Abstract
Background & Aims:
Abnormal delivery of bile acids (BAs) to the colon, due to disease or therapy, causes constipation or diarrhea by unknown mechanisms. The G protein-coupled BA receptor TGR5 (or GPBAR1) is expressed by enteric neurons and endocrine cells, which regulate motility and secretion.
Methods:
We analyzed gastrointestinal and colon transit, and defecation frequency and water content, in wild-type, knockout and transgenic mice (trg5-wt, tgr5-ko and tgr5-tg, respectively). We analyzed colon tissues for contractility, peristalsis, and transmitter release.
Results:
Deoxycholic acid inhibited contractility of colonic longitudinal muscle from tgr5-wt but not tgr5-ko mice. Application of deoxycholic acid, lithocholic acid, or oleanolic acid (a selective agonist of TGR5) to the mucosa of tgr5-wt mice caused oral contraction and caudal relaxation, indicating peristalsis. BAs stimulated release of the peristaltic transmitters 5-hydroxytryptamine and calcitonin gene-related peptide; antagonists of these transmitters suppressed BA-induced peristalsis, consistent with TGR5 localization to enterochromaffin cells and intrinsic primary afferent neurons. tgr5-ko mice did not undergo peristalsis or transmitter release in response to BAs. Mechanically induced peristalsis and transmitter release were not affected by deletion of tgr5. Whole-gut transit was 1.4-fold slower in tgr5-ko than tgr5-wt or tgr5-tg mice, whereas colonic transit was 2.2-fold faster in tgr5-tg mice. Defecation frequency was reduced 2.6-fold in tgr5-ko and increased 1.4-fold in tgr5-tg mice, compared to tgr5-wt mice. Water content in stool was lower (37%) in tgr5-ko than tgr5-tg (58%) or tgr5-wt mice (62%).
Conclusions:
The receptor TGR5 mediates the effects of BAs on colonic motility; TGR5 deficiency causes constipation in mice. These findings might mediate the long-known laxative properties of BAs; TGR5 might be a therapeutic target for digestive diseases.
Keywords: digestion, mouse model, intestine, diarrhea
INTRODUCTION
In addition to their role in the digestion and absorption of dietary fat, bile acids (BAs) control intestinal motility and secretion, and abnormal BA delivery to the intestine due to disease or therapy results in defects in intestinal functions 1, 2. The mechanisms of these well recognized patho-physiological actions of BAs are unknown. The primary bile acids, cholic and chenodeoxycholic acid, are synthesized from cholesterol in hepatocytes, secreted into canaliculi and stored in the gallbladder. After secretion into the intestine, primary BAs are actively absorbed in the ileum. The small amounts of primary BAs that normally reach the colon are deconjugated and dehydroxylated by bacteria to form the secondary BAs deoxycholic acid (DCA) and lithocholic acid (LCA), which are passively absorbed. Since BAs are secreted episodically and are efficiently absorbed via the enterohepatic circulation, BA concentrations in the systemic and portal circulation fluctuate with feeding 3.
Luminal BAs exert region-specific actions in the intestine. They inhibit motility of the small intestine, which may contribute to the “ileal brake” that slows transit to allow efficient absorption 4, 5. In contrast, BAs stimulate motility in the large intestine 6, and their effects on colonic transit are related to their prosecretory actions 7, 8. Disease- or therapy-related alterations in BA concentrations in the intestine have a marked impact on digestive functions. Decreased colonic delivery of free BAs as a result of cholestatic disease or after treatment of lipid disorders with BA sequestrants results in constipation 9, 10. Conversely, inflammatory bowel disease or ileal resection lead to impaired ileal BA absorption and increased colonic delivery, which can cause diarrhea 11. Many (20%) cholecystectomy patients develop diarrhea due to continuous delivery of BAs into the intestine 12. These effects of BAs have therapeutic implications. The ingestion of bile has been used to alleviate constipation for millennia 13, and drugs that target the ileal BA transporter can ameliorate constipation by increasing the colonic delivery of BAs 14 The mechanisms by which luminal BAs affect intestinal functions are unknown.
Although the cell types that mediate these actions of BAs have not been identified, studies using neurotoxins and neurotransmitter antagonists suggest that BAs control motility and secretion through effects on the enteric nervous system 15. Endocrine and paracrine mechanisms may also contribute, since DCA acts on enterochromaffin (EC) cells to stimulate release of 5-hydroxytryptamine (5-HT), a major regulator of secretion and motility 16. BAs also induce release of glucagon-like peptide-1 17, an incretin and mediator of the ileal brake 18. How BAs regulate enteric neurons and enteroendocrine cells is unknown.
We report that a G protein-coupled receptor TGR5 (GPR130, GpBAR1) that is expressed by EC cells and myenteric neurons mediates the effects of BAs on intestinal motility. BAs exert hormonal-like effects by activating nuclear and plasma membrane receptors 19. Nuclear receptors mediate many of the genomic effects of BAs. TGR5 is a widely distributed plasma membrane BA receptor 20,21 that controls energy metabolism 22, glucose homeostasis 17, 23, bile composition/secretion 24–27, and inflammation 20, 28, 29. We observed that TGR5 is expressed by >50% of enteric neurons 30. We now report that TGR5 mediates the effects of BAs on peristalsis, an evolutionarily conserved reflex that is essential for normal digestion, and that altered TGR5 expression has a major impact on intestinal transit and defecation. We have thus identified the elusive mechanism that underlies the patho-physiological actions of BAs in the intestine that have been recognized for millennia.
MATERIALS AND METHODS
Mice.
The generation of tgr5-ko, tgr5-tg and tgr5-wt mice in a C57BL/6 background has been described 23. C57BL/6 mice were from Charles River (Wilmington, MA). Mice were killed by sodium pentobarbital (200 mg.kg−1, i.p.) or CO2 inhalation and bilateral thoracotomy. Institutional Animal Care and Use Committees approved all procedures.
Contractility.
Full thickness segments (1 cm) of proximal colon were mounted in organ baths in physiological saline solution under 1 g tension. Longitudinal contractions were recorded using isotonic transducers, and data were processed and analyzed as described 30. Tissues were equilibrated for 30 min, and challenged with carbachol (1 μΜ) to assess viability. Tissues were washed and challenged with DCA (100 μΜ), UDCA (100 μΜ) or vehicle (0.1% ethanol or distilled H2O). The mean amplitude of the basal tension and the frequency of phasic contractions were determined 5 min before and after challenge, and results are normalized to basal values.
Peristalsis.
Peristalsis was examined using a flat sheet preparation of proximal colon 31, 33. A 5- cm segment of colon was opened and pinned mucosal side up in a tissue bath. The segment was divided into three compartments by vertical partitions and 1 ml of a Krebs’-bicarbonate medium was added to each compartment. The mucosa of the central compartment was stimulated by application of DCA, LCA, OA (1–100 μΜ) or vehicle or by stroking with a fine brush (2–8 strokes, 1 stroke.s−1). Ascending contraction of circular muscle was measured in the orad peripheral compartment and descending relaxation was measured in the caudad peripheral compartment using force-displacement transducers attached to the muscle layers. Results are expressed as grams force above or below baseline tone. In some experiments, GR113808 (1 μΜ), CGRP8–37 (10 μΜ) or vehicle (control) were added the central compartment 10 min before mucosal application of BAs 31.
Transmitter release.
Transmitter release into the central compartment was measured using a flat sheet preparation of distal colon. For measurement of neuropeptide and BDNF release, the medium contained bovine serum albumin (0.1%), amastatin (10 μM) and phosphoramidon (1 μM). For measurement of 5-HT release, the medium contained pargyline (10 μΜ). The mucosa of the central compartment was stimulated by application of DCA, OA or vehicle for 15 min, and medium was collected for immunoassays of CGRP, 5-HT and BDNF 31,33,34. Results are expressed as ng.g−1 wet tissue.
Gastrointestinal transit.
Mice were fasted overnight with free access to water. Evans blue (5%) and methyl cellulose (1.5%) solution was administered by gavage (100 μ1). The time for expulsion of the first blue pellet was determined.
Colonic transit.
Mice were fasted overnight with free access to water. Colonic transit was measured using a bead expulsion test as described 35. A glass bead (3 mm diameter) was inserted into the colon (2 cm). The time until bead expulsion was measured.
Defecation frequency and water content.
Freely feeding mice were observed for 2 hr. and the frequency of pellet expulsion was determined. Fecal water content was measured by comparing the weight of the pellets at the end of the experiment and after drying (24 h, 37°C).
Immunofluorescence.
Whole mounts and frozen sections prepared from paraformaldehyde-fixed tissues were processed for indirect immunofluorescence and confocal microscopy as described 30. Tissues were incubated with primary antibodies (overnight or 48 h, 4°C): rabbit anti-TGR5 P87/88 30 (1:200), NLS1937 (Novus Biologicals, Littleton, CO) (1:5,000); chicken anti-NFM (Gene Tex Inc., Irvine, CA) (1:500); sheep anti-CGRP (1:1,000); goat anti-5-HT #20079 (Immunostar, Hudson, WI, UK) (1:1,000).
Statistical analysis.
Results are expressed as mean±SEM and were compared by Student’s t-test (2 comparisons) or ANOVA and Student-Newman-Keuls test (multiple comparisons). P<0.05 was considered significant.
RESULTS
BAs regulate contractility of colonic muscle via TGR5.
DCA is a TGR5 agonist 21 that inhibits spontaneous phasic contractions of longitudinal muscle of the mouse proximal colon by a neurogenic, nitrergic mechanism 30. Consistent with this observation, DCA (100 μΜ) inhibited spontaneous phasic contractions of longitudinal muscle of isolated proximal colon from tgr5-wild type (wt) mice, inhibiting both the frequency of contractions and the muscle tension (fold-basal (1.0): frequency (0.25±0.05; tension, 0.78±0.05) (Fig. 1A-C). In marked contrast, DCA had no effect on spontaneous contractility of the colon from tgr5-knockout (ko) mice (fold-basal: frequency (0.91±0.05; tension, 0.92±0.02; P<0.001 frequency, P<0.05 tension to tgr5-wt). UDCA (100 μΜ), a weak agonist of TGR5 that retains the detergent and irritant properties of BAs 21, did not affect contractility of the colon from tgr5-wt mice (Fig. 1A). Under basal conditions, the frequency of spontaneous contractions was higher in tgr5-wt (contractions/min, 11.67±2.08) than in tgr5-ko (7.33±1.48) mice, although the difference was not significant (P=0.12, n=6) (tension was set to 1 g). The response to carbachol was similar (1 μΜ carbachol, 3 min: tension, fold-basal, tgr5-wt, 1.44±0.08; tgr5-ko, 1.46±0.06, n=6; frequency could not be determined). Thus, the actions of DCA on contractility of colonic longitudinal muscle in the mouse require TGR5 expression.
Figure 1. TGR5-dependent regulation of colonic contractility.
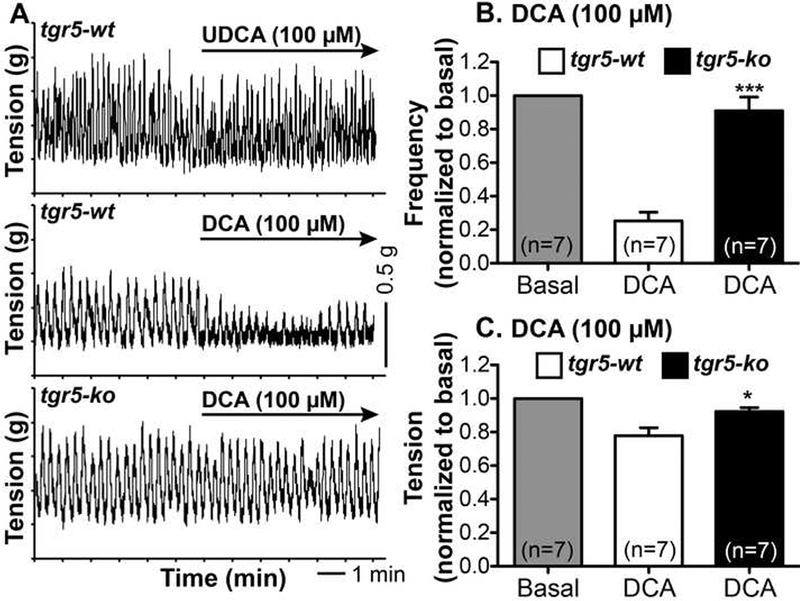
Recordings were made of spontaneous phasic contractions of longitudinal muscle of isolated proximal colon. A. Representative recordings from tgr5-wt and tgr5-ko mice. In tgr5-wt mice, UDCA (100 μΜ) had no effect, whereas DCA (100 μΜ) immediately inhibited spontaneous phasic contractions. In tgr5-ko mice, DCA was inactive. B, C. Mean results of the effects of DCA on frequency (B) and tension (C) normalized to basal values (1.0). DCA reduced the frequency and tension in tgr5-wt but not in tgr5-ko mice. * P< 0.05, *** P<0.001 to tgr5-wt.
BAs promote peristaltic contractions of the colon via TGR5.
A flat sheet preparation of mouse colon divided into three compartments allows assessment of peristaltic contractions of the oral and caudal compartments after application of stimulants to the mucosal side of the central compartment 31. In a flat sheet preparation of proximal colon from tgr5-wt mice, application of DCA (1, 10, 100 μΜ) to the mucosa of the central compartment initiated an immediate and concentration-dependent contraction of the oral compartment and relaxation of the caudal compartment (Fig. 2A, B), consistent with activation of the peristaltic reflex, which was also stimulated by mucosal stroking (Fig. 2C) 31. The effect of the maximal concentration of DCA (100 μΜ) was slightly less than that elicited by maximal mucosal stroking (83.6±6.9% of 8-stroke value for ascending contraction, 61.5±4.1% for descending relaxation). Oleanolic acid (OA, 100 μΜ), a TGR5-selective agonist 36, had a similar effect to DCA (63.6±7.2% of 8-stroke response for ascending contraction, 64.1±6.2% for descending relaxation). In tissue from tgr5-ko mice, these effects were absent (1, 10 μΜ DCA; 100 μΜ OA) or markedly blunted (100 μΜ DCA) (Fig. 2A, B). In contrast, the effects of mucosal stroking on oral contraction and caudal relaxation were the same in tissues from tgr5-wt and tgr5-ko mice (Fig. 2C). Thus, DCA stimulates the ascending contraction and descending relaxation components of the peristaltic reflex of the mouse colon by a TGR5-mediated mechanism. However, TGR5 does not participate in peristalsis induced by mechanical stimulation of the mucosa.
Figure 2. TGR5-dependent stimulation of colonic peristalsis.
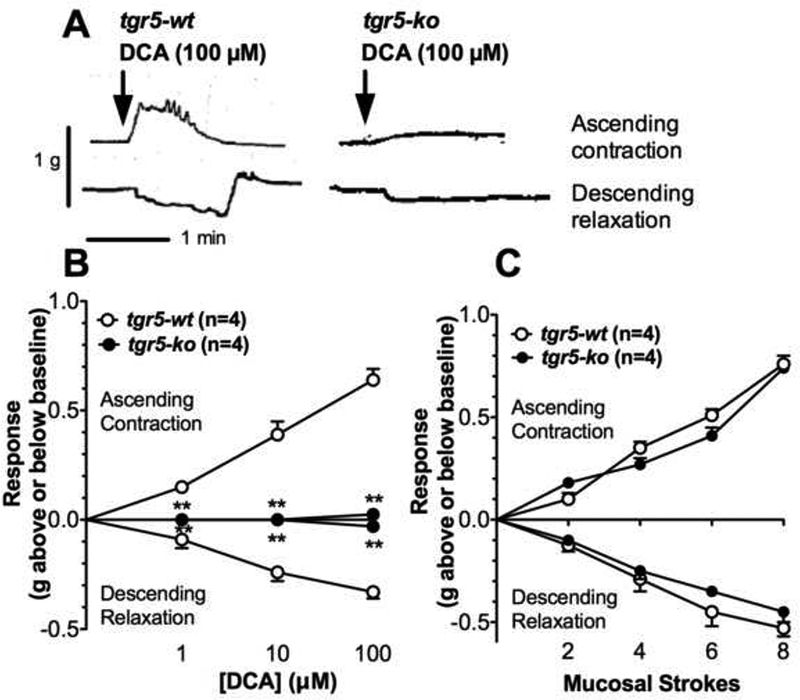
Peristaltic contractions of isolated proximal colon were recorded from tgr5-wt and tgr5-ko mice. A. Representative recordings of ascending contraction and descending relaxation to mucosal application of DCA (100 μΜ), which stimulated peristalsis in tgr5-wt mice, and had diminished effects in tgr5-ko mice. B, C. Mean results of ascending contraction and descending relaxation (grams force above or below baseline tone) in response to mucosal application of graded concentrations of DCA (1– 100 μΜ) (B) or graded mechanical stimulation of the mucosa (2–8 strokes). Compared to responses in tgr5-wt mice, peristaltic contractions to DCA were abolished or attenuated in tgr5-ko mice, whereas responses to mechanical stimulation were unaffected by TGR5 expression. **P<0.005 to tgr5-wt.
Given the irritant actions of BAs and the finding that repeated administration of high concentrations of DCA (4 mM) can cause colonic inflammation 37, we examined whether the highest concentration of DCA (100 μΜ) induced damage to the colonic mucosa. Exposure of the mucosa of the isolated colon to 100 μΜ DCA for 10 min caused no detectable histological damage or neutrophil infiltration to the mucosa (Supporting Information Fig. 1). Moreover, intracolonic administration of DCA (50, μ1, 1 or 3 mM) to mice did not induce granulocyte infiltration, determined by assays of myeloperoxidase activity, or plasma extravasation, determined by measurement of Evans blue leak, within 2–3 h (Supporting Information Fig. 2). Thus, the effects of DCA on peristalsis, which were immediate, are unrelated to mucosal damage or inflammation.
5-HT and CGRP mediate BA-induced peristalsis.
Chemical and mechanical stimulation of the mucosa can release 5-HT from EC cells, which activates 5-HT4 receptors on intrinsic primary afferent neurons (IPANs) to release CGRP 31, 33. CGRP stimulates ascending and descending interneurons, and is thus a critical neuronal transmitter of peristalsis. To investigate the contribution of 5-HT and CGRP to BA-induced peristalsis, antagonists of 5-HT4 receptors (GR113808) or CGRP receptors (CGRP8-37) were added to the central compartment of colon from C57BL/6 mice 10 min before mucosal application of BAs. In tissues treated with antagonists, the effects of DCA on ascending contraction and descending relaxation were abolished (1 μΜ DCA) or markedly blunted (10, 100 μΜ DCA) (Fig. 3A, B). However, CGRP8-37 consistently inhibited DCA-stimulated peristalsis to a greater extent than GR113808 at all DCA concentrations. Thus, CGRP8-37 inhibited DCA (10 μM)-stimulated ascending contraction by 86±7% and descending relaxation by 91±5%, whereas GR113808 inhibited ascending contraction by 33±8% and descending relaxation by 38±7%. Mucosal application of LCA, another secondary BA that activates TGR5 21, stimulated peristalsis to a similar extent to DCA (Fig. 3C). In tissues treated with GR113808 or CGRP8-37, the effects of LCA were also abolished (1 μΜ LCA) or attenuated (10, 100 μΜ LCA). CGRP8-37 inhibited OA (100 μM)-stimulated ascending contraction by 72±5% and descending relaxation by 67±5%, whereas GR113808 inhibited ascending contraction by 30±10% and descending relaxation by 40±5%. Thus, 5-HT and CGRP mediate the effects of BAs on the peristaltic reflex of the mouse colon.
Figure 3. Contributions of 5-HT and CGRP to colonic peristalsis.
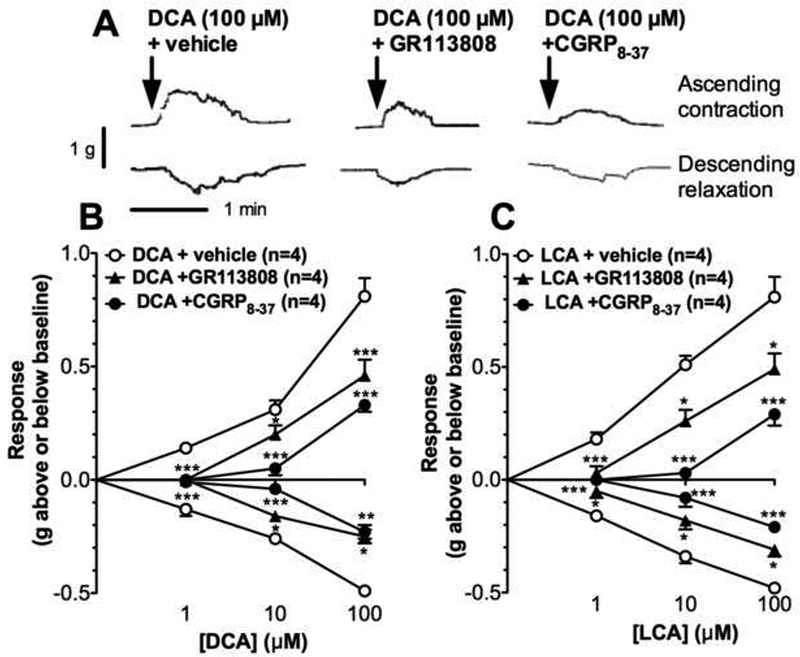
Peristaltic contractions of isolated proximal colon were recorded from C57BL/6 mice. A. Representative recordings of ascending contraction and descending relaxation showing that GR113808 or CGRP8–37 attenuated DCA (100 pM)-stimulated peristalsis. B, C. Mean results of ascending contraction and descending relaxation to graded concentrations of DCA (B) or LCA (C) (1–100 μΜ). GR113808 or CGRP8–37 attenuated DCA- and LCA-stimulated peristalsis, although CGRP8–37 was more effective. *P<0.05, **P<0.005, ***P<0.001 to vehicle.
BAs stimulate the release of 5-HT and CGRP from the colon via TGR5.
To confirm the effects of the antagonists, 5-HT and CGRP release were examined. In preparations of distal colon from tgr5-wt mice, mucosal application of DCA or OA stimulated a concentration-dependent secretion of 5-HT and CGRP immunoreactivity (IR) into the medium of the middle chamber (Fig. 4A, B). Although basal levels of both transmitters were similar between trg5-wt and trg5-ko mice, stimulated release of both transmitters was absent (1,10 μM DCA; 100 μM OA) or blunted (100 μM DCA) in tissues from tgr5-ko mice (Fig. 4A, B), in agreement with the defective BA-stimulated peristalsis (Fig. 2) and the inhibitory effects of antagonists (Fig. 3). BDNF is also released by mechanical stimulation of the mucosa and augments peristalsis by enhancing 5-HT and CGRP release in response to mechanical stimuli 34 Basal BDNF release was the same in tgr5-wt (0.85±0.12 ng/g tissue, n=4) and tgr5-ko (0.98±0.0.19 ng/g) mice (P>0.05). Neither DCA (100 μM, tgr5-wt 0.89±0.20 ng/g; tgr5-ko 1.11±0,22 ng/g, n=4) nor OA (100 μM, tgr5-wt 1.1±0.21 ng/g; tgr5-ko 0.87±0.11 ng/g, n=4) stimulated BDNF release (P>0.05 to basal). However, mechanically-stimulated BDNF release was the same in tgr5-wt (8-strokes=1.66±0.24 ng/g, n=4) and tgr5-ko (8-strokes=1.84±0.18 ng/g) mice (P>0.05 to basal). The lack of BA-stimulated BDNF release may explain the diminished peristaltic contractions in response to DCA compared to mucosal stroking.
Figure 4. TGR5-dependent release of peristaltic transmitters from proximal colon.
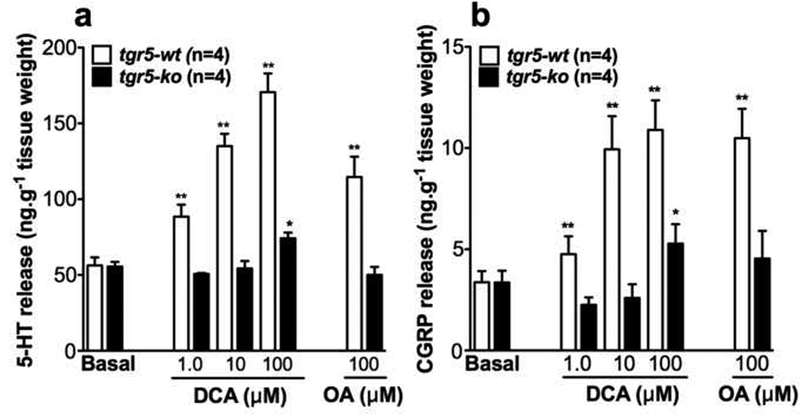
Release of 5-HT-IR (A) or CGRP-IR (B) into the central compartment of the isolated proximal colon from tgr5-wt and trg5-ko mice after mucosal application of DCA (1–100 μΜ) or OA (100 μΜ). Whereas DCA and OA stimulated 5-HT and CGRP release in tgr5-wt mice, responses were absent or attenuated in tgr5-ko mice. *P<0.05, **P<0.01 to basal.
TGR5 is expressed by IPANs and EC cells.
To determine the possible site of action of BAs, we localized TGR5 in the intestine using two antibodies directed to the C-terminus 30. Both antibodies detected human TGR5 heterologously expressed in HEK cells but did not stain non-transfected cells (Supporting Information Fig. 3), and staining of intestinal tissues was abolished by preadsorption with antigen (see 30, indicating specific detection). In sections of colon, TGR5-IR was localized to mucosal epithelial cells and to neurons of the myenteric plexus (Fig. 5A, arrowheads). TGR5-IR colocalized with CGRP-IR in neurons of the myenteric plexus, and CGRP-IR was also detected in mucosal nerve fibers (Fig. 5A, arrowhead with asterisk). In the mucosa, TGR5-IR colocalized with 5-HT-IR in EC cells (Fig. 5B, arrowheads). Wholemounts of myenteric plexus were studied to define the neurochemical coding and identification of neurons expressing TGR5-IR. TGR5-IR colocalized with CGRP-IR and neurofilament M (NFM)-IR, which identify IPANs with Dogiel type II morphology (Fig. 5C, arrowheads). However, TGR5-IR was also detected in other unidentified neurons (Fig. 5C, arrowhead with asterisk). Thus, TGR5 is expressed by cell types that regulate peristalsis.
Figure 5. Localization of TGR5-IR to myenteric neurons and EC cells of mouse colon.
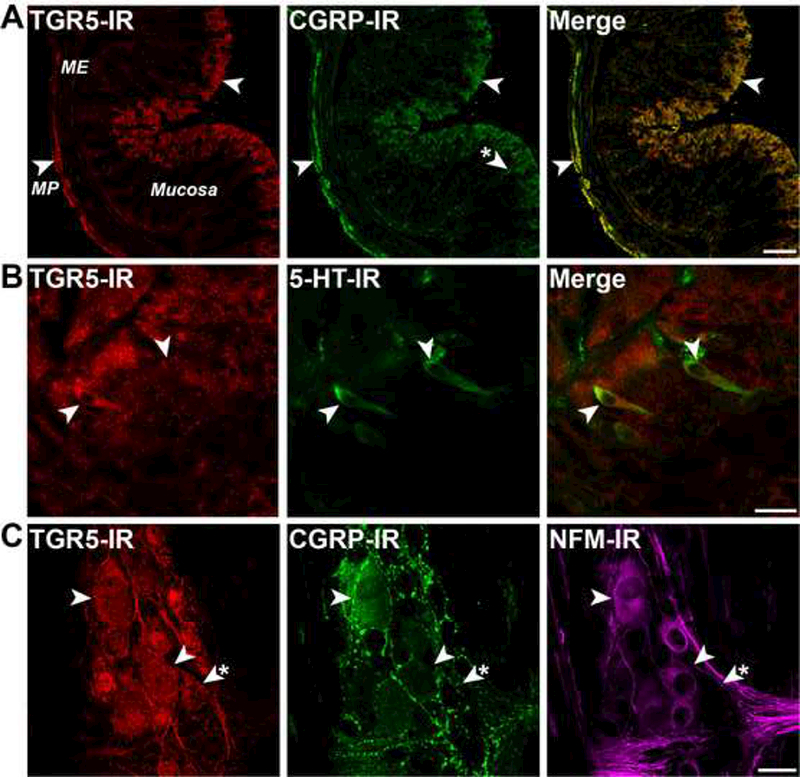
TGR5 was detected in C57BL/6 mice using antibody NLS1937 (A) or P87/8 8 30 (B, C) with similar results. A. In sections of whole thickness colon, TGR5-IR was detected in neurons of the myenteric plexus (MP) within the muscularis externa (ME) and in mucosal epithelial cells (arrowheads). CGRP-IR colocalized with TGR5-IR in myenteric neurons, and was also found in nerve fibers in the mucosa (arrowhead asterisk). B. In sections of the colonic mucosa, TGR5-LI colocalized with 5-HT-IR in EC cells (arrowheads), and was also detected in epithelial cells. C. Analysis of the neurochemical coding of neurons in wholemounts of the myenteric plexus revealed colocalization of TGR5-IR, CGRP-IR and NFM-IR in IPANS (arrowheads), although TGR5-IR was also detected in other neuronal subtypes (arrowhead asterisk). Scale bars: A 100 μm, B, C 20 μm.
TGR5 expression affects gastrointestinal and colonic transit.
The observation that TGR5 expression is required for the effects of DCA on contractility and peristalsis raised the possibility that the level of TGR5 expression per se may affect gastrointestinal and colonic transit. To evaluate this possibility, Evans blue/methyl cellulose was administered by gavage to tgr5-wt, tgr5-ko and tgr5-transgenic (tg, overexpressing mouse TGR5) mice. Gastrointestinal transit, determined by measuring the time for expulsion of the first blue fecal pellet, was 1.4-fold slower in tgr5-ko mice (402±21 min) compared to tgr5-wt (283±30 min) and tgr5-tg (284±27 min) mice (Fig. 6A, P<0.01 tgr5-ko to tgr5-wt and tgr5-tg). BAs can affect gastric emptying as well as motility of the small and large intestines 30. To specifically examine colonic transit, the times for expulsion of a glass bead from the colon of tgr5-wt, tgr5-ko and tgr5-tg mice was determined. Bead expulsion was accelerated 2.2-fold in tgt5-tg mice (879±397 s) compared to tgr5-wt (1939±531 s) or tgr5-ko (1963±434 s) mice (Fig. 6B, P<0.05 tgr5-tg to tgr5-wt and tgr5-ko). Thus, the absence of TGR5 slows gastrointestinal transit, whereas overexpression of TGR5 has no overall effect. TGR5 overexpression accelerates colonic transit, consistent with the pro-secretory and pro-kinetic actions of BAs in the colon.
Figure 6. Gastrointestinal and colonic transit.
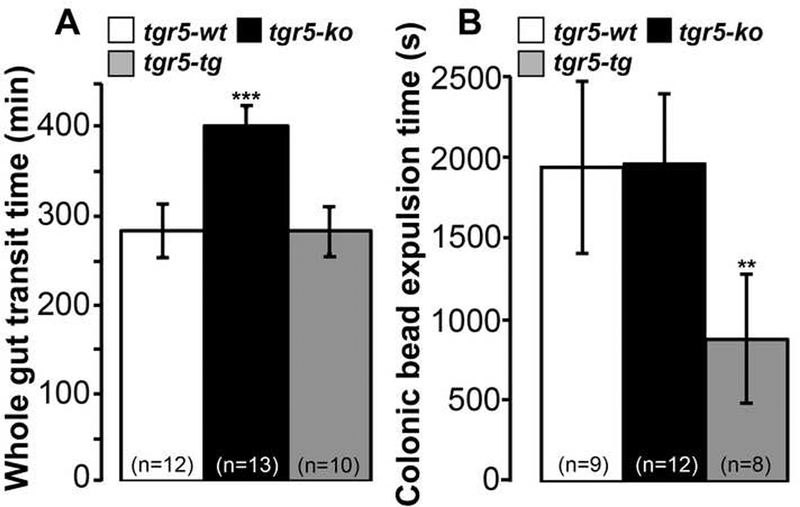
A. Whole gut transit of Evans blue dye. After gavage of dye, the time for expulsion of the first blue pellet was longer in tgr5-ko mice than tgr5-wt or tgr5-tg mice, indicating delayed transit. B. Expulsion of a glass bead from the colon. After insertion of a glass bead into the colon, the time for expulsion was less in tgr5-tg mice than in tgr5-wt or tgr5-ko mice, indicating accelerated colonic transit. **P< 0.01, ***P<0.001 to tgr5-wt.
TGR5 expression affects the frequency of defecation and fecal water content.
To determine if the level of TGR5 expression affects defecation, the rate of pellet excretion in freely-feeding tgr5-wt, tgr5-ko and tgr5-tg mice was determined. Measured over a 2 h period, pellet excretion was increased 1.4-fold in tgr5-tg mice (7.1±0.9 pellets/2 h; P<0.05 to tgr5-wt) but decreased 2.6- fold in tgr5-ko mice (1.9±0.5 pellets/2 h; P<0.01 to tgr5-wt) compared to tgr5-wt mice (4.9±0.7 pellets/2 h) (Fig. 7A). Fecal water content was lower in tgr5-ko mice (37±5%; P<0.001 to tgr5-wt) compared to tgr5-tg mice (57±4%) and tgr5-wt (62±2%) mice, consistent with the decreased frequency of defecation in tgr5-ko mice (Fig. 7B). Thus, tgr5-ko mice are constipated, producing fewer and drier pellets, in accordance with the constipation observed in patients with cholestatic liver disease.
Figure 7. Defecation frequency and water content.
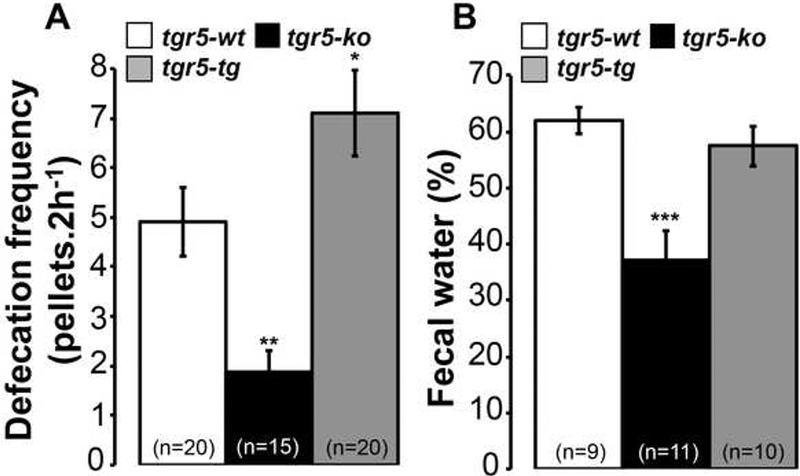
A. Defecation frequency was diminished in tgr5-ko mice but increased in trg5-tg mice compared to tgr5-wt mice. B. Fecal water content was diminished in tgr5-ko mice compared to tgr5-wt or tgr5-tg mice. **P< 0.01, ***P<0.001 to tgr5-wt.
DISCUSSION
We have defined the mechanism underlying the established patho-physiological motor actions of BAs in the colon. Our results show that BAs activate TGR5, which is expressed by EC cells and IPANs, and release 5-HT and CGRP, the major transmitters of the afferent limb of the peristaltic reflex. In keeping with the prokinetic actions of BAs and TGR5, TGR5 deletion delays gastrointestinal transit whereas TGR5 overexpression accelerates colonic transit. Loss of TGR5 function results in excretion of fewer and drier fecal pellets, indicating constipation. Our results identify TGR5 as the key mediator of BA-induced alterations in colonic motility, and suggest that therapies that target TGR5 could be effective treatments for constipation and diarrhea.
TGR5 mediates the effects of BAs on contractility and peristalsis
Our results show that DCA inhibits spontaneous contractility of longitudinal muscle of the isolated mouse colon by a mechanism that requires TGR5 expression. We have previously reported that TGR5 is expressed by nitrergic inhibitory motoneurons of the myenteric plexus of the colon, and that DCA inhibits contractility by a neurogenic, nitrergic mechanism 30. Our present findings support the hypothesis that DCA activates TGR5 on inhibitory motoneurons of the colon that release nitric oxide and inhibit contractility.
A major finding is that mucosal application of DCA and LCA, the major secondary BAs of the colon, stimulated ascending contraction and descending relaxation of colonic circular muscle. DCA and LCA induced peristalsis and stimulated release of peristaltic transmitters at concentrations ranging from 1–100 μΜ. These concentrations are within the physiological range of BAs in the lumen of the human intestine, which spans 10 mM in the proximal small intestine, 2 mM in the terminal ileum and 0.4 mM in the large intestine, where DCA accounts for 34% of BAs 38, 39. The actions of DCA and LCA on peristalsis were immediate and did not cause detectable damage or inflammation in vitro or in vivo, either in the short- (10 min) or long- (2–3 h) term. Thus, BAs are physiological stimulants of colonic peristalsis, which is essential for normal transit and digestion. DCA, like mechanical stimulation of the mucosa, stimulated release of 5-HT and CGRP, and antagonism of 5-HT4 and CGRP receptors attenuated BA-evoked peristalsis. Remarkably, the effects of DCA on peristalsis and transmitter release were absent or blunted in tgr5-ko mice. The involvement of TGR5 is substantiated by the observation that OA, a TGR5-selective agonist 36, stimulated peristalsis and transmitter release via TGR5. In contrast, mechanically-evoked peristalsis was unaffected by TGR5 expression, indicating the selectivity of this process for BAs.
Our results suggest that luminal BAs trigger the afferent limb of the peristaltic reflex by activating TGR5 on EC cells and IPANs, which release 5-HT and CGRP, respectively (Supporting Information Fig. 4). We localized TGR5-IR to EC cells and IPANs, suggesting that DCA may regulate both cell types, and BAs also stimulate 5-HT release from isolated human EC cells 16. Further experimentation is required to determine the relative importance of TRG5 activation on EC cells and IPANs, since 5-HT activates 5-HT4 receptors on IPANs to stimulate CGRP release 31, 33. However, CGRP antagonism was consistently more effective than 5HT4 antagonism in suppressing BA-evoked peristalsis, which supports a major role for CGRP. CGRP activates ascending and descending interneurons that transmit the peristaltic reflex 31. Activation of ascending excitatory motoneurons causes contraction of circular muscle through release of acetylcholine and substance P, whereas activation of descending inhibitory motoneurons induces relaxation of circular muscle via release of nitric oxide and vasoactive intestinal polypeptide 31. When added to the central compartment of a flat sheet preparation, BAs would activate the sensory limb of the peristaltic reflex rather than directly affecting motor neurons. In contrast, BAs could directly regulate motoneurons of muscle strips to affect spontaneous contractility. However, the inhibition of longitudinal muscle contractility is consistent with the reciprocal regulation of circular and longitudinal muscle during peristalsis. Our conclusion that DCA stimulates peristalsis by a TGR5-mediated mechanism is in accordance with reports that luminal BAs stimulate colonic transit 6, 40.
Whereas DCA stimulated release of 5-HT and CGRP, DCA did not affect BDNF release from the colon. BDNF augments peristalsis by enhancing the release of 5-HT and CGRP to mechanical stimulation of the mucosa 34. This inability of DCA to release BDNF may account for the smaller magnitude of DCA-evoked compared to mechanically-evoked peristaltic contractions.
The level of TGR5 expression determines intestinal transit and defecation
By studying mice with loss or gain of TGR5 function, we evaluated the importance of TGR5 in the regulation of intestinal transit and defecation. Gastrointestinal transit, determined by measuring the time for excretion of a marker administered by gavage, was 1.4-fold slower in tgr5-ko mice compared to tgr5-wt and tgr5-tg mice, which were identical. From these studies we were unable to define the major region of the gut that was affected by TGR5 deletion. However, BAs inhibit gastric emptying and slow small intestinal transit 5, 30, possibly by activating TGR5 on intestinal L cells to release glucagon-like peptide 1 17 which slows transit to allow more complete nutrient absorption 18. Thus, it is likely that TGR5 deletion impairs the prokinetic effects of BAs in the colon, resulting in an overall inhibition of whole gut transit.
In contrast to the inhibition of small intestinal transit, BAs and TGR5 agonists promote peristalsis in the colon and accelerate colonic transit 6, 40. Although TGR5 deletion did not affect colon transit, determined by measuring the time for bead expulsion, TGR5 overexpression accelerated colonic transit by 2.2-fold, suggesting a major role for TGR5 in this tissue. This difference between propulsion in mice with loss and gain of TGR5 function is not surprising since bead propulsion is probably initiated by mucosal mechanical stimulation and distension-activated sensory pathways rather than the chemosensitive pathway activated by TGR5. Stimulation of the chemosensitive pathway, such as mediated by overexpression of TGR5, would likely enhance the response to mechanical stimulation as has been shown for the potentiation between luminal fatty acids and mechanical stimulation of the mucosa 41. Loss of TGR5 function reduced the frequency of defecation by 2.6-fold and the fecal water content by 1.7-fold compared to trg5-wt mice, consistent with constipation, whereas gain of TGR5 function was associated with a 1.4-fold increase in defecation frequency.
The impact of TGR5 expression on transit and defecation may be related to a role for TGR5 in control of secretion as well as motility. TGR5 is expressed by cholinergic secretomotor neurons of the submucosal plexus 30, and BAs stimulate fluid and mucus secretion by a neuronal, cholinergic mechanism 15, 42. TGR5 also regulates gall bladder filling 26 and mucosal integrity 43, and TGR5 deficient mice have a reduced BA pool size 26, 27, all of which could influence transit and defecation.
TGR5 as a mediator of and target for digestive diseases
Our results suggest that defects in the colonic delivery of free BAs that are secondary to certain diseases and therapeutic regimens cause constipation or diarrhea due to abnormal activation of TGR5. Decreased colonic delivery of BAs as a result of cholestatic disease or treatment of lipid disorders with BA sequestrants results in constipation 9, 10, possibly due to decreased TGR5 activity. Conversely, excessive delivery of BAs to the colon, which occurs as a result of defective ileal absorption after inflammation or resection, or due to continuous bile secretion after cholecystectomy, can cause diarrhea 11, 12, perhaps by over activation of TGR5. BA have been ingested for millennia to treat constipation 13, and inhibitors of ileal BA transporters relieve constipation by increasing colonic delivery of BAs 14 Our observations that TGR5 activation promotes colonic peristalsis and that TGR5 overexpression accelerates colonic transit, whereas TGR5 deficiency has the opposite effects and results in constipation, suggest that therapeutic targeting of TGR5 is a new strategy to treat constipation and diarrhea. TGR5 agonists may be therapies for constipation, whereas antagonists may relieve diarrhea. Future studies will evaluate these possibilities.
Supplementary Material
ACKNOWLEDGEMENTS.
We thank Louise Pontell and John Furness (University of Melbourne) for help with the histological analyses.
Grant support: Supported by Northern California Institute for Research and Education, Veterans Health Administration (CUC); NHMRC 63303, 103188 (NWB), 454858 (DPP); NIH DK39957, DK43207, DK57840 (NWB), DK34153 (JRG); Monash University (NWB); Ecole Polytechnique Fédérale de Lausanne, Swiss National Science Foundation (SNF 31003A_125487) (KS).
Abbreviations
- BA
bile acid
- BDNF
brain-derived neurotropic factor
- CGRP
calcitonin gene-related peptide
- DCA
deoxycholic acid
- EC
enterochromaffin
- 5-HT
5-hydroxytryptamine
- IPANs
intrinsic primary afferent neurons
- IR
immunoreactivity
- LCA
lithocholic acid
- NFM
neurofilament M
- OA
oleanolic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
Author contributions: FA, DPP, JC and FC performed the experiments; KS generated TGR5 knockout and transgenic mice; JRG, NWB and CUC designed experiments, analyzed data and wrote the manuscript.
REFERENCES
- 1.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci 2008;65:2461–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajor A, Gillberg PG, Abrahamsson H. Bile acids: short and long term effects in the intestine. Scandinavian journal of gastroenterology 2010;45:645–664. [DOI] [PubMed] [Google Scholar]
- 3.Angelin B, Bjorkhem I, Einarsson K, et al. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest 1982;70:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong DN, Krenz HK, Modlin IM, et al. Bile salt inhibition of motility in the isolated perfused rabbit terminal ileum. Gut 1993;34:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penagini R, Misiewicz JJ, Frost PG. Effect of jejunal infusion of bile acids on small bowel transit and fasting jejunal motility in man. Gut 1988;29:789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snape WJ Jr., Shiff S, Cohen S Effect of deoxycholic acid on colonic motility in the rabbit. Am J Physiol 1980;238:G321–325. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M, Murphy R, Chadwick VS. Dose-related effects of chenodeoxycholic acid in the rabbit colon. Dig Dis Sci 1980;25:433–438. [DOI] [PubMed] [Google Scholar]
- 8.Chadwick VS, Gaginella TS, Carlson GL, et al. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med 1979;94:661–674. [PubMed] [Google Scholar]
- 9.Ghaffari K, Savadkuhi ST, Honar H, et al. Obstructive cholestasis alters intestinal transit in mice: role of opioid system. Life Sci 2004;76:397–406. [DOI] [PubMed] [Google Scholar]
- 10.Knodel LC, Talbert RL. Adverse effects of hypolipidaemic drugs. Med Toxicol 1987;2:10–32. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen TA. The role of bile salts in diarrhoea of patients with ulcerative colitis. Gut 1971;12:632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arlow FL, Dekovich AA, Priest RJ, et al. Bile acid-mediated postcholecystectomy diarrhea. Arch Intern Med 1987;147:1327–1329. [PubMed] [Google Scholar]
- 13.Thorner RS. The effect of exclusion of the bile upon gastrointestinal motility. Am J Roentgenol Radium Ther Nucl Med 1955;74:1096–1122. [PubMed] [Google Scholar]
- 14.Camilleri M Pharmacology of the new treatments for lower gastrointestinal motility disorders and irritable bowel syndrome. Clin Pharmacol Ther 2012;91:44–59. [DOI] [PubMed] [Google Scholar]
- 15.Karlstrom L, Cassuto J, Jodal M, et al. Involvement of the enteric nervous system in the intestinal secretion induced by sodium deoxycholate and sodium ricinoleate. Scand J Gastroenterol 1986;21:331–340. [DOI] [PubMed] [Google Scholar]
- 16.Kidd M, Modlin IM, Gustafsson BI, et al. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol 2008;295:G260–272. [DOI] [PubMed] [Google Scholar]
- 17.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 2005;329:386–390. [DOI] [PubMed] [Google Scholar]
- 18.Schirra J, Goke B. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul Pept 2005;128:109–115. [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiological reviews 2009;89:147–191. [DOI] [PubMed] [Google Scholar]
- 20.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem 2003;278:9435–9440. [DOI] [PubMed] [Google Scholar]
- 21.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 2002;298:714–719. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006;439:484–489. [DOI] [PubMed] [Google Scholar]
- 23.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009;10:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keitel V, Cupisti K, Ullmer C, et al. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology 2009;50:861–870. [DOI] [PubMed] [Google Scholar]
- 25.Lavoie B, Balemba OB, Godfrey C, et al. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of KATP channels. J Physiol 2010;588:3295–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T, Holmstrom SR, Kir S, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol 2011;25:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruyama T, Tanaka K, Suzuki J, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol 2006;191:197–205. [DOI] [PubMed] [Google Scholar]
- 28.Pols TW, Nomura M, Harach T, et al. TGR5 Activation Inhibits Atherosclerosis by Reducing Macrophage Inflammation and Lipid Loading. Cell Metab 2011;14:747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YD, Chen WD, Yu D, et al. The G-Protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-kappaB) in mice. Hepatology 2011;54:1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 2010;22:814–825, e227–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grider JR. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther 2003;307:460–467. [DOI] [PubMed] [Google Scholar]
- 32.Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology 1998;115:370–380. [DOI] [PubMed] [Google Scholar]
- 33.Grider JR, Kuemmerle JF, Jin JG. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. The American journal of physiology 1996;270:G778–782. [DOI] [PubMed] [Google Scholar]
- 34.Grider JR, Piland BE, Gulick MA, et al. Brain-derived neurotrophic factor augments peristalsis by augmenting 5-HT and calcitonin gene-related peptide release. Gastroenterology 2006;130:771–780. [DOI] [PubMed] [Google Scholar]
- 35.Poole DP, Pelayo JC, Cattaruzza F, et al. Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology 2011;141:565–575, 575 e561–564. [DOI] [PubMed] [Google Scholar]
- 36.Sato H, Genet C, Strehle A, et al. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun 2007;362:793–798. [DOI] [PubMed] [Google Scholar]
- 37.Traub RJ, Tang B, Ji Y, et al. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology 2008;135:2075–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton JP, Xie G, Raufman JP, et al. Human cecal bile acids: concentration and spectrum. Am J Physiol Gastrointest Liver Physiol 2007;293:G256–263. [DOI] [PubMed] [Google Scholar]
- 39.Northfield TC, McColl I. Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut 1973;14:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirwan WO, Smith AN, Mitchell WD, et al. Bile acids and colonic motility in the rabbit and the human. Gut 1975;16:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. American journal of physiology. Gastrointestinal and liver physiology 2007;292:G429–437. [DOI] [PubMed] [Google Scholar]
- 42.Camilleri M, Murphy R, Chadwick VS. Pharmacological inhibition of chenodeoxycholate-induced fluid and mucus secretion and mucosal injury in the rabbit colon. Dig.Dis.Sci. 1982;27:865–869. [DOI] [PubMed] [Google Scholar]
- 43.Cipriani S, Mencarelli A, Chini MG, et al. The Bile Acid Receptor GPBAR-1 (TGR5) Modulates Integrity of Intestinal Barrier and Immune Response to Experimental Colitis. PLoS One 2011;6:e25637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


