Abstract
Existing evidence suggests a link between the inflammatory potential of diet and risk of cancer. This study aimed to test the linear and potential nonlinear dose-response associations of the Dietary Inflammatory Index (DII), as being representative of inflammatory features of the diet, and site-specific cancer risk. A systematic search was conducted with the use of PubMed and Scopus from 2014 to November 2017. Prospective cohort or case-control studies reporting the risk estimates of any cancer type for ≥3 categories of the DII were selected. Studies that reported the association between continuous DII score and cancer risk were also included. Pooled RRs were calculated by using a random-effects model. Eleven prospective cohort studies (total n = 1,187,474) with 28,614 incident cases and 29 case-control studies with 19,718 cases and 33,229 controls were identified. The pooled RRs for a 1-unit increment in the DII were as follows: colorectal cancer, 1.06 (95% CI: 1.04, 1.08; I2 = 72.5%; n = 9); breast cancer, 1.03 (95% CI: 1.00, 1.07; I2 = 84.0%; n = 7); prostate cancer, 1.06 (95% CI: 0.97, 1.15; I2 = 56.2%; n = 6); pancreatic cancer, 1.16 (95% CI: 1.05, 1.28; I2 = 61.6%; n = 2); ovarian cancer, 1.08 (95% CI: 1.03, 1.13; I2 = 0%; n = 2); esophageal squamous cell carcinoma, 1.24 (95% CI: 1.10, 1.38; I2 = 64.3%; n = 2); renal cell carcinoma, 1.08 (95% CI: 1.02, 1.13; I2 = 0%; n = 2); and esophageal adenocarcinoma, 1.26 (95% CI: 1.13, 1.39; I2 = 0%; n = 2). A nonlinear dose-response meta-analysis showed that, after a somewhat unchanged risk within initial scores of the DII, the risk of colorectal cancer increased linearly with increasing DII score. In the analyses of breast and prostate cancers, the risk increased with a very slight trend with increasing DII score. In conclusion, the results showed that dietary habits with high inflammatory features might increase the risk of site-specific cancers.
Keywords: cancer, diet, dietary patterns, inflammation, meta-analysis
Introduction
The association between diet and cancer has been well investigated. The World Cancer Research Fund International/American Institute for Cancer Research Continuous Update Project Reports on diet, nutrition, and physical activity reported that there is strong evidence that higher consumption of red and processed meat and alcoholic drinks increases the risk of colorectal cancer (CRC); on the other hand, higher intakes of dairy products, whole grains, and fiber-containing foods decrease the risk of CRC (1). The reports also showed that there is strong evidence for the association between higher consumption of meat and processed meat, high-salt foods, and alcoholic drinks and a higher risk of stomach cancer (2); a higher consumption of alcoholic drinks and a higher risk of breast, kidney, and liver cancers (3–5); higher glycemic load and a higher risk of endometrial cancer (6); and a higher consumption of coffee and a lower risk of liver and endometrial cancers (4, 6). There is a crucial need to explore the underlying mechanisms that drive dietary associations with site-specific cancers. However, due to the role of inflammatory pathways in the pathogenesis and progression of several cancer types, such as stomach, colon, and prostate cancers (7–9), it has been suggested that the association between diet and cancer, at least in part, is mediated through diet-induced inflammation (10).
It has been proposed that some dietary components, such as saturated fats, refined carbohydrates, and red meat, may have proinflammatory properties (11–13), whereas some others, including soy products and phytochemicals, may have anti-inflammatory features (14, 15). Previous investigations have indicated that the consumption of flavonoids, whole grains, and legumes was associated with serum concentrations of low-grade inflammatory biomarkers, such as TNF-α, C-reactive protein (CRP), or cell adhesion molecules (16–19). Furthermore, predefined dietary patterns, such as the Mediterranean diet (20, 21), as well as data-driven dietary patterns (22, 23) have been shown to be associated with circulating inflammatory biomarkers and markers of vascular inflammation.
To better understand the inflammatory potential of diet, a diet quality index integrating information on the inflammatory potential of multiple specific foods has been developed to represent the inflammatory potential of the diet as a whole. This Dietary Inflammatory Index (DII) is a literature-derived, population-based diet quality index and is based on the positive or negative effects of different dietary factors on serum concentrations of 6 inflammatory biomarkers (24). The DII consists of 45 dietary components, of which 9 components, including energy, carbohydrates, cholesterol, total fats, saturated fats, trans FAs, protein, iron, and vitamin B-12, have proinflammatory properties, and another 36 components have been shown to have anti-inflammatory features (24). A higher score on the DII represents higher dietary inflammatory potential. It has been shown that higher DII scores were positively and significantly associated with serum concentrations of inflammatory biomarkers, including CRP, TNF-α, IL-6, and homocysteine (25–27). Higher DII scores have also been inversely associated with predefined diet quality indexes, such as the Healthy Eating Index 2010 (HEI-2010), the Alternate Healthy Eating Index (AHEI), and Dietary Approaches to Stop Hypertension (DASH) (28).
Two recent systematic reviews indicated that a higher DII was associated with a higher risk of CRC (29, 30); however, the shape of the dose-response relation has not been determined. The DII consists of 45 evidence-based, inflammation-related dietary components, compared with 11 components in the Mediterranean diet score (31), 8 components in the DASH diet score (32), 10 components in the HEI-2010 (33), 9 components in the AHEI (34), and 23 components in the Recommended Food Score (35). The unique features of the DII include the fact that it integrates information on many foods that have been shown to be anti-inflammatory or proinflammatory and thus the DII 1) accounts for correlated food intakes and 2) focuses on a specific biological mechanism. The Mediterranean Diet Score is similarly based on epidemiologic studies of health benefit from the literature, but focuses on cardiovascular health. The Healthy Eating Index and other regionally defined dietary patterns (e.g., the Baltic Sea Diet) are based on healthy eating guidelines and do not target specific mechanisms. Thus, it seems that the DII provides a relatively more specific and comprehensive understanding of inflammatory features of the diet as a whole than other diet quality indexes. Our study objective was to investigate both linear and nonlinear dose-response associations between the DII and site-specific cancers.
Methods
Search strategy
We systematically searched PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) and Scopus (https://www.scopus.com/) for studies published from 2014 [the DII was developed in 2014 (24)] up to September 2016, and an updated search was performed up to 3 November 2017 using the following key words: [“inflammatory” OR “inflammation” OR “anti-inflammatory” OR “pro-inflammatory”] AND [“diet-related” OR “diet” OR “dietary”] AND [“cancer” OR “carcinoma” OR “neoplasm” OR “adenoma”]. Reference lists of all relative reviews and articles were also manually searched. The search was restricted to the articles published in English. Two independent authors (AJ, AE) reviewed titles and abstracts of all obtained articles and selected observational studies, either prospective cohort or case-control studies, that 1) were conducted among adults aged ≥18 y; 2) reported the newly developed DII by Shivappa et al. (24) as exposure and in ≥3 quantitative categories; 3) reported risk estimates such as ORs, HRs, or RRs and their corresponding 95% CIs of any cancer type in relation to the DII; and 4) reported the number of cases and participants or noncases in each category of the DII. Studies that reported the association between continuous DII score and cancer risk were also included. We excluded the following: 1) cross-sectional studies, 2) studies conducted in patients with specific diseases, and 3) studies without number of cases and noncases or DII ranges in each category of the DII. The present meta-analysis conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist (36) for writing the systematic review and reporting the results.
Data extraction and quality assessment
Two independent investigators (AJ, AE) extracted the following information from eligible studies: first author's name, publication year, study name, country, study design, number of participants, outcome of interest, age range or mean age (years), dietary assessment method, number of DII components, number of cases and participants/controls, reported risk estimates and their 95% CIs for each category of the DII, and covariates adjusted in the multivariate model. We included effect estimates based on models with the most comprehensive covariate adjustment. Components of the DII score in each study were also extracted and are presented in Supplemental Materials. The same 2 authors assessed the quality of included studies using a 9-point scoring system according to the Newcastle-Ottawa Scale, and studies with ≥7 points were considered high quality (37). Any discrepancy was resolved through discussion under supervision of a third author (SS-B).
Statistical analysis
RRs and 95% CIs were considered as the effect size for all studies. The reported ORs in case-control studies and HRs in cohort studies were considered equal to RRs. We measured the linear dose-response relation by using generalized least-squares trend estimation, according to the methods developed by Berlin et al. (38) and Orsini et al. (39). We used the 2-stage generalized least-squares trend estimation method, which first estimated study-specific slope lines and then combined with studies in which the slopes were directly reported to obtain an overall average slope (39). Study-specific results were combined by using a random-effects model. The median point in each category of the DII was assigned. If medians were not reported, we estimated approximate medians by using the midpoint of the lower and upper boundaries. If the upper boundary of the highest category or the lower boundary of the lowest category was not reported, we used the reported maximum and minimum range of the DII, respectively. If the maximum and minimum range had not been reported, we estimated it from the reported mean and SD values (mean ± 3 SDs) in the study (this method was used in 1 study). If studies reported results separately for men and women or other subgroups, we combined the subgroup-specific estimates by using a fixed-effects model and included the combined effect size in the main analysis. Meta-analysis was conducted separately for each cancer type when ≥2 studies reported risk estimates for the same cancer type. Potential nonlinear association was examined by modeling the DII levels with the use of restricted cubic splines with 4 knots at fixed percentiles (5%, 35%, 65%, and 95%) of the distribution (39). Then the study-specific estimates were combined by using the restricted maximum likelihood method in a multivariate random-effects meta-analysis (40). P values for nonlinearity of the meta-analysis were calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero.
Subgroup analyses were conducted whenever possible, based on study design (cohort compared with case-control), sex, study quality, the number of the DII components, geographical region, and menopausal status. To test the potential effect of each study on pooled effect size, sensitivity analyses were conducted by sequential exclusion of each study at a time. There were no quality criteria for study inclusion in the primary meta-analysis, but we conducted a sensitivity analysis by restricting only to studies of high quality in a secondary analysis. Publication bias was tested by funnel plots and by Egger's asymmetry test and Begg's test (P < 0.10) (41). Between-study heterogeneity was explored with the use of Cochrane's Q test of heterogeneity and the I2 statistic (P < 0.05) (42), and I2 statistics of 25%, 50%, and 75% were considered to show low, moderate, and high levels of heterogeneity, respectively. All analyses were conducted with Stata software, version 13 (StataCorp). A P value <0.05 was considered significant.
Results
Our systematic search identified 5603 studies. After limiting the results to studies that were published after 2014 (the DII was developed in 2014), 1619 studies remained. After removal of 204 duplicates and 1368 nonrelevant articles, 47 full-text articles were assessed for eligibility. Of these, 40 studies met the inclusion criteria for inclusion in the present meta-analysis. Detailed reasons for exclusions are provided in Figure 1. Eleven studies, with a total of 1,187,474 participants and 28,614 incident cases, were prospective cohort studies (43–53), and 29 studies involving 19,718 cases and 33,229 controls had a case-control design (54–82). Nine studies were conducted in the United States (45, 47–49, 51–54, 59), 2 studies were from Canada (60, 78), 5 studies were from Asia (55, 57, 64, 67, 73), 2 studies were from Australia (43, 46), 1 was from Mexico (81), 1 was from Jamaica (77), and 20 studies were from Europe (14 from Italy) (44, 50, 56, 58, 61–63, 65, 66, 68–72, 74–76, 79, 80, 82). All of the studies, except for 1 study (44), used FFQs to assess dietary intakes, and all of the studies followed the method developed by Shivappa et al. (24) to calculate the DII (using 18–37 dietary components). Ten studies reported the energy-adjusted DII as the exposure (45, 54, 56, 57, 59, 60, 63, 76, 80, 81). Three studies reported the results of the Iowa Women's Health Study on CRC (49), breast cancer (48), and renal cell carcinoma (47) and were separately included in the relevant analyses. Two studies reported the results of the Melbourne Collaborative Cohort Study on urothelial cell carcinoma (43) and lung cancer (46) and were included in the relevant analyses. Another prospective cohort study (44) reported risk estimates of breast and prostate cancer and its results were separately included in the analyses. In addition, results of the Women's Health Initiative on breast cancer and CRC were separately reported in 2 studies (51, 52), which were included separately in the relevant analyses.
FIGURE 1.
Literature search and study selection process for inclusion in meta-analysis of dietary inflammatory index and cancer risk.
The systematic search found 19 different cancer types for which respective risk estimates have been reported in relation to the DII. General characteristics of the included studies are presented in Table 1, dietary components of the DII in each study are provided in Supplemental Table 1, and numbers of cases and participants/controls and the reported risk estimates of site-specific cancers across different categories of the DII in each study are presented in Supplemental Table 2. Finally, 9 studies reported results on CRC, 7 studies on breast cancer, and 6 studies on prostate cancer, which were included in the dose-response meta-analysis. We also included 2 studies in the analyses of ovarian, pancreatic, renal cell, esophageal squamous cell, and esophageal adenocarcinoma; 11 other cancer sites were evaluated in relation to the DII in only 1 study; thus, study-specific results are shown in Table 2. Different anti-inflammatory and proinflammatory components of the DII are also presented in Table 3.
TABLE 1.
General characteristics of included studies in the meta-analysis of DII and cancer risk1
| Author (ref), year, study name, country | Design (years of follow-up in cohort studies) | Cases/participants (controls), n/n | Sex | Mean age or age range, y | Outcome | Dietary assessment tool (no. of DII components) | DII score2 | Quality score (maximum: 9 points) |
|---|---|---|---|---|---|---|---|---|
| Harmon et al. (45), 2017, Multiethnic Cohort, USA | Prospective cohort (20) | 4388/190,963 | M/F | 45–75 | Colorectal cancer | FFQ (28) | −6.64 to 4.95 | 9 |
| Shivappa et al. (49), 2014, Iowa Women's Health Study, USA | Prospective cohort (19.7) | 1636/34,703 | F | 55–69 | Colorectal cancer | FFQ (37) | −0.87 ± 2.023 | 9 |
| Tabung et al. (52), 2015, Women's Health Initiative, USA | Prospective cohort (11.3) | 1920/152,536 | F | 50–79 | Colorectal cancer | FFQ (32) | −7.05 to 5.63 | 8 |
| Wirth et al. (53), 2015, NIH-AARP Diet and Health Study, USA | Prospective cohort (9.1) | 6944/489,422 | M/F | 50–74 | Colorectal cancer | FFQ (33) | 1.27 ± 2.47 | 9 |
| Cho et al. (55), 2016, National Cancer Center, Korea | Case-control | 923/1846 | M/F | 56 | Colorectal cancer | FFQ (36) | 1.55 ± 2.18 | 5 |
| Sharma et al. (60), 2017, Canada | Case-control | 547/685 | M/F | 61 | Colorectal cancer | FFQ (29) | Cases: −0.73 ± 1.50; controls: −0.89 ± 1.60 | 6 |
| Shivappa et al. (73), 2017, Jordan | Case-control | 153/202 | M/F | 53 | Colorectal cancer | FFQ (18) | Cases: 1.80 ± 0.80; controls: 1.50 ± 1.00 | 5 |
| Shivappa et al. (79), 2015, Italy | Case-control | 1953/4154 | M/F | 56 | Colorectal cancer | FFQ (31) | Cases: 0.14 ± 1.39; controls: −0.06 ± 1.38 | 6 |
| Zamora-Ros et al. (82), 2015, Bellvitge Colorectal Cancer Study, Spain | Case-control | 424/401 | M/F | 65 | Colorectal cancer | FFQ (33) | Cases: 1.44 (−0.88 to 3.18); controls: 1.06 (−0.73 to 3.05) | 6 |
| Graffouillere et al. (44), 2016, SU.VI.MAX cohort, France | Prospective cohort (12.6) | 158/3613 | F | 49 | Breast cancer | 24-h dietary records (36) | 1.00 ± 1.80 | 6 |
| 123/2648 | M | Prostate cancer | 0.30 ± 1.80 | |||||
| Shivappa et al. (48), 2017, Iowa Women's Health Study, USA | Prospective cohort (25) | 2910/34,700 | F | 55–69 | Breast cancer | FFQ (29) | −0.87 ± 2.02 | 9 |
| Shivappa et al. (50), 2015, SWLH, Sweden | Prospective cohort (20) | 1895/49,258 | F | 40 | Breast cancer | FFQ (29) | 2.67 ± 1.47 | 8 |
| Tabung et al. (51), 2016, Women's Health Initiative, USA | Prospective cohort (16) | 7495/122,788 | F | 50–79 | Breast cancer | FFQ (32) | −0.78 ± 2.61 | 8 |
| Ge et al. (56), 2015, Germany | Case-control | 2887/5512 | F | 50–74 | Breast cancer | FFQ (25) | Cases: 0.86 ± 1.30; controls: 0.85 ± 1.29 | 6 |
| Huang et al. (57), 2017, China | Case-control | 867/824 | F | 47 | Breast cancer | FFQ (33) | Cases: −1.80 ± 1.70; controls: −1.20 ± 1.90 | 6 |
| Shivappa et al. (68), 2017, Italy | Case-control | 2569/2588 | F | 23–74 | Breast cancer | FFQ (31) | −0.39 ± 1.86 | 7 |
| Shivappa et al. (50), 2015, Italy | Case-control | 1294/1451 | M | 46–74 | Prostate cancer | FFQ (31) | <21.98 to ≥0.49 | 6 |
| Shivappa et al. (64), 2017, Iran | Case-control | 50/100 | M | 40–78 | Prostate cancer | FFQ (25) | NA | 6 |
| Shivappa et al. (77), 2015, Jamaica | Case-control | 229/250 | M | 40–70 | Prostate cancer | FFQ (21) | −1.05 ± 1.11 | 6 |
| Shivappa et al. (78), 2017, Canada | Case-control | 72/302 | M | 64 | Prostate cancer | FFQ (18) | –8.87 to 7.98 | 7 |
| Vazquez-Salas et al. (81), 2016, Mexico | Case-control | 394/794 | M | 42–94 | Prostate cancer | FFQ (27) | Cases: 0.43 ± 2.02; controls: 0.52 ± 1.53 | 7 |
| Dugue et al. (43), 2016, MCCS, Australia | Prospective cohort (21.9) | 379/37,442 | M/F | 27–76 | Urothelial cell carcinoma | FFQ (29) | Cases: −0.84 (−2.05 to 0.61); noncases: −0.98 (−2.14 to 0.40) | 7 |
| Hodge et al. (46), 2016, MCCS, Australia | Prospective cohort (18) | 403/35,303 | M/F | 27–75 | Lung cancer | FFQ (29) | −4.91 to 4.86 | 7 |
| Shivappa et al. (47), 2017, Iowa Women's Health Study, USA | Prospective cohort (25) | 263/33,817 | F | 55–69 | Renal cell carcinoma | FFQ (29) | −0.87 ± 2.02 | 9 |
| Antwi et al. (54), 2016, SPORE, USA | Case-control | 817/1756 | M/F | >18 | Pancreatic cancer | FFQ (28) | Cases: −0.82 ± 1.80; controls: −1.51 ± 1.72 | 6 |
| Lu et al. (58), 2016, Sweden | Case-control | 181/820 | M/F | 19–80 | Esophageal adenocarcinomas | FFQ (36) | NA | 6 |
| 255/820 | M/F | Gastroesophageal junctional adenocarcinomas | ||||||
| 158/820 | M/F | Esophageal squamous cell carcinomas | ||||||
| Peres et al. (59), 2017, AACES, USA | Case-control | 493/662 | F | 20–79 | Ovarian cancer | FFQ (27) | Cases: −0.43 ± 1.843; controls: −0.57 ± 1.89 | 7 |
| Shivappa et al. (62), 2015, Italy | Case-control | 326/652 | M/F | 63 | Pancreatic cancer | FFQ (31) | Cases: 0.26 ± 1.44; controls: −0.13 ± 1.40 | 6 |
| Shivappa et al. (63), 2017, Ireland | Case-control | 224/256 | M/F | 64 | Esophageal adenocarcinomas | FFQ (25) | −3.08 to 4.74 | 6 |
| Shivappa et al. (65), 2016, Italy | Case-control | 230/547 | M/F | 22–80 | Gastric cancer | FFQ (31) | −4.78 to 4.71 | 6 |
| Shivappa et al. (66), 2016, Italy | Case-control | 185/404 | M/F | <85 | Hepatocellular cancer | FFQ (31) | Cases: 0.24 ± 1.40; controls: −0.11 ± 1.37 | 6 |
| Shivappa et al. (67), 2015, Iran | Case-control | 47/96 | M/F | 40–75 | Esophageal squamous cell cancer | FFQ (27) | Cases: 1.81 ± 1.23; controls: 0.76 ± 1.35 | 6 |
| Shivappa et al. (69), 2017, Italy | Case-control | 690/665 | M/F | <60 to >75 | Bladder cancer | FFQ (31) | Cases: −0.63 ± 1.94; controls: −0.93 ± 2.00 | 5 |
| Shivappa et al. (70), 2017, Italy | Case-control | 767/1534 | F/M | 22–79 | Renal cell carcinoma | FFQ (31) | Cases: 0.13 ± 1.39; controls: −0.06 ± 1.38 | 6 |
| Shivappa et al. (71), 2016, Italy | Case-control | 1031/2411 | F | 56–57 | Ovarian cancer | FFQ (31) | −6.20 to 6.00 | 5 |
| Shivappa et al. (72), 2016, Italy | Case-control | 460/1088 | M/F | 30–80 | Laryngeal cancer | FFQ (31) | Cases: 0.44 ± 1.41; controls: −0.17 ± 1.41 | 6 |
| Shivappa et al. (74), 2017, Italy | Case-control | 536/984 | M/F | 56 | Non-Hodgkin lymphoma | FFQ (30) | −4.23 to 3.62 | 6 |
| Shivappa et al. (75), 2016, Italy | Case-control | 198/594 | M/F | 18–76 | Nasopharyngeal carcinoma | FFQ (31) | Cases: 0.28 ± 1.49; controls: −0.09 ± 1.40 | 6 |
| Shivappa et al. (76), 2016, Italy | Case-control | 454/908 | M/F | 18–79 | Endometrial cancer | FFQ (31) | Cases: 0.05 ± 1.403; controls: −0.03 ± 1.48 | 7 |
| Shivappa et al. (80), 2015, Italy | Case-control | 304/743 | M/F | 39–77 | Esophageal squamous cell cancer | FFQ (31) | Cases: 0.47 ± 1.503; controls: −0.19 ± 1.40 | 6 |
AACES, African American Cancer Epidemiology Study; DII, Dietary Inflammatory Index; MCCS, Melbourne Collaborative Cohort Study; NA, not available; ref, reference; SPORE, Specialized Program of Research Excellence; SU.VI.MAX, Supplementation en Vitamines et Minéraux Antioxydants; SWLH, Swedish Women's Lifestyle Study.
Values are means ± SDs, ranges, or medians (IQRs).
From foods and supplements.
TABLE 2.
RRs/ORs of site-specific cancers associated with a 1-unit increment in the DII1
| Cancer type | Studies, n | Cases, n | Pooled RR (95% CI) | I 2, % | P-heterogeneity |
|---|---|---|---|---|---|
| Colorectal cancer | |||||
| All studies | 9 | 18,888 | 1.06 (1.04, 1.08) | 72.5 | <0.001 |
| Study type | |||||
| Cohort | 4 | 14,888 | 1.04 (1.03, 1.05) | 0 | 0.67 |
| Case-control | 5 | 4000 | 1.12 (1.08, 1.16) | 23.4 | 0.67 |
| Sex | |||||
| Men | 4 | 8278 | 1.09 (1.04, 1.14) | 76.6 | 0.005 |
| Women | 6 | 8767 | 1.05 (1.02, 1.08) | 67.8 | 0.008 |
| Region | |||||
| United States+Canada | 5 | 15,435 | 1.04 (1.03, 1.05) | 0 | 0.54 |
| Europe | 2 | 2377 | 1.11 (1.07, 1.16) | 27.9 | 0.24 |
| Asia | 2 | 1076 | 1.25 (0.97, 1. 25) | 59.9 | 0.11 |
| Study quality | |||||
| ≥7 (high) | 4 | 14,888 | 1.04 (1.03, 1.05) | 0 | 0.67 |
| <7 | 5 | 4000 | 1.12 (1.08, 1.16) | 23.4 | 0.67 |
| DII components | |||||
| <30 | 3 | 5088 | 1.09 (0.99, 1.19) | 69.1 | 0.04 |
| ≥30 | 6 | 13,800 | 1.07 (1.04, 1.10) | 77.2 | <0.001 |
| Breast cancer | |||||
| All studies | 7 | 18,781 | 1.03 (1.00, 1.07) | 84.0 | <0.001 |
| Study type | |||||
| Cohort | 4 | 12,458 | 1.01 (0.99, 1.02) | 16.0 | 0.31 |
| Case-control | 3 | 6323 | 1.12 (1.00, 1.25) | 93.3 | <0.001 |
| Menopausal status | |||||
| Postmenopause | 5 | — | 1.01 (0.99, 1.03) | 61.4 | 0.05 |
| Pre-/perimenopause | 2 | — | 1.21 (0.65, 1.76) | 95.8 | <0.001 |
| Region | |||||
| Europe | 4 | 7509 | 1.03 (0.98, 1.08) | 76.2 | 0.006 |
| United States | 2 | 10,405 | 1.00 (0.99, 1.02) | 0 | 0.50 |
| Asia | 1 | 867 | 1.40 (1.25, 1.59) | — | — |
| Study quality | |||||
| ≥7 (high) | 4 | 14,869 | 1.03 (1.00, 1.06) | 81.1 | 0.001 |
| <7 | 3 | 3912 | 1.11 (0.92, 1.30) | 90.7 | <0.001 |
| DII components | |||||
| <30 | 3 | 7692 | 1.01 (0.99, 1.04) | 41.3 | 0.18 |
| ≥30 | 4 | 11,089 | 1.09 (0.99, 1.18) | 91.2 | <0.001 |
| Prostate cancer | |||||
| All studies | 6 | 2162 | 1.06 (0.97, 1.15) | 56.2 | 0.04 |
| Study type | |||||
| Cohort | 1 | 123 | 0.94 (0.83, 1.07) | — | — |
| Case-control | 5 | 2039 | 1.10 (0.99, 1.20) | 50.6 | 0.09 |
| Study quality | |||||
| ≥7 (high) | 2 | 466 | 1.20 (0.69, 1.71) | 62.7 | 0.10 |
| <7 | 4 | 1696 | 1.08 (0.94, 1.22) | 65.0 | 0.30 |
| DII components | |||||
| <30 | 4 | 745 | 1.22 (0.98, 1.47) | 62.9 | 0.04 |
| ≥30 | 2 | 1417 | 1.01 (0.90, 1.13) | 66.3 | 0.08 |
| Pancreatic cancer | |||||
| Case-control | 2 | 1143 | 1.16 (1.05, 1.28) | 61.6 | 0.10 |
| Sex | |||||
| Men | 2 | 635 | 1.11 (1.04, 1.18) | 0 | 0.48 |
| Women | 2 | 508 | 1.19 (0.97, 1.41) | 71.5 | 0.06 |
| Ovarian cancer | |||||
| Case-control | 2 | 3442 | 1.08 (1.03, 1.13) | 0 | 1.00 |
| Menopausal status | |||||
| Postmenopause | 2 | 1038 | 1.12 (1.05, 1.18) | 0 | 0.64 |
| Pre-/perimenopause | 2 | 484 | 1.02 (0.92, 1.12) | 16.3 | 0.27 |
| Esophageal squamous cell | |||||
| Case-control | 2 | 351 | 1.24 (1.10, 1.38) | 64.3 | 0.09 |
| Renal cell carcinoma | |||||
| All studies | 2 | 1030 | 1.08 (1.02, 1.13) | 0 | 0.85 |
| Study type | |||||
| Cohort | 1 | 263 | 1.07 (1.00, 1.15) | — | — |
| Case-control | 1 | 767 | 1.08 (1.01, 1.15) | — | — |
| Esophageal adenocarcinoma | |||||
| Case-control | 2 | 405 | 1.26 (1.13, 1.39) | 0 | 0.55 |
| Lung cancer | |||||
| Cohort | 1 | 403 | 1.08 (1.00, 1.18) | — | — |
| Endometrial cancer | |||||
| Case-control | 1 | 454 | 1.07 (0.98, 1.17) | — | — |
| Bladder cancer | |||||
| Case-control | 1 | 690 | 1.11 (1.03, 1.20) | — | — |
| Gastric cancer | |||||
| Case-control | 1 | 230 | 1.19 (1.06, 1.34) | — | — |
| Hepatocellular cancer | |||||
| Case-control | 1 | 185 | 1.24 (1.02, 1.51) | — | — |
| Nasopharyngeal cancer | |||||
| Case-control | 1 | 198 | 1.09 (1.02, 1.16) | — | — |
| Laryngeal cancer | |||||
| Case-control | 1 | 460 | 1.27 (1.15, 1.40) | — | — |
| Esophageal squamous cell | |||||
| Case-control | 1 | 158 | 1.29 (1.13, 1.45) | — | — |
| Gastroesophageal junctional adenocarcinoma | |||||
| Case-control | 1 | 255 | 1.19 (1.00, 1.30) | — | — |
| Urothelial cell carcinoma | |||||
| Cohort | 1 | 379 | 1.04 (0.98, 1.10) | — | — |
| Non-Hodgkin lymphoma | |||||
| Case-control | 1 | 536 | 1.14 (1.02, 1.27) | — | — |
DII, Dietary Inflammatory Index.
TABLE 3.
Food variables included in the DII1
| Inflammatory potential | |||
|---|---|---|---|
| Number | Food variables | Negative | Positive |
| 1 | Alcohol | * | |
| 2 | Vitamin B-6 | * | |
| 3 | β-Carotene | * | |
| 4 | Caffeine | * | |
| 5 | Eugenol | * | |
| 6 | Fiber | * | |
| 7 | Folate | * | |
| 8 | Garlic | * | |
| 9 | Ginger | * | |
| 10 | Magnesium | * | |
| 11 | Monounsaturated fat | * | |
| 12 | Polyunsaturated fat | * | |
| 13 | Niacin | * | |
| 14 | n–3 FAs | * | |
| 15 | n–6 FAs | * | |
| 16 | Onion | * | |
| 17 | Riboflavin | * | |
| 18 | Saffron | * | |
| 19 | Selenium | * | |
| 20 | Thiamin | * | |
| 21 | Turmeric | * | |
| 22 | Vitamin A | * | |
| 23 | Vitamin C | * | |
| 24 | Vitamin D | * | |
| 25 | Vitamin E | * | |
| 26 | Zinc | * | |
| 27 | Green/black tea | * | |
| 28 | Flavan-3-ol | * | |
| 29 | Flavones | * | |
| 30 | Flavonols | * | |
| 31 | Flavonones | * | |
| 32 | Anthocyanidins | * | |
| 33 | Isoflavones | * | |
| 34 | Pepper | * | |
| 35 | Thyme/oregano | * | |
| 36 | Rosemary | * | |
| 37 | Vitamin B-12 | * | |
| 38 | Carbohydrate | * | |
| 39 | Cholesterol | * | |
| 40 | Energy | * | |
| 41 | Total fat | * | |
| 42 | Saturated fat | * | |
| 43 | trans Fat | * | |
| 44 | Iron | * | |
| 45 | Protein | * | |
DII, Dietary Inflammatory Index.
CRC
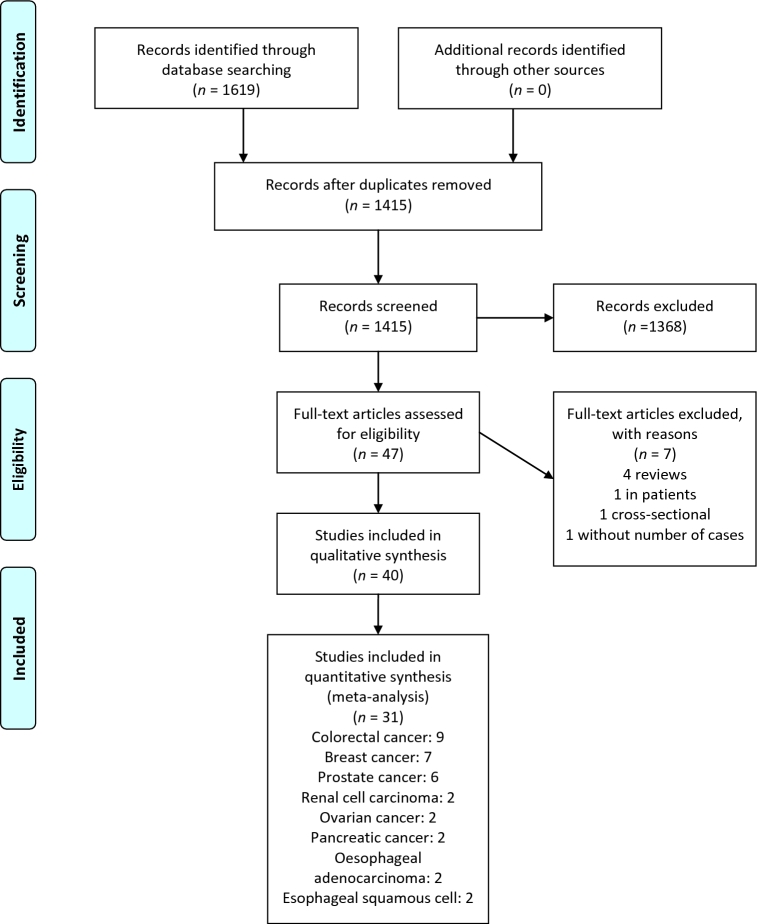
Four prospective cohort studies (45, 49, 52, 53) (867,624 participants and 14,888 incident cases) and 5 case-control studies (55, 60, 73, 79, 82) (involving 4000 cases and 7288 controls) reported results on the association between the DII and risk of CRC. All of the included studies reported the E-DII or took energy intake into account to calculate the DII or took energy intake into account in the multivariate analyses. The linear trend estimation indicated that a 1-unit increment in the DII was associated with a 6% higher risk of CRC (RR: 1.06; 95% CI: 1.04, 1.08; P < 0.001; I2 = 72.5%, P-heterogeneity < 0.001; Figure 2A).
FIGURE 2.
(A) Summary risk estimates for the association between a 1-unit increment in the DII score and risk of colorectal cancer. (B) Dose-response associations between the DII and risk of colorectal cancer (from all studies). (C) Dose-response association between the DII and risk of colorectal cancer (from 4 cohort studies). (B, C) P-nonlinearity < 0.001. The solid lines and dashed lines represent RRs and 95% CIs, respectively. DII, Dietary Inflammatory Index; ES, effect size.
In the sensitivity analysis removing each study at a time, the association changed from 1.05 (95% CI: 1.03, 1.06) with the exclusion of the NIH-AARP Diet and Health Study (53) to 1.08 (95% CI: 1.04, 1.11) with the exclusion of the large Italian case-control study (79). None of the excluded studies explained the between-study heterogeneity; however, when the large Italian case-control study was excluded (79), the heterogeneity was reduced substantially (I2 = 51.4%, P-heterogeneity = 0.04). The association appeared to be stronger for the meta-analysis based on case-control studies compared with cohort studies [pooled RRs (95% CIs): 1.12 (1.08, 1.16) compared with 1.04 (1.03, 1.05), respectively]. The results were more pronounced in men than in women, in European studies than in US and Asian studies, and in those studies with low and moderate quality (<7 scores) than in those with high quality (≥7 scores). Subgroup analysis on the basis of number of the DII components (<30 compared with ≥30) resulted in a significant positive relation only in those with ≥30 components compared with <30 components (Table 2). Some indications of publication bias were found with Egger's regression test (P = 0.01), Begg's rank correlation test (P = 0.06), and a funnel plot (Supplemental Figure 1). An analysis after adjustment with the use of the trim and fill method showed a slightly decreased pooled RR (1.04; 95% CI: 1.02, 1.07; n = 13 studies).
Nonlinear dose-response meta-analysis showed that, after a somewhat unchanged risk within initial scores of the DII, the risk of CRC increased in a linear fashion with increasing the DII score from negative boundary (more healthy, less proinflammatory) toward positive boundary, which represents more proinflammatory features of the diet (P-nonlinearity < 0.001; Figure 2B). Sensitivity analysis with the use of 4 cohort studies showed a nearly similar result with the main analysis (P-nonlinearity < 0.001; Figure 2C).
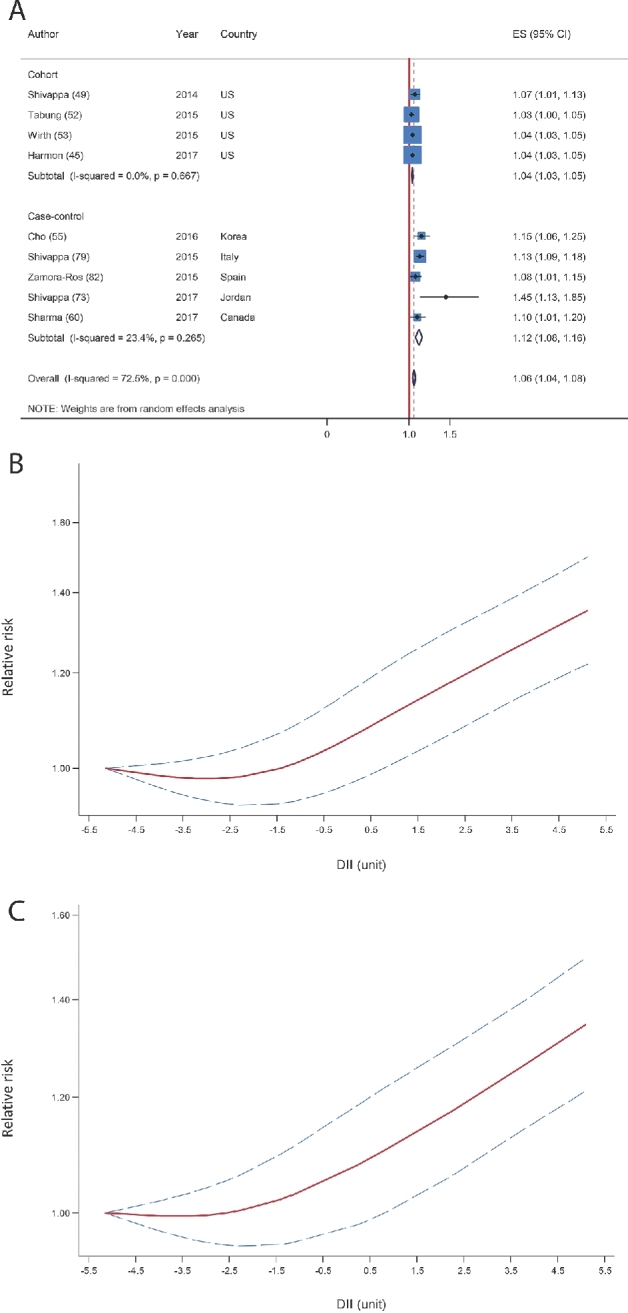
Breast cancer
Four cohort studies (44, 48, 50, 51) (210,362 participants and 12,458 incident cases) and 3 case-control studies (56, 57, 68) (involving 6323 cases and 8924 controls) were included in the analysis of breast cancer. All of the included studies reported the energy-adjusted DII or took energy intake into account in the multivariate analyses or took energy intake into account to calculate the DII. Dose-response meta-analysis indicated that a 1-unit increase in the DII was weakly associated with the risk of breast cancer (pooled RR: 1.03; 95% CI: 1.00, 1.07; P = 0.03, I2 = 84.0%, P-heterogeneity < 0.001; Figure 3A). In the sensitivity analysis, the pooled RR was altered from 1.02 (95% CI: 0.99, 1.04) with the exclusion of the Chinese case-control study (57) to 1.05 (95% CI: 1.00, 1.09) with the exclusion of the German case-control study (56). None of the excluded studies explained the heterogeneity. In the subgroup analyses, a nonsignificant positive association persisted across all subgroups but appeared to be stronger in the case-control subgroup than in cohorts, in Asian studies than in Western studies, in those with ≥30 DII components than in those with <30 components, and among high-quality studies (Table 2). Subgroup analyses yielded study type and number of the DII components as the potential sources of the heterogeneity (Table 2). Begg's test (P = 0.07) but not Egger's asymmetry test (P = 0.10) showed some indications of publication bias. The funnel plot also seemed to be asymmetric (Supplemental Figure 2). Adjustment with the use of the trim and fill method resulted in a nonsignificant association (pooled RR: 1.02; 95% CI: 0.97, 1.06; n = 8 studies).
FIGURE 3.
(A) Summary risk estimates for the association between a 1-unit increment in the DII and risk of breast cancer. (B) Dose-response associations between the DII and risk of breast cancer (from all studies). P- nonlinearity = 0.008. (C) Dose-response associations between the DII and risk of breast cancer (from cohort studies). P-nonlinearity = 0.09. The solid lines and dashed lines represent RRs and 95% CIs, respectively. DII, Dietary Inflammatory Index; ES, effect size; IWHS, Iowa Women's Health Study; SU.VI.MAX, Supplementation en Vitamines et Minéraux Antioxydants; SWLH, Swedish Women's Lifestyle Study; WHI, Women's Health Initiative.
Nonlinear dose-response meta-analysis showed a slight increase in risk along with the increase in the DII from the less proinflammatory boundary toward the more proinflammatory boundary (P-nonlinearity = 0.008; Figure 3B). A sensitivity analysis with the use of 4 prospective cohort studies yielded nearly similar results to the main analysis (P-nonlinearity = 0.09; Figure 3C).
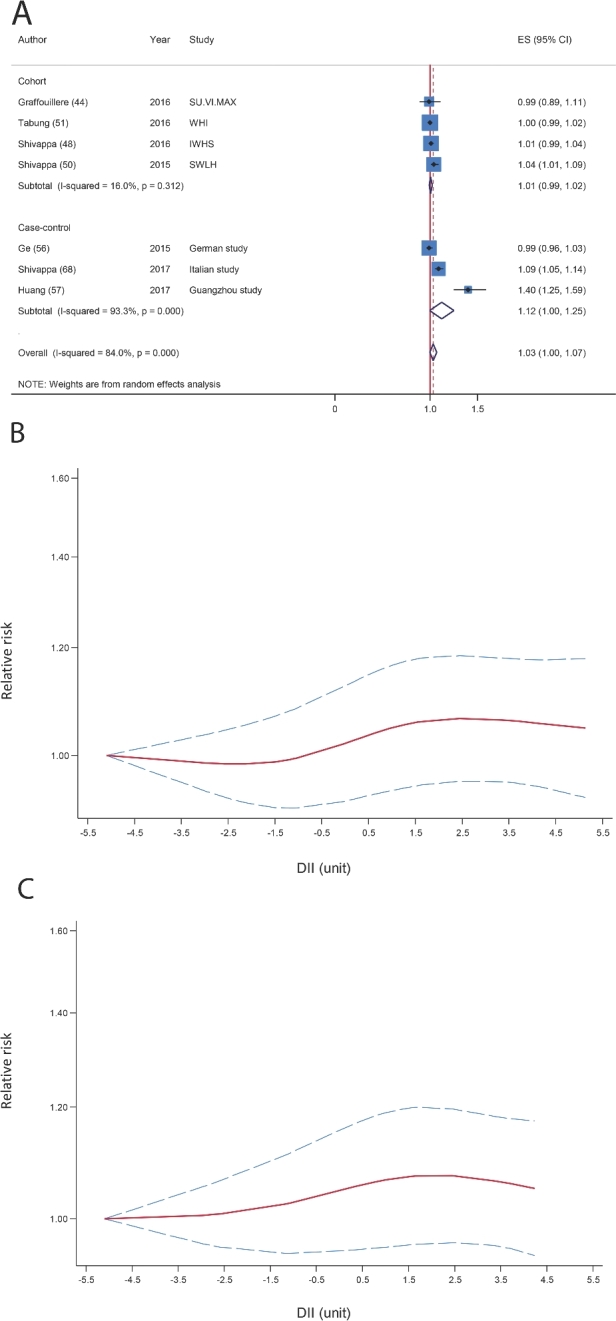
Prostate cancer
The systematic search identified 1 cohort (44) (2648 participants and 123 cases) and 5 case-control studies (61, 64, 77, 78, 81) (2039 cases and 2897 controls) that reported risk estimates on the DII and the risk of prostate cancer. A nonsignificant association was found between a 1-unit increase in the DII and the risk of prostate cancer (pooled RR: 1.06; 95% CI: 0.97, 1.15; P = 0.15, I2 = 56.2%, P-heterogeneity = 0.04; Figure 4A).When analysis was restricted to the 4 case-control studies, the association changed to 1.10 (95% CI: 0.99, 1.20; P = 0.10, I2 = 50.6%, P-heterogeneity = 0.08). In the sensitivity analysis removing each study at a time, the association was altered from 1.04 (95% CI: 0.95, 1.12) with the exclusion of the Jamaican case-control study (77) to 1.10 (95% CI: 0.95, 1.25) with the exclusion of the Italian case-control study (61), and the Jamaican study explained much of the heterogeneity (I2 = 52.2%, P-heterogeneity = 0.08). There was no evidence of publication bias with Egger's test (P = 0.15) and Begg's test (P = 0.13), but the funnel plot seemed to be asymmetric (Supplemental Figure 3).
FIGURE 4.
(A) Summary risk estimates for the association between a 1-unit increment in the DII and risk of prostate cancer. (B) Dose-response associations between the DII and risk of prostate cancer. P-nonlinearity = 0.04. The solid lines and dashed lines represent RRs and 95% CIs, respectively. DII, Dietary Inflammatory Index; ES, effect size.
One study reported the DII only in 2 categories (64), and in another study the range of the DII was very different from that in other studies (61). Therefore, the nonlinear dose-response association was tested by using the results of 4 studies (44, 77, 78, 81), and we observed that the risk of prostate cancer increased very slightly with increasing inflammatory potential of diet (P-nonlinearity = 0.04; Figure 4B).
Other types of cancers
Results of 16 other types of cancer are shown in Table 2. Significant positive associations were found in the analyses of pancreatic cancer (54, 62), ovarian cancer (59, 71), renal cell carcinoma (47, 70), esophageal squamous cell carcinoma (67, 80), and esophageal adenocarcinoma (58, 63). With regard to 11 other cancer types for which only 1 study was available, significant positive associations were observed for bladder (69), gastric (65), laryngeal (72), nasopharyngeal (75), hepatocellular (66), and esophageal squamous cell (58) carcinomas and non-Hodgkin lymphoma (74) but not for urothelial cell carcinoma (43), endometrial cancer (76), lung cancer (46), and gastroesophageal junctional adenocarcinoma (58) (Table 2). The included studies did not report on total cancer outcome; therefore we did not evaluate total cancer in the meta-analysis.
Discussion
The present meta-analysis examined the association between inflammatory features of diet- and site-specific cancer risk with the use of data from 11 cohort and 29 case-control studies in >1,200,000 participants. Our findings indicated that a 1-unit increment in the DII score was associated with a 6% higher risk of CRC, and was nominally associated with the risk of breast cancer (by 3%) and prostate cancer (by 6%). For other cancer types reported in only 1 or 2 studies, significant positive associations were found, except for urothelial cell carcinoma, lung cancer, endometrial cancer, and gastroesophageal junctional adenocarcinoma. Our results are consistent with previous findings that indicated that a healthier diet, higher diet quality, eating more fruit and vegetables, and having dietary habits consistent with dietary guidelines were significantly and inversely associated the risk of different cancer types (83–87).
Our findings reflect those of 2 other recent meta-analyses of the association between the DII and CRC (30, 88). A recent meta-analysis of 4 studies suggested that more-proinflammatory diet scores were associated with a 12–65% higher CRC risk compared with a more anti-inflammatory diet (30). Another recent meta-analysis of 4 cohort and 5 case-control studies indicated that a 1-unit increment in the DII was associated with a 7% higher risk of CRC (88), compared with 6% in the present meta-analysis. Inflammatory pathways are highly involved in the pathogenesis of CRC (8), and a recent meta-analysis of 18 prospective cohort studies indicated that a 1-unit increase in the natural logarithm (ln) CRP was associated with a 12% higher risk of CRC (89). In accordance with our findings, existing evidence suggests that a higher consumption of inflammation-related dietary components such as whole grains, fiber, and vegetables are inversely, and red meat is positively associated with the risk of CRC (90–94). A recent meta-analysis of 40 cohort and case-control studies also indicated that healthy dietary patterns may have protective effects against CRC, whereas a Western-style diet with more proinflammatory features might increase the risk (92).
In the analysis of breast cancer, we found a marginally significant positive association. It has previously been reported that some individual anti-inflammatory components of the DII, such as fiber and omega-3 FAs could significantly reduce the risk of breast cancer (95, 96), and some proinflammatory components such as total fat and red meat (represented by iron and protein in the DII scoring systems) could significantly increase the risk (97, 98). However, we observed that a higher DII score, as representative of a more proinflammatory and less anti-inflammatory diet, did not show such an increased risk. Of 7 included studies in the analysis of breast cancer, only 3 studies reported a significant positive association (48, 57, 68). Thus, it is possible that that the association between diet and breast cancer is mediated by mechanisms other than inflammation. However, we may have been underpowered to observe a significant effect. A possible explanation is the role of alcohol consumption, which has been identified as one of the well-established risk factor for breast cancer (99) but in the DII scoring system has been considered as an anti-inflammatory component. The different impact of diet on the risk of breast cancer phenotypes must also be considered (51). Of 7 included studies, only 3 addressed this issue; 2 studies showed a worse prognosis for an estrogen receptor–positive/progesterone receptor–positive phenotype (56, 57), whereas another showed a somewhat opposite outcome (52).
In the present meta-analysis, results for 19 different cancer types were reported; of these, significant positive associations were found for 13 cancer types, and a marginally significant association was found for another cancer type (breast cancer). However, some important limitations need to be considered. In the analyses of colorectal and breast cancers, the associations were attenuated substantially in the subgroup of cohort studies and among studies with a fewer number of components used to calculate the DII (<30 compared with ≥30). It seems that the increment in the number of components strengthened the associations. However, due to the low number of studies, we could not appropriately test the associations across different combinations of the DII. It has not been clearly determined how the associations change along with the change in the DII composition. A recent meta-analysis of 17 prospective cohort studies and clinical trials suggested an individual role for specific Mediterranean diet components in determining disease risk outcome, in which individual components were not equally associated with the risk of cardiovascular outcomes (100). However, with regard to the DII we have not such summarized evidence and future investigations may be needed to fully investigate the possible effects of each DII component or group of components, such as flavonoids on the strength of the associations, especially in different geographical regions with different dietary habits and different intakes of each component. We also have only 1 study for 11 cancer types, of which 9 studies had a case-control design and were mainly low quality (score of <7). Case-control studies are subject to recall bias, selection bias, and reverse causation bias. In addition, 14 of 29 case-control studies were conducted in Italy. Existing evidence suggests a possible interaction between dietary factors and genetic variants on the risk of cancers (101, 102). Thus, it may be helpful to conduct more high-quality studies for some of the cancer types with only 1 study, especially among more diverse populations with possible different genetic susceptibilities.
There are several potential mechanisms through which a possible link between diet and cancer risk has been proposed. In the present review, we focused on inflammation. It has been indicated that there is a powerful link between chronic inflammation and different types of cancers (7, 103, 104), in a way that ∼20% of all cases of cancer mortality are attributable to the inflammation and chronic infections (105). However, the underlying mechanisms of these relations are not fully characterized. Leukocyte infiltration, accumulation of macrophages, and activation of transcription factors (mainly NF-κB) are some of the suggested pathways that may lead to inappropriate gene expression, enhanced proliferation, and resistance to apoptosis of initiated cells, which, in turn, can result in the development and spread of tumor cells (105). In this regard, activation of NF-κB pathways is a crucial mechanism that plays a key mediatory role between inflammation and carcinogenesis (106).
The possible mechanisms by which dietary features are associated with inflammatory status are not completely well specified. However, some possible explanations were proposed. In the DII scoring system, energy, carbohydrates, and total fats are proinflammatory components, and through increasing body weight, they might lead to worse inflammatory status (107). SFAs through mechanisms such as activation of NF-κB, protein kinase C, and mitogen-activated protein kinases, and subsequent induction of inflammatory genes (108), trans FAs through increasing the activation of the TNF system (109), polyphenols through decreasing the activation of NF-κB pathways (10), magnesium through direct effects on glucose and insulin homeostasis (110), vitamin C through decreasing oxidative stress (111), vitamin E through reducing proinflammatory cytokine expression (112), and n–3 FAs by decreasing the baseline production of hydrogen peroxide and subsequent activation of NF-κB (113) are some of the anti-inflammatory and proinflammatory components of the DII, all of which have been proposed to be associated with inflammatory status. In addition, the DII consists of several antioxidant components and it is thought that oxidative stress plays a role in development and progression of inflammation (114).
The present meta-analysis has some strengths. First, we were able to comprehensively examine the association between the inflammatory potential of diet and site-specific cancer risk. Second, we used the DII as an index of dietary inflammatory properties, which confers a more comprehensive understanding from inflammatory features of the diet than does a single food or nutrient. Third, we could show the shapes of the dose-response relations, which clarified how the risk of colorectal, breast, and prostate cancers change along with the increase in the inflammatory features of the diet.
Some limitations also should be considered when interpreting our results. Most of the included studies had a case-control design. Thus, some limitations of these types of studies must be considered. In addition, 14 studies were from Italy. Thus, we were not able to examine the associations in different geographical regions. Third, our results were accompanied by some evidence of heterogeneity, potentially due to different study designs (cohort compared with case-control), different dietary components used to calculate the DII (18–37 variables), and different adjustment models. Fourth, some evidence of publication bias was found in the analyses of colorectal and breast cancers. In addition, publication bias tests were conducted only in 6 studies in the analysis of prostate cancer. Thus, the results with regard to no evidence of bias may simply be due to chance. Thus, our results may have been affected by publication bias. Finally, the DII consists of 45 different dietary components, with various, and possibly unknown, biological functions. Existing evidence suggests that these nutritional compounds through mechanisms other than inflammation are also linked to cancer risk. Thus, the confounding effects of these pathways should be considered.
In conclusion, the present meta-analysis suggested that greater dietary inflammatory properties, represented by the DII were associated with higher risk of 15 site-specific cancers. It may be helpful to replicate the investigations with the use of more high-quality studies to test the association between the DII and cancer risk in different geographical regions with different dietary habits and possibly different genetic susceptibilities.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—AJ: designed the research, screened articles, extracted data, analyzed data, and wrote the manuscript; AE: conducted the systematic search, screened articles, extracted data, and wrote the manuscript; SS-B: designed the research, analyzed data, wrote the manuscript, and had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: AJ, AE, and SS-B, no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used:
- AHEI
Alternative Healthy Eating Index
- CRC
colorectal cancer
- CRP
C-reactive protein
- DII
Dietary Inflammatory Index
- HEI-2010
Healthy Eating Index 2010
References
- 1. World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: diet, nutrition, physical activity and colorectal cancer. 2017. Available from: wcrf.org/colorectal-cancer-2017.
- 2. World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: Diet, nutrition, physical activity and stomach cancer. 2016. Available from: wcrf.org/stomach-cancer-2016.
- 3. World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: diet, nutrition, physical activity and kidney cancer. 2015. Available from: wcrf.org/kidney-cancer-2015.
- 4. World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: diet, nutrition, physical activity and liver cancer. 2015. Available from: wcrf.org/sites/default/files/Liver-Cancer-2015-Report-pdf.
- 5. World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: diet, nutrition, physical activity and breast cancer. 2017. Available from: wcrf.org/breasr-cancer-2017.
- 6. World Cancer Research Fund International/American Institute for Cancer Research. Continuous Update Project Report: food, nutrition, physical activity, and the prevention of endometrial cancer. 2013. Available from: http://www.dietandcancerreport.org.
- 7. Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther 1999;13(Suppl 1):13–8. [DOI] [PubMed] [Google Scholar]
- 8. Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology 2010;138(6):2101–14, e5. [DOI] [PubMed] [Google Scholar]
- 9. Veeranki S. Role of inflammasomes and their regulators in prostate cancer initiation, progression and metastasis. Cell Mol Biol Lett 2013;18(3):355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hardman WE. Diet components can suppress inflammation and reduce cancer risk. Nutr Res Pract 2014;8(3):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dumas JA, Bunn JY, Nickerson J, Crain KI, Ebenstein DB, Tarleton EK, Makarewicz J, Poynter ME, Kien CL. Dietary saturated fat and monounsaturated fat have reversible effects on brain function and the secretion of pro-inflammatory cytokines in young women. Metabolism 2016;65(10):1582–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez-Alarcon M, Perichart-Perera O, Flores-Huerta S. Excessive refined carbohydrates and scarce micronutrients intakes increase inflammatory mediators and insulin resistance in prepubertal and pubertal obese children independently of obesity. Mediators Inflamm 2014;2014:849031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Samraj AN, Pearce OM, Laubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bingman AE, Secrest P, Diaz SL, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA 2015;112(2):542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Islam MA, Alam F, Solayman M, Khalil MI, Kamal MA, Gan SH. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxidative Medicine and Cellular Longevity 2016;2016, Article ID 5137431, 25 pages. doi: 10.1155/2016/5137431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jheng HF, Hirotsuka M, Goto T, Shibata M, Matsumura Y, Kawada T. Dietary low-fat soy milk powder retards diabetic nephropathy progression via inhibition of renal fibrosis and renal inflammation. Mol Nutr Food Res 2017;61(3), doi: 10.1002/mnfr.201600461. [DOI] [PubMed] [Google Scholar]
- 16. Dibaba DT, Xun P, He K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: meta-analysis and systematic review. Eur J Clin Nutr 2014;68(4):510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lotito SB, Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J Biol Chem 2006;281(48):37102–10. [DOI] [PubMed] [Google Scholar]
- 18. Roager HM, Vogt JK, Kristensen M. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut 2017, 1–11, doi: 10.1136/gutjnl-2017-314786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salehi-Abargouei A, Saraf-Bank S, Bellissimo N, Azadbakht L. Effects of non-soy legume consumption on C-reactive protein: a systematic review and meta-analysis. Nutrition 2015;31(5):631–9. [DOI] [PubMed] [Google Scholar]
- 20. Casas R, Sacanella E, Urpi-Sarda M, Chiva-Blanch G, Ros E, Martinez-Gonzalez MA, Covas MI, Salas-Salvado J, Fiol M, Aros F, et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease: a randomized trial. PLoS One 2014;9(6):e100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koloverou E, Panagiotakos DB, Pitsavos C, Chrysohoou C, Georgousopoulou EN, Grekas A, Christou A, Chatzigeorgiou M, Skoumas I, Tousoulis D, et al. Adherence to Mediterranean diet and 10-year incidence (2002-2012) of diabetes: correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab Res Rev 2016;32(1):73–81. [DOI] [PubMed] [Google Scholar]
- 22. Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr 2007;137(4):992–8. [DOI] [PubMed] [Google Scholar]
- 23. Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2004;80(4):1029–35. [DOI] [PubMed] [Google Scholar]
- 24. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17(8):1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios study. Br J Nutr 2015;113(4):665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr 2014;17(8):1825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol 2015;25(6):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wirth MD, Hébert JR, Shivappa N, Hand GA, Hurley TG, Drenowatz C, McMahon D, Shook RP, Blair SN. Anti-inflammatory Dietary Inflammatory Index scores are associated with healthier scores on other dietary indices. Nutr Res 2016;36(3):214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fan Y, Jin X, Man C, Gao Z, Wang X. Meta-analysis of the association between the inflammatory potential of diet and colorectal cancer risk. Oncotarget 2017;8(35):59592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steck SE, Guinter M, Zheng J, Thomson CA. Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv Nutr 2015;6:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Panagiotakos DB, Pitsavos C, Stefanadis C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis 2006;16(8):559–68. [DOI] [PubMed] [Google Scholar]
- 32. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 33. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc 1995;95(10):1103–8. [DOI] [PubMed] [Google Scholar]
- 34. McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76(6):1261–71. [DOI] [PubMed] [Google Scholar]
- 35. Kant AK, Schatzkin A, Graubard BI, Schairer C. A prospective study of diet quality and mortality in women. JAMA 2000;283(16):2109–15. [DOI] [PubMed] [Google Scholar]
- 36. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Ann Intern Med 2009;151(4):264–9. [DOI] [PubMed] [Google Scholar]
- 37. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 38. Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemio-logic dose-response data. Epidemiology 1993;4(3):218–28. [DOI] [PubMed] [Google Scholar]
- 39. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6(1):40. [Google Scholar]
- 40. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29(12):1282–97. [DOI] [PubMed] [Google Scholar]
- 41. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dugue PA, Hodge AM, Brinkman MT, Bassett JK, Shivappa N, Hebert JR, Hopper JL, English DR, Milne RL, Giles GG. Association between selected dietary scores and the risk of urothelial cell carcinoma: a prospective cohort study. Int J Cancer 2016;139(6):1251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Graffouillere L, Deschasaux M, Mariotti F, Neufcourt L, Shivappa N, Hebert JR, Wirth MD, Latino-Martel P, Hercberg S, Galan P, et al. The Dietary Inflammatory Index is associated with prostate cancer risk in French middle-aged adults in a prospective study. J Nutr 2016;146(4):785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harmon BE, Wirth MD, Boushey CJ, Wilkens LR, Draluck E, Shivappa N, Steck SE, Hofseth L, Haiman CA, Le Marchand L, et al. The Dietary Inflammatory Index is associated with colorectal cancer risk in the Multiethnic Cohort. J Nutr 2017;147(3):430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hodge AM, Bassett JK, Shivappa N, Hebert JR, English DR, Giles GG, Severi G. Dietary inflammatory index, Mediterranean diet score, and lung cancer: a prospective study. Cancer Causes Control 2016;27(7):907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shivappa N, Blair CK, Prizment AE, Jacobs DR Jr., Hebert JR. Dietary inflammatory index and risk of renal cancer in the Iowa Women's Health Study. Eur J Nutr 2017;57(3):1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shivappa N, Blair CK, Prizment AE, Jacobs DR, Hébert JR. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol Nutr Food Res 2017;61(5):1600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shivappa N, Prizment AE, Blair CK, Jacobs DR Jr., Steck SE, Hebert JR. Dietary inflammatory index and risk of colorectal cancer in the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev 2014;23(11):2383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shivappa N, Sandin S, Lof M, Hebert JR, Adami HO, Weiderpass E. Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br J Cancer 2015;113(7):1099–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Caan B, Chlebowski RT, Freudenheim JL, Hou L, Mossavar-Rahmani Y, et al. Association between dietary inflammatory potential and breast cancer incidence and death: results from the Women's Health Initiative. Br J Cancer 2016;114(11):1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, Hou L, Johnson KC, Mossavar-Rahmani Y, Shivappa N, et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women's Health Initiative. Cancer Causes Control 2015;26(3):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wirth MD, Shivappa N, Steck SE, Hurley TG, Hebert JR. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Br J Nutr 2015;113(11):1819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Antwi SO, Oberg AL, Shivappa N, Bamlet WR, Chaffee KG, Steck SE, Hébert JR, Petersen GM. Pancreatic cancer: associations of inflammatory potential of diet, cigarette smoking, and long-standing diabetes. Carcinogenesis 2016;37(5):481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cho YA, Lee J, Oh JH, Shin A, Kim J. Dietary Inflammatory Index and risk of colorectal cancer: a case-control study in Korea. Nutrients 2016;8(8):469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ge I, Rudolph A, Shivappa N, Flesch-Janys D, Hebert JR, Chang-Claude J. Dietary inflammation potential and postmenopausal breast cancer risk in a German case-control study. Breast 2015;24(4):491–6. [DOI] [PubMed] [Google Scholar]
- 57. Huang WQ, Mo XF, Ye YB, Shivappa N, Lin FY, Huang J, Hebert JR, Yan B, Zhang CX. A higher Dietary Inflammatory Index score is associated with a higher risk of breast cancer among Chinese women: a case-control study. Br J Nutr 2017;117(10):1358–67. [DOI] [PubMed] [Google Scholar]
- 58. Lu Y, Shivappa N, Lin Y, Lagergren J, Hebert JR. Diet-related inflammation and oesophageal cancer by histological type: a nationwide case-control study in Sweden. Eur J Nutr 2016;55(4):1683–94. [DOI] [PubMed] [Google Scholar]
- 59. Peres LC, Bandera EV, Qin B, Guertin KA, Shivappa N, Hebert JR, Abbott SE, Alberg AJ, Barnholtz-Sloan J, Bondy M, et al. Dietary Inflammatory Index and risk of epithelial ovarian cancer in African American women. Int J Cancer 2017;140(3):535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sharma I, Zhu Y, Woodrow JR, Mulay S, Parfrey PS, McLaughlin JR, Hebert JR, Shivappa N, Li Y, Zhou X, et al. Inflammatory diet and risk for colorectal cancer: a population-based case-control study in Newfoundland, Canada. Nutrition 2017;42:69–74. [DOI] [PubMed] [Google Scholar]
- 61. Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, Hebert JR. Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr 2015;113(2):278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. Br J Nutr 2015;113(2):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shivappa N, Hebert JR, Anderson LA, Shrubsole MJ, Murray LJ, Getty LB, Coleman HG. Dietary inflammatory index and risk of reflux oesophagitis, Barrett's oesophagus and oesophageal adenocarcinoma: a population-based case-control study. Br J Nutr 2017;117(9):1323–31. [DOI] [PubMed] [Google Scholar]
- 64. Shivappa N, Hebert JR, Askari F, Kardoust Parizi M, Rashidkhani B. Increased inflammatory potential of diet is associated with increased risk of prostate cancer in Iranian men. Int J Vitam Nutr Res 2017:1–8. [DOI] [PubMed] [Google Scholar]
- 65. Shivappa N, Hebert JR, Ferraroni M, La Vecchia C, Rossi M. Association between dietary inflammatory index and gastric cancer risk in an Italian case-control study. Nutr Cancer 2016;68:1262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shivappa N, Hebert JR, Polesel J, Zucchetto A, Crispo A, Montella M, Franceschi S, Rossi M, La Vecchia C, Serraino D. Inflammatory potential of diet and risk for hepatocellular cancer in a case-control study from Italy. Br J Nutr 2016;115(2):324–31. [DOI] [PubMed] [Google Scholar]
- 67. Shivappa N, Hebert JR, Rashidkhani B. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Iran. Nutr Cancer 2015;67(8):1253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shivappa N, Hebert JR, Rosato V, Montella M, Serraino D, La Vecchia C. Association between the dietary inflammatory index and breast cancer in a large Italian case-control study. Mol Nutr Food Res 2017;61:1600500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shivappa N, Hebert JR, Rosato V, Rossi M, Libra M, Montella M, Serraino D, La Vecchia C. Dietary inflammatory index and risk of bladder cancer in a large Italian case-control study. Urology 2017;100:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shivappa N, Hebert JR, Rosato V, Rossi M, Montella M, Serraino D, La Vecchia C. Dietary inflammatory index and renal cell carcinoma risk in an Italian case-control study. Nutr Cancer 2017;69(6):833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shivappa N, Hébert JR, Rosato V, Rossi M, Montella M, Serraino D, La Vecchia C. Dietary inflammatory index and ovarian cancer risk in a large Italian case–control study. Cancer Causes Control 2016;27:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shivappa N, Hebert JR, Rosato V, Serraino D, La Vecchia C. Inflammatory potential of diet and risk of laryngeal cancer in a case-control study from Italy. Cancer Causes Control 2016;27(8):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shivappa N, Hebert JR, Steck SE, Hofseth LJ, Shehadah I, Bani-Hani KE, Al-Jaberi T, Al-Nusairr M, Heath D, Tayyem R. Dietary inflammatory index and odds of colorectal cancer in a case-control study from Jordan. Appl Physiol Nutr Metab 2017;42(7):744–9. [DOI] [PubMed] [Google Scholar]
- 74. Shivappa N, Hebert JR, Taborelli M, Montella M, Libra M, Zucchetto A, Crispo A, Grimaldi M, La Vecchia C, Serraino D, et al. Dietary inflammatory index and non-Hodgkin lymphoma risk in an Italian case-control study. Cancer Causes Control 2017;28(7):791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shivappa N, Hebert JR, Zucchetto A, Montella M, Libra M, Garavello W, Rossi M, La Vecchia C, Serraino D. Increased risk of nasopharyngeal carcinoma with increasing levels of diet-associated inflammation in an Italian case-control study. Nutr Cancer 2016;68(7):1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shivappa N, Hebert JR, Zucchetto A, Montella M, Serraino D, La Vecchia C, Rossi M. Dietary inflammatory index and endometrial cancer risk in an Italian case-control study. Br J Nutr 2016;115(1):138–46. [DOI] [PubMed] [Google Scholar]
- 77. Shivappa N, Jackson MD, Bennett F, Hebert JR. Increased Dietary Inflammatory Index (DII) is associated with increased risk of prostate cancer in Jamaican men. Nutr Cancer 2015;67(6):941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shivappa N, Miao Q, Walker M, Hebert JR, Aronson KJ. Association between a dietary inflammatory index and prostate cancer risk in Ontario, Canada. Nutr Cancer 2017;69(6):825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shivappa N, Zucchetto A, Montella M, Serraino D, Steck SE, La Vecchia C, Hebert JR. Inflammatory potential of diet and risk of colorectal cancer: a case-control study from Italy. Br J Nutr 2015;114(1):152–8. [DOI] [PubMed] [Google Scholar]
- 80. Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Italy. Cancer Causes Control 2015;26(10):1439–47. [DOI] [PubMed] [Google Scholar]
- 81. Vazquez-Salas RA, Shivappa N, Galvan-Portillo M, Lopez-Carrillo L, Hebert JR, Torres-Sanchez L. Dietary inflammatory index and prostate cancer risk in a case-control study in Mexico. Br J Nutr 2016;116(11):1945–53. [DOI] [PubMed] [Google Scholar]
- 82. Zamora-Ros R, Shivappa N, Steck SE, Canzian F, Landi S, Alonso MH, Hebert JR, Moreno V. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge Colorectal Cancer Case-Control Study. Genes Nutr 2015;10(1):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Baena Ruiz R, Salinas Hernandez P. Diet and cancer: risk factors and epidemiological evidence. Maturitas 2014;77(3):202–8. [DOI] [PubMed] [Google Scholar]
- 84. Kohler LN, Garcia DO, Harris RB, Oren E, Roe DJ, Jacobs ET. Adherence to diet and physical activity cancer prevention guidelines and cancer outcomes: a systematic review. Cancer Epidemiol Biomarkers Prev 2016;25(7):1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu X, Wang X, Lin S, Yuan J, Yu IT. Dietary patterns and oesophageal squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer 2014;110(11):2785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2015;115(5):780–800, e5. [DOI] [PubMed] [Google Scholar]
- 87. Smith-Warner SA, Spiegelman D, Yaun SS, Albanes D, Beeson WL, van den Brandt PA, Feskanich D, Folsom AR, Fraser GE, Freudenheim JL, et al. Fruits, vegetables and lung cancer: a pooled analysis of cohort studies. Int J Cancer 2003;107(6):1001–11. [DOI] [PubMed] [Google Scholar]
- 88. Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, Grosso G. Dietary inflammatory index and colorectal cancer risk—a meta-analysis. 2017;9(9):1043–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhou B, Shu B, Yang J, Liu J, Xi T, Xing Y. C-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysis. Cancer Causes Control 2014;25(10):1397–405. [DOI] [PubMed] [Google Scholar]
- 90. Alexander DD, Weed DL, Cushing CA, Lowe KA. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. Eur J Cancer Prev 2011;20(4):293–307. [DOI] [PubMed] [Google Scholar]
- 91. Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Feng YL, Shu L, Zheng PF, Zhang XY, Si CJ, Yu XL, Gao W, Zhang L. Dietary patterns and colorectal cancer risk: a meta-analysis. Eur J Cancer Prev 2017;26(3):201–11. [DOI] [PubMed] [Google Scholar]
- 93. Kashino I, Mizoue T, Tanaka K, Tsuji I, Tamakoshi A, Matsuo K, Wakai K, Nagata C, Inoue M, Tsugane S, et al. Vegetable consumption and colorectal cancer risk: an evaluation based on a systematic review and meta-analysis among the Japanese population. Jpn J Clin Oncol 2015;45(10):973–9. [DOI] [PubMed] [Google Scholar]
- 94. Zhu B, Sun Y, Qi L, Zhong R, Miao X. Dietary legume consumption reduces risk of colorectal cancer: evidence from a meta-analysis of cohort studies. Sci Rep 2015;5:8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen S, Chen Y, Ma S, Zheng R, Zhao P, Zhang L, Liu Y, Yu Q, Deng Q, Zhang K. Dietary fibre intake and risk of breast cancer: a systematic review and meta-analysis of epidemiological studies. Oncotarget 2016;7(49):80980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013;346:f3706. [DOI] [PubMed] [Google Scholar]
- 97. Cao Y, Hou L, Wang W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: a meta-analysis of prospective cohort studies. Int J Cancer 2016;138(8):1894–904. [DOI] [PubMed] [Google Scholar]
- 98. Guo J, Wei W, Zhan L. Red and processed meat intake and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat 2015;151(1):191–8. [DOI] [PubMed] [Google Scholar]
- 99. Suzuki R, Orsini N, Mignone L, Saji S, Wolk A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status—a meta-analysis of epidemiological studies. Int J Cancer 2008;122(8):1832–41. [DOI] [PubMed] [Google Scholar]
- 100. Grosso G, Marventano S, Yang J, Micek A, Pajak A, Scalfi L, Galvano F, Kales SN. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: are individual components equal? Crit Rev Food Sci Nutr 2017;57(15):3218–32. [DOI] [PubMed] [Google Scholar]
- 101. Figueiredo JC, Hsu L, Hutter CM, Lin Y, Campbell PT, Baron JA, Berndt SI, Jiao S, Casey G, Fortini B. Genome-wide diet-gene interaction analyses for risk of colorectal cancer. PLoS Genet 2014;10(4):e1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rock CL, Lampe JW, Patterson RE. Nutrition, genetics, and risks of cancer. Annu Rev Public Health 2000;21:47–64. [DOI] [PubMed] [Google Scholar]
- 103. Hamada S, Masamune A, Shimosegawa T. Inflammation and pancreatic cancer: disease promoter and new therapeutic target. J Gastroenterol 2014;49(4):605–17. [DOI] [PubMed] [Google Scholar]
- 104. Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci 2012;13(9):11071–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, Sica A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology 2009;214(9–10):761–77. [DOI] [PubMed] [Google Scholar]
- 106. Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005;5(10):749–59. [DOI] [PubMed] [Google Scholar]
- 107. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 2011;29:415–45. [DOI] [PubMed] [Google Scholar]
- 108. Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr 2009;139(1):1–4. [DOI] [PubMed] [Google Scholar]
- 109. Mozaffarian D, Pischon T, Hankinson SE, Rifai N, Joshipura K, Willett WC, Rimm EB. Dietary intake of trans fatty acids and systemic inflammation in women. Am J Clin Nutr 2004;79(4):606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Song Y, Li TY, van Dam RM, Manson JE, Hu FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr 2007;85(4):1068–74. [DOI] [PubMed] [Google Scholar]
- 111. Chien C-T, Chang W-T, Chen H-W, Wang T-D, Liou S-Y, Chen T-J, Chang Y-L, Lee Y-T, Hsu S-M. Ascorbate supplement reduces oxidative stress in dyslipidemic patients undergoing apheresis. Arterioscler Thromb Vasc Biol 2004;24(6):1111–7. [DOI] [PubMed] [Google Scholar]
- 112. Murphy RT, Foley JB, Tome MT, Mulvihill NT, Murphy A, McCarroll N, Crean P, Walsh MJ. Vitamin E modulation of C-reactive protein in smokers with acute coronary syndromes. Free Radic Biol Med 2004;36(8):959–65. [DOI] [PubMed] [Google Scholar]
- 113. Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, Willett WC, Hu FB. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr 2004;134(7):1806–11. [DOI] [PubMed] [Google Scholar]
- 114. Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem 2014;395(2):203–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.