Abstract
3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) are extremely well tolerated but are associated with a range of mild-to-moderate statin-associated muscle symptoms (SAMS). Estimates of SAMS incidence vary from <1% in industry-funded clinical trials to 10–25% in nonindustry-funded clinical trials and ∼60% in some observational studies. SAMS are important because they result in dose reduction or discontinuation of these life-saving medications, accompanied by higher healthcare costs and cardiac events. The mechanisms that produce SAMS are not clearly defined. Statins block the production of farnesyl pyrophosphate, an intermediate in the mevalonate pathway, which is responsible for the production of coenzyme Q10 (CoQ10). This knowledge has prompted the hypothesis that reductions in plasma CoQ10 concentrations contribute to SAMS. Consequently, CoQ10 is popular as a form of adjuvant therapy for the treatment of SAMS. However, the data evaluating the efficacy of CoQ10 supplementation has been equivocal, with some, but not all, studies suggesting that CoQ10 supplementation mitigates muscular complaints. This review discusses the rationale for using CoQ10 in SAMS, the results of CoQ10 clinical trials, the suggested management of SAMS, and the lessons learned about CoQ10 treatment of this problem.
Keywords: statin, myalgia, muscle pain, ubiquinone, myopathy
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States, affecting 1 in 3 (or 81.1 million) adults (1). Reducing LDL cholesterol by 1 mmol/L through lifestyle or pharmacologic intervention reduces CVD-related events by 22% (2). 3-Hydroxy-3-methylglutaryl CoA reductase inhibitors (statins) decrease LDL cholesterol by ≤60% and lower the overall CVD risk by 25–50% (3). Approximately 26% of Americans ≥45 y old are currently prescribed a statin, making statins among the most widely prescribed drugs in the United States—and in the world (3, 4).
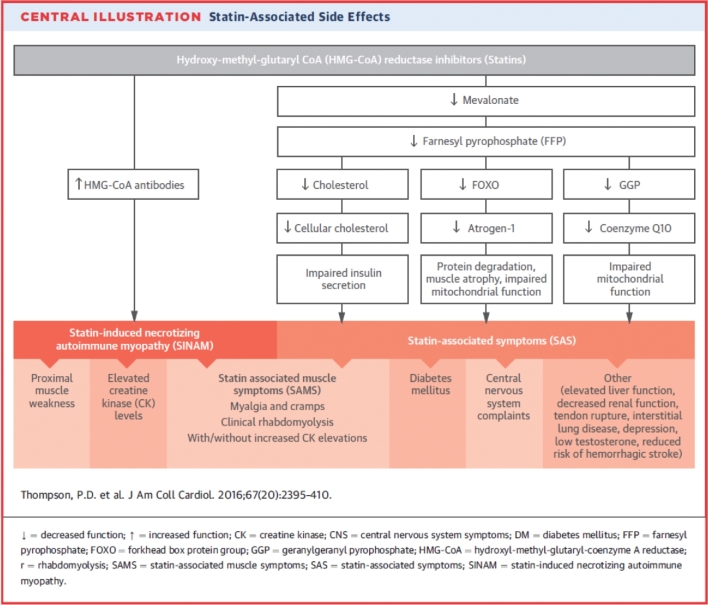
Statins are generally well tolerated, but patient surveys suggest that 30–62% of statin-prescribed patients discontinue therapy because of muscle fatigue, weakness and pain (Figure 1) (5–7). The incidence of these statin-associated muscle symptoms (SAMS) ranges widely, from <1% in industry-funded trials to 10–25% in nonindustry-funded clinical trials (8–10) and 60% in some observational studies (5). High rates of statin discontinuation or nonadherence because of muscle complaints emphasize the need to understand the mechanisms producing SAMS, as suboptimal statin use increases healthcare costs by increasing the risk of cardiac events (5–7) and increasing the use of more expensive medications, such as proprotein convertase subtilisin kexin type 9 (PCSK9 inhibitors). Multiple mechanisms for SAMS have been proposed (11), including the depletion of coenzyme Q10 (CoQ10). This review will discuss why CoQ10 depletion has been suggested as a cause of SAMS and the results of clinical trials testing this hypothesis.
FIGURE 1.
Statin-associated side effects. Reprinted from reference 11 with permission.
The Rationale for CoQ10 Depletion as a Cause of SAMS
CoQ10 is a naturally occurring, fat-soluble coenzyme that resides in the hydrophobic portions of mammalian cellular membranes (12). Approximately half of the body's supply of CoQ10 is obtained by endogenous synthesis and half by fat consumption (12). CoQ10 plays a key role in energy production via mitochondrial electron transport during oxidative phosphorylation. CoQ10 can carry 1 or 2 electrons through the transport chain (13), so can be fully oxidized, partially oxidized, or fully reduced as ubiquinone, ubisemiquinone, or ubiquinol, respectively. CoQ10 also acts as an antioxidant by scavenging free radicals. CoQ10 is concentrated in the cells of organs with the highest energy requirements, such as the kidney, liver, and heart (13). Primary deficiency of CoQ10 is associated with nephrotic syndrome, heart failure, neuropathy, and/or muscular and neurological disorders (14, 15). CoQ10 depletion is a logical candidate as a cause of statin myopathy for the following reasons:
Statins alter lipid metabolism by inhibiting HMG-CoA reductase, the rate-limiting enzyme responsible for the synthesis of cholesterol in the mevalonate pathway. This pathway also produces isoprenylated proteins and CoQ10 (Figure 2).
CoQ10 is a critical enzyme in mitochondrial energy production, and some evidence suggests that mitochondrial dysfunction contributes to SAMS. Muscle biopsies in 3 patients with SAMS and normal creatine kinase (CK) concentrations demonstrated histopathology findings consistent with mitochondrial dysfunction, including ragged red fibers, increased intramuscular lipid, and reduced cytochrome oxidase staining. The latter indicates lower mitochondrial function, as cytochrome oxidase is an important metabolic enzyme in mitochondria (10). Statins may also lessen the increase in mitochondrial function produced by exercise training (16). Maximal oxygen uptake increased by 10% in 19 subjects after undergoing aerobic exercise training for 12 wk, but only by 1.5% in 18 subjects who exercise trained during treatment with 40 mg simvastatin/d (P < 0.01). Citrate synthase activity, a marker of mitochondrial content, measured in vastus lateralis muscle biopsies, increased by 13% in the exercise-only subjects, but decreased by 4.5% in the exercise and statin subjects (P < 0.05). Mitochondrial complexes I, II, III, and IV increased with exercise training in the exercise-only group but not in the exercise training and statin group. Similar reductions in the mitochondrial response to exercise training have been demonstrated in mice (17) treated with simvastatin.
The blood CoQ10 concentration decreases during statin therapy. An analysis of 8 placebo-controlled trials measuring CoQ10 found an average reduction of −0.44 μmol/L (95% CI: −0.52, −0.37 μmol/L), which was statistically significant overall and significant in all but one of the studies (18). This has generally been attributed to the fact that CoQ10 is transported in LDL and VLDL because there is generally no reduction in CoQ10 concentrations when adjusting for the reduction in LDL, suggesting that statin-induced reductions in LDL and VLDL could reduce CoQ10 concentrations. The biological significance of these decreases in CoQ10 concentrations is not clear.
Muscle biopsy studies have shown reductions in intramuscular CoQ10 during statin therapy in some (19), but not all, studies (20–22). Reductions in muscle CoQ10, however, do not prove that CoQ10 causes SAMS. CoQ10 is a mitochondrial protein, so other factors, such as myalgia from statin use, could decrease physical activity, which would reduce the muscle mitochondrial content and lower the CoQ10 concentrations. This makes it impossible to determine whether statin therapy produces CoQ10 depletion, leading to mitochondrial dysfunction and SAMS, or whether SAMS lead to reduced physical activity, reduced muscle mitochondria, and reduced CoQ10 concentrations. The latter could explain why only a small number of patients treated with statin therapy experience SAMS, despite the much more-universal decreases in circulating CoQ10 that occur with statin therapy.
Genetic studies suggest that SAMS are more frequent in individuals with inherited defects in CoQ10 synthesis. The CoQ2 gene encodes for para-hydroxybenzoate-polyprenyl transferase, the second enzyme in the CoQ10 synthetic pathway (23). A genetic comparison of 133 statin-intolerant and 158 statin-tolerant subjects found that the ORs were 2.42 (P = 0.047) and 2.33 (P = 0.019) for 2 single nucleotide polymorphisms in the CoQ2 gene and 2.58 for the haplotype (P = 0.007) (23). We compared the frequency of 31 candidate genes for statin myopathy in 377 patients with SAMS and 416 asymptomatic statin-treated patients (24). Three genes were statistically different between the groups: ATP2B1, which encodes for a calcium transporting ATPase (P < 0.00079); DMPK, which encodes for a protein kinase implicated in myotonic dystrophy (P < 0.0016); and COQ2 (P < 0.000041). CoQ2 was also identified as possibly contributing to SAMS in a hypothesis-free, genome-wide association study in the same subjects (24). This study examined 865,483 single nucleotide polymorphisms; because of the large number of comparisons performed, none were significantly different between the groups, including CoQ2.
FIGURE 2.
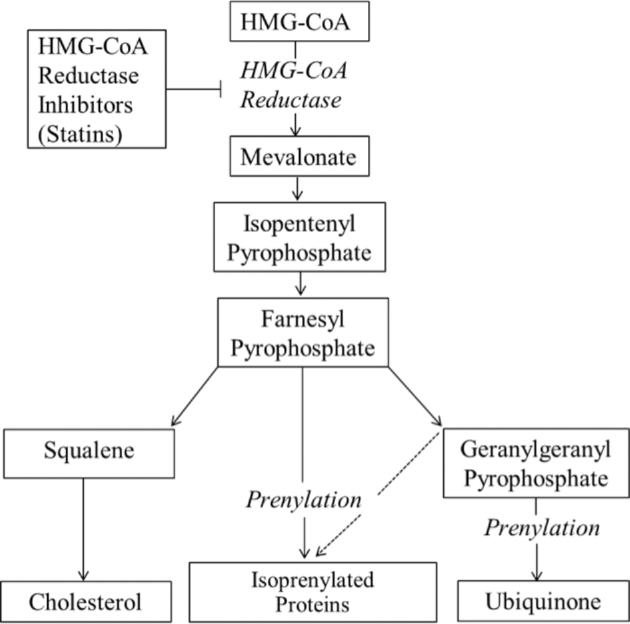
Products of the mevalonate pathway possibly affected by HMG-CoA reductase inhibitors (statins). HMG-CoA, 3-hydroxy-3-methylglutaryl CoA.
The Failure of CoQ10 in Clinical Trials
We are aware of only 6 trials that have examined the effect of CoQ10 supplementation of SAMS. Five of these trials, involving a total of 302 patients, were evaluated by meta-analysis (18). There were no differences in muscle pain (P = 0.20) or plasma CK concentrations (P = 0.38) between individuals who did or did not receive CoQ10 supplementation.
There are no diagnostic tests for SAMS, so it is unclear in these studies which subjects actually had muscle complaints owing to statins. Consequently, we performed trial 6, an NIH-funded study (RC1 AT005836), designed to answer definitively whether CoQ10 treatment resolved SAMS (25). We recruited subjects with a history of SAMS from our cholesterol management clinic. Definite SAMS was diagnosed using a prestudy, run-in protocol. Specifically, subjects were randomized to either 20 mg simvastatin/d or to placebo for 8 wk. Subjects then entered a 4-wk no-treatment washout phase before being assigned to the alternative treatment; subjects who were randomly assigned to receive simvastatin first were crossed over to placebo, and vice versa. We recruited 120 subjects; however, 43 (35.8%) developed muscle pain only during the simvastatin treatment, a group we termed confirmed myalgics. Only 35.8% of patients experienced myalgia on simvastatin and did not experience it on placebo, what we term true or confirmed statin myalgia, and 17.5% of patients had no symptoms on simvastatin or placebo which could have been because the dose we selected was too low. However, 29.2% experienced pain on placebo but not on simvastatin and 17.5% experienced pain on both simvastatin and placebo during the confirmation phase.
This protocol was designed to select only individuals with confirmed myalgia for the CoQ10 treatment arm of the study. Following this lead-in phase, the confirmed myalgics were randomized to either placebo or 600 mg CoQ10/d. This dosage was chosen because the usually recommended dosage of ubiquinol or CoQ10 is 200 mg/d, and prior studies have used 100 or 200 mg/d. We sought to ensure adequate tissue concentrations throughout the trial, as this was a criticism of the prior studies. Consequently, before commencing simvastatin therapy in the CoQ10 protocol, we “loaded” subjects with either CoQ10 600 mg/d or placebo for 2 wk before statin reinitiation, to ensure adequate CoQ10 concentrations before treatment. Subjects continued this dosage of either placebo or CoQ10 and received 20 mg simvastatin/d.
We measured muscle pain according to the Brief Pain Inventory, time to pain onset, arm and leg muscle strength, and maximal oxygen uptake before and after each treatment. Serum CoQ10 increased from 1.3 ± 0.4 to 5.2 ± 2.3 µg/mL with simvastatin and CoQ10, but did not change with simvastatin and placebo treatment (from 1.3 ± 0.3 to 0.8 ± 0.2 µg/mL) (P < 0.05 between groups). The Brief Pain Inventory pain severity and interference scores increased with simvastatin therapy (both P < 0.01), irrespective of CoQ10 assignment (P = 0.53 and 0.56). There were no changes in muscle strength or aerobic fitness with simvastatin with or without CoQ10 (all P > 0.10), and more subjects actually tended to report pain with CoQ10 (14/20 compared with 7/18; P = 0.05). We consider this to be the most definitive study to date evaluating the effect of CoQ10 in treating SAMS, and it demonstrates that CoQ10 does not improve skeletal muscle symptoms or performance in patients with SAMS.
Managing Patients with SAMS
The goal in managing patients with SAMS is to get the patient on the highest tolerated statin dose, as statins are life-saving medications, and to combine statin treatment with other agents that lower LDL cholesterol and reduce atherosclerotic CVD risk (11). Patients should be reassured that SAMS resolve with statin cessation. The only exception is “statin-induced necrotizing myositis,” in which patients develop antibodies against 3-hydroxy-3-methylglutaryl CoA reductase and may require immunosuppression to resolve the disease (11). Statin-induced necrotizing myositis is rare, with a prevalence of 1 in 100,000 (26). Many patients are able to tolerate the drugs once they know that their symptoms will resolve with cessation. We measure CK concentrations in all patients to document muscle injury if present and to ensure that patients who are willing to tolerate their symptoms do not have significant muscle injury. We also measure vitamin D concentrations, as low vitamin D concentrations have been associated with statin myopathy (27); to our knowledge, however, there are no randomized, placebo-controlled studies documenting that treating low vitamin D reduces SAMS. We then stop the statin until the patient is asymptomatic. Failure of the symptoms to resolve after 2–3 mo in a patient with a normal CK concentration argues against the statin as the cause of the symptoms. Once the patient is asymptomatic, we try the same statin at a lower dose or try another statin. We often combine the lower dose statin with ezetimibe or use ezetimibe alone in patients unable to tolerate any statin. Some patients tolerate the over-the-counter supplement red rice yeast, which contains lovastatin, possibly because this is viewed this as a “natural product”. Red rice yeast is less effective than pharmacologic-grade statins and has a variable effect because of variability in its statin content. Statins with longer half-lives, such as atorvastatin, rosuvastatin, and pitavastatin, can be used every other day or even twice weekly, and are often well tolerated in patients with prior SAMS. We also use other agents, such as the new PCSK9 inhibitors, in patients who qualify for these drugs.
CoQ10 administration remains a popular therapy for treatment of SAMS among both physicians and the lay public, with 1.3% of US adults (or 3.3 million) reporting use of CoQ10 supplements in 2015 (28–31). Despite the lack of effect in our and other studies, we also occasionally recommend CoQ10 supplementation to patients who inquire about it or in whom we question if statins are the cause of their symptoms. We recommend 200 mg/d at bedtime, but first inform the patient that CoQ10 has not been effective in clinical trials, even though some patients have found it effective. This approach works well in some patients, but may simply be a placebo effect (32).
It should be mentioned that CoQ10 is influenced by dietary factors, such as dietary fat consumption, vitamin E supplementation, and alcohol intake, and may influence the effectiveness of CoQ10 treatment in patients with SAMS; however, this possibility has not been comprehensively explored in research studies. Food sources with the highest concentrations of CoQ10 include organ meats, beef, pork, fatty fishes, chicken, and nuts (33). Further, an additional uncertainty associated with supplementation of CoQ10 for the treatment of SAMS is whether oral administration of ubiquinone or its reduced form, ubiquinol, augments skeletal muscle CoQ10 to the same extent. The majority (95%) of CoQ10 exists in reduced form in the human body, and this ratio is not affected by oral ingestion of CoQ10 either as ubiquinone or as ubiquinol, as the pharmacokinetic profiles of the 2 are almost identical. Despite this, data from human studies indicate that the effectiveness of CoQ10 for the treatment of SAMS is not affected by the redox status of CoQ10 (34).
Lessons Learned
This experience with CoQ10 provides 3 potentially useful lessons for research and clinical practice. First, a hypothesis deemed possible by several lines of deductive reasoning may still be wrong when tested in carefully conducted clinical trials. Second, it is critically important to determine that subjects enrolled in a clinical trial of a certain condition, in this case SAMS, actually have the phenotype to be examined. Third, even when scientific studies demonstrate that any intervention is ineffective, the intervention, in this case CoQ10, may still be useful in some patients, perhaps through the placebo effect.
Conclusions
Mechanistic studies and deductive reasoning suggest that CoQ10 dysregulation could be the cause, or could at least contribute, to SAMS. Clinical studies, however, have not documented its effectiveness in treating SAMS. Consequently, the present role of CoQ10 supplementation in managing SAMS is limited.
Acknowledgements
All authors have read and approved the final manuscript.
Notes
Published in a supplement to Advances in Nutrition. Presented at the symposium, “Micronutrient Status: Modifying Factors–Drugs, Chronic Disease, Surgery,” held at Columbia University, Institute of Human Nutrition, New York, New York, 17 June 2017. The conference was organized by Columbia University's Institute of Human Nutrition (its contents are solely the responsibility of the authors and do not necessarily represent the official views of Columbia University), and with the aid of an unrestricted grant from Pharmavite, LLC. The Supplement Coordinator for this supplement was Densie Webb. Supplement Coordinator disclosure: Densie Webb was compensated for overseeing the development and publication of the supplement. Airfare and hotel to attend the conference in New York were covered, in addition to payment for work completed. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the author(s) and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Author disclosures: ALZ, no conflicts of interest. BAT has served on and received an honorarium from Amgen's Pharmacovigilance Monitoring Board and research support from Regeneron. PDT has received research grant support from Sanofi, Regeneron, Esperion, and Amarin; has served as a consultant for Esperion, Amgen, Regeneron, and Sanofi; has received speaker honoraria from Regeneron, Sanofi, Amarin, and Amgen; owns stock in Abbvie, Abbott Labs, CVS, General Electric, Johnson & Johnson, Medtronic, and JA Willey; and has provided expert legal testimony on exercise-related cardiac events and statin myopathy.
Abbreviations used:
- CVD
cardiovascular disease
- CoQ10
coenzyme Q10
- CK
creatine kinase
- SAMS
statin-associated muscle symptoms
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C et al. Heart Disease and Stroke Statistics—2017 Update: a report from the American Heart Association. Circulation 2017;135(10):e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep 2012;14(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S1–45. [DOI] [PubMed] [Google Scholar]
- 4. Pencina MJ, Navar-Boggan AM, D'Agostino RB Sr., Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014;370(15):1422–31. [DOI] [PubMed] [Google Scholar]
- 5. Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6(3):208–15. [DOI] [PubMed] [Google Scholar]
- 6. Rosenbaum D, Dallongeville J, Sabouret P, Bruckert E. Discontinuation of statin therapy due to muscular side effects: a survey in real life. Nutr Metab Cardiovasc Dis 2013;23(9):871–5. [DOI] [PubMed] [Google Scholar]
- 7. Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, Stricker B, Mendis S, Hofman A, Mant J et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J 2013;34(38):2940–8. [DOI] [PubMed] [Google Scholar]
- 8. Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19(6):403–14. [DOI] [PubMed] [Google Scholar]
- 9. Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, Chipkin S, Pescatello LS, Simpson K, White CM et al. Effect of statins on skeletal muscle function. Circulation 2013;127(1):96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, Vladutiu GD, England JD, Scripps Mercy Clinical Research Center. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002;137(7):581–5. [DOI] [PubMed] [Google Scholar]
- 11. Thompson PD, Panza G, Zaleski A, Taylor B. Statin-associated side effects. J Am Coll Cardiol 2016;67(20):2395–410. [DOI] [PubMed] [Google Scholar]
- 12. Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol 2007;49(23):2231–7. [DOI] [PubMed] [Google Scholar]
- 13. Aberg F, Appelkvist EL, Dallner G, Ernster L. Distribution and redox state of ubiquinones in rat and human tissues. Arch Biochem Biophys 1992;295(2):230–4. [DOI] [PubMed] [Google Scholar]
- 14. Salviati L, Trevisson E, Doimo M, Navas P. Primary coenzyme Q10 deficiency. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2018. [PubMed] [Google Scholar]
- 15. Saha SP, Whayne TF Jr.. Coenzyme Q-10 in human health: supporting evidence? South Med J 2016;109(1):17–21. [DOI] [PubMed] [Google Scholar]
- 16. Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol 2013;62(8):709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chung HR, Vakil M, Munroe M, Parikh A, Meador BM, Wu PT, Jeong JH, Woods JA, Wilund KR, Boppart MD. The impact of exercise on statin-associated skeletal muscle myopathy. PLoS One 2016;11(12):e0168065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banach M, Serban C, Sahebkar A, Ursoniu S, Rysz J, Muntner P, Toth PP, Jones SR, Rizzo M, Glasser SP et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc 2015;90(1):24–34. [DOI] [PubMed] [Google Scholar]
- 19. Päivä H, Thelen KM, Van Coster R, Smet J, De Paepe B, Mattila KM, Laakso J, Lehtimäki T, von Bergmann K, Lütjohann D et al. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther 2005;78(1):60–8. [DOI] [PubMed] [Google Scholar]
- 20. Lamperti C, Naini AB, Lucchini V, Prelle A, Bresolin N, Moggio M, Sciacco M, Kaufmann P, DiMauro S. Muscle coenzyme Q10 level in statin-related myopathy. Arch Neurol 2005;62(11):1709–12. [DOI] [PubMed] [Google Scholar]
- 21. Laaksonen R, Jokelainen K, Sahi T, Tikkanen MJ, Himberg JJ. Decreases in serum ubiquinone concentrations do not result in reduced levels in muscle tissue during short-term simvastatin treatment in humans. Clin Pharmacol Ther 1995;57(1):62–6. [DOI] [PubMed] [Google Scholar]
- 22. Laaksonen R, Jokelainen K, Laakso J, Sahi T, Harkonen M, Tikkanen MJ, Himberg JJ. The effect of simvastatin treatment on natural antioxidants in low-density lipoproteins and high-energy phosphates and ubiquinone in skeletal muscle. Am J Cardiol 1996;77(10):851–4. [DOI] [PubMed] [Google Scholar]
- 23. Oh J, Ban MR, Miskie BA, Pollex RL, Hegele RA. Genetic determinants of statin intolerance. Lipids Health Dis 2007;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruano G, Windemuth A, Wu AH, Kane JP, Malloy MJ, Pullinger CR, Kocherla M, Bogaard K, Gordon BR, Holford TR et al. Mechanisms of statin-induced myalgia assessed by physiogenomic associations. Atherosclerosis 2011;218(2):451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor BA, Lorson L, White CM, Thompson PD. A randomized trial of coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis 2015;238(2):329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamann PD, Cooper RG, McHugh NJ, Chinoy H. Statin-induced necrotizing myositis - a discrete autoimmune entity within the “statin-induced myopathy spectrum”. Autoimmun Rev 2013;12(12):1177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Michalska-Kasiczak M, Sahebkar A, Mikhailidis DP, Rysz J, Muntner P, Toth PP, Jones SR, Rizzo M, Hovingh GK, Farnier M et al. Analysis of vitamin D levels in patients with and without statin-associated myalgia—a systematic review and meta-analysis of 7 studies with 2420 patients. Int J Cardiol 2015;178:111–6. [DOI] [PubMed] [Google Scholar]
- 28. Deichmann RE, Lavie CJ, Asher T, DiNicolantonio JJ, O'Keefe JH, Thompson PD. The Interaction between statins and exercise: mechanisms and strategies to counter the musculoskeletal side effects of this combination therapy. Ochsner J 2015;15(4):429–37. [PMC free article] [PubMed] [Google Scholar]
- 29. Mancini GB, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, Gupta M, Hegele RA, Ng D, Pearson GJ et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Consensus Working Group Update (2016). Can J Cardiol 2016;32(7 Suppl):S35–65. [DOI] [PubMed] [Google Scholar]
- 30. Rosenson RS, Baker S, Banach M, Borow KM, Braun LT, Bruckert E, Brunham LR, Catapano AL, Elam MB, Mancini GB et al. Optimizing cholesterol treatment in patients with muscle complaints. J Am Coll Cardiol 2017;70(10):1290–301. [DOI] [PubMed] [Google Scholar]
- 31. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report 2015;(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 32. Thompson PD. What to believe and do about statin-associated adverse effects. JAMA 2016;316(19):1969–70. [DOI] [PubMed] [Google Scholar]
- 33. Mattila P, Kumpulainen J. Coenzymes Q9 and Q10: contents in foods and dietary intake. J Food Compost Anal 2001;14:409–17. [Google Scholar]
- 34. Taylor BA. Does coenzyme Q10 supplementation mitigate statin-associated muscle symptoms? Pharmacological and methodological considerations. Am J Cardiovasc Drugs 2017Oct 12. doi: 10.1007/s40256-017-0251-2 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]