Abstract
This study aimed to validate previously reported dosimetric parameters, including thyroid volume, mean dose, and percentage thyroid volume, receiving at least 40, 45 and 50 Gy (V40, V45 and V50), absolute thyroid volume spared (VS) from 45, 50 and 60 Gy (VS45, VS50 and VS60), and clinical factors affecting the development of radiation-induced hypothyroidism (RHT). A post hoc analysis was performed in 178 euthyroid nasopharyngeal cancer (NPC) patients from a Phase III study comparing sequential versus simultaneous-integrated boost intensity-modulated radiation therapy. RHT was determined by increased thyroid-stimulating hormone (TSH) with or without reduced free thyroxin, regardless of symptoms. The median follow-up time was 42.5 months. The 1-, 2- and 3-year freedom from RHT rates were 78.4%, 56.4% and 43.4%, respectively. The median latency period was 21 months. The thyroid gland received a median mean dose of 53.5 Gy. Female gender, smaller thyroid volume, higher pretreatment TSH level (≥1.55 μU/ml) and VS60 < 10 cm3 were significantly associated with RHT in univariate analyses. Only pretreatment TSH ≥ 1.55 μU/ml and VS60 < 10 cm3 were significant predictors in multivariate analysis. Our results suggested that patients with pretreatment TSH ≥ 1.55 μU/ml should be cautious about the risk of RHT. The VS60 ≥ 10 cm3 is recommended for treatment planning.
Keywords: intensity-modulated radiation therapy, nasopharyngeal cancer, hypothyroidism, dosimetric predictors
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) is a mainstay treatment and has become a standard of care for nasopharyngeal cancer (NPC) because it provides conformal dose distribution to the target volume and rapid dose fall-off to critical structures, such as the parotid glands, brainstem, and spinal cord [1–3]. The locoregional control for NPC patients treated with IMRT has also improved. However, significant toxicities, especially radiation-induced hypothyroidism (RHT), may increase because of higher thyroid dose from IMRT compared with 3D radiation therapy (3D-RT) [4, 5]. The thyroid gland is frequently affected by radiation therapy in NPC patients because of its radiosensitivity and its proximity to the elective nodal irradiation field [6]. The incidence of RHT was 39.9–44.5% at 2 years after IMRT [5, 7].
Many studies have recommended various dose–volume constraints for the thyroid gland in IMRT treatment of head and neck cancer. Sachdev revealed that the thyroid volume receiving at least 50 Gy (V50) ≥60% was associated with 6.76 times increased risk of clinical hypothyroidism compared with V50 < 60% [8]. To minimize the risk of hypothyroidism, Kim suggested lowering the thyroid V45 to <50% [9], while Chyan recommended that the absolute thyroid volume spared from doses ≥50 Gy (VS50) ≥ 6 cm3, VS45 ≥ 3 cm3 and V50 < 45%, and keeping the mean thyroid dose to <49 Gy [10]. Other parameters predicting a higher rate of hypothyroidism included younger age [5, 11, 12], female sex [13, 14], mean radiation dose to the thyroid [6] and small thyroid volume [5, 6, 10, 12, 15]. The use of chemotherapy, and autoimmune disease, may also be associated with hypothyroidism [16–18].
In NPC, IMRT encompassed the thyroid gland; therefore, different dose–volume constraints were recommended. Sommat recommended thyroid V40 < 85% [7], while Zhai recommended keeping the thyroid V45 < 50% and V50 < 35% [12]. Other parameters recommended by Lee were absolute thyroid volume spared from 60 Gy (VS60) ≥ 10 cm3 and 45 Gy (VS45) ≥ 5 cm3, respectively [19]. Huang suggested the importance of thyroid V40 but did not recommend dose–volume constraints [11].
Based on these controversies regarding the dose–volume constraint to thyroid and the clinical parameters, our primary aim was to validate previously reported dosimetric parameters and clinical factors affecting the development of RHT in our NPC patients treated with IMRT and concurrent chemotherapy.
MATERIALS AND METHODS
This study was a post hoc analysis of a prospective randomized controlled trial comparing the utility of sequential (SEQ) or simultaneous-integrated boost (SIB) IMRT in non-metastatic NPC, treated between October 2010 and September 2015. This study was approved by the institutional review board (IRB no. 424/53). Informed consent was obtained from every patient before study entrance. There were 209 patients in the analysis of the primary endpoints. After excluding 17 patients whose pretreatment thyroid function tests (TFTs) were not available, 5 patients who had abnormal baseline TFTs, and 9 patients who had insufficient follow-up data to determine TFTs, a total of 178 patients were included in this study. TFTs, including measurement of free thyroxine (FT4) and thyroid-stimulating hormone (TSH), were carried out every 4 months for the first 2 years, every 6 months up to the fifth year and then annually. Radiation-induced hypothyroidism (RHT) was defined as a TSH above the upper limit of our institutional reference range (0.3–4.2 μU/ml), with or without reduced FT4 (normal range 0.8–1.8 ng/dl), regardless of symptoms.
Chemotherapy consisted of weekly treatments of 40 mg/m2 cisplatin given concurrently with 70 Gy IMRT in 33–35 fractions to patients with >T1 or positive nodal disease for a maximum of seven cycles. Adjuvant chemotherapy, consisting of 80 mg/m2 cisplatin and 1000 mg/m2/ day 5-fluorouracil (5-FU) over a 96-h continuous infusion, was given at 4-week intervals for three cycles.
The treatment plan of the randomized trial was previously described in detail [20, 21]. Briefly summarized, all patients were immobilized in the supine position with a tailored head–shoulder thermoplastic mask. A CT simulation was performed and transferred to the Eclipse planning system version 8.6 (Varian Medical Systems, Palo Alto, CA, USA). Magnetic resonance simulation was performed on every patient and was transferred for co-registration with the CT images. Whole-neck IMRT was used in our study. Two planning target volumes (PTVs) were designated: PTV-high risk (PTV-HR), defined as the gross tumor and the pathologic lymph node, and PTV-low risk (PTV-LR), defined as the subclinical disease and the elective lymph node region. These PTVs received doses of 70 Gy and 50–56 Gy, respectively. At the time of this study, we did not delineate the thyroid gland as an organ-at-risk. Thus, for this study, we retrospectively contoured the thyroid gland on the CT and MRI simulation images of the original treated IMRT plan and collected the dose–volume histograms data for the thyroid gland.
Based on the previous dose–volume effect predicting RHT [7, 19], we did not make any attempt to find new dose–volume parameters, but rather validated the effect of previous recommended parameters in our patient population. Dosimetric parameters included thyroid volume (as a continuous value, because Chyan previously reported that patients with thyroid volume <8 cm3 had 3-year freedom from hypothyroidism of only 25% [10], and we decided to keep the 8-cm3 cut-off value instead of identifying a new cut-off value [10]), mean dose, percent thyroid volume receiving at least 40, 45 and 50 Gy (V40, V45 and V50), and absolute thyroid volume spared from 45, 50 and 60 Gy (VS45, VS50 and VS60). Various cut-off dosimetric values for the thyroid gland included V40 (85% [7]), V45 (50% [9, 12]), V50 (35% [12] and 45% [10]), VS45 (5 cm3 [19]), VS50 (6 cm3 [10]) and VS60 (10 cm3 [19]) were analyzed.
Statistical analysis
For comparison of the patient characteristics, the chi-squared test (or Fisher’s exact test, if indicated) was used to compare categorical data, while the independent t-test was used for continuous data. A Mann–Whitney test was performed to compare the dosimetric data between the RHT and euthyroid groups. The median latency period of RHT was determined from the end of radiotherapy to the first detected abnormal TFT. Deceased patients without abnormal TFTs were censored at the time of death. Freedom from RHT was analyzed using the Kaplan–Meier method and the log-rank test. Cox proportional hazard models with univariate and multivariate analyses were performed to identify the predictors for RHT. The optimal cut-off was determined using a receiver operating characteristics (ROC) analysis. Youden’s index was performed on pretreatment TSH and age in order to identify the cut-off values that maximized the difference in RHT between the sensitivity and specificity and between the real-positive and false-positive subjects [22]. Age, gender, stage, pretreatment TSH, and the thyroid dosimetric parameters were included as covariates in the exploratory analysis. Factors with a P-value of <0.05 in the univariate analysis were entered into the multivariate Cox regression model with backward selection. All tests were two-sided, and a P-value of <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS statistics (version 22.0, SPSS Inc., Chicago, Ill).
RESULTS
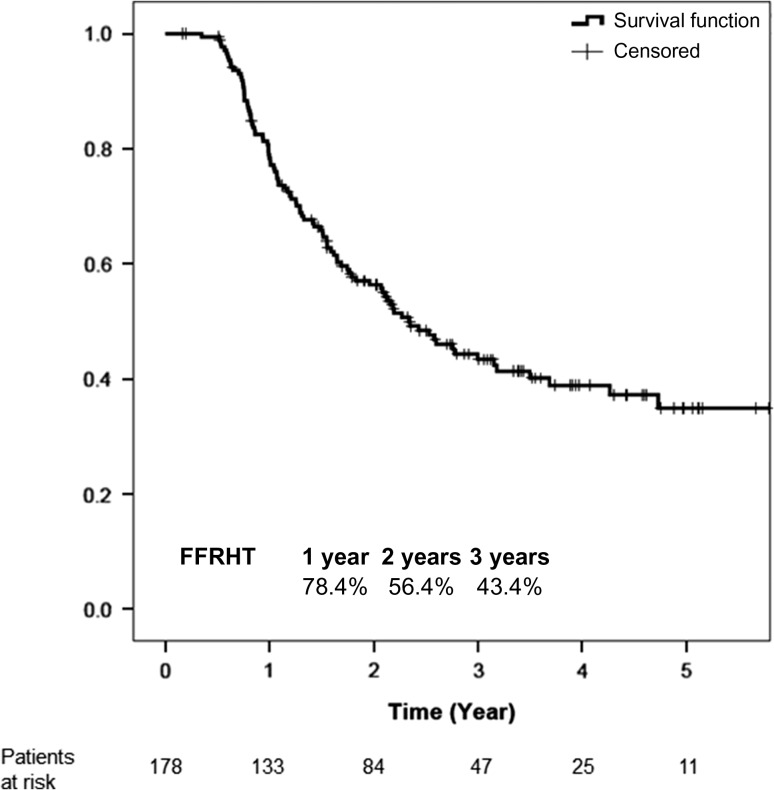
The median follow-up duration was 42.5 months. Patient and treatment characteristics are outlined in Table 1. The patient characteristics were well-balanced between two groups, except that the patients in the RHT group had a higher proportion of females and they were younger. Moreover, the thyroid gland was significantly smaller, but the pretreatment TSH level was significantly higher in the RHT group. Overall, the mean and median thyroid volumes were 15.4 cm3 and 13.1 cm3, respectively. The mean cycles of concurrent chemotherapy and adjuvant chemotherapy were approximately 5.6 cycles and 2.6 cycles, respectively. There were 96 patients (53.9%) who developed RHT, with a median latency period of 21 months (interquartile range 11–36.25 months). There was no clinical hypothyroidism in our population due to regular TFTs during the follow-up period, prompting early detection in any abnormal parameter; thus, RHT in this study referred to subclinical hypothyroidism. Thyroid replacement therapy was prescribed for 44 patients (24.7%). In our clinical practice, thyroid replacement therapy is given when there is continuously increased TSH, or FT4 less than the lower normal limit, irrespective of symptoms. The estimated 1-, 2- and 3-year cumulative incidences of RHT were 21.6%, 43.6% and 56.6%, with corresponding freedom from RHT (FFRHT) rates of 78.4%, 56.4% and 43.4%, respectively (Fig. 1).
Table 1.
Patient and treatment characteristics
| Parameters | Euthyroid (n = 82) | Hypothyroidism (n = 96) | P-value | |
|---|---|---|---|---|
| Age (years) | mean (SD) | 51.7 (10.2) | 47.9 (10.7) | 0.017a |
| Gender | ||||
| Male | N (%) | 71 (86.6) | 70 (72.9) | 0.025a |
| Female | N (%) | 11 (13.4) | 26 (27.1) | |
| T-classification | ||||
| T1–2 | N (%) | 48 (58.5) | 63 (65.6) | 0.331 |
| T3–4 | N (%) | 34 (41.5) | 33 (34.4) | |
| N-classification | ||||
| N0–1 | N (%) | 25 (30.5) | 22 (22.9) | 0.253 |
| N2–3 | N (%) | 57 (69.5) | 74 (77.1) | |
| WHO type | ||||
| Nonkeratinizing CA | N (%) | 20 (24.4) | 15 (15.6) | 0.142 |
| Undifferentiated CA | N (%) | 62 (75.6) | 81 (84.4) | |
| Stage | ||||
| I–II | N (%) | 12 (14.6) | 15 (15.6) | 0.395 |
| III | N (%) | 41 (50.0) | 56 (58.3) | |
| IVA–IVB | N (%) | 29 (35.4) | 25 (26.0) | |
| IMRT technique | ||||
| SIB | N (%) | 40 (48.8) | 52 (54.2) | 0.474 |
| SEQ | N (%) | 42 (51.2) | 44 (45.8) | |
| Pretreatment | ||||
| FT4 (ng/dl) | mean (SD) | 1.3 (0.3) | 1.3 (0.2) | 0.780 |
| TSH (μU/ml) | mean (SD) | 1.4 (0.8) | 2.0 (0.9) | <0.001a |
| Thyroid gland | ||||
| Volume (cm3) | mean (SD) | 17.2 (12.1) | 13.9 (6.9) | 0.025a |
| median (IQR) | 13.9 (11.7–18.8) | 12.1 (9.5–16.6) | 0.005a | |
| Dmean (Gy) | median (IQR) | 52.7 (49.1–55.0) | 53.6 (50.0–56.8) | 0.205 |
| Dmin (Gy) | median (IQR) | 34.5 (30.6–37.9) | 34.7 (31.1–38.8) | 0.417 |
| Dmax (Gy) | median (IQR) | 64.0 (61.3–70.2) | 68.2 (62.9–73.9) | 0.021a |
| V40 (%) | median (IQR) | 96.0 (87.0–100.0) | 96.0 (90.0–100.0) | 0.596 |
| V45 (%) | median (IQR) | 88.2 (78.7–94.3) | 87.7 (75.0–95.8) | 0.770 |
| V50 (%) | median (IQR) | 70.0 (57.3–79.9) | 73.8 (53.7–85.2) | 0.215 |
| VS45 (cm3) | median (IQR) | 1.8 (0.8–3.7) | 1.5 (0.5–3.2) | 0.543 |
| VS50 (cm3) | median (IQR) | 4.2 (2.7–6.8) | 3.7 (1.5–7.0) | 0.084 |
| VS60 (cm3) | median (IQR) | 12.5 (9.1–15.5) | 9.7 (6.9–13.9) | 0.006a |
| Concurrent chemotherapy | mean (SD) | 5.6 (1.4) | 5.7 (1.0) | 0.375 |
| <5 cycles | N (%) | 30 (36.6) | 39 (40.6) | 0.581 |
| ≥6 cycles | N (%) | 52 (63.4) | 57 (59.4) | |
| Adjuvant chemotherapy | mean (SD) | 2.4 (1.0) | 2.7 (0.7) | 0.059 |
| 0–2 cycles | N (%) | 25 (30.5) | 19 (19.8) | 0.099 |
| 3 cycles | N (%) | 57 (69.5) | 77 (80.2) |
WHO = World Health Organization, CA = carcinoma, IMRT = intensity-modulated radiotherapy, IQR = interquartile range, SIB = simultaneous-integrated boost, SEQ = sequential, FT4 = free levothyroxine, TSH = thyroid-stimulating hormone, Dmean = mean dose, Dmin = minimal dose, Dmax = maximal dose, Vx = volume receiving dose x Gy, VSx = volume-sparing dose x Gy, SD = standard deviation.
aP-value of <0.05 was considered statistically significant.
Fig. 1.
Freedom from radiation-induced hypothyroidism curve in the overall population.
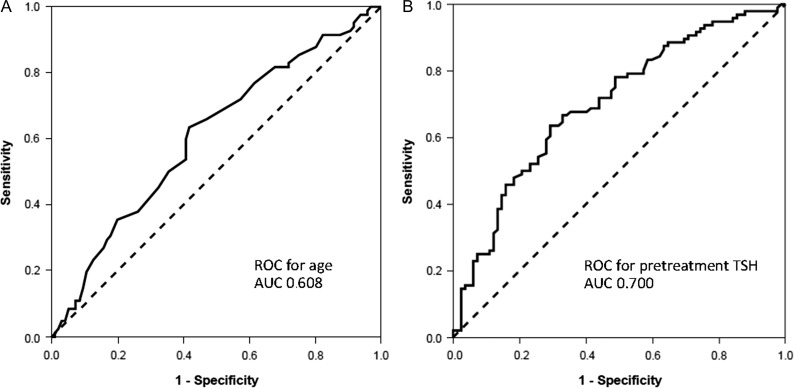
Using the ROC analysis and Youden’s index, we identified a cut-off of patient age at 48.5 years, and pretreatment TSH level of 1.55 μU/ml as a good predictor for RHT, with an area under the curve of 0.608 and 0.700, respectively (Fig. 2). When differentiating RHT among patients, the 48.5-year age cut-off showed 63.4% sensitivity and 58.3% specificity, while the cut-off of TSH level had 63.5% sensitivity and 70.7% specificity. These cut-off points were applied as dichotomous variables along with their continuous value in univariate analysis. The univariate analyses for clinical and dosimetric parameters are presented in Table 2. Female gender, smaller thyroid volume (<8 cm3), higher pretreatment TSH level (≥1.55 μU/ml) and VS60 < 10 cm3 were associated with higher incidence of RHT in univariate analysis. The Kaplan–Meier curves for all significant parameters in univariate analysis are shown in Fig. 3A–D.
Fig. 2.
ROC curve for age and pretreatment TSH.
Table 2.
Univariate analysis and multivariate analysis for freedom from radiation induced hypothyroidism using Cox regression analysis
| Parameters | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | ||
| Age (years) | Continuous | 0.99 (0.97–1.01) | 0.138 | ||
| <48.5 vs ≥48.5 years | 1.38 (0.92–2.07) | 0.123 | |||
| Gender | Male vs female | 0.59 (0.37–0.92) | 0.020a | 0.68 (0.42–1.08) | 0.104 |
| T-classification | T1–2 vs T3–4 | 0.96 (0.63–1.46) | 0.849 | ||
| N-classification | N0–1 vs N2–3 | 0.85 (0.53–1.36) | 0.492 | ||
| Stage group | I–II vs III–IVB | 1.01 (0.58–1.75) | 0.251 | ||
| WHO type | Nonkeratinizing CA vs undifferentiated CA | 0.72 (0.42–1.26) | 0.251 | ||
| IMRT technique | SIB vs SEQ | 1.20 (0.80–1.79) | 0.386 | ||
| Pretreatment TSH | Continuous | 1.64 (1.35–2.01) | <0.001a | ||
| <1.55 vs ≥1.55 μU/ml | 0.37 (0.25–0.57) | <0.001a | 0.40 (0.26–0.60) | <0.001a | |
| Thyroid gland | |||||
| Volume | Continuous | 0.96 (0.93–0.99) | 0.020a | ||
| <8 vs ≥8 cm3 | 2.27 (1.26–4.09) | 0.006a | 1.25 (0.67–2.34) | 0.486 | |
| Dmean (Gy) | Continuous | 1.03 (0.99–1.07) | 0.159 | ||
| <45 vs ≥45 Gy | 0.89 (0.28–2.8) | 0.838 | |||
| V40 | <85 vs ≥85% | 0.64 (0.35–1.18) | 0.154 | ||
| V45 | <50 vs ≥50% | 0.05 (0.00–1564.4) | 0.569 | ||
| V50 | <35 vs ≥35% | 0.99 (0.50–1.96) | 0.973 | ||
| V50 | <45 vs ≥45% | 1.10 (0.63–1.90) | 0.745 | ||
| VS45 | Continuous | 0.97 (0.91–1.03) | 0.328 | ||
| VS45 | <3 vs ≥3 cm3 | 1.21 (0.77–1.90) | 0.402 | ||
| VS45 | <5 vs ≥5 cm3 | 1.04 (0.58–1.86) | 0.903 | ||
| VS50 | Continuous | 0.95 (0.90–1.00) | 0.058 | ||
| VS50 | <6 vs ≥6 cm3 | 1.25 (0.79–1.97) | 0.343 | ||
| VS60 | Continuous | 0.95 (0.92–0.99) | 0.008a | ||
| VS60 | <10 vs ≥10 cm3 | 2.01 (1.33–3.02) | 0.001a | 1.83 (1.21–2.76) | 0.004a |
WHO = World Health Organization, CA = carcinoma, IMRT = intensity-modulated radiotherapy, SIB = simultaneous-integrated boost, SEQ = sequential, FT4 = free levothyroxine, TSH = thyroid-stimulating hormone, Dmean = mean dose, Dmin = minimal dose, Dmax = maximal dose, Vx = volume receiving dose x Gy, VSx = volume-sparing dose x Gy, CI = confidence interval.
a P-value of <0.05 was considered statistically significant.
Fig. 3.
Kaplan–Meier curves for freedom from radiation-induced hypothyroidism for significant predictors in univariate analysis including (A) gender, (B) pretreatment TSH, (C) thyroid volume and (D) thyroid VS60.
Gender, pretreatment TSH, thyroid volume and thyroid VS60 were entered into the multivariate Cox regression model. To prevent multicollinearity, one thyroid parameter was included at a time. Only thyroid VS60 ≥ 10 cm3 and pretreatment TSH < 1.55 μU/ml were identified as protective variables for RHT in multivariate analysis. Patients with TSH < 1.55 and ≥1.55 μU/ml had 3-year FFRHT of 58.8% and 27.6%, respectively (hazard ratio = 0.40, 95% CI 0.26–0.60). The 3-year FFRHT rate was 33.5% in patients with thyroid VS60 < 10 cm3, while it was 50.8% in patients with thyroid VS60 ≥ 10 cm3 (hazard ratio = 1.83, 95% CI 1.21–2.76). The exploratory univariate and multivariate analyses for time to receive thyroid replacement therapy were in accordance with those for RHT (Table 1 supplement). There were significant differences in time to receive thyroid replacement therapy between patients, with TSH < 1.55 vs ≥1.55 μU/ml (P < 0.001), and with thyroid VS60 ≥ 10 vs <10 cm3 (P = 0.004) in multivariate analysis (Fig. 1 supplement).
DISCUSSION
The tolerance dose of the thyroid gland varied significantly between studies with different primary cancer sites (i.e. NPC vs other head and neck cancer) and different endpoints (i.e. subclinical hypothyroidism or clinical hypothyroidism) [5, 8]. In the pre-IMRT era, Emami estimated that the tolerance dose, with 8% probability of clinical hypothyroidism at 5 years and when given radiation treatment to the entire thyroid gland, was 45 Gy [23]. Grande observed the incidence of hypothyroidism in 22.4% and 56% when the thyroid received <60 Gy and >60 Gy, respectively [24]. Others recommended keeping the mean thyroid dose less than <30 Gy [25] or <45 Gy [26].
In the present study, we performed a post hoc analysis of NPC patients treated with chemotherapy concurrent with IMRT. A total of 53.9% of our patients developed RHT, with a median latency period of 21 months. The estimated 1-, 2- and 3-year cumulative incidences of RHT were 21.6%, 43.6% and 56.6%, respectively, which compared favorably with the 1- and 2-year cumulative incidence of 33% and 44.5% reported by Sommat in NPC patients [7]. The high incidence of RHT was because a specific dose–volume constraint for the thyroid gland was not implemented in IMRT planning. By contrast, without dose–volume constraint to the thyroid gland, Lee reported the 1-, 2- and 3-year hypothyroidism (subclinical hypothyroidism + clinical hypothyroidism) incidence at 5.3%, 17.5% and 36.2%, respectively [19]. One possible explanation for this more favorable result may be the relatively short median follow-up time of 3.1 years, since hypothyroidism is time dependent [27]. In a systematic review, Boomsma found an incidence of subclinical hypothyroidism of 23–53%, while the incidence of clinical hypothyroidism ranged from 11–33% and the cumulative incidence of hypothyroidism increased with a longer follow-up period [28]. In a prospective cohort of 135 NPC patients, Zhai revealed a 2-year cumulative RHT incidence of 29.6% [12]. There was no detail as to whether the investigators placed a dose–volume constraint on the thyroid gland in Zhai’s study. A summary of dosimetric predictors for hypothyroidism among previously reported studies is detailed in Table 3.
Table 3.
Comparison of dosimetric predictors for hypothyroidism in the literature
| Authors | Number/primary site/RT technique | Median follow-up | HT definition | HT rate | Univariate variables | Multivariate variables | Suggestion by authors |
|---|---|---|---|---|---|---|---|
| Sommat et al. [7] | 102/NPC/IMRT | 48.8 months |
|
|
|
|
Thyroid V40 ≤ 85% |
| Lee et al. [19] | 149/NPC/IMRT | 3.1 years |
|
|
Thyroid volume, Dmin, Dmean, D05, V30, V35, V40, V45, VS 30, VS35, VS40, VS45, VS50, VS55, VS60 | Thyroid volume, VS45, VS60 |
|
| Zhai et al. [12] | 135/NPC/IMRT | 34.1 months |
|
|
Female, Younger age (49 years), Thyroid volume, Dmin, Dmean, V30, V35, V40, V45, V50, thyrotoxicosis |
|
|
| Diaz et al. [5] | 128/HN/IMRT | 28.3 months |
|
|
|
|
Apply DVC to thyroid gland |
| Chyan et al. [10] | 123/HN/IMRT | 4.6 years |
|
|
Thyroid volume, Dmean, V10, V20, V30, V40, V50, V60, V70, VS10, VS20, VS30, VS50 | Thyroid V50, Dmean, VS45, VS50 |
|
| Kim et al. [9] | 114/HN/3D-RT or IMRT | 25 months |
|
|
Thyroid Dmin, Dmean, V35, V40, V45, V50 | Thyroid V45 | V45 < 50% |
| Present study | 178/NPC/IMRT | 42.5 months |
|
|
Female, pretreatment TSH, thyroid volume, VS60 |
|
VS60 ≥ 10 cm3 |
RT = radiotherapy, IMRT = intensity-modulated radiotherapy, 3D-RT = 3-dimensional radiotherapy, NPC = nasopharyngeal carcinoma, HN = head and neck, HT = hypothyroidism, TSH = thyroid-stimulating hormone, Dmin = minimal dose, Dmax = maximal dose, Dmean = mean dose, Vx = volume receiving dose x Gy, VSx = volume-sparing dose x Gy, DVC = dose–volume constraint, LNL = lower normal limit, UNL = upper normal limit, FT4 = FT4 = free levothyroxine, T4 = levothyroxine.
Typically, extended-field or whole-neck IMRT could achieve mean thyroid doses of 28.6 ± 2.4 Gy, which are much lower than thyroid doses of 44.7 ± 3.7 Gy when using split-field IMRT with 2-step blocking [29]. Contouring of the thyroid gland as an organ-at-risk significantly reduced the median thyroid dose, V30, V40 and V50 [5]. However, since we did not define the thyroid volume or dose constraint when treating the patients, the median of the thyroid mean dose of 53.5 Gy resulted in a considerable risk of RHT in patients who had small thyroid volumes. While Sommat [7] recommended the thyroid V40 ≤ 85%, we were unable to confirm the value of the thyroid V40, since most of the thyroid glands in our study received doses of >50 Gy, and V40 was >90%. An estimated 3-year RHT in Sommat’s study was 8% vs 51% in those with V40 ≤ 85% vs >85% (P = 0.007), respectively, while the patients in our study experienced more 3-year RHT at 42.4% vs 60.8% (P = 0.150), respectively (Fig. 2 supplement). With a slightly shorter median follow-up in our study than in Sommat’s study (42.5 months vs 48.8 months), the number of RHT events/patient in our study was 12/29 (41.4%) [vs 3/14 (21%) in Sommat’s] in those with V40 ≤ 85% and 84/149 (56.4%) [vs 54/88 (61%) in Sommat’s] in those with V40 > 85%. Note that the estimated 3-year RHT and the cumulative rates of RHT between Sommat’s study and our study were almost comparable in patients who received V40 > 85%. The lower 3-year RHT in patients with V40 ≤ 85% in Sommat’s study might result from a low number of patients or from a high proportion of larger thyroid volume in their patient subgroup. No VS parameter was evaluated in their study.
Multivariate analysis of clinical risk factors in recent studies included younger age [7, 12], early T stage [7] and small thyroid glands [12, 19]. Although female gender was significant in univariate analysis in ours and other reports [12, 14], gender lost its significance in multivariate analysis because it is related to the smaller thyroid gland. Previous studies reported that the median and mean volumes of thyroid glands were 18.3 cm3 [11] and 20.5 cm3 [19], while the median and mean volumes of thyroid glands in our study were 13.1 cm3 and 15.4 cm3, respectively. Median and mean thyroid volume in patients with RHT was significantly lower than in those with normal thyroid function (euthyroid) (Table 1). Our finding was in line with other studies [5, 12, 19] that reported smaller thyroid volume increased the risk of RHT in univariate analysis. For the first time, we found that patients with pretreatment TSH of ≥1.55 μU/ml had 2.5 times higher risk of developing RHT and time to receive thyroid replacement therapy in multivariate analysis. Data regarding the significance of pretreatment TSH on RHT are scarce. According to our data, high pretreatment TSH correlated with thyroid volume <8 cm3. We postulate that when the thyroid volume is small, there may not be enough functional thyroid subunit to produce thyroid hormone, thus, TSH increases as a normal feedback mechanism from the pituitary gland, and these patients are prone to developing RHT after receiving radiation treatment. Chyan reported that a pretreatment thyroid volume <8 cm3 gave an incidence of hypothyroidism as high as 75% at 3 years and recommended keeping thyroid VS45 ≥ 3 cm3 and VS50 ≥ 6 cm3 in patients with thyroid volume >8 cm3 [10]. In concordance with Chyan, 18 patients with thyroid volume <8 cm3 in our study had an estimated 3-year RHT of 80.8%. We could not perform any statistical analysis to identify predictors for RHT due to the small number of patients. However, we recommend keeping the mean thyroid dose as low as possible in this patient subgroup.
In multivariate analysis, thyroid VS60 ≥ 10 cm3 and pretreatment TSH < 1.55 μU/ml were significant protective factors. We agreed with Lee [19] that the thyroid VS60 ≥ 10 cm3 constraint should be met. Chyan recommended keeping thyroid VS45 ≥ 3 cm3 and VS50 ≥ 6 cm3 in patients with thyroid volume >8 cm3 [10]. However, thyroid VS45 and VS50 were not significant parameters in our study.
Limitations of our study included follow-up time, which was not relatively long enough to thoroughly assess late RHT. Hypothyroidism was not the primary outcome in this randomized trial comparing different IMRT techniques. Thus, the power to detect significant dose–volume parameters may not have been adequate. Nevertheless, the strength of our study was that various dose–volume constraints were compared and validated in a relatively large NPC population compared with other studies (Table 3). The patient population was derived from a prospective randomized trial with a uniform curative dose with IMRT technique and concurrent cisplatin-based chemotherapy followed by adjuvant cisplatin and 5FU. Moreover, a range of patient characteristics, including pretreatment TSH for the first time, was analyzed in our study.
In conclusion, in patients with pretreatment TSH ≥ 1.55 μU/ml, one should be cautious because of the risk of RHT. A thyroid VS60 ≥ 10 cm3 is recommended for treatment planning. A prospective study with a larger population and longer follow-up is warranted.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Radiation Research online.
ACKNOWLEDGEMENTS
This research article was made possible through help and support from significant advisors and industrious colleagues. Thanks to the Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Thai Red Cross Society for providing the resources and funding for quantitative measurements for the TFTs.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
FUNDING
This work was supported by Ratchadapisek Sompoch Endowment Fund, Chulalongkorn University [RA 8/54].
Supplementary Material
REFERENCES
- 1. Eisbruch A, Haken RK, Kim HM et al. Dose, volume, and function relationships in parotid salivary glands following conformal, and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys 1999;45:577–87. [DOI] [PubMed] [Google Scholar]
- 2. Xia P, Fu KK, Wong GW et al. Comparison of treatment plans involving intensity-modulated radiotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2000;48:329–37. [DOI] [PubMed] [Google Scholar]
- 3. Hunt MA, Zelefsky MJ, Wolden S et al. Treatment planning and delivery of intensity-modulated radiation therapy for primary nasopharynx cancer. Int J Radiat Oncol Biol Phys 2001;49:623–32. [DOI] [PubMed] [Google Scholar]
- 4. Peng G, Wang T, Yang KY et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012;104:286–93. [DOI] [PubMed] [Google Scholar]
- 5. Diaz R, Jaboin JJ, Morales-Paliza M et al. Hypothyroidism as a consequence of intensity-modulated radiotherapy with concurrent taxane-based chemotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2010;77:468–76. [DOI] [PubMed] [Google Scholar]
- 6. Ronjom MF, Brink C, Bentzen SM et al. Hypothyroidism after primary radiotherapy for head and neck squamous cell carcinoma: normal tissue complication probability modeling with latent time correction. Radiother Oncol 2013;109:317–22. [DOI] [PubMed] [Google Scholar]
- 7. Sommat K, Ong WS, Hussain A et al. Thyroid V40 predicts primary hypothyroidism after intensity modulated radiation therapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2017;98:574–80. [DOI] [PubMed] [Google Scholar]
- 8. Sachdev S, Refaat T, Bacchus ID et al. Thyroid V50 highly predictive of hypothyroidism in head-and-neck cancer patients treated with intensity-modulated radiotherapy (IMRT). Am J Clin Oncol 2017;40:413–7. [DOI] [PubMed] [Google Scholar]
- 9. Kim MY, Yu T, Wu HG. Dose–volumetric parameters for predicting hypothyroidism after radiotherapy for head and neck cancer. Jpn J Clin Oncol 2014;44:331–7. [DOI] [PubMed] [Google Scholar]
- 10. Chyan A, Chen J, Shugard E et al. Dosimetric predictors of hypothyroidism in oropharyngeal cancer patients treated with intensity-modulated radiation therapy. Radiat Oncol 2014;9:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang S, Wang X, Hu C et al. Hypothalamic–pituitary–thyroid dysfunction induced by intensity-modulated radiotherapy (IMRT) for adult patients with nasopharyngeal carcinoma. Med Oncol 2013;30:710. [DOI] [PubMed] [Google Scholar]
- 12. Zhai RP, Kong FF, Du CR et al. Radiation-induced hypothyroidism after IMRT for nasopharyngeal carcinoma: clinical and dosimetric predictors in a prospective cohort study. Oral Oncol 2017;68:44–9. [DOI] [PubMed] [Google Scholar]
- 13. Wu YH, Wang HM, Chen HH et al. Hypothyroidism after radiotherapy for nasopharyngeal cancer patients. Int J Radiat Oncol Biol Phys 2010;76:1133–9. [DOI] [PubMed] [Google Scholar]
- 14. Vogelius IR, Bentzen SM, Maraldo MV et al. Risk factors for radiation-induced hypothyroidism: a literature-based meta-analysis. Cancer 2011;117:5250–60. [DOI] [PubMed] [Google Scholar]
- 15. Alterio D, Jereczek-Fossa BA, Franchi B et al. Thyroid disorders in patients treated with radiotherapy for head-and-neck cancer: a retrospective analysis of seventy-three patients. Int J Radiat Oncol Biol Phys 2007;67:144–50. [DOI] [PubMed] [Google Scholar]
- 16. Norris AA, Amdur RJ, Morris CG et al. Hypothyroidism when the thyroid is included only in the low neck field during head and neck radiotherapy. Am J Clin Oncol 2006;29:442–5. [DOI] [PubMed] [Google Scholar]
- 17. Fan CY, Lin CS, Chao HL et al. Risk of hypothyroidism among patients with nasopharyngeal carcinoma treated with radiation therapy: a population-based cohort study. Radiother Oncol 2017;123:394–400. [DOI] [PubMed] [Google Scholar]
- 18. Luo R, Li M, Yang Z et al. Nomogram for radiation-induced hypothyroidism prediction in nasopharyngeal carcinoma after treatment. Br J Radiol 2017;90:20160686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee V, Chan SY, Choi CW et al. Dosimetric predictors of hypothyroidism after radical intensity-modulated radiation therapy for non-metastatic nasopharyngeal carcinoma. Clin Oncol 2016;28:e52–60. [DOI] [PubMed] [Google Scholar]
- 20. Songthong AP, Kannarunimit D, Chakkabat C et al. A randomized phase II/III study of adverse events between sequential (SEQ) versus simultaneous integrated boost (SIB) intensity modulated radiation therapy (IMRT) in nasopharyngeal carcinoma; preliminary result on acute adverse events. Radiat Oncol 2015;10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lertbutsayanukul C, Prayongrat A, Kannarunimit D et al. A randomized phase III study between sequential versus simultaneous integrated boost intensity-modulated radiation therapy in nasopharyngeal carcinoma. Strahlenther Onkol 2018;194:375–85. [DOI] [PubMed] [Google Scholar]
- 22. Yin J, Tian L. Joint confidence region estimation for area under ROC curve and Youden index. Stat Med 2014;33:985–1000. [DOI] [PubMed] [Google Scholar]
- 23. Emami B, Lyman J, Brown A et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109–22. [DOI] [PubMed] [Google Scholar]
- 24. Grande C. Hypothyroidism following radiotherapy for head and neck cancer: multivariate analysis of risk factors. Radiother Oncol 1992;25:31–6. [DOI] [PubMed] [Google Scholar]
- 25. Fujiwara M, Kamikonya N, Odawara S et al. The threshold of hypothyroidism after radiation therapy for head and neck cancer: a retrospective analysis of 116 cases. J Radiat Res 2015;56:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhandare N, Kennedy L, Malyapa RS et al. Primary and central hypothyroidism after radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys 2007;68:1131–9. [DOI] [PubMed] [Google Scholar]
- 27. Tell R, Lundell G, Nilsson B et al. Long-term incidence of hypothyroidism after radiotherapy in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys 2004;60:395–400. [DOI] [PubMed] [Google Scholar]
- 28. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol 2011;99:1–5. [DOI] [PubMed] [Google Scholar]
- 29. Yu Y, Chen J, Leary CI et al. Split-field vs extended-field intensity-modulated radiation therapy plans for oropharyngeal cancer: Which spares the larynx? Which spares the thyroid? Med Dosim 2016;41:148–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.