is possible that other adhesion systems compensate for loss of the normal end-to-end connection between the myocytes. For instance, increased β1-integrin at the sarcolemma of the N-cadherin-deficient cardiomyocytes suggests enhanced cell-ECM interactions that may provide additional structural stability in the absence of AJ and Des (Kostetskii et al., 2005).
The N-cad CKO mice exhibit sudden cardiac death (SCD) beginning about 5 weeks after deleting the gene. Electrocardiographic recordings from transmitters implanted into freely mobile animals showed that SCD in N-cad CKO mice was the result of spontaneous ventricular fibrillation in the absence of any clinical signs of heart failure (Kostetskii et al., 2005). The investigation into the mechanism(s) by which N-cadherin depletion leads to an arrhythmogenic substrate and SCD is complicated by the fact that multiple proteins involved in electrical activity in the heart are affected by disassembly of the ID. Evidence from diseased human myocardium and animal models indicate that alterations in electrical coupling via gap junctions are a critical determinant in the development of the arrhythmogenic substrate (Remo et al., 2012). Both the number and size of Cx43- and Cx40-containing GJ plaques are reduced in the ventricular and atrial myocardium of the N-cad CKO, respectively (Li et al., 2005). The carboxy-terminus of Cx43 is phosphorylated at specific serine residues when it forms an active gap junction complex at the cell surface. In contrast, it is dephosphorylated during trafficking/endocytosis in the cytoplasm. Researchers have taken advantage of a series of antibodies generated by the Lampe lab that recognize the phosphorylation status of Cx43 (Sosinsky et al., 2007). N-cad CKO hearts exhibit an increase in dephosphorylated Cx43 consistent with less functional gap junctions at the sarcolemma. Consistent with this idea, optical mapping studies revealed slowing of conduction velocity in the N-cad CKO hearts (Li et al., 2005). Of note, the reduction in epicardial conduction velocity is similar to that observed in the Cx43 CKO mice which also die from spontaneous lethal arrhythmias (Gutstein et al., 2001). Based on these studies, gap junction remodeling is considered a major determinant of the arrhythmogenesis observed in the N-cad CKO animals.
N-cadherin regulation of gap junction communication
Previous studies demonstrated that N-cadherin-mediated cell-cell contacts are a prerequisite for the aggregation of gap junction channels at the sarcolemma (Hertig et al., 1996, Luo and Radice, 2003). More recent evidence indicates N-cadherin regulates connexon assembly, trafficking and functional gap junction cellular coupling. It is becoming better appreciated that N-cadherin maintains gap junction homeostasis by multiple mechanisms. AJ stabilize the plasma membrane of adjacent cardiomyocytes, which then helps to form an environment at the ID that protects the GJ from mechanical stress. Interestingly, in response to pulsatile stretch neonatal rat ventricular cardiomyocytes markedly upregulate N-cadherin and Cx43 which increases propagation velocity (Zhuang et al., 2000). N-cadherin/catenin complex also organizes the underlying cytoskeleton and associated scaffold proteins thus facilitating the aggregation of connexons into large arrays of gap junctions. The actin-binding scaffold protein, zonula occludens-1 (ZO-1), interacts with Cx43 via a PDZ-binding motif found in the large carboxy terminus (Giepmans and Moolenaar, 1998). ZO-1 has been shown to regulate Cx43 plaque size and distribution (Hunter et al., 2005). N-cadherin-deficient hearts exhibit a loss of ZO-1 from the myocyte termini providing a possible explanation for the reduced Cx43 plaque size in the mutant hearts (Li et al., 2008). Recent data indicate that vinculin, another actin-binding scaffold protein, and ZO-1 together regulate GJ in the heart (Zemljic-Harpf et al., 2014). Lastly, efficient delivery of connexons to the cell-cell border is dependent on tethering of microtubule plus-ends via N-cadherin and β-catenin at AJ (Shaw et al., 2007). The plus-end binding protein EB1 binds to Cx43 and facilitates its transport along the microtubules to the AJ. In the mouse heart, it was shown experimentally that the EB1/Cx43/AJ association can be disrupted by ischemia-reperfusion injury or oxidative stress induced by treatment with hydrogen peroxide (Smyth et al., 2010). Interestingly, N-cadherin association with Cx43 and EB1 is disrupted by stress whereas N-cadherin-β-catenin interaction at the membrane remains intact.
In addition to gap junctions, ion channels are also dependent on proper arrangement of the cytoskeleton. Remodeling of the actin cytoskeleton including downregulation of the actin-binding scaffold protein cortactin is likely responsible for decreased Kv1.5 function in the N-cad CKO model (Cheng et al., 2011). Reduction of the voltage-gated potassium channel Kv1.5 at the sarcolemma likely causes electrical heterogeneity thus facilitating the development of arrhythmia in the N-cad CKO mice. Further studies will be necessary to determine if trafficking of Kv1.5 to the ID is dependent on N-cadherin-mediated cytoskeletal organization.
N-cad CKO mice exhibit a severe cardiac phenotype and SCD. In comparison, N-cadherin heterozygous null mice expressing half the normal level of N-cadherin display normal ID structure, cardiac pathology, and cardiac function (Li et al., 2008). Further examination revealed a reduction in the size of the large GJ plaques and an increase of the dephosphorylated or less active isoform of Cx43 in the N-cad −/+ mice. The gap junction remodeling was accompanied by an increased susceptibility to induced arrhythmias in the N-cad −/+ compared to control littermates. Interestingly, the susceptibility to arrhythmias was similar in N-cad −/+ compared to Cx43 −/+ mice. The generation of N-cad −/+; Cx43 −/+ compound mutants showed a further decrease in gap junction plaque size and increased susceptibility to inducible arrhythmias in comparison to the single mutant mice demonstrating that these two genes function synergistically to regulate gap junction communication in the heart.
To date, no human CDH2 mutations have been identified in people suffering from heart disease. However, recent evidence suggests that N-cadherin and Cx43 expression may be generally affected in heart failure patients. Based on optical mapping data, end-stage non-ischemic human hearts (n=10) exhibit slower transmural activation compared with non-failing hearts (Glukhov et al., 2012). The conduction slowing was associated with decreased total Cx43, phosphorylated Cx43 isoform, as well as reduced co-localization with N-cadherin.
Functional overlap and distinct roles for β-catenin and plakoglobin
β-catenin and plakoglobin (PG) are members of the armadillo (arm) protein family (Zhurinsky et al., 2000). The central arm domain contains 13 imperfect repeats of 42 amino acids that mediate numerous protein-protein interactions. The central arm domain is the most conserved region of the two proteins with 83% amino acid similarity while the N- and C-terminal tails share 57% and 15% similarity, respectively. The relatively low homology between the terminal regions of these proteins likely contributes to the differences in binding partners and functional activity of these otherwise closely related arm family members. In addition to their adhesive roles as part of the junctional complex, these proteins regulate gene expression by interacting with members of the T-cell factor (TCF)/lymphoid enhancing-binding factor (LEF) family of transcription factors (Zhurinsky et al., 2000). In contrast to β-catenin, current opinions regarding the physiological significance of PG as a transcriptional regulator remain controversial. It has been proposed that PG functions primarily as a competitive inhibitor of β-catenin transcriptional activity by sequestering TCF/LEF. This review will focus on β-catenin and PG as both structural proteins of the ID as well as their roles in signal transduction in the heart. Evidence from animal models illustrates the functional interdependence of β-catenin and PG in cardiac homeostasis and disease.
β-catenin function in the heart
Although β-catenin interacts with N-cadherin, there is no evidence supporting an essential role for β-catenin as a structural protein in the heart. Cardiac-specific deletion of β-catenin in mice has revealed important signaling roles for β-catenin at different stages of cardiac development, however these lethal phenotypes appear to be independent of any myocardial cell adhesion defect (Qu et al., 2007, Piven et al., 2011). To overcome the requirement for β-catenin during cardiac morphogenesis, an inducible cardiac-specific β-catenin (β-cat) CKO was generated similar to the N-cad CKO model. Induced deletion of the β-catenin (Ctnnb1) gene in the adult heart has no apparent effect on ID structure, cardiac pathology, or cardiac function. The authors suggested that the lack of a structural defect in the β-cat CKO mice was due to replacement of β-catenin by PG in AJs (Zhou et al., 2007). This hypothesis was recently confirmed by generating a β-cat/PG double knockout (DKO) mouse model (Swope et al., 2012). In the absence of both arm proteins, the N-cadherin/catenin complex is no longer stable at the plasma membrane leading to disassembly of the ID structure and SCD of the β-cat/PG DKO mice.
β-catenin is a well-established downstream effector of the Wnt signaling cascade (Clevers and Nusse, 2012). The cytoplasmic pool of β-catenin is regulated by canonical Wnt signaling and ubiquitin-proteasome-dependent degradation. The targeting of β-catenin degradation is achieved through the phosphorylation of N-terminal serine residues by a multi-protein “destruction complex” containing glycogen synthase kinase 3β (GSK3β) and scaffold proteins adenomatous polyposis coli (APC) and axin. Activation of the Frizzle receptor by Wnt increases the stability of β-catenin and its cytosolic accumulation. β-catenin is then able to translocate into the nucleus where it can interact with TCF/LEF transcription factors and promote the transcription of specific genes involved in cellular growth, differentiation and cell fate.
Upon pathological stress, the heart reactivates several signaling pathways involved in cardiac development including Wnt/β-catenin signaling (Bergmann, 2010). Several groups have subjected β-catenin mutant mice to various stress or injury protocols in order to investigate the requirement for β-catenin in pathological remodeling (Table I). Following transverse aortic constriction (TAC) to induce pressure overload, β-catenin mice exhibit reduced Wnt target gene expression and the pathological hypertrophy response is blunted (Chen et al., 2006). Consistent with a transcriptional mechanism, cardiac-specific expression of dominant negative Lef1 also failed to exhibit hypertrophy following TAC. The role of β-catenin signaling in angiotensinII-induced hypertrophy appears to differ from those reported for TAC or ischemic injury (i.e. myocardial infarction) suggesting involvement of other signaling pathways (Baurand et al., 2007). Although β-catenin inhibition appears to be cardio-protective following injury, the mechanism behind increased cardiac function and reduced infarct size is controversial. A different group reported that loss of β-catenin results in enhancement of cardiac progenitor cell differentiation post injury as opposed to inhibition of hypertrophy (Zelarayan et al., 2008).
Plakoglobin function in the heart
Plakoglobin (PG, γ-catenin, JUP) was initially described as a component of multiple intercellular mechanical junctions (Cowin and Burke, 1996). PG is a unique ID protein as it binds to the cytoplasmic domain of both classical cadherins and desmosomal cadherins thus mediating linkage to the actin and intermediate filaments, respectively. Although PG shares many binding partners with β-catenin, its function outside the junctional complex is less well understood compared to β-catenin.
The first genetic mutation identified in AC patients was a homozygous 2 base pair deletion (2057del2) in the JUP gene causing a premature truncation of the PG protein (McKoy et al., 2000). The disease was originally identified in patients on the Greek island of Naxos hence it is referred to as “Naxos disease” (Protonotarios et al., 1986). In addition to heart problems, Naxos patients exhibit palmoplantar keratoderma and woolly hair. Despite the fact that JUP was the first AC mutation identified, JUP mutations are the least common (<1%) among all the desmosomal mutations identified in AC patients over the past 15 years (Fressart et al., 2010, van Tintelen et al., 2007). The relatively low mutation frequency compared to the other desmosomal genes supports a critical role for this multi-junctional protein in tissue homeostasis. Interestingly, PG is usually diminished at the ID of AC patients regardless of the desmosomal gene mutation making PG a potential diagnostic tool for AC in affected individuals (Asimaki et al., 2009).
Over the past several years, several groups have studied various animal models to understand the molecular mechanisms underlying PG dysfunction in the heart. Interestingly, both cardiac-restricted PG loss-of-function (LOF) and gain-of-function (GOF) studies have shown that altering PG expression can cause AC-like pathology in mice. Two groups recently reported that cardiac-specific deletion of the PG gene cause altered desmosome ultrastructure and decreased cardiac function as determined by echocardiography (Li et al., 2011a, Li et al., 2011b). Expression of AJ proteins is not affected by loss of PG presumably due to upregulation of β-catenin in the mutant hearts. PG CKO mice recapitulate many features of AC pathogenesis that include progressive loss of myocytes, extensive inflammatory infiltration, and replacement fibrosis. In contrast to human AC, adipocytes were not observed in the PG CKO hearts. A more severe phenotype including arrhythmias was observed in one of the two models where PG was deleted early in the perinatal period instead of the adult heart (Li et al., 2011a).
A hallmark of AC is the loss of cardiomyocytes that are replaced with fibroblasts and adipocytes. The molecular mechanism(s) underlying this unique fibro-fatty pathology highlight the dual function of these arm proteins as both structural components of mechanical junctions and transcriptional regulators. The canonical Wnt/β-catenin signaling is an important regulator of myogenesis versus adipogenesis (Ross et al., 2000). The Marian lab showed that DP knockdown caused translocation of PG to the nucleus of cardiomyocytes where it interferes with β-catenin/TCF transcriptional activity resulting in an adipogenic switch (Garcia-Gras et al., 2006). In a subsequent study, the same group performed genetic fate-mapping experiments to demonstrate that adipocytes in the DP CKO model originate from a cardiac progenitor cell population (Lombardi et al., 2009). The current LOF and GOF animal models have provided important insight into the pathogenesis of AC. However, the next generation murine models will require knock-in of specific human mutations to further investigate the molecular mechanism(s) responsible for this unique cardiac pathology.
α-catenin function in the heart
α-catenins are AJ proteins that directly link the cadherin cytoplasmic domain at the sarcolemma to the actin/tubulin cytoskeleton, and have been shown to act as tension transducers that translates mechanical stimuli into a chemical response (Twiss and de Rooij, 2013). In the heart, there are two α-catenins expressed: the ubiquitously expressed αE-catenin and the cardiac-restricted αT-catenin. Both α-catenin isoforms contain three vinculin homology domains, and share 57% amino acid identity (Janssens et al., 2001). The N-terminal domain of α-catenin binds β-catenin (or PG) while its C-terminal domain interacts with F-actin allowing the linkage of the classical cadherins with the actin cytoskeleton. α-catenin binds directly with actin-binding proteins that include vinculin, ZO-1, afadin, and EPLIN. α-catenin functions as a platform for F-actin remodeling machinery and is required for the mechanical coupling between cadherin and actomyosin (Twiss and de Rooij, 2013).
The cardiac-specific αE-catenin CKO model presents with progressive left ventricular dilatation associated with a thinning right ventricular anterior wall leading to a high susceptibility to cardiac rupture post-MI (Sheikh et al., 2006). Loss of αE-catenin did not affect the expression of junctional components located in the AJ, Des or GJ and no arrhythmias were reported in these mice. However, vinculin, a binding partner of αE-catenin, was decreased in the αE-catenin CKO heart. The relatively late onset of cardiomyopathy (>8 months of age) suggests αT-catenin may compensate, at least to some degree, for loss of αE-catenin. In another study, significant mortality was observed in αE-catenin heterozygous null mice post-MI (van den Borne et al., 2008).
αT-catenin is the newest member of the α-catenin family of proteins. Using yeast two-hybrid and co-immunoprecipitation, αT-catenin was shown to bind the desmosomal protein PKP2 (Goossens et al., 2007). By contrast, αE-catenin lacks PKP2 binding capacity. As demonstrated by immunoelectron microscopy αT-catenin and PKP2 localize in the area composita but not the Des (Figure 2). The unique ability of αT-catenin to interact with PKP2 provides a new paradigm for understanding the molecular integration of the junctional components including GJs and ion channels.
Recently, an αT-catenin KO mouse model confirmed the link between αT-catenin and PKP2 in the area composita and its essential role in cardiac function (Li et al., 2012). The AJ components discussed earlier are all required for embryonic development, however αT-catenin-null mice are viable and fertile. Loss of αT-catenin in the area composita leads to early onset of dilated cardiomyopathy, gap junction remodeling and an increased susceptibility to ventricular arrhythmia in the setting of ischemia/reperfusion injury. The expression and distribution of AJ and Des components are not affected in the αT-catenin KO heart, with the exception of PKP2. The more severe cardiac phenotype in the αT-catenin KO compared to the αE-catenin CKO model reveals a unique role for αT-catenin in cardiac homeostasis (Li et al., 2012). The disruption of the αT-catenin-PKP2 interaction may affect the spatial organization of additional junctional components located in the area composita. The Delmar lab has shown that PKP2 interacts with Cx43 as well as the sodium channel Nav1.5 in cardiomyocytes (Oxford et al., 2007, Sato et al., 2011, Sato et al., 2009). Further characterization of the αT-catenin KO model is warranted to determine the molecular mechanism(s) responsible for arrhythmogenesis in these animals.
Recently, two mutations in the human αT-catenin (CTNNA3) gene were identified in AC patients suggesting that perturbation of the area composita may play a critical role in the etiology of this disease (van Hengel et al., 2012). One αT-catenin mutation found in this screen of 76 AC patients inhibits the interaction between αT-catenin and β-catenin (or PG) leading to a mislocalization of αT-catenin into the cytoplasm of HL1 myocardial cells. The second αT-catenin mutation increases dimerization of αT-catenin, which might create aggresomes and disturb its function. Animal models will be necessary to elucidate the consequences of these mutations in the working myocardium.
Conclusions
The paramount importance of N-cadherin in the regulation of ID structure and function in the normal heart are well established. It is likely that the N-cadherin/catenin complex and its associated cytoskeleton will be altered during cardiac remodeling and progression to heart failure. Loss-of-function studies have been informative, however new approaches will be necessary to investigate changes in N-cadherin function during disease progression. The N-cadherin haploinsufficiency model may be useful in this regard, since the mice are healthy and only show signs of arrhythmia after programmed stimulation.
The recent discovery of a novel, exclusive type of ‘hybrid adhering junction’ or ‘area composita’ in the mammalian heart has important implications for our understanding of the molecular mechanisms underlying AC as well as other forms of heart disease. The exact mechanism(s) by which adhesion proteins influence connexon trafficking, channel assembly and/or stability at the ID cannot be fully understood until the functional interdependence of the junctional proteins is known. A ‘snap shot’ of the junctional protein interactome illustrates the complexity of the protein-protein interactions that occur at the ID (Figure 4). Most proteins such as Cx43 are capable of interacting with multiple proteins and thus have the potential to form large macromolecular complexes. Recent data indicate that oxidative stress can disrupt EB1/Cx43 interactions critical for connexon transport to the ID (Smyth et al., 2010). It will be of interest to examine the effects of stress on other junction protein interactions illustrated in this interactome.
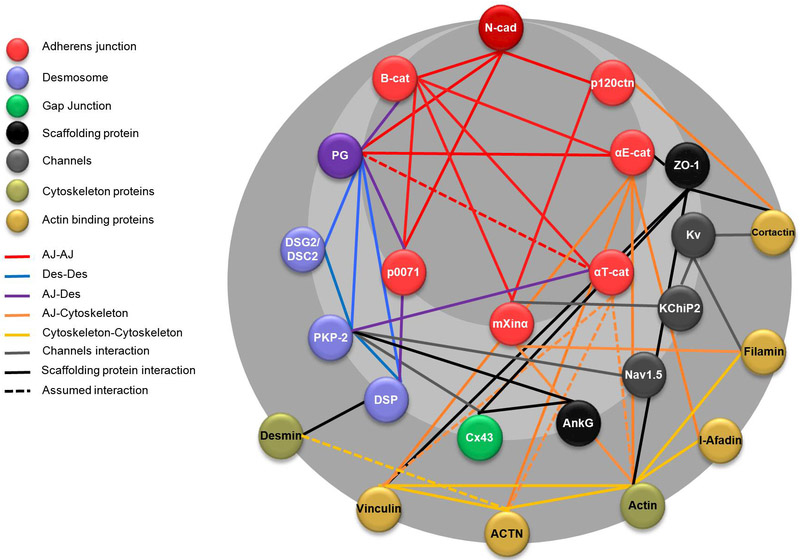
Figure 4: Junctional proteins interactome.
The intercalated disc protein-protein interaction network is based on STRING v9.1 analysis (http://string-db.org/) using the default settings (Franceschini et al., 2013). Black lines represent direct interactions, while dotted lines represent assumed interactions based on studies of the structures homology. (Ncad: N-cadherin; B-cat: β-catenin; p120ctn: p120-catenin; PG: Plakoglobin; PKP2: Plakophillin-2; DSG2: Desmoglein-2; DSC2: Desmocollin-2; DSP: Desmoplakin; Cx43: Connexin-43; AnkG: Ankyrin G; Kv: Potassium channels; kChiP2: Kv channel-interacting protein 2; ACTN: Actinin).
Finally, AC may be considered a disease of the area composita where the AJ and Des join forces to create a unique hybrid junction found exclusively in the four-chamber heart.
Acknowledgments
We thank Karsten Peppel for comments on the manuscript. Work in the authors’ laboratory is supported by the National Institutes of Health (HL111788, CA176097 to GR) and American Heart Association (AHA) Grant-in-Aid (GRNT12030343 to GR).
Footnotes
Conflict of interest
None
References
- ANGST BD, KHAN LU, SEVERS NJ, WHITELY K, ROTHERY S, THOMPSON RP, MAGEE AI & GOURDIE RG 1997. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res, 80, 88–94. [DOI] [PubMed] [Google Scholar]
- ASIMAKI A, TANDRI H, HUANG H, HALUSHKA MK, GAUTAM S, BASSO C, THIENE G, TSATSOPOULOU A, PROTONOTARIOS N, MCKENNA WJ, CALKINS H & SAFFITZ JE 2009. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med, 360, 1075–84. [DOI] [PubMed] [Google Scholar]
- BASSO C, BAUCE B, CORRADO D & THIENE G 2012. Pathophysiology of arrhythmogenic cardiomyopathy. Nat Rev Cardiol, 9, 223–33. [DOI] [PubMed] [Google Scholar]
- BAUCE B, NAVA A, BEFFAGNA G, BASSO C, LORENZON A, SMANIOTTO G, DE BORTOLI M, RIGATO I, MAZZOTTI E, STERIOTIS A, MARRA MP, TOWBIN JA, THIENE G, DANIELI GA & RAMPAZZO A 2010. Multiple mutations in desmosomal proteins encoding genes in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm, 7, 22–9. [DOI] [PubMed] [Google Scholar]
- BAURAND A, ZELARAYAN L, BETNEY R, GEHRKE C, DUNGER S, NOACK C, BUSJAHN A, HUELSKEN J, TAKETO MM, BIRCHMEIER W, DIETZ R & BERGMANN MW 2007. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res, 100, 1353–62. [DOI] [PubMed] [Google Scholar]
- BERGMANN MW 2010. WNT signaling in adult cardiac hypertrophy and remodeling: lessons learned from cardiac development. Circ Res, 107, 1198–208. [DOI] [PubMed] [Google Scholar]
- BORRMANN CM, GRUND C, KUHN C, HOFMANN I, PIEPERHOFF S & FRANKE WW 2006. The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur J Cell Biol, 85, 469–85. [DOI] [PubMed] [Google Scholar]
- CERRONE M, NOORMAN M, LIN X, CHKOURKO H, LIANG FX, VAN DER NAGEL R, HUND T, BIRCHMEIER W, MOHLER P, VAN VEEN TA, VAN RIJEN HV & DELMAR M 2012. Sodium current deficit and arrhythmogenesis in a murine model of plakophilin-2 haploinsufficiency. Cardiovasc Res, 95, 460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN X, SHEVTSOV SP, HSICH E, CUI L, HAQ S, ARONOVITZ M, KERKELA R, MOLKENTIN JD, LIAO R, SALOMON RN, PATTEN R & FORCE T 2006. The beta-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol Cell Biol, 26, 4462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG L, YUNG A, COVARRUBIAS M & RADICE GL 2011. Cortactin is required for N-cadherin regulation of Kv1.5 channel function. J Biol Chem, 286, 20478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEVERS H & NUSSE R 2012. Wnt/beta-catenin signaling and disease. Cell, 149, 1192–205. [DOI] [PubMed] [Google Scholar]
- COWIN P & BURKE B 1996. Cytoskeleton-membrane interactions. Curr Opin Cell Biol, 8, 56–65. [DOI] [PubMed] [Google Scholar]
- DEN HAAN AD, TAN BY, ZIKUSOKA MN, LLADO LI, JAIN R, DALY A, TICHNELL C, JAMES C, AMAT-ALARCON N, ABRAHAM T, RUSSELL SD, BLUEMKE DA, CALKINS H, DALAL D & JUDGE DP 2009. Comprehensive desmosome mutation analysis in north americans with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Cardiovasc Genet, 2, 428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES MS & SPERELAKIS N 1985. Intercalated discs of mammalian heart: a review of structure and function. Tissue Cell, 17, 605–48. [DOI] [PubMed] [Google Scholar]
- FRANCESCHINI A, SZKLARCZYK D, FRANKILD S, KUHN M, SIMONOVIC M, ROTH A, LIN J, MINGUEZ P, BORK P, VON MERING C & JENSEN LJ 2013. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res, 41, D808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKE WW, BORRMANN CM, GRUND C & PIEPERHOFF S 2006. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol, 85, 69–82. [DOI] [PubMed] [Google Scholar]
- FRESSART V, DUTHOIT G, DONAL E, PROBST V, DEHARO JC, CHEVALIER P, KLUG D, DUBOURG O, DELACRETAZ E, COSNAY P, SCANU P, EXTRAMIANA F, KELLER D, HIDDEN-LUCET F, SIMON F, BESSIRARD V, ROUX-BUISSON N, HEBERT JL, AZARINE A, CASSET-SENON D, ROUZET F, LECARPENTIER Y, FONTAINE G, COIRAULT C, FRANK R, HAINQUE B & CHARRON P 2010. Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: spectrum of mutations and clinical impact in practice. Europace, 12, 861–8. [DOI] [PubMed] [Google Scholar]
- GARCIA-GRAS E, LOMBARDI R, GIOCONDO MJ, WILLERSON JT, SCHNEIDER MD, KHOURY DS & MARIAN AJ 2006. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest, 116, 2012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEPMANS BN & MOOLENAAR WH 1998. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol, 8, 931–4. [DOI] [PubMed] [Google Scholar]
- GLUKHOV AV, FEDOROV VV, KALISH PW, RAVIKUMAR VK, LOU Q, JANKS D, SCHUESSLER RB, MOAZAMI N & EFIMOV IR 2012. Conduction remodeling in human end-stage nonischemic left ventricular cardiomyopathy. Circulation, 125, 1835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOOSSENS S, JANSSENS B, BONNE S, DE RYCKE R, BRAET F, VAN HENGEL J & VAN ROY F 2007. A unique and specific interaction between alphaT-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs. J Cell Sci, 120, 2126–36. [DOI] [PubMed] [Google Scholar]
- GREEN KJ, GETSIOS S, TROYANOVSKY S & GODSEL LM 2010. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol, 2, a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTSTEIN DE, MORLEY GE, TAMADDON H, VAIDYA D, SCHNEIDER MD, CHEN J, CHIEN KR, STUHLMANN H & FISHMAN GI 2001. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res, 88, 333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTIG CM, BUTZ S, KOCH S, EPPENBERGER-EBERHARDT M, KEMLER R & EPPENBERGER HM 1996. N-cadherin in adult rat cardiomyocytes in culture. II. Spatio-temporal appearance of proteins involved in cell-cell contact and communication. Formation of two distinct N-cadherin/catenin complexes. J Cell Sci, 109 ( Pt 1), 11–20. [DOI] [PubMed] [Google Scholar]
- HIRSCHY A, SCHATZMANN F, EHLER E & PERRIARD JC 2006. Establishment of cardiac cytoarchitecture in the developing mouse heart. Dev Biol, 289, 430–41. [DOI] [PubMed] [Google Scholar]
- HUNTER AW, BARKER RJ, ZHU C & GOURDIE RG 2005. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell, 16, 5686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSENS B, GOOSSENS S, STAES K, GILBERT B, VAN HENGEL J, COLPAERT C, BRUYNEEL E, MAREEL M & VAN ROY F 2001. alphaT-catenin: a novel tissue-specific beta-catenin-binding protein mediating strong cell-cell adhesion. J Cell Sci, 114, 3177–88. [DOI] [PubMed] [Google Scholar]
- KOSTETSKII I, LI J, XIONG Y, ZHOU R, FERRARI VA, PATEL VV, MOLKENTIN JD & RADICE GL 2005. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res, 96, 346–54. [DOI] [PubMed] [Google Scholar]
- KOSTIN S, RIEGER M, DAMMER S, HEIN S, RICHTER M, KLOVEKORN WP, BAUER EP & SCHAPER J 2003. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem, 242, 135–44. [PubMed] [Google Scholar]
- KWON YS, PARK TI, CHO Y, BAE MH & KIM S 2013. Clinical usefulness of immunohistochemistry for plakoglobin, N-cadherin, and connexin-43 in the diagnosis of arrhythmogenic right ventricular cardiomyopathy. Int J Clin Exp Pathol, 6, 2928–35. [PMC free article] [PubMed] [Google Scholar]
- LI D, LIU Y, MARUYAMA M, ZHU W, CHEN H, ZHANG W, REUTER S, LIN SF, HANELINE LS, FIELD LJ, CHEN PS & SHOU W 2011. a. Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy. Hum Mol Genet, 20, 4582–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J, GOOSSENS S, VAN HENGEL J, GAO E, CHENG L, TYBERGHEIN K, SHANG X, DE RYCKE R, VAN ROY F & RADICE GL 2012. Loss of alphaT-catenin alters the hybrid adhering junctions in the heart and leads to dilated cardiomyopathy and ventricular arrhythmia following acute ischemia. J Cell Sci, 125, 1058–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J, LEVIN MD, XIONG Y, PETRENKO N, PATEL VV & RADICE GL 2008. N-cadherin haploinsufficiency affects cardiac gap junctions and arrhythmic susceptibility. J Mol Cell Cardiol, 44, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J, PATEL VV, KOSTETSKII I, XIONG Y, CHU AF, JACOBSON JT, YU C, MORLEY GE, MOLKENTIN JD & RADICE GL 2005. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circ Res, 97, 474–81. [DOI] [PubMed] [Google Scholar]
- LI J, SWOPE D, RAESS N, CHENG L, MULLER EJ & RADICE GL 2011b. Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling. Mol Cell Biol, 31, 1134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMBARDI R, DONG J, RODRIGUEZ G, BELL A, LEUNG TK, SCHWARTZ RJ, WILLERSON JT, BRUGADA R & MARIAN AJ 2009. Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res, 104, 1076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO Y, FERREIRA-CORNWELL M, BALDWIN H, KOSTETSKII I, LENOX J, LIEBERMAN M & RADICE G 2001. Rescuing the N-cadherin knockout by cardiac-specific expression of N- or E-cadherin. Development, 128, 459–69. [DOI] [PubMed] [Google Scholar]
- LUO Y, HIGH FA, EPSTEIN JA & RADICE GL 2006. N-cadherin is required for neural crest remodeling of the cardiac outflow tract. Dev Biol, 299, 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO Y, KOSTETSKII I & RADICE GL 2005. N-cadherin is not essential for limb mesenchymal chondrogenesis. Dev Dyn, 232, 336–44. [DOI] [PubMed] [Google Scholar]
- LUO Y & RADICE GL 2003. Cadherin-mediated adhesion is essential for myofibril continuity across the plasma membrane but not for assembly of the contractile apparatus. J Cell Sci, 116, 1471–9. [DOI] [PubMed] [Google Scholar]
- MATSUSHITA T, OYAMADA M, FUJIMOTO K, YASUDA Y, MASUDA S, WADA Y, OKA T & TAKAMATSU T 1999. Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res, 85, 1046–55. [DOI] [PubMed] [Google Scholar]
- MCKOY G, PROTONOTARIOS N, CROSBY A, TSATSOPOULOU A, ANASTASAKIS A, COONAR A, NORMAN M, BABOONIAN C, JEFFERY S & MCKENNA WJ 2000. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet, 355, 2119–24. [DOI] [PubMed] [Google Scholar]
- MENG W & TAKEICHI M 2009. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol, 1, a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OXFORD EM, MUSA H, MAASS K, COOMBS W, TAFFET SM & DELMAR M 2007. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res, 101, 703–11. [DOI] [PubMed] [Google Scholar]
- PIEPERHOFF S & FRANKE WW 2007. The area composita of adhering junctions connecting heart muscle cells of vertebrates - IV: coalescence and amalgamation of desmosomal and adhaerens junction components - late processes in mammalian heart development. Eur J Cell Biol, 86, 377–91. [DOI] [PubMed] [Google Scholar]
- PIEPERHOFF S & FRANKE WW 2008. The area composita of adhering junctions connecting heart muscle cells of vertebrates. VI. Different precursor structures in non-mammalian species. Eur J Cell Biol, 87, 413–30. [DOI] [PubMed] [Google Scholar]
- PIVEN OO, KOSTETSKII IE, MACEWICZ LL, KOLOMIETS YM, RADICE GL & LUKASH LL 2011. Requirement for N-cadherin-catenin complex in heart development. Exp Biol Med (Maywood), 236, 816–22. [DOI] [PubMed] [Google Scholar]
- PROTONOTARIOS N, TSATSOPOULOU A, PATSOURAKOS P, ALEXOPOULOS D, GEZERLIS P, SIMITSIS S & SCAMPARDONIS G 1986. Cardiac abnormalities in familial palmoplantar keratosis. Br Heart J, 56, 321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QU J, ZHOU J, YI XP, DONG B, ZHENG H, MILLER LM, WANG X, SCHNEIDER MD & LI F 2007. Cardiac-specific haploinsufficiency of beta-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. J Mol Cell Cardiol, 43, 319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADICE GL, RAYBURN H, MATSUNAMI H, KNUDSEN KA, TAKEICHI M & HYNES RO 1997. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol, 181, 64–78. [DOI] [PubMed] [Google Scholar]
- REMO BF, GIOVANNONE S & FISHMAN GI 2012. Connexin43 cardiac gap junction remodeling: lessons from genetically engineered murine models. J Membr Biol, 245, 275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS SE, HEMATI N, LONGO KA, BENNETT CN, LUCAS PC, ERICKSON RL & MACDOUGALD OA 2000. Inhibition of adipogenesis by Wnt signaling. Science, 289, 950–3. [DOI] [PubMed] [Google Scholar]
- SAFFITZ JE 2009. Arrhythmogenic cardiomyopathy and abnormalities of cell-to-cell coupling. Heart Rhythm, 6, S62–5. [DOI] [PubMed] [Google Scholar]
- SATO PY, COOMBS W, LIN X, NEKRASOVA O, GREEN KJ, ISOM LL, TAFFET SM & DELMAR M 2011. Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circ Res, 109, 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO PY, MUSA H, COOMBS W, GUERRERO-SERNA G, PATINO GA, TAFFET SM, ISOM LL & DELMAR M 2009. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res, 105, 523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAPER J, FROEDE R, HEIN S, BUCK A, HASHIZUME H, SPEISER B, FRIEDL A & BLEESE N 1991. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation, 83, 504–14. [DOI] [PubMed] [Google Scholar]
- SEVERS NJ, BRUCE AF, DUPONT E & ROTHERY S 2008. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res, 80, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW RM, FAY AJ, PUTHENVEEDU MA, VON ZASTROW M, JAN YN & JAN LY 2007. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell, 128, 547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEIKH F, CHEN Y, LIANG X, HIRSCHY A, STENBIT AE, GU Y, DALTON ND, YAJIMA T, LU Y, KNOWLTON KU, PETERSON KL, PERRIARD JC & CHEN J 2006. alpha-E-catenin inactivation disrupts the cardiomyocyte adherens junction, resulting in cardiomyopathy and susceptibility to wall rupture. Circulation, 114, 1046–55. [DOI] [PubMed] [Google Scholar]
- SMYTH JW, HONG TT, GAO D, VOGAN JM, JENSEN BC, FONG TS, SIMPSON PC, STAINIER DY, CHI NC & SHAW RM 2010. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest, 120, 266–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOSINSKY GE, SOLAN JL, GAIETTA GM, NGAN L, LEE GJ, MACKEY MR & LAMPE PD 2007. The C-terminus of connexin43 adopts different conformations in the Golgi and gap junction as detected with structure-specific antibodies. Biochem J, 408, 375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWOPE D, CHENG L, GAO E, LI J & RADICE GL 2012. Loss of cadherin-binding proteins beta-catenin and plakoglobin in the heart leads to gap junction remodeling and arrhythmogenesis. Mol Cell Biol, 32, 1056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAVORA F, ZHANG M, CRESSWELL N, LI L, FOWLER D, FRANCO M & BURKE A 2013. Quantitative Immunohistochemistry of Desmosomal Proteins (Plakoglobin, Desmoplakin and Plakophilin), Connexin-43, and N-cadherin in Arrhythmogenic Cardiomyopathy: An Autopsy Study. Open Cardiovasc Med J, 7, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASELLI GF, BEUCKELMANN DJ, CALKINS HG, BERGER RD, KESSLER PD, LAWRENCE JH, KASS D, FELDMAN AM & MARBAN E 1994. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation, 90, 2534–9. [DOI] [PubMed] [Google Scholar]
- TWISS F & DE ROOIJ J 2013. Cadherin mechanotransduction in tissue remodeling. Cell Mol Life Sci, 70, 4101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEN BORNE SW, NARULA J, VONCKEN JW, LIJNEN PM, VERVOORT-PETERS HT, DAHLMANS VE, SMITS JF, DAEMEN MJ & BLANKESTEIJN WM 2008. Defective intercellular adhesion complex in myocardium predisposes to infarct rupture in humans. J Am Coll Cardiol, 51, 2184–92. [DOI] [PubMed] [Google Scholar]
- VAN HENGEL J, CALORE M, BAUCE B, DAZZO E, MAZZOTTI E, DE BORTOLI M, LORENZON A, LI MURA IE, BEFFAGNA G, RIGATO I, VLEESCHOUWERS M, TYBERGHEIN K, HULPIAU P, VAN HAMME E, ZAGLIA T, CORRADO D, BASSO C, THIENE G, DALIENTO L, NAVA A, VAN ROY F & RAMPAZZO A 2012. Mutations in the area composita protein alphaT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy. Eur Heart J, 34, 201–10. [DOI] [PubMed] [Google Scholar]
- VAN TINTELEN JP, HOFSTRA RM, WIESFELD AC, VAN DEN BERG MP, HAUER RN & JONGBLOED JD 2007. Molecular genetics of arrhythmogenic right ventricular cardiomyopathy: emerging horizon? Curr Opin Cardiol, 22, 185–92. [DOI] [PubMed] [Google Scholar]
- WANG X & GERDES AM 1999. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. Intercalated disc remodeling. J Mol Cell Cardiol, 31, 333–43. [DOI] [PubMed] [Google Scholar]
- XU T, YANG Z, VATTA M, RAMPAZZO A, BEFFAGNA G, PILICHOU K, SCHERER SE, SAFFITZ J, KRAVITZ J, ZAREBA W, DANIELI GA, LORENZON A, NAVA A, BAUCE B, THIENE G, BASSO C, CALKINS H, GEAR K, MARCUS F, TOWBIN JA & MULTIDISCIPLINARY STUDY OF RIGHT VENTRICULAR DYSPLASIA, I. 2010. Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol, 55, 587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELARAYAN LC, NOACK C, SEKKALI B, KMECOVA J, GEHRKE C, RENGER A, ZAFIRIOU MP, VAN DER NAGEL R, DIETZ R, DE WINDT LJ, BALLIGAND JL & BERGMANN MW 2008. Beta-Catenin downregulation attenuates ischemic cardiac remodeling through enhanced resident precursor cell differentiation. Proc Natl Acad Sci U S A, 105, 19762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEMLJIC-HARPF AE, GODOY JC, PLATOSHYN O, ASFAW EK, BUSIJA AR, DOMENIGHETTI AA & ROSS RS 2014. Vinculin directly binds zonula occludens-1 and is essential for stabilizing connexin-43-containing gap junctions in cardiac myocytes. J Cell Sci, 127, 1104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU J, QU J, YI XP, GRABER K, HUBER L, WANG X, GERDES AM & LI F 2007. Upregulation of gamma-catenin compensates for the loss of beta-catenin in adult cardiomyocytes. Am J Physiol Heart Circ Physiol, 292, H270–6. [DOI] [PubMed] [Google Scholar]
- ZHUANG J, YAMADA KA, SAFFITZ JE & KLEBER AG 2000. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res, 87, 316–22. [DOI] [PubMed] [Google Scholar]
- ZHURINSKY J, SHTUTMAN M & BEN-ZE’EV A 2000. Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci, 113 ( Pt 18), 3127–39. [DOI] [PubMed] [Google Scholar]