Hypocotyl cell elongation related to photomorphogenesis in Arabidopsis seedlings is regulated by a network involving ethylene, auxin, and brassinosteroid signalling that is mediated by interactions among ERF72, ARF6, and BZR1, forming a revised BZR-ARF-PIF/DELLA-ERF (BAP/DE) module.
Keywords: ERF72, ARF6, BZR1, hypocotyl elongation, photomorphogenesis, Arabidopsis thaliana
Abstract
The phytohormones brassinosteroid (BR), auxin, and gibberellin (GA) regulate photomorphogenesis-related hypocotyl elongation in Arabidopsis via the co-operative interaction of BZR-ARF-PIF/DELLA (BAP/D) transcription factors/regulators. In addition, ethylene activates the PIF3 or ERF1 pathway through EIN3/EIL1 to balance hypocotyl elongation in Arabidopsis seedlings. However, the mechanism by which ethylene is co-ordinated with other phytohormones to produce light-regulated hypocotyl growth remains elusive. In this study, we found that hypocotyl cell elongation is regulated by a network involving ethylene, auxin, and BR signalling, which is mediated by interactions among ERF72, ARF6, and BZR1. ERF72 interacted directly with ARF6 and BZR1 in vitro and in vivo, and it antagonised regulation by ARF6 and BZR1 of the transcription of BEE3 and XTH7. In addition, light modulated the subcellular localisation of ERF72 and transcription of ERF72 through the EIN2-EIN3/EIL1 pathway, facilitating the function of ERF72 in photomorphogenesis. The expression of BEE3 and XTH7 was also regulated by the EIN2-EIN3/EIL1 pathway. Our findings indicate that a revised BZR-ARF-PIF/DELLA-ERF (BAP/DE) module integrates light and hormone signals to regulate hypocotyl elongation in Arabidopsis.
Introduction
After germination, plants exhibit two distinct adaptations to the surrounding environment. Seedlings grown in darkness exhibit elongated hypocotyls and the formation of an apical hook, while development of the cotyledons, apical meristem, and root system is inhibited; this is termed skotomorphogenesis. In contrast, seedlings grown in light and undergoing photomorphogenesis are characterised by inhibited hypocotyl elongation, accelerated root growth, opened and green cotyledons, and no apical hook (Von Arnim and Deng, 1996). Both skotomorphogenesis and photomorphogenesis are driven by cell division in the apical meristems followed by cell elongation, resulting in growth of the hypocotyl and the roots (Chaiwanon et al., 2016; de Wit et al., 2016). Light signals are perceived by different photoreceptors and are transmitted to downstream transcription factors in order to regulate cell elongation during seedling morphogenesis. There are two classes of primary photoreceptor-regulated transcription factors, which play opposite roles in regulating cell elongation. The transcription factors ELONGATED HYPOCOTYL5 (HY5) and GATA2/4, which are degraded by the E3 ubiquitin ligase CONSTITUTIVE PHOTOMORPHOGENIC1 (COP1) under dark conditions, negatively regulate cell elongation (Luo et al., 2010; Huang et al., 2014). Many targets of HY5 are regulators of hormone signalling, including those of abscisic acid (ABA), gibberellin (GA), ethylene, auxin, brassinosteroid (BR), cytokinin, and jasmonic acid (Lau and Deng, 2010; Wang et al., 2012; Oh et al., 2014). Another class of transcription factors, PHYTOCHROME INTERACTION FACTORS (PIFs), positively regulate cell elongation in darkness and are rapidly degraded upon exposure to light (Leivar et al., 2008; Leivar and Monte, 2014). Previous studies have shown that PIFs integrate light signals, hormone-signalling pathways, and the circadian clock to optimise cell elongation and seedling photomorphogenesis (Leivar and Monte, 2014; Paik et al., 2017).
The signalling pathways centred on the plant growth-promoting hormones auxin, BR, GA, and ethylene have been studied extensively (Kim and Wang, 2010; Sun, 2010; Merchante et al., 2013; Weijers and Wagner, 2016). Auxin is perceived by a co-receptor comprising a TRANSPORT INHIBITOR-RESISTANT 1/AUXIN SIGNALLING F-BOX (TIR1/AFB) protein and an AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) transcriptional co-regulator (Calderón Villalobos et al., 2012), leading to polyubiquitination and degradation of the Aux/IAA protein and the release of AUXIN RESPONSE FACTOR (ARF) family transcription factors to activate auxin-responsive transcription (Weijers and Wagner, 2016). SHORT HYPOCOTYL2 (SHY2)/IAA3 is a member of the Aux/IAA family in Arabidopsis, and 1-week-old seedlings of shy2-2 grown in the dark show short hypocotyls, expanded cotyledons, open apical hooks, and true leaf primordia, suggesting an important role for auxin in regulating photomorphogenesis (Tian and Reed, 1999). In addition, a double-mutant of ARF6 and its close homolog ARF8, arf6 arf8, shows a short-hypocotyl phenotype in darkness, suggesting that ARF6 and ARF8 regulate hypocotyl elongation redundantly (Nagpal et al., 2005).
BR binds the receptor kinase BRASSINOSTEROID-INSENSITIVE1 (BRI1) at the cell surface and transduces the signal to activate the transcription factors BRASSINOZALE-RESISTANT1 (BZR1) and BZR2 (also named BES1 for BRI1-EMS-SUPPRESSOR1), which regulate the expression of BR-responsive genes (He et al., 2005; Kim and Wang, 2010). Arabidopsis BR-insensitive or -deficient mutants display a de-etiolated phenotype under dark conditions (Clouse et al., 1996; Li et al., 1996; Clouse, 2002), and the light-induced genes were de-repressed in BR-deficient mutants under dark conditions (Szekeres et al., 1996; Song et al., 2009). In addition, the constitutive photomorphogenesis phenotype of BR-deficient or -insensitive mutants is suppressed by the dominant bzr1-1D and bes1-D mutations, indicating that activation of BZR1 and BZR2 is essential for skotomorphogenesis in Arabidopsis (Wang et al., 2002; Yin et al., 2002).
GA, similar to auxin, regulates cell elongation by de-repressing its signalling pathway via intracellular receptor GIBBERELLIN-INSENSITIVE DWARF1 (GID1)-induced degradation of DELLA proteins via the ubiquitin-proteasome pathway (Sun, 2010). DELLAs were first found to interact with PIFs and inhibit their DNA-binding activity (de Lucas et al., 2008; Feng et al., 2008), and subsequently reported to inhibit the DNA-binding activities of many transcription factors, including BZR1 and ARF6 (Bai et al., 2012; Oh et al., 2014). The interactions of ARF6, BZR1, and PIF4 enhance their target-binding and transcriptional activities, and these factors synergistically promote hypocotyl elongation by co-activating numerous shared target genes with known functions in cell elongation (EXP8, BIM1, BEE1/3, PREs, HAT2, IBH1, HFR1, PAR1/2, and EXO) (Oh et al., 2014). The interactions among ARF6, BZR1, and PIF4, and the inhibition of their function by DELLAs, is known as the BZR-ARF-PIF/DELLA (BAP/D) transcriptional module, and it elegantly mediates the co-operative regulation by BR, auxin, GA, and light signals of cell elongation during seedling morphogenesis (Bai et al., 2012; Oh et al., 2014; Chaiwanon et al., 2016).
Ethylene is perceived by five endoplasmic reticulum-localised receptors, ETHYLENE RECEPTOR1 (ETR1), ETR2, ETHYLENE RESPONSE SENSOR1 (ERS1), ERS2, and ETHYLENE-INSENSITIVE4 (EIN4), that relieve repression by CONSTITUTIVE TRIPLE RESPONSE1 (CTR1) of the downstream signalling component EIN2 (Gallie, 2015). EIN2 is cleaved and translocates to the nucleus, where it stabilises EIN3 and its homolog EIN3-LIKE1 (EIL1) and thus activates the transcription of ethylene-responsive genes in Arabidopsis (Ju et al., 2012; Qiao et al., 2012; Wen et al., 2012). Ethylene activates two pathways through EIN3/EIL1 to synchronously regulate hypocotyl growth and cotyledon development in response to different light and soil conditions in Arabidopsis seedlings (Zhong et al., 2012). One of these pathways depends on the induction of ETHYLENE RESPONSE FACTOR1 (ERF1), and the other depends on the induction of PIF3; both are direct targets of EIN3. Under dark conditions, PIFs promote hypocotyl elongation, and soil-dependent ethylene accumulation leads to ERF1-mediated thickening of the hypocotyl cell wall and inhibition of etiolated hypocotyl elongation. The increased level of ethylene also represses biosynthesis of the chlorophyll precursor protochlorophyllide in the cotyledons via the PIF3 pathway. Protochlorophyllide, when present at high levels, can cause photo-oxidative damage after light absorption. However, when seeds germinate in the presence of light or when seedlings emerge from the soil, PIFs are degraded and the ethylene-induced increased abundance of PIF3 stimulates hypocotyl elongation. In contrast, ERF1 is stabilised in the presence of light, and further stimulation of ERF1 by ethylene has no effect on growth (Zhong et al., 2012). These results explain the ethylene-induced response in dark-grown seedlings, and suggest that ethylene plays an important role in co-ordinating growth and development to guide emergence from the soil and produce the shift to photoautotrophic growth (Ecker, 1995; Benavente and Alonso, 2006; de Wit et al., 2016). However, the links between ethylene and other hormones that regulate photomorphogenesis are unclear.
In this study, we found that transgenic Arabidopsis seedlings overexpressing stable ERF72 (35S::MAERF) exhibited the constitutive photomorphogenesis phenotype of a short hypocotyl, no apical hook, and open cotyledons in darkness, compared with the wild-type, the erf mutant, and plants overexpressing wild-type ERF72 (35S::MCERF). Transcriptomic analyses showed that light-regulated genes and ARF6- and BZR1-target genes were enriched among ERF72-related differentially expressed genes. We further demonstrated that ERF72 interacted with ARF6 and BZR1 to repress their activation of the transcription of target genes. Moreover, light modulated the subcellular localisation of ERF72 and the transcription of ERF72 through the EIN2-EIN3/EIL1 pathway. The expression of BEE3 and XTH7 was also regulated by the EIN2-EIN3/EIL1 pathway under any light regimen. Our results suggest that a revised BZR-ARF-PIF/DELLA-ERF (BAP/DE) module is involved in integrating light and hormone signalling pathways to control cell elongation in Arabidopsis hypocotyls.
Materials and methods
Plant growth and phenotypic analyses
The Arabidopsis thaliana plants used in this study were of the Col-0 ecotype. The T-DNA insertion mutant erf (Stock No: CS849696) was obtained from The Arabidopsis Information Resource (TAIR; https://www.arabidopsis.org/) and confirmed by genotyping, PCR, and RT-PCR (Supplementary Fig. S1 at JXB online). All seeds were surface-sterilised and sown on half-strength Murashige and Skoog (MS) medium containing 1.5% sucrose and 0.7% agar, and incubated at 4 °C under dark conditions for 3 d. The plates were then irradiated with white light for 6 h to promote germination and subsequently kept either in darkness set periods of time or under continuous red light (60 µmol m–2 s–1), far-red light (5 µmol m–2 s–1), blue light (7 µmol m–2 s–1), or white light (60 µmol m–2 s–1) for 5 d. For the light-to-dark transition experiments, seedlings were grown under continuous light or dark conditions for 7 d, and then transferred to the opposite conditions for set periods of time. For cycloheximide (CHX) treatment, seedlings were grown in continuous light for 7 d, transferred to plates containing half-strength MS medium with or without 100 μM CHX, and then placed in the light or darkness for set periods of time. The seedlings were then collected for experimental use.
Images of the hook and hypocotyl phenotypes of seedlings were obtained using an EOS60D digital camera (Canon, Japan). Cell length was determined using a confocal laser-scanning microscope (LSM700; Zeiss, Germany). Hypocotyl length and cell length were measured using the ImageJ software (https://imagej.nih.gov/ij/).
Plasmid construction
The full-length cDNAs of the wild-type (WT) ERF72 (MCERF) and stable ERF72 (with the second amino acid, cysteine, of ERF72 mutated to alanine; MAERF) lacking a stop codon were amplified by RT-PCR and inserted into the BamHI and NotI restriction sites of the plasmid pE2c (Addgene; http://www.addgene.org/), which harbours a triple HA-tag, to generate the construct pE2c-MC(/A)ERF72-HA. The MAERF72 fragment was inserted into the same restriction sites of pE6c (Addgene) containing a yellow fluorescent protein (YFP) tag to generate the construct pE6c-MAERF-YFP. In addition, the MC(/A)ERF72 fragment was inserted into pMDC32 using the Gateway LR II kit (Invitrogen, USA) to generate the 35S::MC(/A)ERF-HA and 35S::MAERF-YFP constructs. To generate a native promoter-driven plant expression vector, the 1443-bp DNA fragment upstream of the ATG codon of ERF72 was amplified and used to replace the 35S promoter of the 35S::MAERF construct to generate PERF72::MAERF. The full-length cDNAs of ARF6 and BZR1 lacking a stop codon were amplified by RT-PCR and inserted into the BamHI and NotI restriction sites of pE3c (Addgene), which harbours a triple MYC-tag, to generate the constructs pE3c-ARF6 and pE3c-BZR1, respectively.
For promoter activity assays, the 2553-bp DNA fragment upstream of the ATG codon of BEE3 was amplified and inserted into the BamHI and AscI restriction sites of pGPTV to generate the PBEE3::GUS (β-glucuronidase) construct. In addition, the 2023-bp DNA fragment upstream of the ATG codon of XTH7 was amplified and inserted into the HindIII and AscI restriction sites of pGPTV to generate the PXTH7::GUS construct. The promoter fragment of ERF72 was cut from PERF72::MAERF and inserted into the HindIII and AscI restriction sites of pGPTV to generate the PERF72::GUS construct. For the 4×GCC-like box construct, DNA fragments containing the G1 or G2 box from the BEE3 promoter and G3 or G4 box from the XTH7 promoter were synthesised and inserted into the AscI and XbaI sites or the SalI and XbaI sites of pE1n, respectively. Using two pairs of isocaudomers, SpeI and XbaI in pE1-G1/G2 or SalI and XhoI in pE1-G3/G4, four copies of the GCC-like box fragments were made and inserted into the AscI and XbaI restriction sites of pGPTV to generate the P4×G1::GUS or P4×G2::GUS constructs, or into the HindIII and SalI restriction sites of pGPTV to generate the P4×G3::GUS or P4×G4::GUS constructs.
To produce constructs for yeast two-hybrid assays, truncated fragments of ARF6, ARF6N, and ARF6C were subcloned from pE3c-ARF6 and inserted into the BamHI and NotI restriction sites of pE1c to generate the pE1c-ARF6N and pE1c-ARF6C constructs. The ARF6N/ARF6C fragment was inserted into pDEST-GBKT7 using the Gateway LR II kit to generate the pGBKT7-ARF6N/ARF6C construct. The full-length cDNA of ERF72 was amplified by PCR and inserted into pGADT7 using EcoRI and BamHI restriction sites to generate pGADT7-ERF72.
To construct vectors for the production of recombinant proteins, the BZR1-MYC fragment of pE3c-BZR1 was inserted into p28a-DEST using the Gateway LR II kit to generate the pET28a-BZR1-MYC construct, and the ERF72 fragment from pE2c-MCERF72-HA was inserted into pHIS-6p-MBP using the BamHI and NotI restriction sites to generate pHIS-MBP-ERF.
To construct vectors for firefly luciferase (LUC) complementation imaging (LCI) assays (Chen et al., 2008), ARF6 and BZR1 fragments were digested from pE3c-ARF6/BZR1 by BamHI and XhoI or BamHI and XbaI, and subcloned into 35S::NLuc digested with BamHI and XhoI or BamHI and XbaI to generate 35S::ARF6/BZR1-NLuc. The ERF72 fragment was inserted into the KpnI and SalI restriction sites of 35S::CLuc to generate the 35S::CLuc-ERF vector.
All clones and vectors were validated by sequencing. The primers used are listed in Supplementary Table S1.
Generation of transgenic plants
The constructs 35S::MC(/A)ERF, 35S::MAERF-YFP, and PERF72::MAERF were separately introduced into Agrobacterium GV3101, and then transformed into WT Arabidopsis plants to generate the 35S::MCERF, 35S::MAERF, and 35S::MAERF-YFP transgenic lines, or into the erf mutant to generate the PERF72::MAERF transgenic line, using the floral dip transformation method (Clough and Bent, 1998). The transformants were selected on half-strength MS medium containing 50 mg ml–1 hygromycin B (Sigma-Aldrich, USA). T3-generation homozygous transformants carrying a single insertion were used in subsequent experiments.
RNA-seq analysis
Total RNA was extracted from seedlings grown under dark conditions at 2 d after gemination (DAG) using an RNA Extraction Kit (Promega, USA). A total of 3.0 µg of RNA was used to generate sequencing libraries using the NEBNext Ultra RNA Library Prep Kit following the manufacturer’s instructions (NEB, USA), and index codes were added to attribute sequences to each sample. Solexa sequencing was performed as a commercial service at Novogene (http://www.novogene.com/) with an Illumina HiSeq 2000 sequencer. The sequencing data were deposited in the NCBI’s Sequence Read Archive (SRA; https://www.ncbi.nlm.nih.gov/sra) under accession number SRP125848. Low-quality bases (Q<20) at the ends of the sequencing reads were trimmed using the SolexaQA software (Cox et al., 2010) (ver. 1.10, parameters: –b h 20); our RNA-seq data are shown in Supplementary Table S2. After trimming, all reads were mapped to the Arabidopsis genome (TAIR9; www.arabidopsis.org) using the TopHat software (Trapnell et al., 2009). Read counts were generated using HTSeq in union mode. Differentially expressed genes (DEGs) between samples were defined by Deseq (Anders and Huber, 2010), using a fold-change >2 and adjusted P-value <0.05.
Gene expression analysis
For qRT-PCR analysis, total RNA was isolated using an RNA Extraction Kit (Promega, USA), and first-strand cDNA was synthesised from 3.0 µg of RNA using a reverse transcriptase (TransGen, China). qRT-PCR was performed using TransStart Tip Green qPCR Super Mix following the manufacturer’s instructions (TransGen). Three biological replicates were performed per sample, and expression levels were normalised to that of Actin2 as the control. Value changes of more than two-fold (>2 or <0.5) were considered to indicate a significant difference of target gene expression. The primers used are listed in Supplementary Table S1.
Yeast two-hybrid assay
The GAL4 DNA-binding domain (BD) fusion plasmid pGADT7-ERF72 and the activation domain (AD) fusion plasmid pGBKT7-ARF6N/ARF6C were transformed into Saccharomyces cerevisiae AH109. Different combinations of AD and BD fusion plasmids were generated by mating. Yeast strains were grown on SD/–Trp–Ura–His dropout plates containing 1 mM 3-amino-1,2,4-triazole (3-AT) to confirm their interactions.
Recombinant protein production and pull-down assays
BZR1-MYC and MBP-ERF fusion proteins were expressed in Escherichia coli BL21 (DE3) and induced by isopropyl β-D-1-thiogalactopyranoside (IPTG). Briefly, E. coli BL21 cells containing pET28a-BZR1-MYC and pHIS-MBP-ERF were induced for 8 h with 0.1 mM IPTG at 16 °C, and subjected to ultrasonic treatment in lysis buffer (50 mM Tris-HCl pH 7.4, 200 mM NaCl, 1 mM β-mercaptoethanol, 0.5% Triton X-100, and 10% glycerine). After centrifugation, the supernatant was used as the crude protein extract.
For pull-down assays, crude protein extracts were added to binding buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 2 mM KCl, and 0.1% Tween-20), followed by incubation at 4 °C for 1 h with gentle rotation. Amylose resin (20 μl) was added, followed by incubation for 1 h to precipitate MBP-containing proteins. The resin was washed five times in binding buffer.
Immunoblot assays
Seedlings were ground to powder in liquid nitrogen, and total proteins were extracted in buffer [62.5 mM Tris-HCl pH 6.8, 2% sodium dodecyl sulphate (SDS), 10% glycerine, and 1 mM β-mercaptoethanol]. The protein concentration was determined using a Bio-Rad Protein Assay Kit with bovine serum albumin as the standard. Following resolution by SDS-polyacrylamide gel electrophoresis, proteins were transferred to a polyvinylidene difluoride membrane (Millipore, USA) under 200 mA constant current for 45 min in transfer buffer (12.5 mM Tris-HCl, 192 mM glycine, and 10% methanol; pH 8.3). Anti-HA (Abcam, 1:10 000) and anti-MYC (Cell Signalling Technology, 1:1000) antibodies were used to visualise protein bands.
LCI assays
LCI assays were carried out as described previously (Chen et al., 2008). Agrobacterium cells containing constructs were suspended in infiltration medium (MS medium containing 10 mM MES pH 5.6, and 150 mM acetosyringone) to an optical density at 600 nm (OD600) of 1.0, and every pair of constructs were mixed in equimolar ratios. Luciferase activity was assayed using a Lumazone FA1300 Imaging System (Roper Scientific, USA).
ChIP-qPCR assays
Chromatin immunoprecipitation (ChIP)-qPCR assays were performed as described previously (Cui et al., 2016). Briefly, 5 ml of protoplasts were transfected with ARF6-MYC or BZR1-MYC alone or together with ERF72-HA, and incubated for 24 h. Cell walls were cross-linked in 1% formaldehyde for 20 min and quenched in glycine for 5 min. Chromatin complexes were isolated and sonicated to reduce the average DNA fragment size to ~500 bp. An anti-MYC antibody (1:50) was used to pull down DNA-protein complexes. The precipitated DNA fragments were recovered and quantified by qPCR using SYBR Premix ExTaq Mix. The relative enrichment of DNA was calculated by normalising the amount of target DNA to that of the internal control gene CNX5 (At5g55130), and the input DNA amount. Data are presented as means (SD) of three biological replicates. The primers used for qPCR are listed in Supplementary Table S1.
Transient gene expression assays
Arabidopsis mesophyll protoplasts (2 × 105) were isolated from 4-week-old rosette leaves and transfected with 30 μg of DNA (effector:reporter:internal standard = 5:4:1) and incubated overnight as described previously (Liang et al., 2015). Protoplasts were harvested by centrifugation and lysed in 100 μl of passive lysis buffer (Promega, USA). Firefly luciferase activity (as an internal standard) was measured using a Luciferase Reporter Kit (Promega, USA). GUS activity was measured as described previously (Jefferson et al., 1987).
Statistical analysis
Statistical analyses were performed using the Data Processing System (Tang and Zhang, 2013). One-way ANOVA and Tukey’s multiple range test were conducted to determine the significance of differences (P<0.05).
Accession numbers
The RNA-seq data are deposited in the Sequence Read Archive at NCBI (https://www.ncbi.nlm.nih.gov/sra) under accession number SRP125848. Sequence data can be found in TAIR (https://www.arabidopsis.org/) under the following accession numbers: ERF72 (At3g16770), ARF6 (At1g30330), BZR1 (At1g75080), BEE3 (At1g73830), XTH7 (At4g37800), CNX5 (At5g55130), UBC30 (At5g56150), PP2A (At1g69960), EIN3 (At3g20770), and Actin2 (At3g18780).
Results
Overexpression of stable ERF72 in Arabidopsis triggers a constitutive photomorphogenic-like response under dark conditions
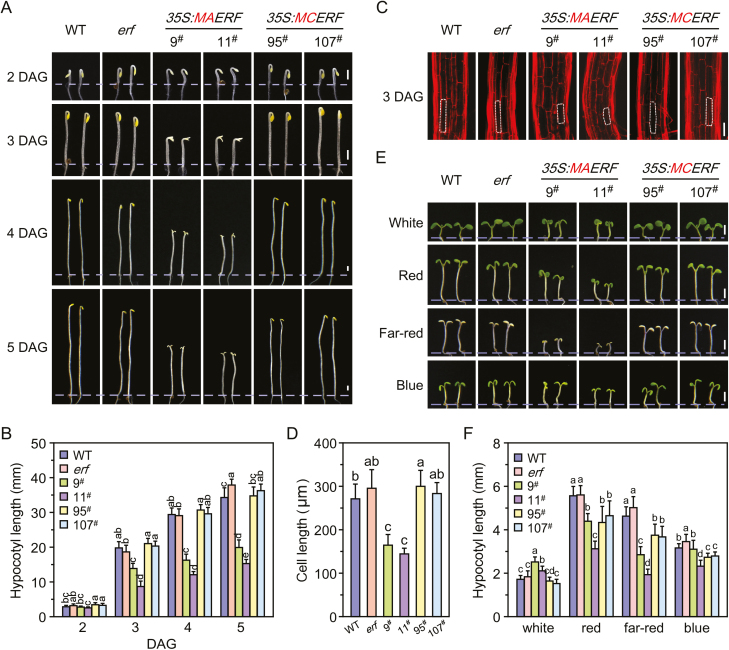
In Arabidopsis, there are five Group-VII ERFs, namely HYPOXIA RESPONSIVE ERF1 (HRE1, ERF73), HRE2 (ERF71), RELATED TO AP2.12 (RAP2.12, ERF74), RAP2.2 (ERF75), and RAP2.3 (ERF72) (Nakano et al., 2006), which are characterised by a conserved N-terminal domain beginning with the residues Met-Cys (MC) and which are involved in oxygen sensing and nitric oxide (NO)-regulated processes, including seed germination, stomatal closure, and hypocotyl elongation, via the N-end rule pathway of targeted proteolysis (Licausi et al., 2011; Gibbs et al., 2014). Here, we generated transgenic plants overexpressing WT ERF72 (35S::MCERF) and proteolysis-resistant ERF72 (with the second amino acid, cysteine, mutated to alanine; 35S::MAERF) (Supplementary Fig. S1), and found that 35S::MAERF plants exhibited a shorter hypocotyl, no apical hook, and more open cotyledons compared with WT, erf mutant, and 35S::MCERF plants after germination under dark conditions (Fig. 1A, B, Supplementary Fig. S2). We determined the hypocotyl cell length of seedlings at 3 DAG and found that the mean length in 35S::MAERF transgenic plants was significantly decreased compared with WT, erf, and 35S::MCERF plants, which was in agreement with their shorter hypocotyls (Fig. 1C, D). These results demonstrated that the overexpression of stabilised ERF72 in WT plants triggered a constitutive photomorphogenic-like response under dark conditions, and the resulting limited cell elongation contributed to the shorter hypocotyls of 35S::MAERF plants.
Fig. 1.
Overexpression of stabilised ERF72 (35S::MAERF) in Arabidopsis triggers a constitutive photomorphogenic-like response. (A) Growth of wild-type (WT), erf, and transgenic seedlings under dark conditions. 9# and 11#, independent transgenic lines overexpressing stable ERF72 driven by the 35S promoter in a WT background (35S::MAERF); 95# and 107#, independent lines overexpressing ERF72 in a WT background (35S::MCERF). DAG, days after germination. Scale bars are 1 mm. (B) Hypocotyl length of WT, erf, 35S::MAERF, and 35S::MCERF seedlings under the same conditions as in (A). Data are means (±SD) of at least 20 seedlings. Different letters indicate significant differences at P<0.05 according to Tukey’s test. (C) Propidium iodide staining of hypocotyl cells of 3-DAG seedlings of WT, erf, 35S::MAERF, and 35S::MCERF under the same conditions as in (A). The scale bar is 100 μm. (D) Cell length of 3-DAG seedlings under the same conditions as in (C). Data are means (±SD) of at least 20 seedlings. Different letters indicate significant differences at P<0.05 according to Tukey’s test. (E) Growth of 5-DAG seedlings of WT, erf, 35S::MAERF, and 35S::MCERF under continuous white, red, far-red, and blue light. Transgenic lines are as in (A). Scale bars are 2 mm. (F) Hypocotyl length of WT, erf, 35S::MAERF, and 35S::MCERF seedlings under the light conditions described in (E). Data are means ( SD) of at least 20 seedlings. Different letters indicate significant differences at P<0.05 according to Tukey’s test.
We further evaluated seedling growth at 3 DAG under continuous red, far-red, blue, and white light conditions. The hypocotyl length of WT, erf, 35S::MCERF, and 35S::MAERF plants was significantly shorter under continuous red, far-red, and blue light conditions than under dark conditions, and the hypocotyl lengths of plants grown under white light were shortest compared with those grown under monochromatic light or dark conditions (Fig. 1E, F). More interestingly, the hypocotyls of 35S::MAERF plants were slightly longer than those of WT, erf, and 35S::MCERF plants grown under white light. However, the hypocotyls of 35S::MAERF, similar to that of 35S::MCERF plants, were shorter than those of WT and erf plants under continuous red and far-red light conditions (Fig. 1E, F), suggesting that the hypocotyl growth of 35S::MAERF plants was dependent on the specific light conditions.
RNA-seq analyses to identify ERF72-related DEGs
To evaluate the role of ERF72 in controlling hypocotyl growth, we performed a transcriptomic analysis among WT, erf, and 35S::MAERF plants grown under dark conditions. In total, 21 801 genes in the Arabidopsis genome were functionally annotated according to at least one read in any plant sample. Based on the fragments per kilobase of transcript per million mapped reads (FPKM) value of each gene using a cut-off of a two-fold change and an adjusted P-value <0.05 between two samples, 849 DEGs were identified between 35S::MAERF (overexpressing, OE) and WT plants (256 up-regulated and 593 down-regulated), 779 DEGs between OE and erf plants (216 up-regulated and 563 down-regulated), and 49 DEGs between erf and WT plants (17 up-regulated and 32 down-regulated). Using a Venn diagram to analyse the DEGs of OE versus WT, OE versus erf, and erf versus WT plants based on the RNA-seq data, we identified 674 ERF72-related DEGs, including 176 up-regulated (26%) and 498 down-regulated genes (74%) (Fig. 2A, Supplementary Table S3).
Fig. 2.
Light-regulated genes, ARF6-target genes, and BZR1-target genes are enriched in ERF72-responsive differentially expressed genes (DEGs). (A) Venn diagram of DEGs in dark-grown 35S::MAERF plants (overexpressing, OE) versus wild-type (WT) (OE vs WT), OE vs erf, and erf vs WT based on the RNA-seq data. Black numbers, total numbers of DEGs; red and blue numbers, up-regulated and down-regulated genes, respectively. Genes within the black border are ERF72-responsive DEGs. (B) Venn diagram of ERF72-responsive DEGs, light-regulated genes, ARF6-target genes, and BZR1-target genes. (C) Enrichment of light-regulated genes, ARF6-target genes, and BZR1-target genes in ERF72-responsive DEGs. Genome indicates all genes.
Light-regulated, and ARF6- and BZR1-target genes are enriched in ERF72-related DEGs
Previous studies have shown that ARF6 and its close homolog ARF8 regulate hypocotyl elongation (Nagpal et al., 2005), and BZR1 promotes cell elongation and seedling morphogenesis in response to BR in Arabidopsis (Oh et al., 2012). Therefore, the constitutive photomorphogenic-like phenotype of 35S::MAERF seedlings led us to investigate the relationships between ERF72-related DEGs, light-regulated genes, ARF6-target genes, and BZR1-target genes. In our RNA-seq dataset, we found 2813 light-regulated genes, 2376 ARF6-target genes, and 3803 BZR1-target genes, among which 233 light-regulated genes, 84 ARF6-target genes, and 155 BZR1-target genes were ERF72-responsive DEGs (Fig. 2B, Supplementary Table S3). Furthermore, these three co-regulated gene categories were enriched dramatically among ERF-related DEGs compared to their representation in our RNA-seq dataset (Fig. 2C). More interestingly, the common target genes included several with known functions in cell elongation, such as BEE1/3, PAR1/2, and EXO, which were down-regulated in 35S::MAERF seedlings (Supplementary Table S3). These results suggested that ERF72, ARF6, and BZR1 functioned co-operatively to regulate hypocotyl elongation and seedling photomorphogenesis.
ERF72 physically interacts with ARF6 and BZR1
The significant enrichment of ARF6- and BZR1-target genes in ERF72-responsive DEGs suggested that ERF72 regulates their expression by activating or inhibiting the transcription of ARF6 and BZR1, or by directly interacting with ARF6 and BZR1. We found that the expression levels of ARF6 and BZR1 in 35S::MAERF plants were similar to those in WT and erf plants (fold-change <2) (Supplementary Fig. S3A), indicating that ERF72 had no effect on the transcription of ARF6 and BZR1. Yeast two-hybrid assays using truncated fragments of ARF6 or BZR1 showed that ERF72 interacted with the C-terminal domain of ARF6 (Fig. 3A, B), while truncated fragments of BZR1 were capable of self-activation and did not interact with ERF72 (data not shown). Furthermore, a pull-down assay showed that MPB-tagged ERF72, but not MBP alone, pulled down recombinant MYC-tagged BZR1 in vitro (Fig. 3C), which demonstrated that ERF72 interacted with BZR1 in vitro. To confirm the interaction of ERF72 with ARF6 or BZR1 in vivo, a firefly LCI assay was performed in tobacco leaves. Co-expression of ARF6-nLUC fusion proteins with ERF72-cLUC or BZR1-nLUC with ERF72-cLUC yielded a strong fluorescence signal (Fig. 3D, E), suggesting that the functional luciferase was reconstituted via direct interactions between ERF72 and ARF6 or ERF72 and BZR1. These results suggested that ERF72 interacted with ARF6 or BZR1 to regulate the expression of ARF6- and BZR1-target genes.
Fig. 3.
ERF72 interacts with ARF6 and BZR1. (A) Domain structures of ARF6 and its truncated fragments (ARF6N and ARF6C). (B) Yeast two-hybrid assay of activation domain (AD)-tagged ERF72 with binding domain (BD)-tagged ARF6N, and ARF6C or BD alone. (C) Pull-down assays of BZR1 and ERF72. Recombinant BZR1-MYC was used as prey and pulled down using protein crude extract of MBP or MBP-ERF72 as bait. (D, E) Firefly luciferase complementation imaging assays of the interaction of ERF72 with ARF6 (D) or BZR1 (E) in tobacco leaves. Full-length ERF72 was fused to the C-terminal fragment of luciferase (c-LUC), and the full-length sequence of ARF6 or BZR1 was fused to the N-terminus of luciferase (n-LUC). Empty vectors were used as negative controls.
ERF72 antagonises regulation by ARF6 and BZR1 of the transcription of BEE3 and XTH7
To evaluate the role of ERF72 in the transcriptional activity of ARF6 and BZR1, we selected the ARF6-target gene BR-enhanced expression 3 (BEE3) and the BZR1-target gene xyloglucan endo/transglycosidase hydrolase 7 (XTH7), which are related to cell elongation (Rose et al., 2002; Baumann et al., 2007; Cifuentes-Esquivel et al., 2013). Using the BEE3 promoter reporter system transiently transformed into Arabidopsis mesophyll protoplasts, we found that ARF6 or ERF72 alone activated the BEE3 promoter under both light and dark conditions (Fig. 4A, B). However, when ARF6 was co-transformed with ERF72 activation of the reporter gene driven by the BEE3 promoter was enhanced under light conditions but weakened under dark conditions (Fig. 4B). BZR1 is a transcriptional repressor (He et al., 2005), and it inhibited XTH7 promoter activity under both light and dark conditions (Fig. 4A, C). However, this repression was disrupted by its co-transformation with ERF72. Moreover, ERF72 was capable of activating the XTH7 promoter under both light and dark conditions (Fig. 4C). In addition, the expression of BEE3 and XTH7 was decreased in 2-DAG seedlings of 35S::MAERF plants compared with the wild type and erf under dark conditions (Supplementary Fig. S3B). These results indicated that ERF72 antagonised the transcriptional function of ARF6 and BZR1 in Arabidopsis in a light-/dark-dependent manner. Furthermore, four GCC-like boxes in the promoter regions of BEE3 (G1 and G2) and XTH7 (G3 and G4) were predicted to bind ERF72 (Supplementary Fig. S4A). However, the GUS activities of G1 to G4 (Supplementary Fig. S4B) were negligible (Supplementary Table S4), indicating that these GCC-like boxes were not functional. Therefore, ERF72 may regulate the transcription of BEE3 and XTH7 by interacting directly with ARF6 or BZR1 in Arabidopsis.
Fig. 4.
ERF72 antagonises the regulation by ARF6 and BZR1 of the transcription of BEE3 and XTH7, respectively. (A) Schematic diagram of the BEE3 promoter, XTH7 promoter, and constructs used. LUC, firefly luciferase. GUS, beta-glucuronidase. Black and grey lines in the BEE3 promoter region indicate the AuxRE (TGTCTC) and TGTCGG elements, respectively. Boxes B1–B3 indicate fragments that contain the AuxRE element, TGTCGG element, or both. Light grey and grey lines in the XTH7 promoter region indicate the BRRE element (GGTGTG) and G-Box element (CACGTG), respectively. (B) Effect of ERF72 and ARF6 on transcriptional regulation of the BEE3 promoter in Arabidopsis mesophyll protoplasts under light or dark conditions for 48 h. Promoter activity is expressed as the ratio of GUS to LUC activity. Data are means (±SD) (n=3). Different letters indicate significant differences at P<0.05 according to Tukey’s test. (C) Effect of ERF72 and BZR1 on transcriptional regulation of the XTH7 promoter in Arabidopsis mesophyll protoplasts under light or dark conditions for 48 h. Promoter activity is expressed as the ratio of GUS to LUC activity. Data are means (±SD) (n=3). Different letters indicate significant differences at P<0.05 according to Tukey’s test. (D) Binding of the B1–B3 boxes to ARF6 was suppressed by ERF72. Relative enrichment of fragments of B1–B3 and a region of PP2A (At1g69960) used as a control were detected using chromatin immunoprecipitation (ChIP)-qPCR. (E) Binding of the BRRE and G-Box elements to BZR1 was not affected by ERF72. Relative enrichment of the BRRE and G-Box elements and a region of UBC30 (At5g56150) used as a control were detected using ChIP-qPCR. Data in (D, E) are means (±SD) of three biological replicates. Significant differences were determined by one-way ANOVA: *P<0.05,; **P<0.01.
ARF6 binds to the AuxRE (TGTCTC) and TGTCGG elements in the promoter sequences of target genes, and BZR1 binds to the BRRE (GGTGTG) and G-box (CACGTG) elements (He et al., 2005; Oh et al., 2014). A promoter element analysis revealed that the BEE3 promoter contains two AuxRE elements and three TGTCGG elements (B1, B2, and B3 fragments), and the XTH7 promoter contains one BRRE element and one G-box (Fig. 4A). ChIP-qPCR showed that the B1–B3 fragments were enriched by ARF6-MYC compared with the negative control (PP2A), but this was markedly depressed by the co-expression of ARF6-MYC and ERF72-HA (Fig. 4D), suggesting that in the presence of ARF6, ERF72 down-regulates the expression of BEE3. In addition, the BRRE element-containing fragment, but not the G-box-containing fragment, of the XTH7 promoter was enriched by BZR1-MYC, but unaffected by the co-expression of BZR1-MYC and ERF72-HA (Fig. 4E), indicating that ERF72 ameliorated the inhibition of BZR1 for expression of XTH7 through an unknown pathway, without affecting its binding to the XTH7 promoter.
Light regulates the transcription and subcellular location of ERF72
The role of overexpression of MAERF in photomorphogenesis in the dark led us to investigate the expression of ERF72 in response to different light conditions. As anticipated, the expression level of ERF72 in light-grown seedlings was lower than that in dark-grown seedlings. After seedlings were transferred to dark conditions, ERF72 expression increased gradually for 12 h and then decreased up to 48 h (Fig. 5A). In contrast, after seedlings were transferred from dark to light conditions, the ERF72 mRNA level decreased dramatically within 6 h and a low level was maintained up to 48 h (Fig. 5A). Consistent with our qRT-PCR results, the ERF72 protein level was reduced in PERF72::MAERF-HA seedlings transferred from dark to light conditions and increased when the seedlings were transferred from light to dark conditions (Fig. 5B). A protein synthesis inhibitor, cycloheximide (CHX), was used to determine whether the increased ERF72 protein level was due to the increasing ERF72 mRNA level in seedlings transferred from light to dark conditions. As shown in Fig. 5C, in the presence of CHX, the level of ERF72 protein was decreased in seedlings transferred from light to dark or between different light conditions. These results suggested that the increase in ERF72 protein was caused primarily by the increased mRNA abundance during the light-to-dark transition.
Fig. 5.
Effect of light on the transcription of ERF72 and subcellular localisation of ERF72. (A) The expression level of ERF72 was determined by qRT-PCR. Seedlings were grown under continuous light or dark conditions for 7 d and then transferred to the opposite conditions for the indicated times. ERF72 expression was normalised to that of Actin2. Data are means (±SD) of three biological replicates. (B) Immunoblotting of ERF72 using a HA antibody in PERF72::MAERF72-HA transgenic seedlings in the erf background under the same conditions as in (A). An anti-Actin antibody served as a control. WT, wild-type. (C) Immunoblotting of ERF72 using a HA antibody in PERF72::MAERF72-HA transgenic seedlings treated with 100 µM cycloheximide (+CHX) or buffer only (–CHX). Seedlings were grown in continuous light for 7 d and transferred to half-strength MS medium with or without CHX under light or dark conditions for the indicated times. An anti-Actin antibody served as a control. (D) The subcellular localisation of ERF72 in Arabidopsis roots during the light-to-dark transition. Seedlings overexpressing 35S::MAERF-YFP were grown under continuous light or dark conditions for 4 d and then transferred to the opposite conditions for the indicated times. Scale bars are 50 μm.
A previous study had shown that ERF72 translocates from the cytoplasm to the nucleus in response to hypoxia (Abbas et al., 2015). In a similar manner, the subcellular localisation of stabilised ERF72 (MAERF-YFP) was changed from the cytoplasm to the nucleus in Arabidopsis roots during the dark-to-light transition, and this stabilised ERF72 was removed from the nucleus through a mechanism unrelated to the N-end rule pathway, as the second amino acid of ERF72, which is normally cysteine, was mutated to alanine (Fig. 5D). In summary, these results indicated that light inhibited the transcription of ERF72 and triggered the translocation of ERF72 from the cytoplasm to the nucleus in Arabidopsis seedlings.
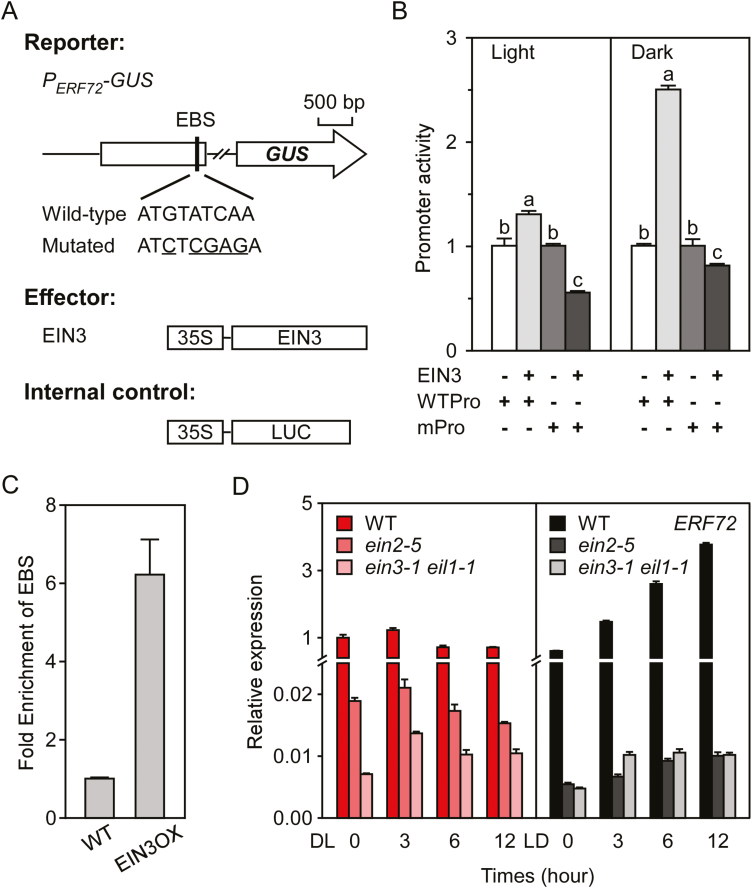
ERF72 is a target gene of EIN3
Previous research has shown that ERF72 is a candidate target gene of EIN3 (Chang et al., 2013), and its promoter region contains a putative EIN3-binding site (EBS) (Fig. 6A). PERF72::GUS activity analysis showed that EIN3 activated the transcription of the WT ERF72 promoter, but not the EBS-mutated promoter. Moreover, the activation activity of EIN3 was greater under dark than light conditions (Fig. 6B). ChIP-qPCR analysis showed that EIN3 binded the EBS fragment of the ERF72 promoter (Fig. 6C). qRT-PCR analysis showed that the expression level of ERF72 was markedly lower in ein2-5 and ein3-1 eil1-1 mutant seedlings than in the WT under both dark and light conditions. However, the expression trend was similar to the WT in ein2-5 and ein3-1 eil1-1 seedlings during the light-to-dark transition (Fig. 6D). These results demonstrated that ERF72 was the target gene of EIN3 and that dark-induced transcription of ERF72 was mediated by the EIN2-EIN3/EIL1 pathway in Arabidopsis.
Fig. 6.
ERF72 is a target gene of EIN3. (A) Schematic diagrams of the ERF72 promoter (pERF72) and constructs used in this assay. EBS, EIN3 binding site. The mutated reporter construct harboured a mutated EBS of the ERF72 promoter. LUC, firefly luciferase. (B) EIN3 activates the ERF72 promoter by binding to the EBS under both light and dark conditions. Promoter activities of the wild-type (WT) or mutated PERF72 are presented as the ratio of GUS to LUC activity. Data are means (±SD) (n=3). Different letters indicate significant differences at P<0.05 according to Tukey’s test. (C) EIN3 binds to the EBS site of the ERF72 promoter. WT and EIN3OX seedlings were grown under dark conditions for 1 week and then subjected to chromatin immunoprecipitation (ChIP)-qPCR of EIN3 binding to the indicated promoter region. Data are means (±SD) of three biological repeats. (D) Relative expression of ERF72 in WT, ein2-5, and ein3-1 eil1-1 plants during the dark-to-light (DL) or light-to-dark (LD) transition. Seedlings were grown under continuous dark or light conditions for 7 d, then transferred to the opposite conditions for the indicated times. ERF72 expression was normalised to that of actin2, and the relative expression of ERF72 under dark conditions was set as 1.0. Data are means (±SD) of three biological replicates.
The expression of BEE3 and XTH7 is also regulated by the EIN2-EIN3/EIL1 pathway
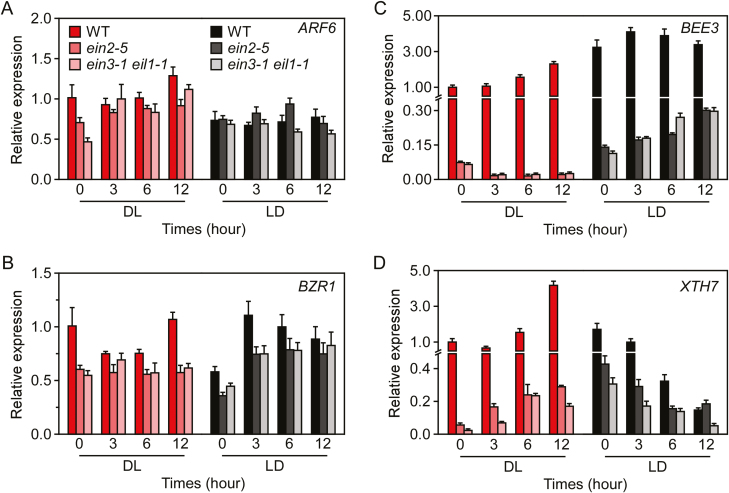
We performed qRT-PCR to investigate the role of the EIN2-EIN3/EIL1 pathway in the transcriptional regulation of ARF6 and BZR1, and their target genes BEE3 and XTH7. The expression level of ARF6 was lower in ein3-1 eil1-1 seedlings than that in the WT in dark conditions, and then it increased to the same level as the WT after transferring to light for 3 h. However, the expression of ARF6 in ein2-5 was not significantly different to that of the WT and ein3-1 eil1-1 because the value change of expression level of ARF6 in ein2-5 was less than two-fold that in the WT and ein3-1 eil1-1 during the dark-to-light transition. In addition, the expression of ARF6 in ein2-5 and ein3-1 eil1-1 seedlings was similar to that in the WT during light-to-dark transition (Fig. 7A). At the same time, the expression of BZR1 in ein2-5 and ein3-1 eil1-1 seedlings showed no significant difference with that in the WT during dark-to-light transition. However, the expression level of BZR1 was lower in light conditions than in the dark in WT, ein2-5, and ein3-1 eil1-1 seedlings, but it increased to the same level after transferring to dark conditions for 3 h (Fig. 7B). These results suggested that ARF6 could be regulated by the ethylene signalling pathway and that BZR1 was mainly regulated by light/dark conditions.
Fig. 7.
The expression of BEE3 and XTH7, but not ARF6 and BZR1, is regulated by the EIN2-EIN3/EIL1 pathway during the dark-to-light (DL) or light-to-dark (LD) transition. Wild-type (WT), ein2-5, and ein3-1 eil1-1 seedlings were grown under continuous dark or light conditions for 7 d, then transferred to the opposite conditions for the indicated times. The relative expression levels of ARF6, BZR1, BEE3, and XTH7 were separately normalised to that of Actin2. The expression level under dark conditions was set as 1.0. Data are means (±SD) of three biological replicates.
In addition, the expression levels of BEE3 and XTH7 were dramatically lower in ein2-5 and ein3-1 eil1-1 seedlings than in the WT under both dark and light conditions (Fig. 7C, D), which was similar to the expression patterns of BEE3 and XTH7 in 2-DAG seedlings of 35S::MAERF plants under dark conditions (Supplementary Fig. S3B). We also found that the expression level of BEE3 in the WT was increased after seedlings were transferred from dark to light conditions, and fluctuated slightly after seedlings were transferred from light to dark conditions. However, the level of BEE3 in ein2-5 and ein3-1 eil1-1 seedlings was gradually decreased during dark-to-light transition and increased during light-to-dark transition (Fig. 7C). In contrast, the expression of XTH7 was increased during dark-to-light transition and decreased during light-to-dark transition in all the genotypes (Fig. 7D). These results indicated that ethylene regulated the transcription of BEE3 and XTH7 in Arabidopsis via the EIN2-EIN3/EIL1 pathway, which was further balanced through the BAP/DE module according to light/dark conditions.
Discussion
Ethylene is responsible for the triple-response phenotype of dark-grown Arabidopsis seedlings, which is characterised by exaggerated curvature of the apical hook, radial swelling of the hypocotyl, and inhibition of hypocotyl and root growth (Ecker, 1995; Benavente and Alonso, 2006). Previous studies have shown that ethylene, through EIN3/EIL1, activates two distinct pathways: the PIF3-dependent growth-promoting pathway and an ERF1-mediated growth-inhibiting pathway, to regulate hypocotyl elongation in Arabidopsis (Smalle et al., 1997; Zhong et al., 2012).Zhong et al. (2014) further showed that the soil overlaying seedlings activates ethylene production to regulate emergence via EIN3/EIL1-conducted PIF3–ERF1 molecular circuitry. Moreover, Shi et al. (2016a) showed that COP1 stabilises EIN3 levels by directly targeting EBF1 and EBF2 for ubiquitination to induce ethylene-mediated hypocotyl elongation and seedling emergence from the soil. In our study, we found that ERF72 is another target gene of EIN3/EIL1 and the expression of ERF72 is up-regulated during light-to-dark transition, indicating that this transition, similar to overlaying soil, induces ethylene production. We further found that ERF72 interacts with BZR1 and ARF6 to connect the ethylene-, BR-, and auxin-signalling pathways in light-regulated hypocotyl elongation of Arabidopsis seedlings. These results provide novel insights into the role of the ethylene signalling pathway in regulating seedling photomorphogenesis.
Previous studies have shown that members of the group-VII ERF (ERF-VII) family are involved in hypoxia-induced shoot elongation and photomorphogenesis in plants (Gibbs et al., 2014; Giuntoli et al., 2014; Sasidharan and Voesenek, 2015; Voesenek and Bailey-Serres, 2015). Under deep-water conditions, ethylene accumulation induces the expression of two ERF-VII genes, SNORKEL1 and SNORKEL2, which triggers GA-mediated internode elongation in rice (Hattori et al., 2009). In addition, SUB1A-1, which is located in the SUBMERGENCE1 (SUB1) locus (another member of the ERF-VII family that also contains SUB1B and SUB1C), is reported to be a primary determinant of enhanced survival of completely submerged rice plants (Xu et al., 2006). SUB1A limits shoot elongation by promoting accumulation of the GA-response transcriptional inhibitors SLENDER RICE1 (SLR1) and SLENDER RICE-LIKE1 (SLRL1) and concomitantly diminishing the expression of GA-inducible genes in submerged conditions in rice (Fukao and Bailey-Serres, 2008). Arabidopsis has five ERF-VII genes, ERF71–75 (Nakano et al., 2006), the products of which contain a characteristic conserved motif, the MC-dipeptide, at the amino terminus that leads to proteolysis by the N-end rule pathway (Gibbs et al., 2011). The stability of ERF-VIIs is enhanced by NO signalling, and these factors are involved in regulation of seed germination, stomatal closure, and hypocotyl elongation (Gibbs et al., 2014), as well as hypoxia-regulated apical hook development after germination under dark conditions in Arabidopsis. Thus, to protect the stem-cell niche, plants monitor soil oxygen content after germination by means of hypoxia-stabilised ERF-VIIs (Abbas et al., 2015). However, not all ERFs that contain the MC-dipeptide are degraded by the N-end rule pathway; for example, SUB1A in rice (Gibbs et al., 2011), which plays different roles in response to abiotic stresses and developmental cues (Fukao et al., 2011, 2012). In our study, transgenic Arabidopsis overexpressing MAERF, but not those expressing MCERF, exhibited photomorphogenesis-related phenotypes under dark conditions (Fig. 1). These results suggest that the degradation of ERF72 through the N-end rule pathway is involved in the regulation of early seedling development in Arabidopsis.
To determine the mechanism by which ERF72 regulates hypocotyl elongation in Arabidopsis seedlings, we performed RNA-seq of dark-grown 35S::MAERF, erf, and WT seedlings (Fig. 2). Light-regulated and ARF6- and BZR1-target genes were enriched in ERF72-responsive DEGs, indicating that ARF6, BZR1, and ERF72 integrate the light and hormone signals that regulate gene expression during skotomorphogenesis. Previous research has shown that ARF6, BZR1, and PIF4 interact with each other to promote hypocotyl elongation by co-activating numerous shared target genes with known functions in cell elongation (Oh et al., 2014). Furthermore, DELLAs, as transcriptional inhibitors, form complexes with ARF6, BZR1, and PIF4 to create the BZR-ARF-PIF/DELLA (BAP/D) transcriptional module that regulates the expression of cell elongation-related genes, which in turn mediates the co-operative regulation of Arabidopsis morphogenesis by BR, auxin, GA, and light signals (Bai et al., 2012; Oh et al., 2014; Chaiwanon et al., 2016). In addition, ethylene stimulates cell wall acidification and induces production of cell wall modification proteins to facilitate rapid elongation of submerged Rumex palustris petioles (Voesenek and Bailey-Serres, 2015). Therefore, we evaluated the promoter activity of the ERF72-responsive DEGs, BEE3 and XTH7, which are target genes of ARF6 and BZR1, respectively. The results showed that ERF72 interacts with ARF6 and BZR1 (Fig. 3), inhibiting the transcriptional activity of ARF6 by suppressing its binding to the BEE3 promoter and directly antagonising the effect of BZR1 on transcription of XTH7. In addition, a previous study showed that DELLAs interact with and inhibit DNA binding by ERF72 (Marín-de la Rosa et al., 2014). Therefore, we propose that the revised BAP/DE (BZR-ARF-PIF/DELLA-ERF) module integrates ethylene, auxin, BR, GA, and light signals to regulate hypocotyl elongation during seedling photomorphogenesis, suggesting that crosstalk among these four proteins affects downstream gene expression related to hypocotyl cell elongation.
ERF72 is a candidate target of EIN3 (Chang et al., 2013). Our results showed that EIN3 binds the EBS fragment of the ERF72 promoter to activate the transcription of ERF72 under dark conditions (Fig. 6); indeed, the expression level of ERF72 was markedly lower in ein2-5 mutant and ein3-1 eil1-1 double-mutant seedlings than in the WT under both dark and light conditions. These results demonstrated that ERF72 is a target gene of EIN3, and the transcription of ERF72 is regulated by the EIN2-EIN3/EIL1 pathway in Arabidopsis. Previous studies have shown that COP1 stabilises EIN3 levels by directly targeting EBF1 and EBF2 for ubiquitination under dark conditions (Shi et al., 2016a). However, phyB interacts with EIN3 and EBF1/EBF2 in a light-dependent manner and stimulates degradation of EIN3 by SCFEBF1/EBF2 E3 ligases to attenuate ethylene-mediated responses (Shi et al., 2016b). In our study, dark conditions induced transcription of ERF72 and increased the abundance of ERF72 in Arabidopsis seedlings (Fig. 5). These results are in agreement with the stability of EIN3 under different light conditions. In addition, Li and Chye (2004) reported that AtEBP/ERF72 is transported to the plasma membrane by interacting with acyl-CoA-binding protein 2 (ACBP2) after transient co-expression in tobacco leaves. Here, we showed that ERF72-YFP fluorescence was dispersed in the cytoplasm under dark conditions and aggregated in the nucleus under light conditions; this nuclear localisation facilitates the function of ERF72. Therefore, we propose a mechanism by which the light, auxin, BR, and ethylene signalling pathways interplay to regulate hypocotyl growth and seedling morphogenesis (Fig. 8). In dark-grown seedlings, EIN3 mediates ethylene-induced expression of ERF72, despite ERF72 being localised in the cytoplasm, while ARF6 and BZR1 in the nucleus regulate the expression of target genes. This results in expression of a large number of cell elongation-related genes to mediate skotomorphogenic growth. Following exposure of dark-grown seedlings to light, ERF72 is translocated to the nucleus by an unknown mechanism, and interacts with ARF6 and BZR1 to attenuate the transcriptional regulation of target genes of ARF6 and BZR1. As a result, hypocotyl growth is inhibited and seedlings undergo photomorphogenesis. In our study, overexpression of MAERF led to accumulation of ERF72 in nuclei in dark-grown transgenic seedlings, which limited regulation by ARF6 and BZR1 of the expression of cell elongation-related genes, resulting in photomorphogenic growth. Our results suggest that light, auxin, BR, and ethylene signalling through the revised BAP/DE module play harmonious roles in the regulation of hypocotyl elongation in Arabidopsis seedlings.
Fig. 8.
Crosstalk among the auxin, brassinosteroid (BR), and ethylene signalling pathways, mediated by ARF6, BZR1, and ERF72, regulates hypocotyl growth and photomorphogenesis in Arabidopsis seedlings.
Interestingly, the expression of BEE3 and XTH7 was dramatically down-regulated in ein2-5 and ein3-1 eil1-1 seedlings compared to the WT under both dark and light conditions. Several putative EBS-like sites were predicted in the region upstream of the ATG site in the BEE3 and XTH7 promoters, which require further confirmation by a GUS activity assay (Supplementary Fig. S5). These results indicated that BEE3 and XTH7 are the potential target genes of EIN3. However, the expression of BEE3 and XTH7 were also regulated by different light conditions in ein2-5 and ein3-1 eil1-1 seedlings. In addition, we found that BEE3 and XTH7 are the target genes of ARF6 and BZR1, respectively. And ERF72, as one of the target genes of EIN3, interacts with ARF6 and BZR1 to separately regulate the transcriptional activity of ARF6 on BEE3 and that of BZR1 on XTH7. We also found that the expression of BEE3 and XTH7 was dramatically decreased in 2-DAG seedlings of 35S::MAERF plants compared with the WT and erf under dark conditions. These results suggested that the expression of BEE3 and XTH7 are fine-tuned by light and phytohormones via merging the EIN2-EIN3/EIL1 pathway and BAP/DE module during seedling photomorphogenesis in Arabidopsis.
Supplementary data
Supplementary data are available at JXB online.
Table S1. List of primers used in this study.
Table S2. RNA-seq data and its alignment to the Arabidopsis reference genome.
Table S3. List of differentially expressed genes.
Table S4. Raw data for promoter activity analysis.
Fig. S1. ERF72 expression in the WT, erf mutant, 35S::MAERF, and 35S::MCERF transgenic lines.
Fig. S2. Cotyledon development of WT, erf, and transgenic plants under dark conditions.
Fig. S3. Expression of ARF6, BZR1, BEE3, and XTH7 detected in seedlings of WT, erf, and 35S::MAERF grown under dark conditions.
Fig. S4. Locations of GCC-like boxes in the BEE3 and XTH7 promoters and the constructs used for GUS assays.
Fig. S5. Locations of the putative EBS-like sites in the BEE3 and XTH7 promoters.
Acknowledgments
We thank Dr Jianmin Zhou (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for providing the plasmids for LCI. We are grateful to Dr Xingwang Deng (State Key Laboratory of Protein and Plant Gene Research, Peking-Tsinghua Center for Life Sciences, School of Advanced Agricultural Sciences and School of Life Sciences, Peking University, China) for providing the facilities for the different light conditions. We thank Dr Hongwei Guo (Department of Biology, Southern University of Science and Technology) for providing seeds from ein2-5, ein3-1 eil1-1, and EIN3-OX plants. We thank Dr Michael J. Holdsworth (Division of Plant and Crop Sciences, School of Biosciences, University of Nottingham, UK) for providing seeds from the erfVII penta mutant. This work was supported by the National Natural Science Foundation of China (Grant no. 31070250) and National Key Basic Research Program of China (973 Program, 2013CB126902). The authors declare that they have no competing interests.
Glossary
Abbreviations:
- ERF72
ethylene response factor 72
- ARF6
auxin response factor 6
- BZR1
brassinazole-resistant 1
- BAP/DE
BZR-ARF-PIF/DELLA-ERF
- BR
brassinosteroid
- GA
gibberellin.
References
- Abbas M, Berckhan S, Rooney DJ, et al. 2015. Oxygen sensing coordinates photomorphogenesis to facilitate seedling survival. Current Biology 25, 1483–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biology 11, R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai MY, Shang JX, Oh E, Fan M, Bai Y, Zentella R, Sun TP, Wang ZY. 2012. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nature Cell Biology 14, 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MJ, Eklöf JM, Michel G, Kallas AM, Teeri TT, Czjzek M, Brumer H III. 2007. Structural evidence for the evolution of xyloglucanase activity from xyloglucan endo-transglycosylases: biological implications for cell wall metabolism. The Plant Cell 19, 1947–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavente LM, Alonso JM. 2006. Molecular mechanisms of ethylene signaling in Arabidopsis. Molecular BioSystems 2, 165–173. [DOI] [PubMed] [Google Scholar]
- Calderón Villalobos LI, Lee S, De Oliveira C, et al. 2012. A combinatorial TIR1/AFB-Aux/IAA co-receptor system for differential sensing of auxin. Nature Chemical Biology 8, 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiwanon J, Wang W, Zhu JY, Oh E, Wang ZY. 2016. Information integration and communication in plant growth regulation. Cell 164, 1257–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KN, Zhong S, Weirauch MT, et al. 2013. Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. eLIFE 2, e00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM. 2008. Firefly luciferase complementation imaging assay for protein–protein interactions in plants. Plant Physiology 146, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes-Esquivel N, Bou-Torrent J, Galstyan A, Gallemí M, Sessa G, Salla Martret M, Roig-Villanova I, Ruberti I, Martínez-García JF. 2013. The bHLH proteins BEE and BIM positively modulate the shade avoidance syndrome in Arabidopsis seedlings. The Plant Journal 75, 989–1002. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Clouse SD. 2002. Arabidopsis mutants reveal multiple roles for sterols in plant development. The Plant Cell 14, 1995–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Langford M, McMorris TC. 1996. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiology 111, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MP, Peterson DA, Biggs PJ. 2010. SolexaQA: at-a-glance quality assessment of illumina second-generation sequencing data. BMC Bioinformatics 11, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Lu F, Qiu Q, et al. 2016. REF6 recognizes a specific DNA sequence to demethylate H3K27me3 and regulate organ boundary formation in Arabidopsis. Nature Genetics 48, 694–699. [DOI] [PubMed] [Google Scholar]
- de Lucas M, Davière JM, Rodríguez-Falcón M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blázquez MA, Titarenko E, Prat S. 2008. A molecular framework for light and gibberellin control of cell elongation. Nature 451, 480–484. [DOI] [PubMed] [Google Scholar]
- de Wit M, Galvão VC, Fankhauser C. 2016. Light-mediated hormonal regulation of plant growth and development. Annual Review of Plant Biology 67, 513–537. [DOI] [PubMed] [Google Scholar]
- Ecker JR. 1995. The ethylene signal transduction pathway in plants. Science 268, 667–675. [DOI] [PubMed] [Google Scholar]
- Feng S, Martinez C, Gusmaroli G, et al. 2008. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. 2008. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proceedings of the National Academy of Sciences, USA 105, 16814–16819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J. 2011. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. The Plant Cell 23, 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao T, Yeung E, Bailey-Serres J. 2012. The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice. Plant Physiology 160, 1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR. 2015. Ethylene receptors in plants – why so much complexity?F1000Prime Reports 7, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Lee SC, Isa NM, et al. 2011. Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs DJ, Md Isa N, Movahedi M, et al. 2014. Nitric oxide sensing in plants is mediated by proteolytic control of group VII ERF transcription factors. Molecular Cell 53, 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuntoli B, Lee SC, Licausi F, Kosmacz M, Oosumi T, van Dongen JT, Bailey-Serres J, Perata P. 2014. A trihelix DNA binding protein counterbalances hypoxia-responsive transcriptional activation in Arabidopsis. PLoS Biology 12, e1001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Nagai K, Furukawa S, et al. 2009. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460, 1026–1030. [DOI] [PubMed] [Google Scholar]
- He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang ZY. 2005. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307, 1634–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Ouyang X, Deng XW. 2014. Beyond repression of photomorphogenesis: role switching of COP/DET/FUS in light signaling. Current Opinion in Plant Biology 21, 96–103. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C, Yoon GM, Shemansky JM, et al. 2012. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proceedings of the National Academy of Sciences, USA 109, 19486–19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Wang ZY. 2010. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annual Review of Plant Biology 61, 681–704. [DOI] [PubMed] [Google Scholar]
- Lau OS, Deng XW. 2010. Plant hormone signaling lightens up: integrators of light and hormones. Current Opinion in Plant Biology 13, 571–577. [DOI] [PubMed] [Google Scholar]
- Leivar P, Monte E. 2014. PIFs: systems integrators in plant development. The Plant Cell 26, 56–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, Huq E, Quail PH. 2008. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Current Biology 18, 1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Chye ML. 2004. Arabidopsis Acyl-CoA-binding protein ACBP2 interacts with an ethylene-responsive element-binding protein, AtEBP, via its ankyrin repeats. Plant Molecular Biology 54, 233–243. [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. 1996. A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272, 398–401. [DOI] [PubMed] [Google Scholar]
- Liang M, Li H, Zhou F, Li H, Liu J, Hao Y, Wang Y, Zhao H, Han S. 2015. Subcellular distribution of NTL transcription factors in Arabidopsis thaliana. Traffic 16, 1062–1074. [DOI] [PubMed] [Google Scholar]
- Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LA, Perata P, van Dongen JT. 2011. Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479, 419–422. [DOI] [PubMed] [Google Scholar]
- Luo XM, Lin WH, Zhu S, et al. 2010. Integration of light- and brassinosteroid-signaling pathways by a GATA transcription factor in Arabidopsis. Developmental Cell 19, 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-de la Rosa N, Sotillo B, Miskolczi P, et al. 2014. Large-scale identification of gibberellin-related transcription factors defines group VII ETHYLENE RESPONSE FACTORS as functional DELLA partners. Plant Physiology 166, 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchante C, Alonso JM, Stepanova AN. 2013. Ethylene signaling: simple ligand, complex regulation. Current Opinion in Plant Biology 16, 554–560. [DOI] [PubMed] [Google Scholar]
- Nagpal P, Ellis CM, Weber H, et al. 2005. Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132, 4107–4118. [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. 2006. Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiology 140, 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Bai MY, Arenhart RA, Sun Y, Wang ZY. 2014. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. eLIFE 3, e03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Zhu JY, Wang ZY. 2012. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nature Cell Biology 14, 802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik I, Kathare PK, Kim JI, Huq E. 2017. Expanding roles of PIFs in signal integration from multiple processes. Molecular Plant 10, 1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Shen Z, Huang SS, Schmitz RJ, Urich MA, Briggs SP, Ecker JR. 2012. Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338, 390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Braam J, Fry SC, Nishitani K. 2002. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant & Cell Physiology 43, 1421–1435. [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Voesenek LA. 2015. Ethylene-mediated acclimations to flooding stress. Plant Physiology 169, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Liu R, Xue C, Shen X, Wei N, Deng XW, Zhong S. 2016a. Seedlings transduce the depth and mechanical pressure of covering soil using COP1 and ethylene to regulate EBF1/EBF2 for soil emergence. Current Biology 26, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Shen X, Liu R, Xue C, Wei N, Deng XW, Zhong S. 2016b. The red light receptor phytochrome B directly enhances substrate-E3 ligase interactions to attenuate ethylene responses. Developmental Cell 39, 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Straeten DVD. 1997. Ethylene can stimulate Arabidopsis hypocotyl elongation in thelight. Proceedings of the National Academy of Sciences, USA 94, 2756–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Zhou XY, Li L, Xue LJ, Yang X, Xue HW. 2009. Genome-wide analysis revealed the complex regulatory network of brassinosteroid effects in photomorphogenesis. Molecular Plant 2, 755–772. [DOI] [PubMed] [Google Scholar]
- Sun TP. 2010. Gibberellin-GID1-DELLA: a pivotal regulatory module for plant growth and development. Plant Physiology 154, 567–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Németh K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell J, Koncz C. 1996. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell 85, 171–182. [DOI] [PubMed] [Google Scholar]
- Tang QY, Zhang CX. 2013. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Science 20, 254–260. [DOI] [PubMed] [Google Scholar]
- Tian Q, Reed JW. 1999. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126, 711–721. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Pachter L, Salzberg SL. 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voesenek LA, Bailey-Serres J. 2015. Flood adaptive traits and processes: an overview. New Phytologist 206, 57–73. [DOI] [PubMed] [Google Scholar]
- Von Arnim A, Deng XW. 1996. Light control of seedling development. Annual Review of Plant Physiology and Plant Molecular Biology 47, 215–243. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Bai MY, Oh E, Zhu JY. 2012. Brassinosteroid signaling network and regulation of photomorphogenesis. Annual Review of Genetics 46, 701–724. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Nakano T, Gendron J, et al. 2002. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Developmental Cell 2, 505–513. [DOI] [PubMed] [Google Scholar]
- Weijers D, Wagner D. 2016. Transcriptional responses to the auxin hormone. Annual Review of Plant Biology 67, 539–574. [DOI] [PubMed] [Google Scholar]
- Wen X, Zhang C, Ji Y, Zhao Q, He W, An F, Jiang L, Guo H. 2012. Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Research 22, 1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ. 2006. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442, 705–708. [DOI] [PubMed] [Google Scholar]
- Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J. 2002. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109, 181–191. [DOI] [PubMed] [Google Scholar]
- Zhong S, Shi H, Xue C, Wang L, Xi Y, Li J, Quail PH, Deng XW, Guo H. 2012. A molecular framework of light-controlled phytohormone action in Arabidopsis. Current Biology 22, 1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Shi H, Xue C, Wei N, Guo H, Deng XW. 2014. Ethylene-orchestrated circuitry coordinates a seedling’s response to soil cover and etiolated growth. Proceedings of the National Academy of Sciences, USA 111, 3913–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.