Abstract
Kappa opioid receptors (KOR) are considered to be a promising therapeutic target for stress-induced psychiatric disorders such as anxiety and depression. Preclinical data show that KOR antagonists have greater efficacy if administered before stressful experiences as opposed to afterwards. However, almost all of these studies use long-acting antagonists, leaving it unclear whether inhibition of KOR after stress is required for efficacy. Here we show that administration of the short-acting KOR antagonist AZ-MTAB before episodes of social defeat stress block the induction of anhedonia (both males and females) and social avoidance responses (females) that persist two weeks after stress. In both males and females pre-stress AZ-MTAB treatment also blunted anticipatory autogrooming behavior immediately prior to the third episode of defeat. In contrast when AZ-MTAB was administered two weeks after defeat (immediately before behavior testing) in female California mice, it was ineffective at reversing anhedonia and social avoidance. These results suggest that short-acting KOR antagonists may have greater therapeutic potential if administered before exposure to psychosocial stressors.
Keywords: kappa opioid receptor, anhedonia, social interaction, social defeat stress, depression
Introduction
Psychosocial stress is an important risk factor for stress-induced psychiatric disorders such as depression, and the activation of kappa-opioid receptors (KOR) facilitates behavioral responses to stress (Knoll et al., 2007; Land et al., 2008; Wiley et al., 2009; Lalanne et al., 2014). Inhibition of KOR receptors can reduce social withdrawal induced by social defeat (Bruchas et al., 2011) as well as stress-induced drug seeking behaviors (Beardsley et al., 2005; McLaughlin et al., 2006). These findings have generated strong interest in KOR as a potential novel therapeutic target for the treatment of depression and anxiety (Knoll and Carlezon, 2010). The majority of studies examining the effects of KOR antagonists on depression- and anxiety-like behavior use drugs such as JDTic or norBNI, which inhibit KOR for an extended period of several weeks (Potter et al., 2011). Most results suggest that long acting KOR antagonists have great efficacy when administered before stressful experiences (Mague et al., 2003; McLaughlin et al., 2003; Land et al., 2008; Carr et al., 2009; Falcon et al., 2016), however because of the long-acting properties of these drugs, it is unclear whether KOR inhibition after stress is required as well. Furthermore, there is growing evidence that stressors alter the behavioral effects of KOR (Kudryavtseva et al., 2004a; Kudryavtseva et al., 2004b; Al-Hasani et al., 2013; Donahue et al., 2015; Laman-Maharg et al., 2017), such that KOR may have different behavioral effects before and after stress. Another gap in the literature is that the majority of preclinical work on KOR antagonists has not considered sex as a biological variable.
Women are more likely to develop mood or anxiety disorders than men (Kessler et al., 2003), yet most preclinical data on KOR are derived from studies on male rodents. A few studies that have directly compared KOR effects in males and females demonstrate important sex differences (Chartoff and Mavrikaki, 2015). For example, the KOR agonist U50,488 induced anhedonia for intracranial self-stimulation at lower doses for male rats compared to females (Russell et al., 2013). Similarly, female rats took longer to discriminate a KOR agonist from vehicle using a fixed ratio schedule of food reinforcement (Craft et al., 1998), suggesting that the aversive effects of KOR agonists are weaker in females. In contrast, female California mice formed a place aversion to a low dose of U50,488 while a high dose of U50,488 was required to induce place aversion in males (Robles et al., 2014; Laman-Maharg et al., 2017). Overall it appears that sex differences in KOR function are context-dependent. To date, no study has tested whether inhibition of KOR before or after social defeat modulates depression- or anxiety-like behaviors in females.
Here we compare the behavioral effects of the short-acting KOR antagonist AZ-MTAB administered before or after social defeat stress. Experiments were conducted on male and female California mice (Peromyscus californicus), which is a monogamous species in which both males and females show territorial aggression (Silva et al., 2010). This allowed us to study the effects of social defeat in both sexes. In previous studies, three episodes of social defeat induces social avoidance behavior in females but not males, and we’ve observed that this effect is stronger 2–4 weeks after defeat versus 1 day after defeat (Trainor et al., 2011; Trainor et al., 2013; Greenberg et al., 2014). Since our previous studies showed that the effects of defeat stress are stronger in females and males in the social interaction test, in the current studies we focused more on females. To validate that AZ-MTAB blocks KOR receptors, in experiment 1 we tested whether AZ-MTAB blocked the effects of the KOR agonist U50,488 on immobility in female California mice tested in the forced swim test. In experiment 2 male and female mice were treated with AZ-MTAB or vehicle immediately before three episodes of social defeat. We quantified both short-term effects and long-term effects of AZ-MTAB on behavior in males and females. Finally, we tested whether acute AZ-MTAB administered two weeks after defeat (immediately before behavior testing) could reverse the effects of defeat stress in female California mice. Overall, our results suggest that short-acting KOR antagonists have stronger behavioral effects when administered before episodes of stress versus after stress when anxiogenic and depression-like behaviors have developed.
Materials and Methods
Experiment 1: Validation of AZ-MTAB inhibition of KOR in the forced swim test in Female California Mice
To test that an efficacious dose of AZ-MTAB published for male rats (Peters et al., 2011) could block the behavioral effects of KOR activation in female California mice, we examined the effects of AZ-MTAB in a two-day forced swim test. Females were randomly assigned to control or defeat conditions and run through social defeat testing. To examine the long-term effects of social defeat stress, forced swim testing occurred two weeks later. All drugs were administered on day 2, with AZ-MTAB (Sigma, St. Louis, MO; 10mg/kg dissolved in 26% DMSO in sterile PBS 10% tween) administered two hours and U50,488 (10 mg/kg dissolved in 10% tween in sterile PBS) administered 30 min before testing. All females received 2 injections: one injection two hours before testing (AZ-MTAB or 26% DMSO vehicle), and a second injection 30 min before testing (U50,488 or PBS). To test whether the relatively high concentration of DMSO had non-specific effects on behavior, we compared DMSO vehicle and PBS treated mice in an open field test.
Experiment 2: Effects of short-term KOR inhibition immediately before defeat in Male and Female California Mice
Mice were randomly assigned to control or stress groups, as well as to AZ-MTAB-treated or vehicle-treated groups, and then treated i.p. with either vehicle (26% DMSO in sterile PBS 10% Tween) or 10mg/kg AZ-MTAB (Sigma, St. Louis, MO) dissolved in DMSO vehicle two hours before each episode of social defeat stress (one injection for three consecutive days). We examined both the short- and long-term effects of acutely inhibiting KOR during social defeat stress. Short-term behavior was assessed through observations of autogrooming behaviors prior to the first and last episode of social defeat stress. To assess long-term effects of acutely inhibiting KOR during social defeat stress, animals were run through the corresponding behavior tests: sucrose preference test (each male and female), social interaction test (each female), and elevated plus maze (each male) two weeks after the last episode of social defeat.
Experiment 3: Effects of short-term KOR inhibition after stress on behavior in Female California Mice
A separate group of females were randomly assigned to control or defeat conditions and run through social defeat stress. To assess the effects of inhibiting KOR inhibition following stressful experiences on depression-like behavior, mice were treated with either a 10mg/kg dose of AZ-MTAB or vehicle two hours before each behavior test (sucrose anhedonia and social interaction). Testing occurs two weeks following stress in order to ascertain the long-term effects of stress. All mice were run through each behavior test.
Experimental Procedures
Animals and housing conditions
Adult male and female California mice (Peromyscus californicus), 3–6 months old, were bred in our laboratory colony and housed in same-sex groups of two to three per cage on Sani-Chips bedding with cotton nestlets in clear polypropylene cages. Mice were kept on a 16-hour light/8-hour dark cycle (lights on at 23:00 h) with Teklad 2016 food (Harlan, Hayward, CA, USA) and water provided ad libitum. All procedures were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of California, Davis. Mice were euthanized after behavior testing by 5% isoflurane administration followed by rapid decapitation. Estrous cycle was assessed postmortem to avoid disrupting behavior (Silva et al., 2010).
Social defeat stress and autogrooming observations
Mice were randomly assigned to social defeat or control handling for 3 consecutive days. Social defeat stress was administered as previously described (Trainor et al., 2013). Mice assigned to social defeat were placed in the cage of an aggressive same-sex mouse. Each episode lasted 7 minutes or until the resident attacked the focal mouse 7 times, whichever occurred first. Control mice were placed in a clean cage for 7 minutes. Immediately following defeat or control conditions mice were returned to their home cage (Greenberg et al., 2014). Autogrooming behavior was observed immediately prior to social defeat episodes on the first and last day of social defeat in experiment 1. To quantify autogrooming, each mouse was transferred from the colony room to the behavior testing room and placed in a clean polypropylene cage for three minutes. Videos were scored for total time spent autogrooming by an observer without knowledge of treatment conditions.
Forced Swim Test
Females were randomly assigned to control or defeat conditions, and then tested in the forced swim test two weeks later. Swim testing took place in an opaque cylinder (25.5 cm tall × 20 cm in diameter) filled with 14 cm of 30°C water during the light phase. Each cylinder was cleaned with Quatricide (1:64, Quatricide PV in water, Pharmacal Research Labs, Inc) between animals. After each trial, mice were dried with paper towels and returned to home cages placed on a heating pad. On day 1, a single swim trial of 15 min was conducted. Immobility was defined as stationary posture for at least 2 seconds with only minor movements to keep the head above water (McLaughlin et al., 2003; Castagne et al., 2011). Immobility was quantified across the entire 15 min test. On day 2, each mouse was tested in a series of four 6 min swim trials each separated by a 6–7 min return to home cage (McLaughlin et al., 2003; Bruchas et al., 2007; Land et al., 2008; Carey et al., 2009). Immobility was live scored by experimenters’ blind to the treatment groups; all videos of testing were recorded from above.
Sucrose anhedonia, social interaction behavior and elevated plus maze
Two-bottle fluid intake choice tasks (water and sucrose) were used to assess anhedonia (Willner et al., 1987; Moreau et al., 1992) in both experiment 2 and experiment 3. Anhedonia was defined as a reduction in sucrose preference relative to control groups. Prior to social defeat or control conditions, all mice were habituated to a two-bottle choice with tap water for two days. Following habituation, a pre-stress sucrose preference test was done; all mice were given the choice between pre-weighed bottles of regular and 1% sucrose water for 24 hours. Measurements of fluid intake were taken by an observer blind to the treatment conditions. Observations were repeated 14 days after defeat or control conditions. Intake was calculated on an absolute basis (sucrose and water intake separately) and as a preference score (sucrose preference relative to total fluid intake: total amount of sucrose water consumed/total amount of water consumed *100).
Two days after sucrose testing females were tested in social interaction as previously described (Greenberg et al., 2014; Trainor et al., 2013) in both experiment 2 and experiment 3. First, each focal mouse was introduced into the open field (89 × 63 × 60 cm) for 3 min (open field phase). Total distance traveled was recorded to assess locomotor behavior (Anymaze, Stoelting). The amount of time that the focal mouse spent within 8 cm of the cage (interaction zone) was recorded for 3 min. Finally, an unfamiliar intact same-sex mouse was placed into the wire cage for 3 min (interaction phase) and the time spent in the interaction zone was recorded. Risk assessment behavior was also scored during the interaction phase based on previous studies which showed that stressed females orient towards the interaction zone when a target mouse is present but not when the cage is empty (Duque-Wilckens et al., 2018). This response resembles vigilance responses seen in rats confronted with a predator (Blanchard et al., 2011) or stretch-attend response observed in hamsters exposed to defeat stress (Gray et al., 2015)
Males were run through an elevated plus maze instead of social interaction because in previous studies we have found that three episodes of defeat did not generate strong responses in male California mice in the social interaction test. All experimental mice were transferred to the behavior room at least 30 minutes before testing to allow for habituation. Experimental animals were placed in the center area of the plus maze (40 × 7 × 25) facing a closed arm, and behavior was recorded for 10 minutes. The arena was cleaned between each trial. The number of entries into and time spent in the open arms were scored. All testing took place during the light cycle (6:00–14:00) in an order randomized for drug treatment.
Statistical analyses
All statistical analyses were performed using R software. Normality of data was assessed using Shapiro-test. A Fligner-Killeen test was used to assess homogeneity of variance. Two-way ANOVA was used to analyze behaviors in autogrooming observations, social interaction tests, elevated plus maze, and sucrose preference tests. For ANOVA analyses that revealed significant interaction effects, pairwise comparisons were used to detect differences between groups. Force swim data on day 2 were square root transformed for analysis to normalize variance between treatment groups and analyzed using a repeated measures ANOVA testing for drug treatment. Cohen’s d is reported for effect size.
3. Results
Experiment 1:Validation of AZ-MTAB inhibition of KOR in the forced swim test in Female California Mice
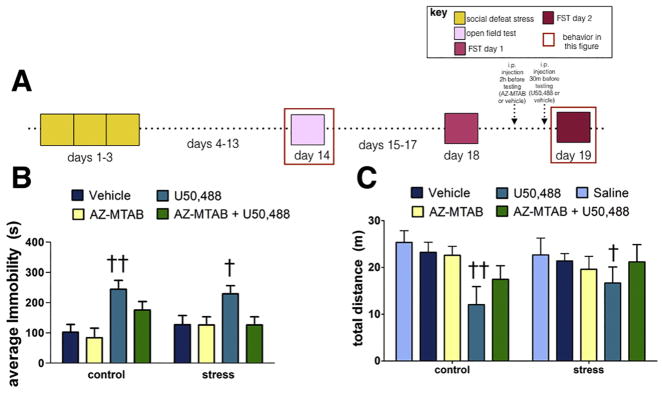
In the forced swim test (Fig. 1A), we detected a main effect of drug treatment on immobility (Fig. 1B; main effect of drug, p<0.01) but no effects of stress and no stress*treatment interaction effect (all p’s > 0.87). Females treated with U50,488 showed more immobility in both control (p < 0.01) and stressed (p < 0.05) females while pretreatment with AZ-MTAB blunted the effects of U50,488 (Fig. 1B). We also assessed whether the vehicle used to deliver AZ-MTAB (which contained 26% DMSO) had nonspecific effects on behavior in an open field test that was conducted 4 days before forced swim testing. We compared these data with females treated with PBS (Duque-Wilckens et al., 2018) that were tested at about the same time as the AZ-MTAB studies. There were no differences in DMSO vehicle and PBS treated mice while U50,488 reduced locomotor behavior in both control (Fig. 1C, p<0.01) and stressed (Fig. 1C, p<0.01) females.
Figure 1. Effects of 10 mg/kg U50,488 and 10 mg/kg AZ-MTAB on immobility in control and stressed female California mice in Experiment 1.
Experimental timeline (Fig. 1A). Average immobility during the FST (Fig. 2B). Locomotor behavior in a novel environment (Fig. 2C). † p < 0.05, †† p < 0.01 compared to vehicle (26% DMSO in saline). Group N’s: control/vehicle n=6, control/U50,488 n=6, control/AZ-MTAB+U50,488 n = 7; stress/vehicle n=6, stress/U50,488 n =6, stress/AZ-MTAB+U50,488 n=6. Error bars are SEM.
Experiment 2: Effects of short-term KOR inhibition immediately before defeat in Male and Female California Mice
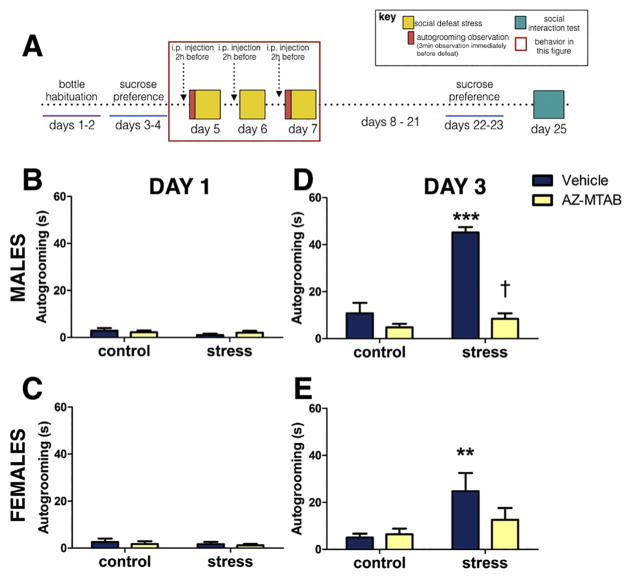
Autogrooming levels prior to defeat or control handling (Fig. 2A) were minimal and there were no differences across groups (Fig. 2B, 2C, all p’s > 0.252). In males, stress increased the amount of time spent grooming relative to control animals (Fig. 2D, stress*treatment, p < 0.0001). Immediately before the third episode of defeat, stressed males treated with vehicle showed a 15-fold increase in autogrooming compared to stressed males treated with AZ-MTAB (planned comparison, p<0.0001, d=5.09), and compared to controls (planned comparison, all p’s <0.0001, d’s >2.56). There were no increases in autogrooming in vehicle or AZ-MTAB treated control males (Fig. 2D, all p’s >0.18, d’s <0.44). Effects of AZ-MTAB were more modest in females. Although the stress*treatment interaction was not significant (p=0.169), a main effect of stress was present (p=0.012), where stress increased the amount of time spent grooming in females treated with vehicle (Fig. 2E). Stressed females treated with vehicle showed more autogrooming than controls (all p’s < 0.012, d=1.14) while stressed females treated with AZ-MTAB were not significantly different from controls (all p’s > 0.39, d=0.72). There were no increases in autogrooming in vehicle or AZ-MTAB treated control females (Fig. 2E, all p’s >0.84, d’s <0.24). There were no systematic biases in the distribution of estrous stage across treatment groups (Table 1).
Fig. 2. Autogrooming behavior in male and female California mice in Experiment 2.
Experimental timeline (Fig. 2A). Time spent displaying autogrooming behavior prior to defeat (day 1, Fig. 2B and 2C), and prior to the last episode of defeat (day 3, Fig. 2D and 2E). ***p<0.0001 compared to controls; **p<0.01, main effect of stress; † p<0.0001 compared to vehicle. Group N’s: Males: control/vehicle: 14, control/AZ-MTAB: 14, stress/vehicle: 14, stress/AZ-TAB: 14. Females: control/vehicle: 8, control/AZ-MTAB: 8, stress/vehicle: 8, stress/AZ-MTAB: 8. All error bars are SEM.
Table 1.
Estrous stage analysis on behavior (3-way ANOVA for stress * treatment * estrous).
| EXPERIMENT 2 | Main effect estrous | Estrous*stress*treatment |
|---|---|---|
| Autogrooming | p=0.2 | p=0.9 |
| Sucrose preference | p=0.1 | p=0.9 |
| Social interaction | p=0.3 | p=0.1 |
| EXPERIMENT 3 | Main effect estrous | Estrous*stress*treatment |
|---|---|---|
| Sucrose preference | p=0.8 | p=0.7 |
| Social interaction | p=0.4 | p=0.3 |
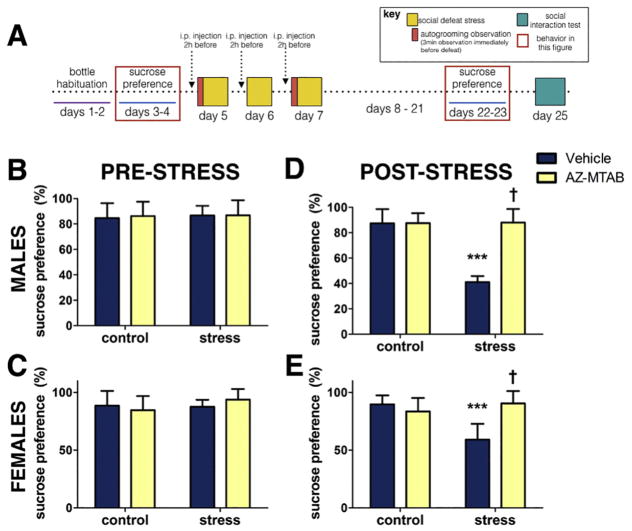
In the sucrose preference test (Fig. 3A), no differences were observed between baseline preference levels for sucrose water before social defeat (Fig. 3B, 3C, all p’s >0.309) or total fluid consumed either before (Table 2, all p’s > 0.32) or following (Table 2, all p’s > 0.21) stress exposure. Stress decreased sucrose preference in both males (Fig. 3D, p<0.0001, d =5.41) and females (Fig. 3E, p<0.0001, d =2.75) treated with vehicle compared to controls. AZ-MTAB treatment before defeat blocked the development of sucrose anhedonia two weeks later in both males (Fig. 3D, stress*treatment, p<0.0001) and females (Fig. 3E, stress*treatment, p<0.0001). In both males (p>0.63, d<0.17) and females (p >0.63, d <0.53) treated with AZ-MTAB, sucrose preference levels did not differ from controls. There were no effects of AZ-MTAB treatment in males or females that were not exposed to defeat (all p’s>0.05). There were no systematic biases in the distribution of estrous stage across treatment groups (Table 1). All drug testing occurred two weeks prior to sucrose preference testing.
Fig. 3. Sucrose preference in male and female California mice in Experiment 2.
Experimental timeline (Fig. 3A). Baseline levels of preference for sucrose water over tap water prior to social defeat stress (Fig. 3B and 3C) and following social defeat stress (Fig. 3D and 3E). ***p<0.0001 compared to control; † p<0.0001 compared to vehicle. Group N’s: Males: control/vehicle: 14, control/AZ-MTAB: 18, stress/vehicle: 18, stress/AZ-MTAB: 14. Females: control/vehicle: 10, control/AZ-MTAB: 10, stress/vehicle: 10, stress/AZ-MTAB: 10. All error bars are SEM.
Table 2.
Total Fluid Consumed in Sucrose Preference Test (Experiment 2).
| Test Group | Pre-test | Post-test |
|---|---|---|
| FEMALES | ||
| Control/AZ-MTAB | 17.78 ± 6.69 | 18.50 ± 7.79 |
| Contro/vehicle | 15.67 ± 4.58 | 17.63 ± 3.70 |
| Stress/AZ-MTAB | 17.22 ± 4.52 | 21.71 ± 7.63 |
| Stress/vehicle | 19.56 ± 9.34 | 17.00 ± 4.47 |
| MALES | ||
| Control/AZ-MTAB | 16.83 ± 4.19 | 17.06 ± 6.22 |
| Contro/vehicle | 16.57 ± 5.68 | 17.21 ± 3.33 |
| Stress/AZ-MTAB | 14.62 ± 6.05 | 18.77 ± 4.66 |
| Stress/vehicle | 14.78 ± 5.49 | 18.50 ± 5.66 |
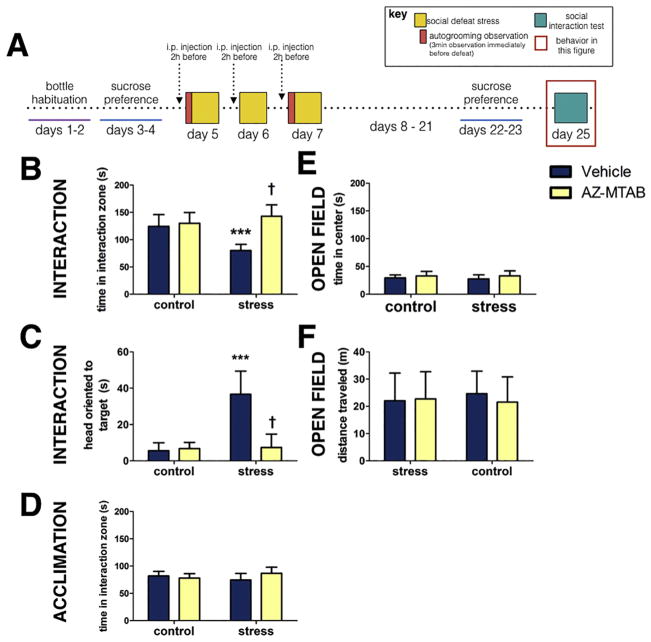
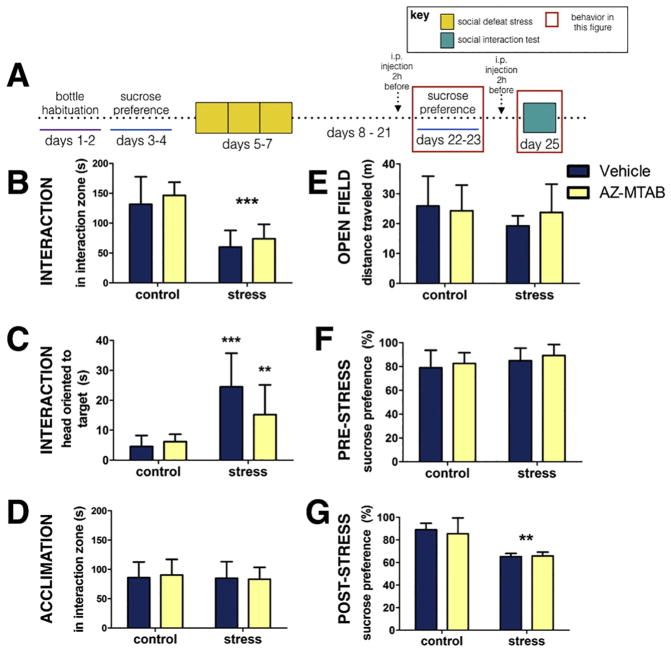
In the social interaction test (Fig. 4A) stress decreased the amount of time spent in the interaction zone in females compared to controls (p<0.0001, d=2.48, Fig. 4B). Treatment with AZ-MTAB before stress blocked effects of stress on social interaction behavior (Fig. 4B, stress*treatment p<0.0001). Stressed females treated with AZ-MTAB before defeat showed more time spent in the interaction zone than stressed females treated with vehicle (p<0.0001, d=3.69, Fig. 4B). Stressed females treated with AZ-MTAB also did not appear different from controls (all p’s>0.13, d’s <0.64, Fig. 4B). Similar effects were seen on head orientation to the target (Fig. 4C, stress*treatment p<0.0001). Social defeat increased head orientation to the target mouse in females treated with vehicle (Fig. 4C, p<0.0001, d=3.28) but not in females treated with AZ-MTAB (Fig. 4C., p=0.85, d=0.12). No differences were observed during the acclimation phase (Fig. 4D, all p’s >0.416). No differences were observed during an open field phase in time spent in the center of the arena (Fig. 4E, all p’s > 0.33) or in distance traveled (Fig. 4F, all p’s >0.53). There were no systematic biases in the distribution of estrous stage across treatment groups (Table 1).
Fig 4. Social interaction behavior in female California mice in Experiment 2.
Experimental timeline (Fig. 4A). Time spent near the interaction zone while a novel mouse was present (Fig. 4B). Head orientation towards the target mouse during the interaction phase (Fig. 4C). Time spent near the cage during an acclimation phase (Fig. 4D). Time spent in the center during the open field phase (Fig. 4E). Locomotor behavior during open field testing (Fig. 4F). ***p<0.0001 compared to controls; † p< 0.0001 compared to vehicle. Groups N’s: control/vehicle: 10, control/AZ-MTAB: 10, stress/vehicle: 9, stress/AZ-MTAB: 10. All error bars are SEM.
Unexpectedly, there were no effects of stress or treatment effects on time spent in the open arms of the elevated plus maze (stress*treatment, all p’s > 0.07), although there was an effect of treatment on entries into the open arms (Table 3, main effect of treatment, p<0.0001), where males treated with AZ-MTAB before stress entered the open arms of the plus maze more frequently than vehicle treated males regardless of stress condition (p’s< 0.0001, d’s >3.16), and more frequently than control animals treated with AZ-MTAB (p=0.02, d=0.78).
Table 3.
General Anxiety in the Elevated Plus Maze in Male California Mice (Experiment 2).
| Test Group | Time spent in open arms | Open arm entries |
|---|---|---|
| Control/AZ-MTAB | 139.48 ± 88.60 | 33.64 ± 11.01 † |
| Control/vehicle | 245.07 ± 175.23 | 7.00 ± 4.03*** |
| Stress/AZ-MTAB | 260.25 ± 132.11 | 44.33 ± 16.97 |
| Stress/vehicle | 277.03 ± 176.22 | 7.33 ± 4.14*** |
p <0.0001 compared to AZ-MTAB treated animals,
p=0.02 compared to stress/AZ-MTAB. Mean ± SEM reported; n= 12 per group.
Experiment 3: Effects of short-term KOR inhibition after stress on behavior in Female California Mice
When mice received vehicle or AZ-MTAB after defeat (before behavior testing) (Fig. 5A), stress decreased the amount of time spent in the interaction zone during a social interaction test for both vehicle (p<0.001, d=1.87) and AZ-MTAB (p<0.001, d=3.11) treated females (Fig. 5B). AZ-MTAB treatment before behavior testing did not blunt the effects of defeat on social interaction behavior (Fig. 5B, main effect of stress p < 0.0001). Stress also increased head orientation behavior (Fig. 5C) in both vehicle (p=0.0001, d=2.38) and AZ-MTAB (p<0.01, d=1.36) treated animals. No differences were seen during the acclimation phase (Fig. 5D, all p’s > 0.662). No groups differences were observed during open field testing (Fig. 5E, all p’s > 0.31). No differences in sucrose preference were observed before stress (Fig. 5F., all p’s > 0.140). Stress decreased sucrose preference in both vehicle (p=0.002, d=5.26) and AZ-MTAB (p<0.001, d=1.94) treated females (Fig. 5G). When AZ-MTAB was administered before sucrose preference testing (one injection immediately before post-stress sucrose preference), it did not reverse the effects of defeat on preference levels (Fig. 5G, main effect of stress p<0.001). There were no differences in total fluid intake across groups before or following social defeat (Table 4). There were no systematic biases in the distribution of estrous stage across treatment groups (Table 1).
Fig. 5. Effects of post-stress AZ-MTAB treatment on sucrose preference and social interaction behavior in female California mice in Experiment 3.
Experimental timeline (Fig. 5A). Time spent in the interaction zone while a novel mouse was present (Fig. 5B) Head orientation towards the target mouse during the interaction phase (Fig. 5C). Time spent near the cage during an acclimation phase (Fig. 5D). Locomotor behavior during open field testing (Fig. 5E). Baseline levels of preference for sucrose water over tap water prior to social defeat stress (Fig. 5F) and following social defeat stress (Fig. 5G). ***p<0.0001, **p<0.001 compared to controls. Groups N’s: control/vehicle: 8, control/AZ-MTAB: 7, stress/vehicle: 6, stress/AZ-MTAB: 9. All error bars are SEM.
Table 4.
Total Fluid Consumed in Sucrose Preference Test in Female California mice (Experiment 3).
| Test Group | Pre-test | Post-test |
|---|---|---|
| Control/AZ-MTAB | 16.71 ± 7.93 | 16.43 ± 3.91 |
| Contro/vehicle | 18.29 ± 6.37 | 17.00 ± 7.14 |
| Stress/AZ-MTAB | 19.25 ± 7.07 | 18.38 ± 6.84 |
| Stress/vehicle | 16.43 ± 6.37 | 17.29 ± 7.14 |
Discussion
Previous studies demonstrated that KOR antagonists administered before stressful experiences blunt the effects of stress on behavior (Mague et al., 2003; McLaughlin et al., 2006; Chartoff et al., 2012). However, since antagonists such as norBNI are long-acting, it was never clear whether KOR inhibition was required both before and after a stressor for behavioral efficacy. Our experiments with a short-acting KOR antagonist show that inhibition of KOR activity during, but not after, social defeat stress is sufficient to prevent anhedonia in both sexes and social avoidance in females. In contrast, KOR inhibition following social defeat was ineffective at inhibiting anhedonia or social avoidance in females. These results suggests that new short-acting KOR antagonists have greater potential if used in a prophylactic approach before stressful experiences as opposed to after traumatic experiences (Van’t Veer and Carlezon Jr, 2013).
Only one previous study has reported in vivo effects of AZ-MTAB (Peters et al., 2011). This study demonstrated that AZ-MTAB has selectivity for KOR and was capable of blocking KOR agonist-induced diuresis within 5 hours but not one week later, indicating the drug to be more short-acting than other antagonists such as norBNI. Previous work showed that immobility, especially on the second day of testing, is enhanced by KOR activation in males (McLaughlin et al., 2003) and that KOR agonists can increase immobility in the forced swim test (Mague et al., 2003). We observed similar results in female California mice, where treatment with the KOR agonist U50,488 induced immobility. We also showed that pre-treatment with a 10mg/kg dose of AZ-MTAB abolished the effect of U50,488, confirming that AZ-MTAB inhibits KOR receptors. This also indicated that this dose of AZ-MTAB works effectively in California mice. Interestingly, AZ-MTAB alone did not decrease immobility. This is consistent with other studies showing that the long acting KOR antagonist norBNI does not inhibit immobility in female California mice or C57Bl6/J mice (Laman-Maharg et al., 2018). This study also did not observe increased immobility in stressed females, consistent with our results. In other species, effects of social defeat on immobility have been inconsistent (Der-Avakian et al., 2014; Iñiguez et al., 2014). This inconsistent effect of defeat on behavior in the forced swim test supports the assertion that immobility may be more of a measure of alternative coping strategies rather than a depression-like behavior (Molendijk and de Kloet, 2015; Colom-Lapetina et al., 2017).
A striking finding was that in both males and females, the behavioral effects AZ-MTAB administered before defeat were evident in behavioral changes observed two weeks later. Vehicle treated males and females showed decreases in sucrose preference after defeat stress, but these changes were blocked by AZ-MTAB pretreatment before defeat. There were no effects of AZ-MTAB on sucrose preference in control mice, indicating that the effects of AZ-MTAB were specific to stressful contexts. The effect sizes for anhedonia were larger in males than females, consistent with previous studies reporting stronger effects of KOR on behavior in males than in females (Russell et al., 2013; Chartoff and Mavrikaki, 2015). For females, we observed strong effects of KOR inhibition in the social interaction test, in which stressed female California mice exhibit social avoidance (Greenberg et al., 2014; Duque-Wilckens et al., 2018). Treatment with AZ-MTAB prevented a decrease in time spent in the interaction zone with the target mouse and also abolished a social vigilance response, in which stressed females orient towards the target mouse while avoiding it (Duque-Wilckens et al., 2018). This response is similar to rats confronted with a predator (Blanchard et al., 2011). The vigilance-avoidance hypothesis posits that individuals with high anxiety detect aversive stimuli more rapidly but then avoid the threat at longer durations (Mogg et al., 2004). In clinical experiments, this is most typically quantified with eyetracking and reaction time data in contexts that do not allow the participant to actively avoid aversive stimuli (Bar-Haim et al., 2007). Using a large arena for social interaction tests allows for the detection of both an attentional and avoidance response in the same individual. Our results suggest that biased attention and behavioral avoidance can be observed in the same individual and that KOR activation during stress facilitates this response. The specificity of AZ-MTAB on behavior were reflected in the absence of differences in the acclimation phase (when the target mouse was absent) and control mice which did not experience social defeat.
In addition to these long-term behavioral effects, AZ-MTAB also abolished autogrooming behavior immediately before the third episode of defeat stress. Increased autogrooming can be induced by stressors such as restraint (Smith and Wang, 2014), chronic mild stress (Harris et al., 2013), and social defeat (Greenberg et al., 2015). California mice increase autogrooming immediately prior to a third episode of defeat, most likely as an anticipatory response to transfer to the behavior testing room. While this could reflect an acute effect of KOR inhibition, other results suggest this effect may not be due to acute KOR inhibition. Stressed females treated with AZ-MTAB immediately before the social interaction test still showed strong social avoidance responses. Acute oxytocin receptor antagonist treatment after defeat but before social interaction testing increased time spent in the interaction zone and decreased vigilance responses (Duque-Wilckens et al., 2018). This demonstrates that these behavioral responses are sensitive to acute pharmacological treatments. Furthermore, when male mice exposed to defeat were treated with the short-acting KOR antagonist (CERC-501) immediately prior to a social interaction test, social avoidance was still observed (Browne et al., 2017). Together these results suggest that KOR inhibition is less effective when administered after stressful experiences. Also supporting the notion that KOR activation induces long-term behavioral changes, the short acting KOR antagonist, LY2444296, blocks U69,593-induced place aversion (Valenza et al., 2017).
Overall our results indicate that activation of KOR during stressful experiences induces long term behavioral changes in both males and females, and that prolonged KOR activation following stress may be a less important mechanism for these behavioral phenotypes. Some research suggests that stress changes the effects of KOR on behavior (Kudryavtseva et al., 2004a; Kudryavtseva et al., 2004b; Al-Hasani et al., 2013; Donahue et al., 2015; Laman-Maharg et al., 2017), which may be part of why we observed a lack of efficacy for KOR antagonists after stress had occurred. One limitation of this study is that AZ-MTAB shows less selectivity for KOR than norBNI, so it is possible that other opioid-receptors could have been affected in our studies. However, previous studies have clearly established that KOR receptors are engaged during stressful experiences, including social stress (Mague et al., 2003; McLaughlin et al., 2003; Carr et al., 2009; Falcon et al., 2016). Furthermore our results are significant in light of the obstacles that long-lasting KOR antagonists have faced in clinical trials (Buda et al., 2015). Accumulating evidence suggests that future studies with short acting KOR antagonists will be more successful if administration occurs in close proximity with stressful contexts as opposed to after stress-induced behavioral phenotypes have emerged.
Manuscript highlights.
Short-acting KOR antagonists given before stress block depression-like behaviors.
Blocking KOR after stress does not affect stress phenotypes.
Drugs targeting KOR may be more useful given in a prophylactic approach.
Acknowledgments
The authors have no conflicts of interest to disclose. We thank Cindy Clayton for coordinating animal care. This work was supported by R01 MH103322.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hasani R, McCall JG, Bruchas MR. Exposure to chronic mild stress prevents kappa opioid-mediated reinstatement of cocaine and nicotine place preference. Front Pharmacology. 2013;4:96. doi: 10.3389/fphar.2013.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, Blanchard RJ. Risk assessment as an evolved threat detection and analysis process. Neurosci Biobehav Rev. 2011;35:991–998. doi: 10.1016/j.neubiorev.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Browne CA, Falcon E, Robinson SA, Berton O, Lucki I. Reversal of stress-induced social interaction deficits by buprenorphine. International Journal of Neuropsychopharmacology. 2017 doi: 10.1093/ijnp/pyx079. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38a MAPK Deletion in Serotonergic Neurons Produces Stress Resilience in Models of Depression and Addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buda JJ, Carroll FI, Kosten TR, Swearingen D, Walters BB. A double-blind, placebo-controlled trial to evaluate the safety, tolerability, and pharmacokinetics of single, escalating oral doses of JDTic. Neuropsychopharmacology. 2015;40:2059–2065. doi: 10.1038/npp.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Lyons AM, Shay CF, Dunton O, McLaughlin JP. Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:4293–4300. doi: 10.1523/JNEUROSCI.6146-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-Like Effects of [kappa]-Opioid Receptor Antagonists in Wistar Kyoto Rats. Neuropsychopharmacology. 2009;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] Chapter. 2011;8:10A.11–18. doi: 10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62:167–176. doi: 10.1016/j.neuropharm.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Mavrikaki M. Sex differences in kappa opioid receptor function and their potential impact on addiction. Front Neurosci. 2015;9:1–16. doi: 10.3389/fnins.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom-Lapetina J, Begley SL, Johnson ME, Bean KJ, Kuwamoto WN, Shansky RM. Strain-dependent sex differences in long-term forced swim paradigm. Behav Neurosci. 2017 doi: 10.1037/bne0000215. in press. [DOI] [PubMed] [Google Scholar]
- Craft RM, Kruzich PJ, Boyer JS, Harding JW, Hanesworth JM. Sex differences in discriminative stimulus and diuretic effects of the kappa opioid agonist U69,593 in the rat. Pharmacol Biochem Behav. 1998;61:395–403. doi: 10.1016/s0091-3057(98)00124-5. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Mazei-Robison MS, Kesby JP, Nestler EJ, Markou A. Enduring defiits in brain reward function after chronic social social defeat in rats: susceptibility, resilience, and antidepressant response. Biol Psychiatry. 2014;76:542–549. doi: 10.1016/j.biopsych.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, Carlezon WA. Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opiod receptor system. Behav Pharmacol. 2015;26:654–663. doi: 10.1097/FBP.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M, Laredo SA, Hao R, Perkeybile AM, Minie VA, Tan PB, Bales KL, Trainor BC. Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female california mice. Biological Psychiatry. 2018 doi: 10.1016/j.biopsych.2017.08.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, Kirby LG, Lucki I. Antidepressant-like effects of buprenorphine are mediated by kappa opioid receptors. Neuropsychopharmacology. 2016;41:2344–2351. doi: 10.1038/npp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CL, Norvelle A, Larkin T, Huhman KL. Dopamine in the nucleus accumbens modulates the memory of social defeat in syrian hamsters (mesocricetus auratus) Behavioural Brain Research. 2015;286:22–28. doi: 10.1016/j.bbr.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, de Jong TR, Yang V, Saltzman W. Chronic variable stress in fathers alters paternal and social behavior but not pup development in the biparental California mouse (Peromyscus californicus) Horm Behav. 2013;64:799–811. doi: 10.1016/j.yhbeh.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B, Warren BL. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17:247–255. doi: 10.3109/10253890.2014.910650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. National comorbidity survey replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of k-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bondar NP, Avgustinovich DF. Effects of Repeated Experience of Aggression on the Aggressive Motivation and Development of Anxiety in Male Mice. Neurosci Behav Physiol. 2004a;34:721–730. doi: 10.1023/b:neab.0000036013.11705.25. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Gerrits MAFM, Avgustinovich DF, Tenditnik MV, Van Ree JM. Modulation of anxiety-related behaviors by m- and k-opioid receptor agonists depends on the social status of mice. Peptides. 2004b;25:1355–1363. doi: 10.1016/j.peptides.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa-opioid receptor: from addiction to depression, and back. Front Psychiatry. 2014;5:1–17. doi: 10.3389/fpsyt.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman-Maharg A, Williams AV, Zufelt MD, Minie VA, Ramos-Maciel S, Hao R, Ordones Sanchez E, Copeland T, Silverman JL, Leigh A, Snyder R, Carroll FI, Fennell TR, Trainor BC. Kappa opioid receptor antagonists reduce immobility in male but not female rodents in the forced swim test. Front Pharmacol. 2018;9:1–11. doi: 10.3389/fphar.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laman-Maharg AR, Copeland T, Ordones Sanchez E, Campi KL, Trainor BC. The long-term effects of stress and kappa opioid receptor activation on conditioned place aversion in male and female California mice. Behavioural Brain Research. 2017;332:299–307. doi: 10.1016/j.bbr.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Therapeutics. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley B, Miles F, Dixon R. Time course of attentional bias for threat scenes: testing the vigilance-avoidance hypothesis. Cognition and Emotion. 2004;18:689–700. [Google Scholar]
- Molendijk ML, de Kloet ER. Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology. 2015;62:389–391. doi: 10.1016/j.psyneuen.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Moreau J-L, Jenck F, Martin JR, Mortas P, Haefely WE. Antidepressant treatment prevents chronic mild stress-induced anhedonia as assessed by ventral tegmentum self-stimulation behaviour in rats. Eur Neuropsychopharmacol. 1992;2:43–49. doi: 10.1016/0924-977x(92)90035-7. [DOI] [PubMed] [Google Scholar]
- Peters MF, Zacco A, Gordon J, Maciag CM, Litwin LC, Thompson C, Schroeder P, Sygowski LA, Piser TM, Brugel TA. Identification of short-acting k-opioid receptor antagonists with anxiolytic-like activity. Eur J Pharmacol. 2011;661:27–34. doi: 10.1016/j.ejphar.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Potter DN, Damez-Werno D, Carlezon WA, Jr, Cohen BM, Chartoff EH. Repeated exposure to the k-opioid receptor agonist salvinorin a modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiatry. 2011;70:744–753. doi: 10.1016/j.biopsych.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles CF, McMackin MZ, Campi KL, Doig IE, Takahashi EY, Pride MC, Trainor BC. Effects of kappa opioid receptors on conditioned place aversion and social interaction in males and females. Behav Brain Res. 2014;262:84–93. doi: 10.1016/j.bbr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SE, Rachlin AB, Smith KL, Muschamp JW, Berry L, Zhao Z, Chartoff EH. Sex difference in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry. 2013;76:213–222. doi: 10.1016/j.biopsych.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AL, Fry WH, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res. 2010;208:528–534. doi: 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76:281–288. doi: 10.1016/j.biopsych.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLOS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav. 2013;63:543–550. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza M, Butelman ER, Kreek MJ. Effects of the novel relatively short-acting kappa opioid receptor antagonist LY2444296 in behaviors observed after chronic extended-access cocaine self-administration in rats. Psychopharmacology. 2017;234:2219–2231. doi: 10.1007/s00213-017-4647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology. 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Bolaños Guzmán CA. k-opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology. 2009;34:1339–1350. doi: 10.1038/npp.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of Sucrose Preference by Chronic Unpredictable Mild Stress, and Its Restoration by a Tricyclic Antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]