Abstract
To review the current status of, and labor expended for (in terms of time required), intracavitary brachytherapy (ICBT) in definitive radiotherapy/chemoradiotherapy for cervical cancer patients, two national surveys were performed. The first survey was conducted between July and August 2016 and consisted of a questionnaire of 12 items regarding ICBT procedures for cervical cancer, which was sent to 173 centers installed with high-dose-rate remote after-loading brachytherapy systems. Between November and December 2016, another survey was performed in 79 centers to evaluate labor required for ICBT procedures in terms of time spent and number of staff involved. In the first survey, the response rate was 77% of the 173 centers. ICBT was performed for cervical cancer in 118 (89%) centers. Imaging modalities used after applicator insertion were X-ray alone in 46 (40%), computed tomography in 69 (60%) and magnetic resonance imaging in 5 (4%) centers. Three-dimensional (3D) planning was performed in 55 centers (48%). Fifty-five (70%) centers responded to the second survey regarding ICBT-mandated labor. The median cumulative duration of the entire ICBT procedure was 330 min (the sum of the times spent by each staff member) and was longer in the 3D image–guided brachytherapy (3D-IGBT) (405 min) than in the X-ray group (230 min). This trend was significant for the specific processes of image acquisition and treatment planning, especially for radiation oncologists. In definitive radiotherapy/chemoradiotherapy for cervical cancer patients, 3D-IGBT use has been gradually spreading in Japan. The present survey revealed that ICBT, especially 3D-IGBT, requires substantial labor and time from staff.
Keywords: cervix neoplasms, radiotherapy, image-guided, brachytherapy, surveys and questionnaires
INTRODUCTION
In the past few decades, the prevalence of uterine cervical cancer has been increasing, especially in young women in Japan [1]. In clinical practice in Japan, radical hysterectomy with/without postoperative adjuvant treatment has been the first treatment of choice for operable Stage I–IIB patients for a long time [2]. Recently, clinical application of definitive radiotherapy (RT) or concurrent chemoradiotherapy (CCRT) has been increasing for patients with bulky tumors or Stage IIB disease as well as Stage III and IVA patients [2] according to the current guidelines [3, 4].
Definitive RT/CCRT for cervical cancer patients consists of external beam radiotherapy (EBRT) and intracavitary brachytherapy (ICBT). Excellent oncologic outcomes with acceptable toxicities after RT/CCRT have been reported. Recently, some physicians have been applying highly precise EBRT, such as intensity-modulated radiotherapy (IMRT) and stereotactic body radiotherapy (SBRT) as alternative treatments to ICBT. However, data from the National Cancer Database indicated that IMRT/SBRT as a boost is associated with significantly poorer oncologic outcomes compared with ICBT [5]. The NCCN Clinical Practice Guidelines in Oncology for Cervical Cancer, version 1.2018, clearly state that ‘conformal external beam radiotherapies (such as IMRT) should not be used as routine alternatives to brachytherapy for treatment of central disease of an intact cervix’ [3]. Therefore, it is very important to appropriately provide ICBT as an essential treatment for definitive RT/CCRT for cervical cancer patients.
ICBT requires substantial labor and time from multidisciplinary medical staff. A shortage of staff is one of the major issues in most Japanese radiotherapy centers. Recently, treatment planning for ICBT has shifted rapidly and globally from 2D to 3D modalities, belatedly to EBRT [6–10]. Three-dimensional image-guided ICBT (3D-IGBT) can be individualized according to the tumor size/shape and anatomy of each patient, while minimizing the dose to surrounding normal organs. As a result, improved oncologic outcomes as well as decreasing toxicities of 3D-IGBT compared with 2D-ICBT have been reported [11, 12]. However, clinical dissemination of 3D-IGBT has been slow and limited in Japan [13], probably because of the abovementioned structural issues in Japanese radiotherapy centers.
A working group was organized by the Japanese Group of Brachytherapy/Japan Society for Radiation Oncology in 2016 to review the current status of ICBT and its associated issues in Japan. In this paper, we will present the results from surveys performed by the working group and attempt to propose solutions for the issues identified.
MATERIALS AND METHODS
A survey regarding intracavitary brachytherapy performance
A questionnaire containing 12 items regarding ICBT procedures for cervical cancer (Appendix 1) was sent by postal mail to 173 radiotherapy centers installed with high-dose-rate (HDR) remote after-loading brachytherapy systems (RALSs) installed. A list of these centers was provided by the Database Committee of the Japan Society for Radiation Oncology (JASTRO). The survey was conducted between July and August 2016.
Measurement of labor expended during the intracavitary brachytherapy procedure
Another survey was conducted to measure the labor required by staff involved in ICBT procedures that use tandem and ovoid applicators for patients treated with definitive RT/CCRT. Cases of postoperative ICBT (using a vaginal cylinder or ovoid applicators alone) and ICBT with interstitial needles were excluded from the evaluation. Between November and December 2016, measurements were conducted at 79 centers that accepted our request at the time of the first questionnaire survey. The centers and cases were divided into two groups according to the type of imaging conducted after applicator insertion: X-rays only (X-ray group) and computed tomography (CT) and/or magnetic resonance imaging (MRI) (3D-IGBT group). The survey was performed between November and December 2016. Each center was asked to record the actual time in minutes and number of staff members (radiation oncologist, radiotherapist, medical physicist, and nurse) involved in each process of ICBT. The processes included preparation before the patient entered the HDR-RALS suite, preparation after the patient entered, applicator insertion, image acquisition, treatment planning, treatment (source delivery), applicator removal, and clean-up after treatment. Times were measured for one to three ICBT interventions at each center. The total time in minutes for each process was calculated as the sum of the times spent by each staff member involved in the procedure. For radiation oncologists, the values used to rank the physician’s experience were multiplied by the actual times measured. The physician experience values were proposed by the Japanese Health Insurance Federation for Surgery (2014) as follows: 1 for physicians with 1–4 years, 1.238 for those with 5–9 years, 1.52 for those with 10–14 years, and 1.747 for those with over 15 years of experience [14].
RESULTS
A survey of intracavitary brachytherapy performance
Of the 173 centers contacted in the first survey, 133 (77%) responded and completed the questionnaires. The results were analyzed in October 2016.
Outline
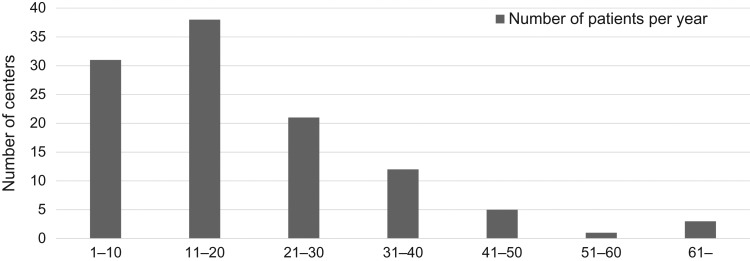
ICBT in definitive RT/CCRT for cervical cancer was performed at 118 (89%) of the 133 centers. Figure 1 shows the distribution of the centers according to the total number of patients treated with ICBT per year. The median numbers of patients and ICBT treatment sessions per year were 18 (range: 1–126) and 59 (range: 2–378), respectively. The mean numbers of ICBT fractions per patient were three in 20 centers, four in 74 centers, five in 9 centers, six in 4 centers, three or four in 7 centers, two to four in 2 centers, and five or six in 1 center.
Fig. 1.
Numbers of centers according to the total number of patients treated with intracavitary brachytherapy (ICBT) per year (n = 111). Patients treated with ICBT postoperatively (using a vaginal cylinder or ovoid applicator alone) were excluded.
Methods of treatment planning
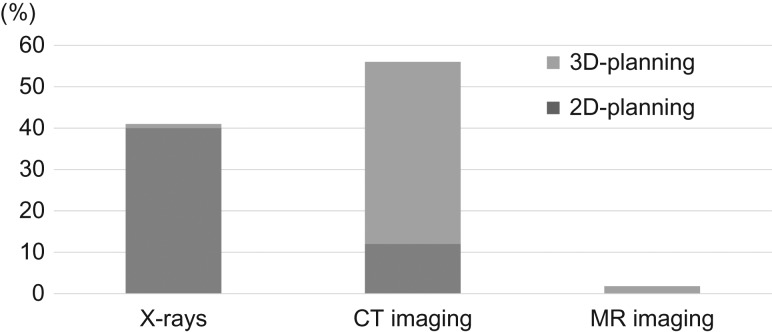
Figure 2 shows the imaging modalities and planning methods utilized for ICBT. In the analysis, 3D-planning was based on both high-risk clinical target volume (HR-CTV) contouring and dose evaluation using dose–volume histogram (DVH) parameters, while 2D-planning was based on X-rays and/or CT images, but prescription and evaluation were based on points alone (e.g. point A and other reference points). In the survey, 46 of 115 (40%) centers responded that they utilized X-rays alone for ICBT planning. In 69 (60%) centers, CT images were acquired after applicator insertion. 3D-planning was performed in 55 (48%) of 115 centers, of which 5 (4%) also acquired and utilized MRI for 3D-planning. Of the 55 centers that perform 3D-planning, 46 (84%) responded that 3D-planning is performed during every ICBT session. Of the 69 centers using CT, 30 (43%) have CT scanners installed in the same suite as the HDR-RALS machine. Of the 46 centers that use X-rays only, 24 (52%) plan to start 3D-IGBT within 3 years.
Fig. 2.
Acquired imaging modalities and treatment methods for ICBT (n = 115).
Physician-reported issues with the reimbursement system for intracavitary brachytherapy services for cervical cancer
Table 1 lists the issues regarding reimbursement for ICBT services reported by physicians in the survey. Most physicians responded that the current reimbursement system for ICBT was insufficient for various reasons.
Table 1.
Issues regarding reimbursement for ICBT costs for cervical cancer
| Issues | Number of centers |
|---|---|
| Low reimbursement for treatment | 35 |
| Low reimbursement for management | 30 |
| Limited number of times to calculate management feea | 31 |
| Low reimbursement for source replacement | 24 |
| None | 4 |
Multiple answers were allowed. ICBT = intracavitary brachytherapy.
aIn the current rule, the calculation is permited twice in maximum.
A survey of the labor expended during intracavitary brachytherapy
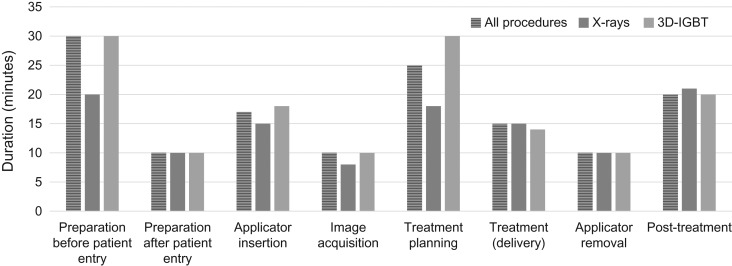
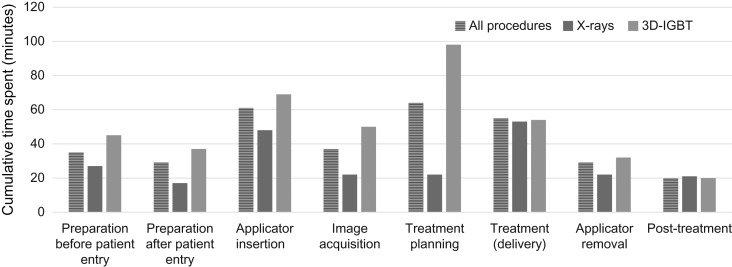
Fifty-five (70%) centers responded and provided data from 146 ICBT sessions. The characteristics of the patients who were monitored and the time spent conducting the ICBT procedures are shown in Table 2. In the 3D-IGBT group, 3D-planning was performed in 81 patients (87%). The median total duration for all ICBT process was 147 min. The duration was longer in the 3D-IGBT group (155 min) compared with in the X-ray group (141 min). Figure 3 shows the median duration for each specific process of ICBT. The numbers of staff involved in each ICBT process are shown in Table 3. For some processes (preparation after the patient entry, applicator insertion, image acquisition, and treatment planning), the numbers of radiation oncologists involved were greater in the 3D-IGBT group compared with in the X-ray group. In contrast, no significant difference in the number of radiotherapists/medical physicists or nurses was observed between the groups. The cumulative time spent by all staff members in all ICBT processes was 330 min. The cumulative time spent was longer in the 3D-IGBT group (405 min) than in the X-ray group (230 min). Figure 4 shows the cumulative time spent by all staff during each ICBT process. Other than the specific processes of treatment delivery and post-treatment, longer times were observed in the 3D-IGBT than in the X-ray group. Tables 4–6 show the median cumulative time spent for each procedure according to occupation. Although a similar trend was observed for radiation oncologists as that for overall staff, there were no differences between the X-ray and 3D-IGBT groups in the other staff categories. Seven patients from three centers were treated with MRI-based IGBT. The median cumulative time spent by staff on these seven patients was 911 min: 471 min by radiation oncologists and 440 min by other staff. Substantial differences were observed in the process of applicator insertion (3D-IGBT overall: 69 min, MRI-based: 171 min), image acquisition (3D-IGBT overall: 50 min, MRI-based: 185 min) and treatment planning (3D-IGBT overall: 98 min, MRI-based: 194 min).
Table 2.
Characteristics of patients who were monitored and the times required for ICBT
| Total (n = 146) | X-rays (n = 53) | 3D-IGBT (n = 93) | |
|---|---|---|---|
| FIGO stage | |||
| I | 33 | 11 | 22 |
| II | 46 | 19 | 27 |
| III | 45 | 16 | 29 |
| IV | 16 | 6 | 10 |
| Unknown | 6 | 1 | 5 |
| Age (median) | 65 (25–93) | 65 (25–93) | 65 (29–86) |
| Sedation/analgesia | |||
| IV conscious | 71 | 19 | 52 |
| General anesthesia | 9 | 0 | 9 |
| Oral/suppository | 53 | 27 | 26 |
| None | 8 | 6 | 2 |
| Unknown | 5 | 1 | 4 |
ICBT = intracavitary brachytherapy, 3D-IGBT = three-dimensional image-guided brachytherapy, IV = intravenous.
Fig. 3.
Median duration for each specific ICBT process (n = 146).
Table 3.
Mean numbers of staff involved in each ICBT process (range)
| Process | Total (n = 146) | X-rays (n = 53) | 3D-IGBT (n = 93) |
|---|---|---|---|
| Preparation before the patient entry | |||
| RO | 0.9 (0–3) | 0.8 (0–2) | 0.9 (0–3) |
| RT/P | 1 (0–2) | 0.9 (0–2) | 1 (0–2) |
| N | 1.1 (0–2) | 1.1 (0–2) | 1.2 (0–2) |
| Preparation after the patient entry | |||
| RO | 0.9 (0–4) | 0.6 (0–4) | 1.2 (0–4) |
| RT/P | 0.8 (0–2) | 0.8 (0–2) | 0.8 (0–2) |
| N | 1.3 (1–2) | 1.2 (1–2) | 1.3 (1–2) |
| Applicator insertion | |||
| RO | 1.6 (1–4) | 1.35 (1–3) | 1.8 (1–4) |
| RT/P | 0.8 (0–3) | 0.8 (0–3) | 0.8 (0–3) |
| N | 1.2 (0–2) | 1.1 (0–2) | 1.3 (1–2) |
| Image acquisition | |||
| RO | 1.3 (0–4) | 0.9 (0–3) | 1.4 (0–4) |
| RT/P | 1.5 (1–3) | 1.5 (1–3) | 1.5 (1–3) |
| N | 0.9 (0–2) | 0.6 (0–2) | 1 (0–2) |
| Treatment planning | |||
| RO | 1.4 (0–3) | 1.2 (0–3) | 1.6 (1–3) |
| RT/P | 1.6 (0–3) | 1.6 (0–3) | 1.6 (0–3) |
| N | 0.5 (0–2) | 0.4 (0–1) | 0.6 (0–2) |
| Treatment (delivery) | |||
| RO | 1.3 (0–4) | 1.3 (0–4) | 1.3 (0–3) |
| RT/P | 1.5 (0–3) | 1.6 (1–3) | 1.5 (0–2) |
| N | 1.5 (0–3) | 1.6 (1–3) | 1.5 (0–2) |
| Applicator removal | |||
| RO | 1.4 (1–4) | 1,5 (1–4) | 1.2 (1–3) |
| RT/P | 0.7 (0–2) | 0.6 (0–2) | 0.7 (0–2) |
| N | 1.1 (0–2) | 1.2 (1–2) | 1.1 (0–2) |
| Post-treatment | |||
| RO | 0.1 (0–2) | 0 | 0.2 (0–2) |
| RT/P | 0.5 (0–2) | 0.5 (0–2) | 0.5 (0–2) |
| N | 1.2 (0–3) | 1.2 (0–2) | 1.2 (1–3) |
RO = radiation oncologist, RT/P = radiotherapist and/or medical physicist, N = nurse.
Fig. 4.
Median cumulative times spent by all staff during each ICBT process (n = 146). The ‘cumulative times’ refers to the sum of the times spent by each staff member involved.
Table 4.
Median time expended by radiaton oncologists during each ICBT process (min)
| Process | Totala (n = 146) | X-raysa (n = 53) | 3D-IGBTa (n = 93) |
|---|---|---|---|
| Preparation before the patient entry | 0 | 0 | 0 |
| Preparation after the patient entry | 9 | 0 | 16 |
| Applicator insertion | 36 | 28 | 39 |
| Image acquisition | 18 | 9 | 25 |
| Treatment planning | 42 | 9 | 55 |
| Treatment (delivery) | 25 | 22 | 25 |
| Applicator removal | 17 | 12 | 18 |
| Post-treatment | 0 | 0 | 0 |
| Total | 147 | 80 | 178 |
ICBT = intracavitary brachytherapy, 3D-IGBT = three dimensional image-guided brachytherapy.
aSum of the times required by each staff involved in the procedure.
Table 6.
Median time expended by nurses during each ICBT process (min)
| Process | Totala (n = 146) | X-raysa (n = 53) | 3D-IGBTa (n = 93) |
|---|---|---|---|
| Preparation before the patient entry | 20 | 15 | 30 |
| Preparation after the patient entry | 15 | 12 | 15 |
| Applicator insertion | 20 | 20 | 20 |
| Image acquisition | 7 | 3 | 10 |
| Treatment planning | 0 | 0 | 0 |
| Treatment (delivery) | 13 | 11 | 14 |
| Applicator removal | 10 | 10 | 10 |
| Post-treatment | 20 | 15 | 20 |
| Total | 105 | 86 | 119 |
ICBT = intracavitary brachytherapy, 3D-IGBT = three-dimensional image-guided brachytherapy.
aSum of the times required by each staff involved in the procedure.
Table 5.
Median time expended by radiotherapists and/or medical physicists during each ICBT process (min)
| Process | Totala (n = 146) | X-raysa (n = 53) | 3D-IGBTa (n = 93) |
|---|---|---|---|
| Preparation before the patient entry | 15 | 12 | 15 |
| Preparation after the patient entry | 5 | 5 | 5 |
| Applicator insertion | 5 | 0 | 10 |
| Image acquisition | 15 | 10 | 15 |
| Treatment planning | 30 | 26 | 30 |
| Treatment (delivery) | 17 | 20 | 15 |
| Applicator removal | 2 | 0 | 4 |
| Post-treatment | 0 | 0 | 0 |
| Total | 89 | 73 | 94 |
ICBT = intracavitary brachytherapy, 3D-IGBT = three-dimensional image-guided brachytherapy.
aSum of the times required by each staff involved in the procedure.
DISCUSSION
The present study demonstrated the current status of and issues regarding ICBT for patients with uterine cervical cancer in Japan. As shown in Table 7, whereas the dissemination of 3D-IGBT in Japan has been slow compared with in other developed countries, current research has revealed that the number of centers applying 3D-IGBT has been increasing gradually [13]. Further expansion is expected over the next few years. The GEC-ESTRO guidelines and experts from overseas recommend the use of MRI for 3D-IGBT [15, 16]. However, our survey showed that only 4% of centers in Japan perform MRI-based IGBT.
Table 7.
ICBT treatment planning for cervical cancer
| Surveillance | Number | 2D-ICBT | 3D-IGBT | ||
|---|---|---|---|---|---|
| Country | Year | of centers | X-ray | CT | MRI |
| US [6, 7] | 2007 | 133 | 43% | 55% | 2% |
| 2014 | 219 | 15% | 95% | 34% | |
| Canada [8, 9] | 2009 | 22 | 50% | 45% | 5% |
| 2012 | 24 | 21% | 75% | 38% | |
| 2015 | 28 | 4% | 96% | 57% | |
| The Netherlands [10] | 2015 | 16 | 0% | 55% | 100% |
| Japan [13] (present study) | 2012 | 171 | 80% | 14% | 1% |
| 2016 | 133 | 40% | 44% | 4% | |
ICBT = intracavitary brachytherapy, IGBT = image-guided intracavitary brachytherapy, CT = computed tomography, MRI = magnetic resonance imaging.
In addition to the questionnaire survey, we performed another survey to measure the labor required for ICBT procedures. This investigation demonstrated that the level of labor, which was expressed as the time required by staff, to perform ICBT procedures was large. The survey also revealed that 3D-IGBT required more time than did X-ray-based ICBT, especially for treatment planning. Although the data were limited, the study showed that the labor required for MRI-based IGBT is substantial. These findings suggest that there are some barriers to utilizing ICBT, especially 3D-IGBT, in Japanese radiotherapy centers because of limited man-power and time. Whereas some centers employ MRI-based IGBT [17], most centers cannot afford to do so. For such situations in Japan, consensus-based recommendations for HR-CTV with CT were recently developed to minimize the variation in CT-based HR-CTV and deviation from MRI-based HR-CTV [18].
Besides these issues of man-power and machine operation time, the high cost necessary for installing and maintaining the HDR-brachytherapy system is another major challenge. The current reimbursement system is far from sufficient to cover the full costs of ICBT, especially 3D-IGBT. In our present questionnaire survey, opinions regarding reimbursement issues for ICBT for cervical cancer were also assessed. Most centers indicated problems relating to the treatment fees and reimbursement funds. In a Korean survey, similar to our results, 27 of 28 centers indicated that they encounter difficulties in maintaining their brachytherapy facilities [19]. That Korean study also reported that the number of centers installing HDR-brachytherapy systems decreased by 28.2% from 2006 to 2014, while the number of total radiotherapy systems increased. According to the Korean Central Cancer Registry, the age-standardized incidence rate of cervical cancer per 100 000 persons decreased from 16.3 in 1999 to 10.6 in 2010 [19]. In contrast, this figure increased from 7.0 in 1999 to 11.2 in 2010 in Japan [1]. This suggests that a decrease in the number of institutions housing HDR-brachytherapy systems would be a more serious problem in Japan than in Korea. To overcome this issue, increasing the reimbursement funds for ICBT, especially 3D-IGBT, is essential.
Recently, Bauer-Nilsen and their colleagues reported the costs of administering brachytherapy and EBRT using a time-driven activity-based costing methodology [20]. They compared the costs with the United States Medicare reimbursement and relative value units (RVUs) [20]. They demonstrated that brachytherapy is costlier to deliver and requires more time from the attending radiation oncologist and medical physicist compared with EBRT, and their analyses of the reimbursement indicated the payments to physicians do not account for the substantial time requirements and expertise in the USA [20]. In our present analyses, actual costs and reimbursement in yen and/or US dollars were not estimated. Future research is needed to suggest the appropriate reimbursement for conducting steady ICBT performance for cervical cancer patients in Japan.
From the perspective of efficiency, limiting ICBT treatment to specialized centers housing HDR-brachytherapy systems may be an appropriate future direction. However, that should be carefully discussed before execution. First, it would be essential for patients who receive EBRT in radiotherapy departments lacking brachytherapy equipment to be transferred to HDR-brachytherapy centers smoothly and securely. Prolonging the overall treatment time negatively affects oncologic outcomes in cervical cancer patients treated with definitive RT/CCRT [21, 22]. Second, the number of patients who receive an EBRT boost (e.g. IMRT or SBRT) as an alternative to ICBT may increase. Third, it is mandatory to ensure sufficient manpower in centers performing HDR-brachytherapy where patients would be transferring from other institutions. Therefore, adequate reimbursement to sufficiently cover labor costs, including those for quality assurance, would be particularly important in these centers.
In summary, the present study demonstrated that the use of 3D-IGBT in definitive RT/CCRT for patients with cervical cancer is gradually spreading in Japan. The survey also revealed that ICBT, especially 3D-IGBT, requires substantial labor and time from various staff members. These results suggest that adequate reimbursement is crucial for providing ICBT as an essential treatment for definitive RT/CCRT for cervical cancer patients.
ACKNOWLEDGEMENTS
The authors thank all staff members of the Japanese radiation oncology centers who kindly responded to the surveys. Results from this research were presented at the 30th Annual Meeting of the Japanese Society for Radiation Oncology, Osaka, November 17–19, 2017.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
FUNDING
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI [Grant No. JP16K10398].
APPENDIX 1
Questions:
Q1. Performance of ICBT for patients with intact uterine cervical cancer
Yes or No
If the answer to Q1 is ‘No’, please answer the following question:
Q2. Reason for not performing ICBT:
No patients indicated
Center uses EBRT instead of ICBT
Referral to other centers that perform ICBT
Other
If the answer to Q1 is ‘Yes’, please answer the following questions:
Q3. How many patients were treated with definitive ICBT from Jan. 2015 to Dec. 2015?
Q4. How many ICBT procedures were conducted from Jan. 2015 to Dec. 2015?
Q5. How many ICBT procedures were conducted per patient (the institutional standard)?
Q6. What method is used for ICBT treatment planning?
2D-planning with X-rays
2D-planning with CT images (no contouring, DVH evaluation)
3D-planning with CT images (with both contouring and DVH evaluation)
3D-planning with MRI (with both contouring and DVH evaluation)
Other
If the answer to Q6 is ‘2’ or ‘3’, please answer the following question:
Q7. Is an in-suite CT scanner used?
Yes or No
If the answer to Q6 is ‘3’ or ‘4’, please answer the following question:
Q8. Is 3D-planning performed for every ICBT procedure?
Yes or No
If the answer to Q8 is ‘No’, please answer the following question:
Q9. What is the reason for not performing 3D-planning for every procedure?
No need
Time issue
Man-power issue
Insufficient reimbursement
Other
If the answer to Q6 is ‘1’, please answer the following question:
Q10. Is there a plan to start 3D-IGBT within 3 years?
Yes or No
If the answer to Q10 is ‘No’, please answer the following question:
Q11. What is the reason for no plan?
No need
Time issue
Man-power issue
Equipment issue
Poor access to CT/MRI suite
Insufficient reimbursement
Other
Q12. Problems regarding reimbursement of ICBT for cervical cancer
Low treatment fee
Low management fee
Limited number of times to calculate management feea
Low reimbursement for source replacement
Low additional fee for 3D-planning
No problem
aIn the current rule, the calculation is permitted for twice in maximum.
Additional comments:
REFERENCES
- 1. Cancer Information Service. Cancer Statistics in Japan; table download https://ganjoho.jp/reg_stat/statistics/dl/index.html#incidencehttp://plaza.umin.ac.jp/~jsog-go/ (4 January 2018, date last accessed).
- 2. Japan Society of Obstetrics and Gynecology Gynecologic Tumor Committee (in Japanese). http://plaza.umin.ac.jp/~jsog-go/ (4 January 2018, date last accessed).
- 3. National Comprehensive Cancer Network Cervical Cancer (2018) NCCN Clinical Practice Guidelines in Oncology https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (4 January 2018, date last accessed).
- 4. Ebina Y, Yaegashi N, Katabuchi H et al. Japan Society of Gynecologic Oncology guidelines 2011 for the treatment of uterine cervical cancer. Int J Clin Oncol 2015;20:240–8. [DOI] [PubMed] [Google Scholar]
- 5. Gill BS, Lin JF, Krivak TC et al. National Cancer Database analysis of radiation therapy consolidation modality for cervical cancer: the impact of new technological advancements. Int J Radiat Oncol Biol Phys 2014;90:1083–90. [DOI] [PubMed] [Google Scholar]
- 6. Viswanathan AN, Erickson BA. Three-dimensional imaging in gynecologic brachytherapy: a survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys 2010;76:104–9. [DOI] [PubMed] [Google Scholar]
- 7. Grover S, Harkenrider MM, Cho LP et al. Image guided cervical brachytherapy: 2014 survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys 2016;94:598–604. [DOI] [PubMed] [Google Scholar]
- 8. Pavamani S, D’Souza DP, Portelance L et al. Image-guided brachytherapy for cervical cancer: a Canadian Brachytherapy Group survey. Brachytherapy 2011;10:345–51. [DOI] [PubMed] [Google Scholar]
- 9. Taggar AS, Phan T, Traptow L et al. Cervical cancer brachytherapy in Canada: a focus on interstitial brachytherapy utilization. Brachytherapy 2017;16:161–6. [DOI] [PubMed] [Google Scholar]
- 10. de Boer P, Jürgenliemk-Schulz IM, Westerveld H et al. Patterns of care survey: radiotherapy for women with locally advanced cervical cancer. Radiother Oncol 2017;123:306–11. [DOI] [PubMed] [Google Scholar]
- 11. Rijkmans EC, Nout RA, Rutten IH et al. Improved survival of patients with cervical cancer treated with image-guided brachytherapy compared with conventional brachytherapy. Gynecol Oncol 2014;135:231–8. [DOI] [PubMed] [Google Scholar]
- 12. Ohno T, Noda SE, Okonogi N et al. In-room computed tomography–based brachytherapy for uterine cervical cancer: results of a 5-year retrospective study. J Radiat Res 2017;58:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohno T, Toita T, Tsujino K et al. A questionnaire-based survey on 3D image–guided brachytherapy for cervical cancer in Japan: advances and obstacles. J Radiat Res 2015;56:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Draft Proposal from the Japanese Health Insurance Federation for Surgery (JHIFS) in 2014. Igakutushinsya, 2014, 8. (in Japanese).
- 15. Pötter R, Haie-Meder C, Van Limbergen E et al. ; GEC ESTRO Working Group Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image–based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67–77. [DOI] [PubMed] [Google Scholar]
- 16. Harkenrider MM, Alite F, Silva SR et al. Image-based brachytherapy for the treatment of cervical cancer. Int J Radiat Oncol Biol Phys 2015;92:921–34. [DOI] [PubMed] [Google Scholar]
- 17. Nemoto MW, Iwai Y, Togasaki G et al. Preliminary results of a new workflow for MRI/CT-based image-guided brachytherapy in cervical carcinoma. Jpn J Radiol 2017;35:760–5. [DOI] [PubMed] [Google Scholar]
- 18. Ohno T, Wakatsuki M, Toita T et al. ; The Working Group of the Gynecological Tumor Committee of the Japanese Radiation Oncology Study Group (JROSG) Recommendations for high-risk clinical target volume definition with computed tomography for three-dimensional image-guided brachytherapy in cervical cancer patients. J Radiat Res 2017;58:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H, Kim JY, Kim J et al. Current status of brachytherapy in Korea: a national survey of radiation oncologists. J Gynecol Oncol 2016;27:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bauer-Nilsen K, Hill C, Trifiletti DM et al. Evaluation of delivery costs for external beam radiation therapy and brachytherapy for locally advanced cervical cancer using time-driven activity-based costing. Int J Radiat Oncol Biol Phys 2018;100:88–94. [DOI] [PubMed] [Google Scholar]
- 21. Petereit DG, Sarkaria JN, Chappell R et al. The adverse effect of treatment prolongation in cervical carcinoma. Int J Radiat Oncol Biol Phys 1995;32:1301–7. [DOI] [PubMed] [Google Scholar]
- 22. Song S, Rudra S, Hasselle MD et al. The effect of treatment time in locally advanced cervical cancer in the era of concurrent chemoradiotherapy. Cancer 2013;119:325–31. [DOI] [PubMed] [Google Scholar]