Abstract
We measured HIV incidence rate, trend and risk factors in 564 HIV-negative young people (<30 years) who inject drugs (PWID) in San Francisco between 2000 and 2014. HIV incidence was 0.93/100 person-years (PY; 95% CI: 0.50, 1.73). Incidence varied between 0.62/100 PY in 2000–2002 and 1.06/100 PY in 2012–2014 (P for trend =1.0). HIV incidence varied significantly (P < 0.01) by race/ethnicity: among Hispanics it was 8.19/100 PY (95% CI: 3.41, 19.68), African-Americans 4.59/100 PY (95%CI: 1.15, 18.37), and Whites 0.26/100 PY (95% CI: 0.06, 1.03). Male participants who reported sex with men (MSM) had higher HIV incidence (2.63/100 PY; 95%CI: 1.31, 5.25) compared to males who did not report MSM (0.50/100 PY; 95%CI: 0.12, 1.99) (P = 0.01). Despite an overall stable HIV incidence trend, incidence was elevated among African-American and Hispanic PWID, and men who have sex with men. Addressing prevention needs in these key populations is critical for the goal of eliminating HIV transmission.
Keywords: HIV, Incidence, Epidemiology, Drug Users, Injection, Young Adults, Cohort Studies, San Francisco
Author summary
Medimos la tasa de incidencia y factores de tendencia y de riesgo del VIH en 564 jóvenes (menores de 30 años) VIH-negativos que se inyectan drogas en San Francisco entre el 2000 y el 2014. La incidencia de VIH fue de 0.93/100 personas-años (IC 95%: 0.50, 1.73). La incidencia varió entre 0.62/100 personas-años entre el 2000 y el 2002 y 1.06/100 personas-años entre el 2012 y el 2014 (P de tendencia =1.0). La incidencia del VIH varió significativamente (p<0.001) por raza/etnicidad: entre Hispanos fue de 8.19/100 personas-años (IC 95%: 3.41, 19.68), Afroamericanos 4.59/100 personas-años (IC 95%: 1.15, 18.37), y Blancos 0.26/100 personas-años (IC 95%: 0.06, 1.03). Los participantes masculinos que reportaron sexo con hombres tuvieron la mayor incidencia de VIH (2.63/100 personas-años; IC 95%: 1.31, 5.25) en comparación con participantes masculinos que no reportaron sexo con hombres (0.50/100 personas-años; IC 95%: 0.12, 1.99) (P = 0.01). A pesar de una tendencia de incidencia de VIH universalmente estable, la incidencia fue elevada entre personas (que se inyectan) Afroamericanas e Hispanas, y entre hombres que tienen sexo con hombres. Dirigir necesidades de prevención en estas poblaciones clave es vital para la meta de eliminar la transmisión del VIH.
INTRODUCTION
In 2014, an estimated 8.9% (3,954) of all HIV diagnoses in the United States (U.S.) were attributed to injection drug use (1). In San Francisco, California, an early epicenter of the HIV epidemic in the 1980s, there has been a sustained HIV prevention response targeting people who inject drugs (PWID) (2). Widespread publically-funded harm reduction efforts have included the following: needle and syringe programs (NSP) beginning in 1989 (3, 4); non-prescription pharmacy sales of syringes since 2004 (5); medication-assisted treatment programs for opioid use disorder, including publically-funded methadone since the 1970s (6) and buprenorphine since 2005 (7); as well as programs to curb methamphetamine injection (8). HIV counseling and testing programs have been implemented widely in both clinical and community settings in San Francisco (9). While these initiatives have played a significant part in reducing risk of HIV among PWID (10), ongoing high risk sexual and drug-use exposures and sub-optimal access to health and prevention services contribute to continuing risk for infection (2).
In 2014, the San Francisco Department of Public Health estimated that 15,979 residents in San Francisco were living with HIV, and that 6% of these were in PWID (11). Including men who have sex with men (MSM) who inject drugs, the estimated proportion of people living with HIV that have had injection related exposure is 21%. HIV is clustered principally in the MSM population (74% of the reported infections), but there remains potential for emergent or even new outbreaks of HIV in other risk groups (11). Such outbreaks have been shown to occur among PWID in several countries (10) including the U.S., demonstrating how HIV can spread rapidly in populations with poor access to HIV prevention (12). At a national meeting in 2013 focused on the expanding hepatitis C virus (HCV) epidemic in young adult PWID, the potential for HIV outbreaks was raised as a significant concern (13, 14). As a result of the Indiana outbreak in Scotts County where 135 new HIV cases were diagnosed over a three month period, and where annually fewer than five HIV cases have been reported, (12) and other data (15) showing increasing HCV infection in PWID, the U.S. Centers for Disease Control and Prevention (CDC) has urged health departments nationwide enhance surveillance of HIV and HCV cases among PWID as part of the effort to prevent outbreaks elsewhere (16). Young adult PWID are an important high-risk population to monitor and toward whom to direct prevention efforts against both HCV and HIV (15, 17–19).
The primary aim of this study is to assess the trends and epidemiological parameters of HIV infection in young adult PWID in San Francisco. Given the high level of combined HIV prevention services in San Francisco, we hypothesize that although HIV incidence among PWID has remained low, it is likely there are subgroups with elevated incidence. We utilized data collected between 2000 and 2014 from a prospective cohort study in San Francisco to examine HIV incidence overall and among potential high-risk subgroups.
METHODS
Study design and participants
The UFO Study is an ongoing prospective observational cohort study of young adult PWID in San Francisco. The study’s primary goal is to recruit and follow HCV-negative study participants and assess the risk factors, incidence, and natural history of acute HCV infection. The details of the study design and methods have been previously published (4, 20–22). In brief, potential study participants were recruited using street-based outreach. People who reported drug injection in the previous month, were younger than 30 years old, understood and spoke English, did not report being infected with HCV (since 2003), and did not plan to leave San Francisco within three months, were invited to receive an HCV blood test. Participants were tested for anti-HCV and HCV RNA (20) at baseline, and those with negative RNA results who consented were enrolled into a prospective cohort and followed at quarterly visits, which included structured risk behavior interviews and HCV testing. HIV testing was offered at study visits but not required for study participation. Blood specimens were collected at each study visit and stored in a specimen repository. All participants were provided risk reduction counseling concomitant with HCV and HIV testing. Participants received financial compensation for baseline (up to $15) and follow up visits ($20-$40). The study also provided some on-site medical care, immunizations (hepatitis A and B, seasonal influenza, and tetanus/diphtheria/pertussis), and referrals to primary care, mental health, and drug treatment services.
Data collection and questionnaire
A comprehensive, interviewer-administered questionnaire was used at baseline and during quarterly visits to collect demographic information (age, gender, education, race, housing status, incarceration (jail and or prison) lifetime history, and information on injection-related risk exposures (duration, frequency and types of drugs used, and sharing syringes/needles or rigs or cookers), sexual-related risk exposures (including unprotected sex, exchange sex, and number of sexual partners), and the use of prevention services (accessing needles through NSP and/or pharmacies, attending drug treatment program, and other HCV and HIV testing and counseling services). The survey queried if participants ‘ever’ and ‘recently’ (in the previous 3 months) engaged in injection-related exposures.
Primary outcome
The primary outcome of this study was estimated HIV incidence rate. We included participants enrolled between 2000–2014 who tested negative for HIV at baseline and who had one or more follow-up visits that included blood specimen collection (Fig. 1). We included data collected from all three of the recruitment waves. HIV testing was offered, but not conducted systematically as part of the study protocol; therefore, we used stored blood specimens obtained from past study visits to assess HIV infection. HIV infection status was determined using GS HIV Combo Ag/Ab EIA (4th gen assay) with GS HIV-1 Western Blot assay confirmation (Bio-Rad Laboratories, Redmond, WA, USA). Tests were first conducted on the last participant visit. For positive cases, we retrospectively tested the blood samples from previous visits to ascertain the earliest confirmed seropositive sample. The HIV infection date was estimated as the midpoint between last documented seronegative and first confirmed seropositive blood sample. Self-reported data for new infection was used to define the date of new infection for participants who did not have lab results or reported a positive HIV test result after their last study visit. HIV negative participants were censored at the last study visit date.
Figure 1. Study cohort participation.
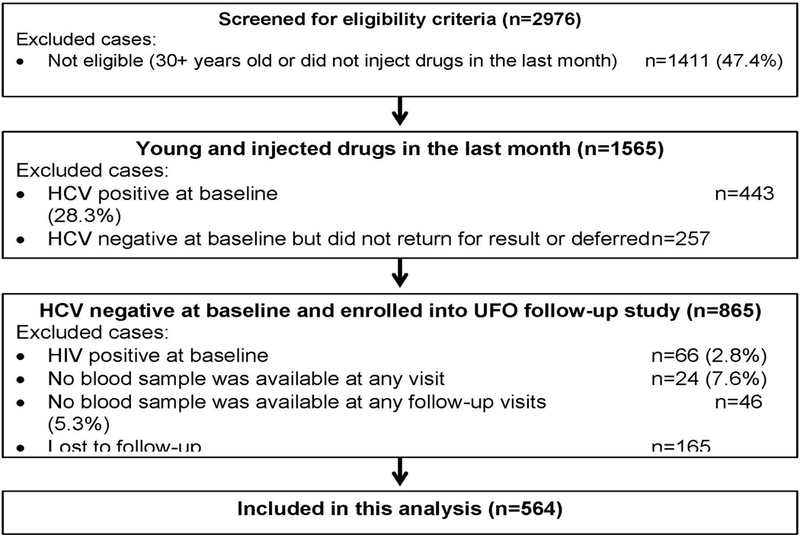
-
Other variables and exposures of interest
Variables of a-priori interest included age, age at first injection, gender (male, female, transgender) and race/ethnicity. These were chosen based on a published systematic review and a national report on HIV risk among PWID (23, 24). Race/ethnicity was categorized as the following: White (anyone reporting Caucasian or White); African-American (including if they reported being Black); Hispanic (those reporting non-Black Latino or Hispanic); and ‘Other’, which primarily due to small numbers included anyone identifying as Asian, Filipino, or Native American. Participants who reported more than one racial-ethnic group were categorized as African-American if multiple-races including African-American was reported, or Hispanic if multiple ethnicities including Hispanic was reported. Male participants who reported ever or recently having (in last 3 months) same-sex sexual contacts were classified as men who have sex with men (MSM). Regarding the type of the injected drug used most in last 30 days, reported drugs at each visit were categorized as follows: (1) opioids (including heroin, morphine, methadone, oxycodone, hydrocodone); (2) stimulants (including methamphetamine, amphetamine, speed, cocaine, and or crack); (3) mixed opioid/stimulant (speedball [cocaine with heroin] and goofball [speed with heroin]); and (4) other miscellaneous drugs among them benzodiazepines and ketamine.
Statistical Analyses
Enrollment characteristics of all eligible study participants were assessed using descriptive statistics. To determine whether the population characteristics varied over time, we conducted the Cuzick nonparametric test for trend (25). We examined demographic characteristics, risk exposures and preventive behaviors at enrollment over five time intervals (2000–2002, 2003–2005, 2006–2008, 2009–2011, 2012–2014). Incidence of HIV infection among defined subgroups of participants were calculated using life table methods based on estimated person-years (PY) of time at risk, with accompanying 95% confidence intervals (95% CI) calculated based on a Poisson distribution. We used the Log-rank test to evaluate marginal, between-group differences in cumulative incidence rates in groups defined by characteristics and risk behavior patterns.
We assessed associations between known confounders (NSP, drug treatment and incarceration), and all key demographic and behavioral variables (listed in Table 2) of HIV incidence in bivariate analyses using Cox proportional hazards regression. Those variables significantly associated with HIV incidence with a p-value < 0.05 were included in the multivariable analyses. The time scale for the analysis was the follow-up time since entry into the study. Potential violations of the proportional hazards (PH) assumption were assessed using log-minus-log survival plots and scaled Schoenfeld residual statistics. All predictors, except ‘ever’ reported sex with men (MSM exposure), satisfied the PH assumption via graphical and formal test evaluation. Because the graphical assessment for sexual behavior revealed no obvious departure from PH (26), the PH assumption was retained for this variable as well. For the two numerical predictors, age at baseline and age at first injection, we considered linear, categorical and nonlinear (restricted cubic spline with five knots) effects in models. The test for nonlinearity effect of both was not significant (p=0.213 for age and p=0.938 for age at first injection). Age at first injection was excluded from the final model since it was not statistically significant in the marginal analysis. Considering the small number of events (only 10 HIV seroconversions were found), the related possibility of violating the positivity assumption (27), and the fact that all models yielded similar interpretations, we retained age as a linear term in subsequent models. We also included baseline male sexual behavior or MSM exposure (a binary indicator of ‘ever’ reported sex with men) as fixed predictor. To further examine the association between MSM exposure and HIV incidence, we compared the Cox regression coefficient (28) for MSM in models with and without two behaviors, “any unprotected sex, in last 3 months” and “any borrowing or sharing of needles and equipment, in last 3 months”. This analysis was performed to assess whether the higher HIV risk among MSM mediated by unprotected sex or unsafe injection or both.
Table 2. – HIV Incidence in by selected demographic characteristics and exposures among young adults who inject drugs; San Francisco, 2000–14.
| Variable | # Events | Person years | HIV Incidence | 95% CI | Pa |
|---|---|---|---|---|---|
| Overall | 10 | 1073.80 | 0.93 | 0.5, 1.73 | ---- |
| Age groups at enrolment, years |
0.01 (X2= 9.12) |
||||
| 15–19 | 0 | 230.48 | 0 | 0 | |
| 20–24 | 9 | 466.60 | 1.93 | 1, 3.71 | |
| 25–29 | 1 | 376.72 | 0.27 | 0.04, 1.88 | |
| Gender | 0.24 (X2=2.84) |
||||
| Male | 9 | 679.72 | 1.32 | 0.69, 2.54 | |
| Female | 1 | 369.92 | 0.27 | 0.04, 1.92 | |
| Transgender | 0 | 9.22 | 0 | ||
| High school and more | 6 | 553.47 | 1.08 | 0.49, 2.41 | 0.57 (X2=0.32) |
| Race/ethnicity |
0.01 (X2=55.47) |
||||
| White | 2 | 778.70 | 0.26 | 0.06, 1.03 | |
| African-American | 2 | 43.53 | 4.59 | 1.15, 18.37 | |
| Hispanic | 5 | 61.03 | 8.19 | 3.41, 19.68 | |
| Other | 1 | 190.53 | 0.52 | 0.07, 3.73 | |
| Homeless, past 3 months | 4 | 534.05 | 0.75 | 0.28, 2 | 0.40 (X2=0.70) |
| Ever incarcerated (prison, jail or juvenile hall) | 8 | 708.15 | 1.13 | 0.56, 2.26 | 0.22 (X2=1.53) |
| Any alcohol use, in past 3 months | 5 | 595.00 | 0.84 | 0.35, 2.02 | 0.56 (X2=0.35) |
| Age at first drug injection, years | 0.62 (X2=0.97) |
||||
| <20 | 8 | 736.18 | 1.09 | 0.54, 2.17 | |
| 20–25 | 1 | 265.55 | 0.38 | 0.05, 2.67 | |
| >25 | 1 | 67.70 | 1.48 | 0.21, 10.49 | |
| Type of drug used most days in past month | 0.09 (X2=6.53) |
||||
| Opioids | 2 | 388.66 | 0.51 | 0.13, 2.06 | |
| Stimulants | 6 | 234.65 | 2.56 | 1.15, 5.69 | |
| Opioids with stimulants | 0 | 49.86 | 0 | 0 | |
| Others | 0 | 12.09 | 0 | 0 | |
| Ever experienced a drug overdose | 2 | 324.62 | 0.62 | 0.15, 2.46 | 0.35 (X2=0.87) |
| Drug overdose in last 3 months | 0 | 43.03 | 0 | 0 | 0.49 (X2=0.48) |
| Injected every day in the last month | 0 | 230.89 | 0 | 0 | 0.09 (X2=2.58) |
| Ever accessed needle and syringe program service | 3 | 394.77 | 0.76 | 0.25, 2.36 | 0.48 (X2=0.50) |
| Recent access of needle and syringe program service, past 30 days | 5 | 552.02 | 0.91 | 0.38, 2.18 | 0.85 (X2=0.04) |
| Borrowed someone else’s rig to inject drug in last 3 months | 0 | 210.65 | 0 | 0 | 0.09 (X2=2.95 |
| Shared cooker for dissolving drugs in last 3 months | 1 | 375.39 | 0.27 | 0.04, 1.89 | 0.08 (X2=3.03) |
| Any history of drug treatment (lifetime) | 3 | 498.33 | 0.60 | 0.19, 1.87 | 0.09 (X2=2.87) |
| Recent drug treatment, in last 3 months | 1 | 245.75 | 0.41 | 0.06, 2.89 | 0.26 (X2=1.27) |
| Recent unprotected sex, in last 3 months | 4 | 481.41 | 0.83 | 0.31, 2.21 | 0.43 (X2=0.62) |
| Ever had sexual relations with same-sex partner (males) | 8 | 304.76 | 2.63 | 1.31, 5.25 |
0.01 (X2=7.45) |
| Recent sexual relations with same-sex partner in last 3 months (males) | 3 | 75.08 | 4.00 | 1.29, 12.39 |
0.01 (X2=9.38) |
| Recent exchange sex for money or drugs, in last 3 months (any gender) | 1 | 71.41 | 1.40 | 0.2, 9.94 | 0.49 (X2=0.48) |
| Number of sexual partners in last 3 months | 0.19 (X2=3.29) |
||||
| 0 (not sexually active) | 2 | 210.12 | 0.95 | 0.24, 3.81 | |
| 1 | 1 | 318.34 | 0.31 | 0.04, 2.23 | |
| 2 or more | 5 | 295.55 | 1.69 | 0.7, 4.06 |
Abbreviations: CI, confidence interval; P, P values. All rates are per 100 person-years observation.
a P values of Log-rank test for equality of survivor functions. P < 0.05 are presented in bold font.
To assess the possible impact of selection bias, we compared the baseline characteristics of those included into this analysis with those excluded (lost to follow-up). We also accounted for the impact of possible informative censoring on regression results using inverse probability of censoring weighted methods (27). Weights were based on the estimated the probability of censoring from a logistic regression model, with predictors and interactions selected using a 1% significance level. We used both baseline fixed characteristics (age, education, age at first injection, ever needle exchange, and ever same-sex relationship) and time-varying covariates with one visit lag (recent alcohol use, recent daily injection and recent drug treatment) to calculate the censoring weights. The goodness of fit and discrimination ability of the model was acceptable (Hosmer-Lemeshow, p=0.522; C-statistic = 0.59). Inverse probability of censoring weights varied from 1.00 to 12.35, and we truncated extreme weights at the 5th (1.03) and 95th (1.93) percentiles. We used weighted, pooled logistic regression to estimate the adjusted odds ratio (AOR) for the effect of different predictors on the HIV incidence rate outcome. The adjusted ORs from this approach provide a good approximation to the HRs obtained from the proportional hazard regression model (29). All analyses were performed with Stata, version 13.1 (Stata Corp, College Station, Texas).
Ethical review
The study protocol was reviewed and approved by the University of California, San Francisco Institutional Review Board. All participants provided written informed consent to participate in the study, for specimen storage and testing for blood-borne viruses including HIV.
RESULTS
Out of 2,976 screened persons, a total of 865 eligible persons were enrolled in the prospective study (Fig. 1). Sixty-six (7.61%) were HIV positive at their enrollment visit, of whom 34 (51.52%) reported that they knew their HIV status. In 94.26% of visits, HIV seronegative people reported their HIV status correctly. Excluding those who did not provide any blood at a baseline visit (2.82%, n=24), who tested HIV positive at enrollment (7.64%, n=66), who had no follow-up visit (19.15%, n=165), and who provided no blood samples in follow-up (5.30%, n=46), 564 participants were included in this analysis.
At enrollment (Table 1), the participants were mostly aged between 20 to 24 years old (43.79%), were male (65.43%), completed high school (60.82%), were White (72.70%), and the majority (69.34%) were homeless during the previous three months. Almost two-thirds (65.24%) started drug injection under 20 years old and 58.08% used opioids as their principally injected drug. In the three months prior to the interview, 34.88% reported injecting daily, 38.10% borrowed someone else’s rig to inject with, and 60.50% shared a cooker for dissolving drugs. The majority (77.90%) reported using a NSP in the past 30 days and 18.93% reported attending a drug treatment program/facility in the past 3 months. Unprotected sex was reported by 78.98% of participants, and 27.5% of men reported ever having male-to-male sexual (MSM) behavior. Participants’ characteristics at enrollment and over the five time intervals are presented in Supplemental Table 1.
Table 1. Baseline demographic characteristics and risk exposures of young adult people who inject drugs in San Francisco (n=564).
–
| Characteristic/Exposure | Categories | No. | % |
|---|---|---|---|
| Age groups, years | 15–19 | 107 | 18.97 |
| 20–24 | 247 | 43.79 | |
| 25–29 | 210 | 37.23 | |
| Gender | Male | 369 | 65.43 |
| Female | 192 | 34.04 | |
| Transgender | 3 | 0.53 | |
| High school or higher education level | 340 | 60.82 | |
| Race/ethnicity | White | 410 | 72.70 |
| African-American | 27 | 4.79 | |
| Hispanic | 39 | 6.91 | |
| Other | 88 | 15.60 | |
| Homeless, past 3 months | 389 | 69.34 | |
| Ever incarcerated (prison, jail or juvenile hall) | 451 | 80.68 | |
| Any alcohol use, in past 3 months | 452 | 80.43 | |
| Age at first drug injection, years | <20 | 366 | 65.24 |
| 20–25 | 159 | 28.34 | |
| > 25 | 36 | 6.42 | |
| Type of drug used most days in past month | Opioids | 320 | 58.08 |
| Stimulants | 184 | 33.39 | |
| Opioids with stimulants | 34 | 6.17 | |
| Others | 13 | 2.36 | |
| Ever experienced a drug overdose | 205 | 36.74 | |
| Drug overdose in last 3 months | 77 | 13.70 | |
| Injected every day in the last month | 196 | 34.88 | |
| Ever accessed needle and syringe program service | 258 | 85.43 | |
| Recent accessed of needle and syringe program, past 30 days | 437 | 77.90 | |
| Borrowed someone else’s rig to inject in last 3 months | 213 | 38.10 | |
| Shared cooker for dissolving drugs in last 3 months | 340 | 60.50 | |
| Any history of drug treatment (lifetime) | 365 | 65.53 | |
| Recent drug treatment, in last 3 months | 106 | 18.93 | |
| Recent unprotected sex, in last 3 months | 372 | 78.98 | |
| Ever had sexual relations with same-sex partner (males) | 151 | 27.50 | |
| Recent sex with same-sex partner, in last 3 months (males) | 69 | 19.71 | |
| Recent exchange sex for money or drugs, in last 3 months (any gender) | 79 | 15.58 | |
| Number of sexual partners in last 3 months | 0 (not sexually active) | 81 | 14.73 |
| 1 | 209 | 38.00 | |
| 2 or more | 260 | 47.27 |
Participants who were lost to follow-up (N=165; 19.1%) after the baseline visit were more likely to be younger (X2=10.85, p=0.004), less educated (X2=3.39, p=0.066), using alcohol (X2=7.46, p=0.006), injecting at younger ages (X2=5.51, p=0.064), injecting less than daily (X2=13.91, p=0.001) (Supplemental Table 1). In addition, they were less likely to have ever accessed a NSP (X2=8.24, p=0.004), recently attended a drug treatment program (X2=4.31, p=0.038), had recent unprotected sex (X2=7.41, p=0.006) and ever MSM exposure (X2=5.49, p=0.019). These are the variables that we used to calculate the censoring weights.
Over the 1073.80 PY, 10 incident HIV cases were identified, for an overall incidence of 0.93/100 PY (95% CI: 0.50, 1.73). We identified seven of the ten new HIV infections from the repository. Of the seven, four were aware of their status (self-reported HIV+ on risk assessment or behavioral questionnaire), two also tested positive at regular study visits; one was lost to follow-up. Fig. 2 presents HIV incidence over five time-intervals since 2000. HIV incidence varied over time but not significantly. The highest incidence was observed during 2003–2005 at 2.25/100 PY (95% CI: 0.94, 5.41) and the lowest during 2009–2011, when no new infections were detected. Most recently (2012–2014), a modest increase was observed. Significant differences (X2=9.12, p=0.001) were observed in HIV incidence rate by race/ethnicity (Table 2): Hispanics had the highest HIV incidence (8.19/100 PY; 95% CI: 3.41, 19.68), followed by African-Americans (4.59/100 PY; 95% CI: 1.15, 18.37), compared to Whites (0.26/100 PY; 95% CI: 0.06, 1.03). Among men who reported any lifetime MSM exposure, incidence was 2.63/100 PY (CI 95%: 1.31, 5.25), lower than the incidence rate (4.0/100 PY, 95% CI: 1.29, 12.39) observed for men who reported recent MSM exposure, but not statistically significantly different (X2=0.21, p=0.648). Both were significantly higher (X2=3.66, p=0.05) than male PWID who did not report MSM exposure (0.50/100 PY; 95% CI 0.12, 1.99). Table 2 shows HIV incidence by several subgroups, including gender, race/ethnicity, and behavioral exposures. The HIV incidence rate ratio for MSM PWID compared to heterosexual male PWID was 9.8 (95% CI: 1.32, 436.07) (X2=7.13, p=0.004).
Figure 2. Trend in HIV incidence per 100 person-years among young people who report injection drug use, UFO study, San Francisco, 2000–14.
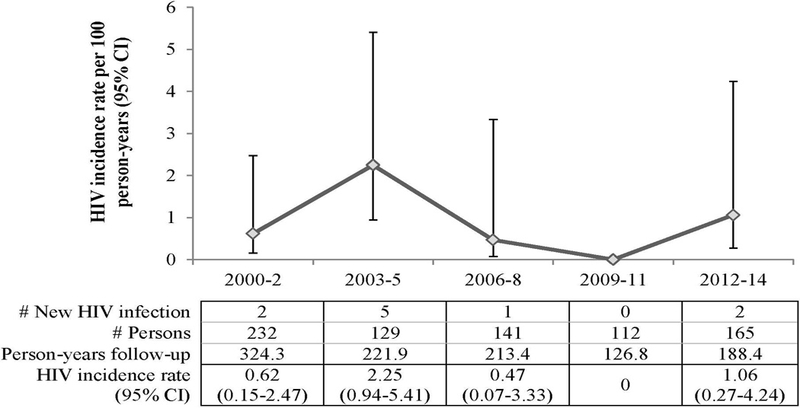
-
Results from the unadjusted and multivariable Cox proportional hazards model are presented in Table 3. In adjusted analyses, African-Americans and Hispanic participants respectively had adjusted hazard ratios (AHR) for HIV acquisition of 39.68 (95% CI: 1.77, 887.27) and 60.29 (95% CI: 4.70, 773.17), compared to White participants. In compare to heterosexual males and females, MSM had significantly higher independent risk for HIV acquisition (AHR 9.83, 95% CI: 1.45, 66.77). After adjustment for loss to follow-up by inverse probability weighting (Supplemental Table 3), African-Americans (AOR = 26.05, 95% CI: 1.39, 1722.31), and Hispanics (AOR 87.58, 95% CI: 2.03, 3761.62) compared to White participants and MSM compared to others (AOR 85.96.1, 95% CI: 6.23, 1186.68) remained at significantly higher risk for HIV acquisition.
Table 3. Predictors of HIV incidence among young adult people who inject drugs; San Francisco, 2000–14.
–
| Variable | Crude HR | 95% CI | Adjusted HR a | 95% CI |
|---|---|---|---|---|
| Age at enrolment, years | 0.97 | 0.81, 1.15 | 0.83 | 0.59, 1.15 |
| Race/ethnicity | ||||
| White | 1 | Referent | 1 | Referent |
| African-American | 26.67 | 3.56, 199.69 | 39.68 | 1.77, 887.27 |
| Hispanic | 40.42 | 7.74, 211.2 | 60.29 | 4.70, 773.17 |
| Other | 2.03 | 0.18, 22.45 | 5.35 | 0.31, 92.80 |
| Sexual behavior | ||||
| Men heterosexual or female | 1 | Referent | 1 | Referent |
| Men who ever had sex with men | 10.25 | 2.16, 48.58 | 9.83 | 1.45, 66.77 |
| Recent needle and syringe program service, past 30 days | ||||
| No | 1 | Referent | 1 | Referent |
| Yes | 0.87 | 0.21, 3.64 | 1.94 | 0.23, 16.31 |
| Recent drug treatment in last 3 months | ||||
| No | 1 | Referent | 1 | Referent |
| Yes | 0.32 | 0.04, 2.61 | 1.34 | 0.10, 18.01 |
| Ever incarcerated (prison, jail or juvenile hall) | ||||
| No | 1 | Referent | 1 | Referent |
| Yes | 0.58 | 0.07, 4.97 | 0.36 | 0.03, 4.72 |
Abbreviations: HR, hazard ratio; CI, confidence interval;
a Includes all variables with significant Log-rank test in Table 2 at the level of 0.05, and needle exchange, drug treatment and incarceration as already known confounders for HIV incidence.
Further analysis indicated that the AHR of MSM exposure on HIV incidence after removing the path through unsafe sex (by including it in the regression model) was reduced to 3.21 (95% CI: 0.19, 54.27). Moreover, the AHR of MSM on HIV incidence after removing path through unsafe injection is 9.41 (95% CI: 1.15, 77.06). The direct effect (measured by AHR) of MSM on HIV incidence after removing the two indirect paths through unsafe sex and injection is 1.11 (95% CI: 0.05, 24.05). Given these results, we estimated that 67.3% [ = (9.83 – 3.21 / 9.83] and 4.2% [ = (9.83 – 9.41 / 9.83] of the MSM total effect on HIV incidence was through unsafe sex or unsafe injection, respectively.
DISCUSSION
This 14-year study of young adult PWID showed HIV incidence ranging from 0.93 to 1.06 /100 PY with no significant time trend. These results are consistent with findings from the study during the previous decade (1990 to 1999) that also found stable HIV incidence, mostly under 1% in PWID in San Francisco (30). Even with a wide range of services and prevention programs offered to PWID in San Francisco (2), and increases in HIV prevention services in the U.S. and San Francisco, (31, 32), our data did not show a declining trend of the HIV incidence, but rather a stabilized and potentially low endemic or steady- state level. Further improvements upon prevention services such as multicomponent interventions (33), network-based interventions (34) to reduce risk at the community level, pre-exposure prophylaxis (35), and novel outreach programs to reach and serve hard-to-reach minorities (36) may have potential to further reduce HIV in young adult PWID. These results are also consistent with other studies in the U.S showing low HIV incidence rates among PWID, including in New York (0.4 per 100 PY, 95% CI: 0.0, 2.5) (37). Low HIV prevalence has been observed in a recent study of PWID in San Diego (4.2%, 95% CI: 2.4, 5.9) (38). Estimates are lower than observed HIV incidence in Montreal (which declined from 3.5 per 100 PY to 1.8 per 100 PY) over similar time periods (39), and in Vancouver (2.49/100 PY (40)) although both of these studies involved older PWID and a higher prevalence of cocaine injection, which has been associated with increased HIV risk (41). The CDC estimated an average annual rate of new HIV infection diagnosis of 3 per 100,000 PWID (40) from data from 34 states in 2004–2007, and Lansky et al., (42) estimated 55 per 100,000 PWID in a literature review of U.S. studies. Our observed rate is higher (equivalent to 930 per 100,000), suggesting that a considerable number of new HIV infections among PWIDs in the U.S. may remain undiagnosed. In order to achieve the goal of an HIV-free generation, interventions to diagnose HIV early among PWID are critical.
An important finding in this study was that African Americans and Hispanics were at much higher risk of HIV compared to White participants. These findings mirror other research in older PWID by others, wherein with both African Americans and Hispanics have been shown to have higher HIV incidence than Whites, and research suggests it is a more significant problem in the U.S. compared to other countries (23, 43). Surprisingly, however we found that HIV incidence among Hispanics was higher than observed among African Americans; which is not reflective of national 2015 surveillance data on HIV diagnosis rates, which show higher diagnosis rates among African Americans than Hispanics (44). Given that California has the largest population of Hispanics (14.4 million in 2011) in the country (45), Hispanic PWID need to be prioritized for HIV prevention, diagnosis and treatment interventions. Our finding of increased risk for HIV in association with race/ethnicity in young adult PWID highlights critical gaps in the reach of prevention interventions. Des Jarlais et al., (43) have documented these important racial/ethnic disparities in HIV prevalence along with low uptake of interventions by minorities. Higher HIV prevalence and associated risk exposures have been reported among African-American (11%) and Hispanic (10%) PWID in the CDC’s National Health Behavior Survey conducted in 20 U.S. cities in 2009 (24), along with lower HIV testing rates, more undiagnosed HIV, and lower access to drug and alcohol treatment (2, 24). Access to and utilization of HIV prevention services among Hispanic men (46) and African American MSM (47) have been reported to be less than for Whites. African American MSM were also reported at greater risk for HIV infection not because of their risk behaviors, but because of several mediating social factors including living neighborhood, having sexual partners from high-risk networks as well as lower access to HIV prevention services due to poverty (47).
Previous studies reported challenges for young African American MSM in accessing to and uptake of PrEP (48, 49), a standard HIV prevention method, despite high levels of PrEP interest and attempts to reduce structural barriers affecting access. While stigma and discrimination have been suggested as one of the main structural barriers (49), further studies needed to understand the underlying causes of suboptimal HIV prevention uptake in young African American MSM and particularly black MSM-PWID who may experience even higher level of stigma and discrimination due to injection. In comparison to Whites, African Americans and Hispanics reported higher level of discrimination due to drug use (50) and injection (51). Developing discrimination- and stigma-free HIV prevention programs targeted to these marginalized populations are needed.
Recent studies in San Francisco among MSM, suggest sexual segregation by African American MSM may contribute to increased HIV transmission in minorities resulting from smaller and highly interconnected social groupings (52). These smaller networks can result in increased risk as a result of smaller degrees of separation with and reduced ‘randomness’ in partner selection (53). Future investigations may elucidate the relative contributions of network structures and individual risk exposures in young adult PWID, and also whether phylogenetic clusters are connected by shorter transmission chains (54).
In San Francisco, we also found that HIV risk is significantly increased among male PWID who have sex with men, which is unsurprising since the HIV epidemic in this city remains centered in MSM. Eight of nine males infected with HIV in this study reported MSM exposure, with a corresponding 20-fold increased risk compared to heterosexual male PWID that has been ongoing since we first began sampling this population in 2000 (55). Our additional analysis suggested that MSM-PWID are at higher risk for HIV incidence through unsafe sex pathway, rather than unsafe injection. Risk exposures known to be associated with HIV in male PWID who have sex with men (55, 56) show little change over time in this prospective analysis, including: no change in the proportion of sex partners or unprotected sex along with concerning increases in homelessness and the proportion of participants who report exchange sex (Supplemental Table 2). These results and other data from San Francisco (57), which show a prevalence ratio of 6.5 to 1 in male PWID who report sex with men compared to heterosexual male PWID, clearly show a need for accelerated and targeted HIV prevention efforts specific to male PWID who have sex with men (57), some of whom may not identify as gay or bisexual (58) and prevention services aimed at MSM.
Our study has some limitations. Close to 20% of participants were lost to follow-up, and those ‘lost’ varied in significant ways including some demographic characteristics (i.e. age and education but not gender and race/ethnicity) and risk behaviors. Since those more likely to be lost were younger, injected drugs less frequently, had less unprotected sex, and less likely to ever have male-to-male sexual contact compared to those who remained in the study, our observed HIV incidence might be overestimated. The UFO Study uses several strategies to maximize retention rate including: screening out participants who plan to travel (19) (initiated in 2003); intensive street-based outreach with contact tracing; tracking and accessing participants who are incarcerated for one month or more: and the use of electronic messaging and social media. Statistical adjustment using inverse probability weighting to adjust for loss to follow-up effects on study measures suggests that our findings regarding the association between race/ethnicity, same-sex relationship and excess HIV risk are robust. The impact of “missingness” is difficult to quantify even with statistical adjustments (59). We used both baseline and follow-up characteristics to predict and assess this impact, however, there may be other, unmeasured factors that account for loss to follow-up. More should be done to understand, prevent, and adjust for such factors, since this is a source of bias that impacts much of our understanding of risk in PWID (60, 61). It is possible that male participants underreported sex with other men; however, the effect of such misclassification would be bias to the null. Finally, only 10 new HIV infections were detected in our cohort, and this small number may have restricted our ability to examine other behavioral risk factors for new HIV infection.
Stable and low HIV incidence rates among young adult PWID in San Francisco are heartening, but our data suggest, as elsewhere (62), that while current HIV prevention efforts may be effective and durable, they are not enough to eliminate the racial/ethnic disparities in excess HIV risk among young adult PWID in urban settings (63). Unlike the HCV epidemic and the HIV outbreak in predominantly White PWID in rural areas like Scott County, Indiana, San Francisco and other urban cities are increasingly responding to preventing infections in minority populations (1, 52, 64). There are clearly important opportunities and a greater need for targeted HIV prevention efforts aimed at African American and Hispanic PWID in this city. All of these efforts will contribute to getting the rate of new HIV infections in San Francisco to zero.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. Meghan Morris for her contributions to this study. We also recognize the important contribution of San Francisco Department of Public Health, Berkeley Free Clinic, Homeless Youth Alliance, San Francisco Community Clinic Consortium Street Outreach Services, and San Francisco AIDS Foundation. This paper is dedicated to the memory of Joep Lange, whose brilliance and passion to HIV prevention will be forever missed.
FUNDING
This study was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number R01DA016017 (K.P.), NIH K24 under award number AA022586 (J.A.H) and NIMH R25 under award number MH064712 (A.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2014; vol. 26. http://www.cdc.gov/hiv/library/reports/surveillance/. Published November 2015. Accessed 07/15/2016.
- 2.Coffin PO, Jin H, Huriaux E, Mirzazadeh A, Raymond HF. Trends in use of health care and HIV prevention services for persons who inject drugs in San Francisco: Results from National HIV Behavioral Surveillance 2005–2012. Drug and alcohol dependence 2015;146:45–51. [DOI] [PubMed] [Google Scholar]
- 3.Bluthenthal RN, Kral AH, Lorvick J, Erringer EA, Edlin BR. Harm reduction and needle exchange programmes. Lancet 1998;351(9118):1819–20. [DOI] [PubMed] [Google Scholar]
- 4.Hahn JA, Page-Shafer K, Lum PJ, Ochoa K, Moss AR. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology (Baltimore, Md) 2001;34(1):180–7. [DOI] [PubMed] [Google Scholar]
- 5.Lutnick A, Cooper E, Dodson C, Bluthenthal R, Kral AH. Pharmacy syringe purchase test of nonprescription syringe sales in San Francisco and Los Angeles in 2010. Journal of urban health : bulletin of the New York Academy of Medicine 2013;90(2):276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaffe JH, O’Keeffe C. From morphine clinics to buprenorphine: regulating opioid agonist treatment of addiction in the United States. Drug and alcohol dependence 2003;70(2 Suppl): S3-11. [DOI] [PubMed] [Google Scholar]
- 7.Tsui JI, Evans JL, Lum PJ, Hahn JA, Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA internal medicine 2014;174(12):1974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Archives of general psychiatry 2011;68(11):1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.City and County of San Francisco DoPH. http://www.sfhiv.org/our-services. Accessed 10 March 2015. [PMC free article] [PubMed]
- 10.Des Jarlais DC, Kerr T, Carrieri P, Feelemyer J, Arasteh K. HIV infection among persons who inject drugs: ending old epidemics and addressing new outbreaks. AIDS (London, England) 2016;30(6):815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.San Francisco Department of Public Health - HIV epidemiology section. HIV epidemiology annual report 2014 (https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/HIV-EpidemiologyAnnualReport-2014.pdf). Aug 2015.
- 12.Conrad C, Bradley HM, Broz D, et al. Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone--Indiana, 2015. MMWR Morbidity and mortality weekly report 2015;64(16):443–4. [PMC free article] [PubMed] [Google Scholar]
- 13.Valdiserri R, Khalsa J, Dan C, et al. Confronting the emerging epidemic of HCV infection among young injection drug users. American journal of public health 2014;104(5):816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services (HHS) Office. Technical consultation on Hepatitis C Virus Infection in Young Persons Who Inject Drugs http://aids.gov/pdf/hcv-and-young-pwid-consultation-report.pdf. 2013.
- 15.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2014;59(10):1411–9. [DOI] [PubMed] [Google Scholar]
- 16.Fraser H, Zibbell J, Hoerger T, et al. Scaling up HCV prevention and treatment interventions in rural USA - model projections for tackling an increasing epidemic. Addiction (Abingdon, England) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. American journal of epidemiology 2008;168(10):1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rondinelli AJ, Ouellet LJ, Strathdee SA, et al. Young adult injection drug users in the United States continue to practice HIV risk behaviors. Drug and alcohol dependence 2009;104(1–2):167–74. [DOI] [PubMed] [Google Scholar]
- 19.Hahn JA, Page-Shafer K, Ford J, Paciorek A, Lum PJ. Traveling young injection drug users at high risk for acquisition and transmission of viral infections. Drug and alcohol dependence 2008;93(1–2):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. The Journal of infectious diseases 2009;200(8):1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. The Journal of infectious diseases 2002;186(11):1558–64. [DOI] [PubMed] [Google Scholar]
- 22.Evans JL, Tsui JI, Hahn JA, et al. Mortality among young injection drug users in San Francisco: a 10-year follow-up of the UFO study. American journal of epidemiology 2012;175(4):302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Des Jarlais DC, Bramson HA, Wong C, et al. Racial/ethnic disparities in HIV infection among people who inject drugs: an international systematic review and meta-analysis. Addiction (Abingdon, England) 2012;107(12):2087–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broz D, Wejnert C, Pham HT, et al. HIV infection and risk, prevention, and testing behaviors among injecting drug users -- National HIV Behavioral Surveillance System, 20 U.S. cities, 2009. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2014;63(6):1–51. [PubMed] [Google Scholar]
- 25.Cuzick J A Wilcoxon-type test for trend. Statistics in Medicine 1985;4:87. [DOI] [PubMed] [Google Scholar]
- 26.Kleinbaum DG, Klein M. Survival Analysis; A Self-Learning Text, Third Edition2012. [Google Scholar]
- 27.Cole SR, Hernán MA. Constructing Inverse Probability Weights for Marginal Structural Models. American journal of epidemiology 2008;168(6):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardnett FP, Pals SL, Borkowf CB, et al. Assessing mediation in HIV intervention studies. Public health reports (Washington, DC : 1974). 2009;124(2):288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Agostino R, Lee M, Belanger A, et al. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med` 1990;9:1501–15. [DOI] [PubMed] [Google Scholar]
- 30.Kral AH, Lorvick J, Martinez A, et al. HIV prevalence and risk among heterosexual methamphetamine injectors in California. Substance use & misuse 2011;46(9):1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Des Jarlais DC, Arasteh K, McKnight C, et al. HIV infection during limited versus combined HIV prevention programs for IDUs in New York City: the importance of transmission behaviors. Drug and alcohol dependence 2010;109(1–3):154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim NJ, Jin H, McFarland W, Raymond HF. Trends in sources and sharing of needles among people who inject drugs, San Francisco, 2005–2012. International Journal of Drug Policy [DOI] [PubMed] [Google Scholar]
- 33.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. The Journal of infectious diseases 2011;204(1):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellard M, Rolls DA, Sacks-Davis R, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology (Baltimore, Md) 2014;60(6):1861–70. [DOI] [PubMed] [Google Scholar]
- 35.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013;381(9883):2083–90. [DOI] [PubMed] [Google Scholar]
- 36.Lampejo T, Turner R, Roberts C, et al. Novel outreach settings to enhance sexually transmitted infection/HIV awareness, diagnosis and treatment in hard-to-reach populations. International journal of STD & AIDS 2017:956462417723816. [DOI] [PubMed] [Google Scholar]
- 37.Des Jarlais DC, Diaz T, Perlis T, et al. Variability in the incidence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus infection among young injecting drug users in New York City. American journal of epidemiology 2003;157(5):467–71. [DOI] [PubMed] [Google Scholar]
- 38.Garfein RS, Rondinelli A, Barnes RF, et al. HCV infection prevalence lower than expected among 18–40-year-old injection drug users in San Diego, CA. Journal of urban health : bulletin of the New York Academy of Medicine 2013;90(3):516–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruneau J, Daniel M, Abrahamowicz M, et al. Trends in human immunodeficiency virus incidence and risk behavior among injection drug users in montreal, Canada: a 16-year longitudinal study. American journal of epidemiology 2011;173(9):1049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ (Clinical research ed) 2009;338:b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SG, Lowe EL, Dixit D, et al. Cocaine-mediated impact on HIV infection in humanized BLT mice. Scientific reports 2015;5:10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lansky A, Finlayson T, Johnson C, et al. Estimating the number of persons who inject drugs in the united states by meta-analysis to calculate national rates of HIV and hepatitis C virus infections. PloS one 2014;9(5):e97596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Des Jarlais DC, McCarty D, Vega WA, Bramson H. HIV infection among people who inject drugs: the challenge of racial/ethnic disparities. The American psychologist 2013;68(4):274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. HIV Surveillance Report, 2015; vol. 27. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2016. Accessed 11/03/2017. 2016.
- 45.Passel J, Cohn DV, Hugo Lopez M. Hispanics Account for More than Half of Nation’s Growth in Past Decade http://www.pewhispanic.org/2011/03/24/hispanics-account-for-more-than-half-of-nations-growth-in-past-decade. Accessed 11/03/2017. 2011.
- 46.Glasman LR, Weinhardt LS, Hackl KL. Disparities in access to HIV prevention among men of Mexican descent living in the Midwestern United States. Journal of immigrant and minority health 2011;13(6):1125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neaigus A, Reilly KH, Jenness SM, et al. Multilevel risk factors for greater HIV infection of black men who have sex with men in New York City. Sexually transmitted diseases 2014;41(7):433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arrington-Sanders R, Morgan A, Oidtman J, et al. A Medical Care Missed Opportunity: Preexposure Prophylaxis and Young Black Men Who Have Sex With Men. The Journal of adolescent health : official publication of the Society for Adolescent Medicine 2016;59(6):725–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolle CP, Rosenberg ES, Siegler AJ, et al. Challenges in Translating PrEP Interest into Uptake in an Observational Study of Young Black MSM. Journal of acquired immune deficiency syndromes (1999) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minior T, Galea S, Stuber J, Ahern J, Ompad D. Racial differences in discrimination experiences and responses among minority substance users. Ethnicity & disease 2003;13(4):521–7. [PubMed] [Google Scholar]
- 51.Cooper H, Friedman SR, Tempalski B, Friedman R, Keem M. Racial/ethnic disparities in injection drug use in large US metropolitan areas. Annals of epidemiology 2005;15(5):326–34. [DOI] [PubMed] [Google Scholar]
- 52.Raymond HF, McFarland W. Racial mixing and HIV risk among men who have sex with men. AIDS and behavior 2009;13(4):630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vieira I, Cheng R, Harper P, de Senna V. Small world network models of the dynamics of HIV infection. Annals of Operations Research 2010;178(1):173–200. [Google Scholar]
- 54.Little SJ, Kosakovsky Pond SL, Anderson CM, et al. Using HIV networks to inform real time prevention interventions. PloS one 2014;9(6):e98443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shafer KP, Hahn JA, Lum PJ, et al. Prevalence and correlates of HIV infection among young injection drug users in San Francisco. Journal of acquired immune deficiency syndromes (1999) 2002;31(4):422–31. [DOI] [PubMed] [Google Scholar]
- 56.Bacon O, Lum P, Hahn J, et al. Commercial sex work and risk of HIV infection among young drug-injecting men who have sex with men in San Francisco. Sexually transmitted diseases 2006;33(4):228–34. [DOI] [PubMed] [Google Scholar]
- 57.Jin H, Huriaux E, Loughran E, Packer T, Raymond HF. Differences in HIV risk behaviors among people who inject drugs by gender and sexual orientation, San Francisco, 2012. Drug and alcohol dependence 2014;145:180–4. [DOI] [PubMed] [Google Scholar]
- 58.Rosario M, Schrimshaw EW, Hunter J. Disclosure of sexual orientation and subsequent substance use and abuse among lesbian, gay, and bisexual youths: critical role of disclosure reactions. Psychology of addictive behaviors : journal of the Society of Psychologists in Addictive Behaviors 2009;23(1):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression Methods in Biostatistics: Springer; US; 2012. [Google Scholar]
- 60.Sordo L, Bravo MJ, Barrio G, et al. Potential bias due to outcome-related loss to follow-up in cohort studies on incidence of drug injection: systematic review and meta-analysis. Addiction (Abingdon, England) 2015;110(8):1247–57. [DOI] [PubMed] [Google Scholar]
- 61.Maher L, Page K. Commentary on Sordo et al. (2015): Reducing bias in prospective observational studies of drug users: The need for upstream and downstream approaches. Addiction (Abingdon, England) 2015;110(8):1259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Des Jarlais DC, Arasteh K, Friedman SR. HIV among drug users at Beth Israel Medical Center, New York City, the first 25 years. Substance use & misuse 2011;46(2–3):131–9. [DOI] [PubMed] [Google Scholar]
- 63.Escudero DJ, Lurie MN, Kerr T, Howe CJ, Marshall BD. HIV pre-exposure prophylaxis for people who inject drugs: a review of current results and an agenda for future research. J Int AIDS Soc 2014;17:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.San Francisco Department of Public Health HIV Prevention Section. 2010 San Francisco HIV Prevention Plan - Chapter 4: Strategies & Interventions San Francisco: 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


