Abstract
Kidney stone disease is a global health care problem, with a high recurrence rate after stone removal. It is thus crucial to develop effective strategies to prevent the formation of new or recurrent stones. Caffeine is one of the main components in caffeinated beverages worldwide (i.e., coffee, tea, soft drinks, and energy drinks). Previous retrospective and prospective studies have reported contradictory effects of caffeine on kidney stone risk. Although it has a diuretic effect on enhancing urinary output, it may slightly increase the stone risk index. However, 3 large cohorts have suggested a preventive role of caffeine in kidney stone disease. In addition, a recent in vitro study has addressed relevant mechanisms underlying the preventive role of caffeine against stone pathogenesis. This review summarizes the relevant data from previous evidence and discusses the association between caffeine consumption and kidney stone risk reduction.
Keywords: calcium oxalate, coffee, crystal adhesion, nephrolithiasis, trigonelline, urolithiasis
Introduction
Caffeine is one of the most common bioactive compounds that is widely consumed around the globe. Caffeinated beverages, including coffee, tea, soft drinks, chocolate products, and energy drinks, are the main sources of dietary caffeine. Its amount in each beverage varies, depending on the type of original plant (1), growing conditions (2), and brewing method (3). Caffeine is frequently used for enhancing mood, alertness, and cognitive performance. It also has physiologic and pharmacologic actions in several biological systems, including nervous (4, 5), cardiovascular (6–8), renal (9–11), and muscular (12, 13) systems. In addition, caffeine can reduce the risk of several diseases, such as Alzheimer disease (14, 15), Parkinson disease (16, 17), and diabetes mellitus (18–20). Moreover, it has a mild diuretic effect by inhibiting fluid reabsorption along proximal renal tubules (21–23). Although the effects of caffeine have been extensively studied in several kidney diseases (24–28), its role in kidney stone disease seems to be overlooked and underinvestigated.
Kidney stone disease (or nephrolithiasis or urolithiasis) is a common disease caused by the deposition of chemical crystals [calcium oxalate (CaOx), calcium phosphate, etc.] within the kidney. The removal of kidney stones can be done by shock wave lithotripsy, ureteroscopy, percutaneous nephrolithotomy, and other surgical procedures (29–32). However, the most important problem related to this disease is its high recurrence rate after stone removal and the continuously increasing number of new cases (33, 34). As a result, ∼$5 billion have been spent annually in the United States for the management of kidney stone disease (34). Furthermore, the cost of stone removal is much higher than the cost for prevention (33, 34). Interestingly, the interval required for recurrent stone formation is shorter than that of new stone formation (35). Therefore, recent kidney stone research has focused on strategies to prevent the formation of new and recurrent stones (29). One of the simple strategies to prevent kidney stone formation is sufficient hydration and increased water intake to enhance urinary output (35–37).
The risk of kidney stones can be reduced by some, but not all, types of fluids that are taken to increase urine volume (38–41). Moreover, caffeine-rich beverages (i.e., coffee and tea) have been recognized to contain oxalate (42) and can increase urinary calcium excretion (43). However, the data from 3 large cohort studies have reported the association between caffeine consumption and a lower risk of kidney stone disease (44). In addition, a recent in vitro study has shown a reduction in adhesion of CaOx crystals on caffeine-treated renal tubular epithelial cells (45). On the basis of these lines of evidence, caffeine (particularly from coffee) may be one of the protective mechanisms against CaOx kidney stone formation (45). This article thus summarizes and discusses the data from previous studies on effects of caffeine in kidney stone disease, particularly the CaOx type, which is the most common form of kidney stones in humans (46).
Overview of Caffeine
Caffeine and its popularity
Caffeine (1,3,7-trimethylxanthine, C8H10N4O2) is a popular stimulant worldwide that is frequently consumed to enhance mood, alertness, muscle endurance, and exercise performance. Caffeine is a naturally occurring alkaloid found in seeds and leaves of several types of plants (i.e., coffee beans, tea leaves, cocoa beans, and kola nuts). The amount of caffeine content in each plant varies, depending on the type of plant, its growth environment, and the preparation or brewing method. For example, Robusta coffee usually has a higher caffeine content than Arabica coffee (47). In addition, the roasted temperature and pressure can influence caffeine content in the coffee (47).
Due to the long historical usage and its popularity, there have been several studies that reported on habitual caffeine consumption around the world. The data from the NHANES showed that 89% of the US adult population (aged >19 y) consume caffeine, of which 98% of the sources are from beverages, including coffee (64%), soft drinks (18%), tea (16%), and energy drinks (<1%) (48). The average amount of caffeine consumed is 186 mg/d (48). In addition, ∼68% of the US child population (aged <12 y) consume 25 mg caffeine/d, whereas ∼75% of the US adolescent population (aged 12–19 y) consume 50 mg caffeine/d (49). The key sources of caffeine consumed by US children and adolescents are soft drinks and tea (49). In Austria, the most common sources of caffeine for adolescents and young-adult Austrians are coffee (60.8%), energy drinks (11.9%), and colas (9.5%), with a consumption amount of ∼357 mg/d (50). In The Netherlands, information from a pencil-and-paper survey from Dutch students (aged 18–30 y) has indicated that 87.8% of those participants are consumers of caffeine from coffee (50.8%), tea (34.8%), energy drinks (9.2%), colas (4.7%), and chocolate milk (0.5%) (51). These data clearly indicate that caffeine has been widely consumed around the globe in almost all age groups, with the highest consumption in adults. The most common sources of dietary caffeine are from caffeinated beverages, such as coffee (the most popular source globally), tea, chocolate products, and soft drinks, as well as energy drinks that have been recently introduced into the market and whose popularity has been increasing continuously and dramatically (52).
Caffeine absorption, metabolism, and elimination
After oral intake, caffeine is rapidly and completely absorbed from the stomach and small intestine into the blood circulation, and is then distributed to almost all tissues and body fluids. The peak plasma concentration of caffeine is ∼8–10 mg/L, which is reached within 15–120 min after its intake (53), whereas its half-life is ∼5 h (54). It is metabolized in the liver by cytochrome P450 to become metabolite products, including paraxanthine (1,7-dimethylxanthine), theobromide (3,4-dimethylxanthine), and theophylline (1,3-dimethylxanthine) (55). These dimethylxanthine compounds are then demethylated to monomethylxanthine and finally oxidized to methyl uric acid and other end products (56). Whereas the majority of the consumed caffeine is metabolized to become various end products (metabolites) as previously mentioned, the remaining amount (<2%) is intact and directly excreted into the urine without accumulating inside human body (57).
Caffeine and the Kidney
The kidney is a main vital organ that plays crucial roles in the regulation of water and electrolyte homeostasis and in the elimination of waste products. Circulating byproducts from metabolism are eliminated by the kidney as urine. The urine volume depends on a balance between glomerular filtration rate (GFR) and tubular reabsorption rate, both of which can be influenced by several factors, including intrinsic and extrinsic stimuli (e.g., fluid intake and some types of substances). Compounds in the methylxanthine family (i.e., caffeine and its metabolites) have been recognized as diuretic substances that increase the urine output by enhancement of GFR, reduction of tubular reabsorption, or both (58, 59). Because of the similarity in chemical structure of caffeine and adenosine, caffeine can bind to adenosine receptor and is recognized as an adenosine receptor antagonist (58, 59). As such, caffeine can act as an adenosine inhibitor to inhibit adenosine activity by competitive binding to its receptor. This mechanism of action of caffeine has been confirmed in an adenosine receptor A1–deficient murine model, in which caffeine did not increase the urine volume (58). In addition to its diuretic action, caffeine also has a natriuretic property because the caffeine-induced increase in urine flow is related to the increased urinary excretion of solutes (i.e., sodium, chloride, calcium, phosphate, and magnesium) (58, 59). In addition, a recent study reported a set of urinary proteins that are altered after caffeine intake (1 cup of coffee contains 152 mg caffeine) in association with the increased urine volume (60). The proteome data have shown the decreased urinary excretion of kininogen, which can cause an increase in intrarenal kinin that, in turn, reduces vasopressin release and promotes renal vasodilation (60). Intrarenal vasodilation thereby enhances glomerular blood flow, GFR, and urine output (60).
On the other hand, as an adenosine receptor antagonist, caffeine can inhibit adenosine activity of juxtaglomerular cells, resulting in the promotion of renin secretion (61–63). As such, some investigations have shown contradictory findings because the secretion of renin, a hormone produced by the kidney that is responsible for the conversion of angiotensinogen into angiotensin, can lead to vasoconstriction as well as tubular reabsorption by activating the release of antidiuretic hormone (also known as vasopressin) (64). Nevertheless, the latter, contradictory effects have been found only in renin-elevated states (e.g., cirrhosis and genetic heart failure), not in the normal physiologic condition (65, 66).
Caffeine and Kidney Stone Disease
Effects of caffeine consumption on kidney stone disease
Although the effects of caffeine on the kidney have been extensively studied, its influence in kidney stone disease seems to be overlooked. The evidence on whether caffeine prevents or promotes kidney stone disease has recently become more clear. Because an increase in fluid intake is widely recommended for the prevention of kidney stone formation, some previous studies during the past 2 decades focused on the relevance of the type of consumed beverages, including caffeinated beverages, in association with kidney stone incidence. In 1996, the first cohort data retrieved from the Health Professionals Follow-Up Study (HPFS) in 45,298 male participants who had no history of kidney stones were reported (38). The findings showed that not all types of beverages affect kidney stone disease. Only caffeinated coffee and decaffeinated coffee and tea, but not caffeinated and noncaffeinated sodas, are associated with an ∼10% lower risk of kidney stone incidence (38).
In 1998, the Nurses’ Health Study (NHS), another cohort study in 81,083 female participants who had no history of kidney stones reported the association between beverage consumption and a lower risk of kidney stone disease (39). The data showed a 10%, 9%, and 8% lower risk of kidney stone formation in the participants who consumed caffeinated coffee, decaffeinated coffee, and tea, respectively (39).
On the other hand, because the restriction of dietary oxalate is one of the recommended ways to reduce the risk of CaOx kidney stone occurrence or recurrence, coffee and tea, which have been recognized as oxalate-rich beverages, are widely recommended as beverages to avoid. Moreover, some studies have speculated that caffeine might be related to an increased risk of kidney stone formation because of its activity in elevating urinary calcium excretion (67). In 2004, the data obtained from 39 kidney stone formers, who consumed caffeine after 14 h of fasting, showed that caffeine slightly increased urinary excretion of calcium, magnesium, sodium, and citrate, but not oxalate (67). In addition, the Tiselius stone risk index in 32 of 39 stone formers was found to be modestly increased after caffeine consumption (67). Note that the Tiselius stone risk index, which was introduced by Tiselius and Larsson (68), is calculated to predict the risk of development of calcium-containing stones based on only some biochemical contents in the urine, including calcium, oxalate, magnesium, and citrate. Moreover, urinary citrate, which is considered to be a strong inhibitor of CaOx kidney stones (69, 70), is also increased in caffeine consumers, and thereby may be able to counterbalance the effects of the high urinary calcium excretion.
In 2013, in a much greater number of participants (194,094 individuals enrolled from the HPFS, NHS I, and NHS II), the data showed a 26%, 16%, and 11% lower risk of kidney stone disease in those who consumed caffeinated coffee, decaffeinated coffee, and tea, respectively (44). With this much larger sample size, the difference between caffeinated and decaffeinated coffee can be observed. Note that the effects of caffeinated and decaffeinated coffee were comparable to those in former reports with much smaller sample sizes (38, 39). Regardless of sample size, decaffeinated coffee is consistently associated with kidney stone risk reduction in all of these 3 reports (38, 39, 44). It is thus possible that other bioactive compounds in coffee (e.g., the second most abundant alkaloid, namely trigonelline) may exert similar protective effects against kidney stone disease (although not as strong as those affected by caffeine). The trigonelline content in coffee varies among different types of coffee on the basis of roasting and brewing methods (3, 71, 72). For example, its amount is greater in Arabica coffee than in Robusta coffee (in which the caffeine content is greater) (3). Nevertheless, trigonelline content does not differ in caffeinated compared with decaffeinated coffee (73). It should be noted that decaffeinated coffee does not really lack caffeine. A recent study reported a range of 0–13.9 mg caffeine/16 ounces (473 mL) of decaffeinated coffee, whereas caffeinated coffee usually contains >100 mg caffeine/16-ounce serving (74). Therefore, the amount of daily caffeine intake should also be taken into account. Similar effects have been observed in colas (also known as caffeinated sodas), which contain 41.5–48.4 mg caffeine/16-ounce serving (75). The 2013 mega-data showed an 8% lower risk of kidney stone disease in the participants who consumed artificially sweetened cola (44). In contrast, artificially sweetened noncola (noncaffeinated soda) is associated with a 17% higher risk of kidney stones (44).
Moreover, the effects of caffeine on kidney stone disease were reanalyzed in 2014 in these 3 large cohorts with >200,000 participants (enrollment of 51,529 men from the HPFS, 121,700 women from the NHS I, and 116,430 women from the NHS II) (76). Regardless of beverage type, this latest mega-data set divided consumers of caffeine into 5 quintiles according to the amount of caffeine consumed per day (Figure 1A). The data showed that the participants who consumed caffeine in the highest quintile (568 ± 185 mg caffeine/d) had a 26%, 29%, and 31% lower risk of stone development in the HPFS, NHS I, and NHS II cohorts, respectively (76) (Figure 1B). On the basis of these collective data from the previously mentioned cohorts, it can be suggested that caffeine consumption is associated with a reduction in kidney stone risk.
FIGURE 1.
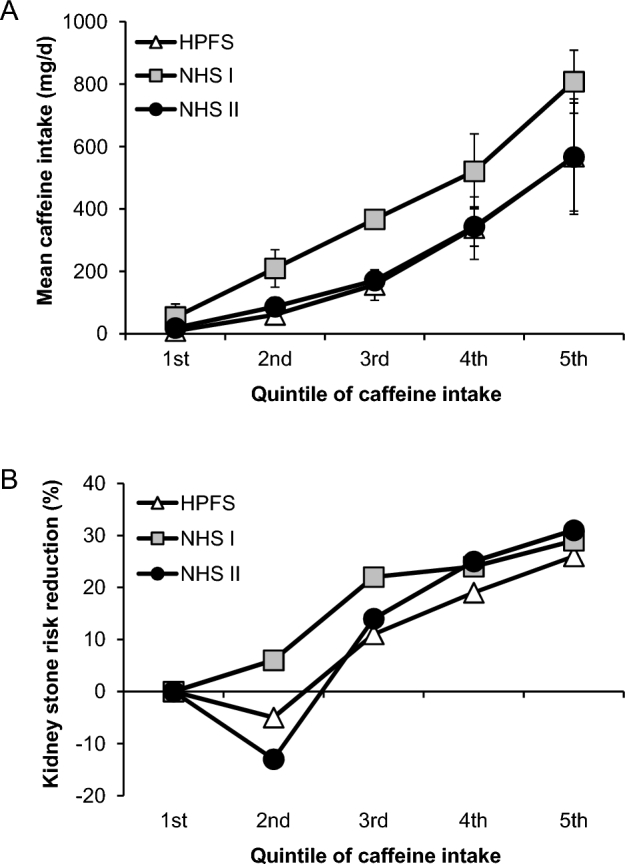
Effect of caffeine dosage on kidney stone risk reduction. (A) Mean caffeine daily intake in each quintile. (B) Percentage of kidney stone risk reduction in each quintile. The data are summarized from the data set of 3 large cohorts with >200,000 participants (enrollment of 51,529 men from the HPFS, 121,700 women from the NHS I, and 116,430 women from the NHS II) reported by Ferraro et al. (76). HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
Although an increase in urinary calcium excretion was found in caffeine consumers in the study by Massey and Sutton (67) in 2004, which had only 39 subjects, the data set from the latest 3 large cohorts with >200,000 participants as previously mentioned showed a decrease in urinary oxalate excretion in the highest-quintile caffeine consumers when compared with the lowest-quintile population (76). In addition, caffeine intake drastically increases the urine volume, which ultimately leads to a significant reduction in net supersaturation of calcium and oxalate ions (60). Moreover, there is a consensus among several studies indicating an elevation in urinary excretion of citrate, one of the most potent CaOx stone inhibitors, in caffeine consumers (69, 70). Overall, the inhibitory factors seem to overcome promoting factors induced by caffeine, leading to a reduction in kidney stone risk.
Investigations of mechanisms underlying the preventive effects of caffeine in kidney stone disease
It is surprising that, to our knowledge, there was no previous in vitro or in vivo study that examined the effects of caffeine on kidney stone formation until 2016, when in vitro evidence of the protective effects of caffeine on CaOx kidney stone formation was reported (45). In that study, caffeine was shown to reduce CaOx crystal adhesion on the apical surface of renal tubular epithelial cells by translocation of a CaOx crystal-binding protein, annexin A1, from apical membranes to cytoplasm (45). This is the only direct evidence that shows cellular mechanisms underlying the inhibitory effects of caffeine against kidney stone disease and strengthens its role as an inhibitor, rather than a promoter, of CaOx kidney stone formation.
Conclusions and Future Perspectives
The ultimate goals of kidney stone management are to reduce new stone formation and to prevent its recurrence after surgical removal. Caffeine is one of the most popular compounds in beverages that are daily consumed worldwide. Three large cohorts have shown the potential of caffeine consumption to reduce kidney stone risk. Moreover, a recent in vitro study showed mechanisms underlying the preventive effects of caffeine against kidney stone formation by translocation of a CaOx crystal-binding protein, annexin A1, from apical membranes to cytoplasm, resulting in a significant reduction of CaOx crystal adhesion on the apical surface of renal tubular epithelial cells. Even with evidence from these in vivo and in vitro studies, more in-depth information and knowledge on the preventive effects of caffeine against kidney stone formation are required. Moreover, the fact that decaffeinated coffee is also associated with a lower risk of kidney stone incidence leads to another hypothesis that other bioactive compounds in coffee (e.g., trigonelline) may also exert similar protective effects against kidney stone disease. Therefore, the field of kidney stone research needs more studies to achieve the ultimate goals of preventing new and recurrent kidney stone formation.
Acknowledgments
Both authors drafted the manuscript, read and approved the final manuscript, and are responsible for all aspects of the manuscript.
Notes
Supported by a Mahidol University research grant and the Thailand Research Fund (IRN60W0004 and IRG5980006). VT is also supported by “Chalermphrakiat” and “Research Staff” grants from the Faculty of Medicine Siriraj Hospital.
Author disclosures: PP and VT, no conflicts of interest.
Abbreviations used:
- CaOx
calcium oxalate
- GFR
glomerular filtration rate
- HPFS
Health Professionals Follow-Up Study
- NHS
Nurses’ Health Study
References
- 1. Campa C, Doulbeau S, Dussert S, Hamon S, Noirot M. Diversity in bean caffeine content among wild coffea species: evidence of a discontinuous distribution. Food Chem 2005;91:633–7. [Google Scholar]
- 2. Ashihara H, Crozier A. Caffeine: a well known but little mentioned compound in plant science. Trends Plant Sci 2001;6:407–13. [DOI] [PubMed] [Google Scholar]
- 3. Casal S, Oliveira MB, Alves MR, Ferreira MA. Discriminate analysis of roasted coffee varieties for trigonelline, nicotinic acid, and caffeine content. J Agric Food Chem 2000;48:3420–4. [DOI] [PubMed] [Google Scholar]
- 4. Nehlig A. Effects of coffee/caffeine on brain health and disease: what should I tell my patients? Pract Neurol 2016;16:89–95. [DOI] [PubMed] [Google Scholar]
- 5. Meeusen R, Roelands B, Spriet LL. Caffeine, exercise and the brain. Nestle Nutr Inst Workshop Ser 2013;76:1–12. [DOI] [PubMed] [Google Scholar]
- 6. Del Brutto OH, Mera RM, Zambrano M. Cardiovascular health and caffeine consumption: a population-based study in rural Ecuador. Int J Cardiol 2014;172:284–5. [DOI] [PubMed] [Google Scholar]
- 7. Temple JL, Ziegler AM, Graczyk A, Bendlin A, Sion T, Vattana K. Cardiovascular responses to caffeine by gender and pubertal stage. Pediatrics 2014;134:e112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zulli A, Smith RM, Kubatka P, Novak J, Uehara Y, Loftus H, Qaradakhi T, Pohanka M, Kobyliak N, Zagatina A, et al. Caffeine and cardiovascular diseases: critical review of current research. Eur J Nutr 2016;55:1331–43. [DOI] [PubMed] [Google Scholar]
- 9. Bolignano D, Coppolino G, Barilla A, Campo S, Criseo M, Tripodo D, Buemi M. Caffeine and the kidney: what evidence right now? J Ren Nutr 2007;17:225–34. [DOI] [PubMed] [Google Scholar]
- 10. Lee J, Ha JH, Kim S, Oh Y, Kim SW. Caffeine decreases the expression of Na+/K+-ATPase and the type 3 Na+/H+ exchanger in rat kidney. Clin Exp Pharmacol Physiol 2002;29:559–63. [DOI] [PubMed] [Google Scholar]
- 11. Luo D, Sun H, Xiao RP, Han Q. Caffeine induced Ca2+ release and capacitative Ca2+ entry in human embryonic kidney (HEK293) cells. Eur J Pharmacol 2005;509:109–15. [DOI] [PubMed] [Google Scholar]
- 12. Trevino MA, Coburn JW, Brown LE, Judelson DA, Malek MH. Acute effects of caffeine on strength and muscle activation of the elbow flexors. J Strength Cond Res 2015;29:513–20. [DOI] [PubMed] [Google Scholar]
- 13. Tazzeo T, Bates G, Roman HN, Lauzon AM, Khasnis MD, Eto M, Janssen LJ. Caffeine relaxes smooth muscle through actin depolymerization. Am J Physiol Lung Cell Mol Physiol 2012;303:L334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghoneim FM, Khalaf HA, Elsamanoudy AZ, Abo El-Khair SM, Helaly AM, Mahmoud HM, Elshafey SH. Protective effect of chronic caffeine intake on gene expression of brain derived neurotrophic factor signaling and the immunoreactivity of glial fibrillary acidic protein and Ki-67 in Alzheimer's disease. Int J Clin Exp Pathol 2015;8:7710–28. [PMC free article] [PubMed] [Google Scholar]
- 15. Dragicevic N, Delic V, Cao C, Copes N, Lin X, Mamcarz M, Wang L, Arendash GW, Bradshaw PC. Caffeine increases mitochondrial function and blocks melatonin signaling to mitochondria in Alzheimer's mice and cells. Neuropharmacology 2012;63:1368–79. [DOI] [PubMed] [Google Scholar]
- 16. Rodrigues TM, Castro CA, Ferreira JJ. Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson's disease: systematic review and meta-analysis. Parkinsonism Relat Disord 2016;27:25–34. [DOI] [PubMed] [Google Scholar]
- 17. Simon DK, Wu C, Tilley BC, Wills AM, Aminoff MJ, Bainbridge J, Hauser RA, Schneider JS, Sharma S, Singer C, et al. Caffeine and progression of Parkinson disease: a deleterious interaction with creatine. Clin Neuropharmacol 2015;38:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Santos RM, Lima DR. Coffee consumption, obesity and type 2 diabetes: a mini-review. Eur J Nutr 2016;55:1345–58. [DOI] [PubMed] [Google Scholar]
- 19. da Silva LA, de Freitas L, Medeiros TE, Osiecki R, Garcia MR, Snak AL, Malfatti CR. Caffeine modifies blood glucose availability during prolonged low-intensity exercise in individuals with type-2 diabetes. Colomb Med (Cali) 2014;45:72–6. [PMC free article] [PubMed] [Google Scholar]
- 20. Omagari K, Sakaki M, Tsujimoto Y, Shiogama Y, Iwanaga A, Ishimoto M, Yamaguchi A, Masuzumi M, Kawase M, Ichimura M, et al. Coffee consumption is inversely associated with depressive status in Japanese patients with type 2 diabetes. J Clin Biochem Nutr 2014;55:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ming Z, Lautt WW. Caffeine-induced natriuresis and diuresis via blockade of hepatic adenosine-mediated sensory nerves and a hepatorenal reflex. Can J Physiol Pharmacol 2010;88:1115–21. [DOI] [PubMed] [Google Scholar]
- 22. Fenton RA, Poulsen SB, de la Mora CS, Soleimani M, Busslinger M, Dominguez Rieg JA, Rieg T. Caffeine-induced diuresis and natriuresis is independent of renal tubular NHE3. Am J Physiol Renal Physiol 2015;308:F1409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Coca A, Casa DJ, Antonio J, Green JM, Bishop PA. Caffeine and diuresis during rest and exercise: a meta-analysis. J Sci Med Sport 2015;18:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vendramini LC, Nishiura JL, Baxmann AC, Heilberg IP. Caffeine intake by patients with autosomal dominant polycystic kidney disease. Braz J Med Biol Res 2012;45:834–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tanner GA, Tanner JA. Chronic caffeine consumption exacerbates hypertension in rats with polycystic kidney disease. Am J Kidney Dis 2001;38:1089–95. [DOI] [PubMed] [Google Scholar]
- 26. Belibi FA, Wallace DP, Yamaguchi T, Christensen M, Reif G, Grantham JJ. The effect of caffeine on renal epithelial cells from patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2002;13:2723–9. [DOI] [PubMed] [Google Scholar]
- 27. Campana C, Griffin PL, Simon EL. Caffeine overdose resulting in severe rhabdomyolysis and acute renal failure. Am J Emerg Med 2014;32:111–4. [DOI] [PubMed] [Google Scholar]
- 28. Bergman EA, Massey LK, Wise KJ, Sherrard DJ. Effects of dietary caffeine on renal handling of minerals in adult women. Life Sci 1990;47:557–64. [DOI] [PubMed] [Google Scholar]
- 29. Barnela SR, Soni SS, Saboo SS, Bhansali AS. Medical management of renal stone. Indian J Endocrinol Metab 2012;16:236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knoll T, Buchholz N, Wendt-Nordahl G. Extracorporeal shockwave lithotripsy vs. percutaneous nephrolithotomy vs. flexible ureterorenoscopy for lower-pole stones. Arab J Urol 2012;10:336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El Husseiny T, Buchholz N. The role of open stone surgery. Arab J Urol 2012;10:284–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srisubat A, Potisat S, Lojanapiwat B, Setthawong V, Laopaiboon M. Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL) or retrograde intrarenal surgery (RIRS) for kidney stones. Cochrane Database Syst Rev 2009;CD007044. [DOI] [PubMed] [Google Scholar]
- 33. Lotan Y. Economics and cost of care of stone disease. Adv Chronic Kidney Dis 2009;16:5–10. [DOI] [PubMed] [Google Scholar]
- 34. Hyams ES, Matlaga BR. Economic impact of urinary stones. Transl Androl Urol 2014;3:278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fink HA, Wilt TJ, Eidman KE, Garimella PS, MacDonald R, Rutks IR, Brasure M, Kane RL, Ouellette J, Monga M. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American College of Physicians Clinical Guideline. Ann Intern Med 2013;158:535–43. [DOI] [PubMed] [Google Scholar]
- 36. Fink HA, Akornor JW, Garimella PS, MacDonald R, Cutting A, Rutks IR, Monga M, Wilt TJ. Diet, fluid, or supplements for secondary prevention of nephrolithiasis: a systematic review and meta-analysis of randomized trials. Eur Urol 2009;56:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qaseem A, Dallas P, Forciea MA, Starkey M, Denberg TD. Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2014;161:659–67. [DOI] [PubMed] [Google Scholar]
- 38. Curhan GC, Willett WC, Rimm EB, Spiegelman D, Stampfer MJ. Prospective study of beverage use and the risk of kidney stones. Am J Epidemiol 1996;143:240–7. [DOI] [PubMed] [Google Scholar]
- 39. Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med 1998;128:534–40. [DOI] [PubMed] [Google Scholar]
- 40. Goldfarb DS, Coe FL. Beverages, diet, and prevention of kidney stones. Am J Kidney Dis 1999;33:398–400. [DOI] [PubMed] [Google Scholar]
- 41. Passman CM, Holmes RP, Knight J, Easter L, Pais V, Assimos DG. Effect of soda consumption on urinary stone risk parameters. J Endourol 2009;23:347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gasinska A, Gajewska D. Tea and coffee as the main sources of oxalate in diets of patients with kidney oxalate stones. Rocz Panstw Zakl Hig 2007;58:61–7. [PubMed] [Google Scholar]
- 43. Massey LK, Whiting SJ. Caffeine, urinary calcium, calcium metabolism and bone. J Nutr 1993;123:1611–4. [DOI] [PubMed] [Google Scholar]
- 44. Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Soda and other beverages and the risk of kidney stones. Clin J Am Soc Nephrol 2013;8:1389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Peerapen P, Thongboonkerd V. Caffeine prevents kidney stone formation by translocation of apical surface annexin A1 crystal-binding protein into cytoplasm: in vitro evidence. Sci Rep 2016;6:38536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schubert G. Stone analysis. Urol Res 2006;34:146–50. [DOI] [PubMed] [Google Scholar]
- 47. Hecimovic I, Belscak-Cvitanovic A, Horzic D, Komes D. Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem 2011;129:991–1000. [DOI] [PubMed] [Google Scholar]
- 48. Fulgoni VL III, Keast DR, Lieberman HR. Trends in intake and sources of caffeine in the diets of US adults: 2001–2010. Am J Clin Nutr 2015;101:1081–7. [DOI] [PubMed] [Google Scholar]
- 49. Ahluwalia N, Herrick K. Caffeine intake from food and beverage sources and trends among children and adolescents in the United States: review of national quantitative studies from 1999 to 2011. Adv Nutr 2015;6:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rudolph E, Faerbinger A, Koenig J. Caffeine intake from all sources in adolescents and young adults in Austria. Eur J Clin Nutr 2014;68:793–8. [DOI] [PubMed] [Google Scholar]
- 51. Mackus M, van de Loo AJ, Benson S, Scholey A, Verster JC. Consumption of caffeinated beverages and the awareness of their caffeine content among Dutch students. Appetite 2016;103:353–7. [DOI] [PubMed] [Google Scholar]
- 52. Drewnowski A, Rehm CD. Sources of caffeine in diets of US children and adults: trends by beverage type and purchase location. Nutrients 2016;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev 1999;51:83–133. [PubMed] [Google Scholar]
- 54. Lang R, Dieminger N, Beusch A, Lee YM, Dunkel A, Suess B, Skurk T, Wahl A, Hauner H, Hofmann T. Bioappearance and pharmacokinetics of bioactives upon coffee consumption. Anal Bioanal Chem 2013;405:8487–503. [DOI] [PubMed] [Google Scholar]
- 55. Tassaneeyakul W, Birkett DJ, McManus ME, Tassaneeyakul W, Veronese ME, Andersson T, Tukey RH, Miners JO. Caffeine metabolism by human hepatic cytochromes P450: contributions of 1A2, 2E1 and 3A isoforms. Biochem Pharmacol 1994;47:1767–76. [DOI] [PubMed] [Google Scholar]
- 56. Ludwig IA, Clifford MN, Lean ME, Ashihara H, Crozier A. Coffee: biochemistry and potential impact on health. Food Funct 2014;5:1695–717. [DOI] [PubMed] [Google Scholar]
- 57. Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb Exp Pharmacol 2011;200:33–91. [DOI] [PubMed] [Google Scholar]
- 58. Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther 2005;313:403–9. [DOI] [PubMed] [Google Scholar]
- 59. Osswald H, Schnermann J. Methylxanthines and the kidney. Handb Exp Pharmacol 2011;200:391–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Peerapen P, Ausakunpipat N, Sutthimethakorn S, Aluksanasuwan S, Vinaiphat A, Thongboonkerd V. Physiologic changes of urinary proteome by caffeine and excessive water intake. Clin Chem Lab Med 2017;55:993–1002. [DOI] [PubMed] [Google Scholar]
- 61. Tofovic SP, Branch KR, Oliver RD, Magee WD, Jackson EK. Caffeine potentiates vasodilator-induced renin release. J Pharmacol Exp Ther 1991;256:850–60. [PubMed] [Google Scholar]
- 62. Tseng CJ, Kuan CJ, Chu H, Tung CS. Effect of caffeine treatment on plasma renin activity and angiotensin I concentrations in rats on a low sodium diet. Life Sci 1993;52:883–90. [DOI] [PubMed] [Google Scholar]
- 63. Tofovic SP, Kusaka H, Rominski B, Jackson EK. Caffeine increases renal renin secretion in a rat model of genetic heart failure. J Cardiovasc Pharmacol 1999;33:440–50. [DOI] [PubMed] [Google Scholar]
- 64. Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol 2016;310:H137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Echeverri D, Montes FR, Cabrera M, Galan A, Prieto A. Caffeine's vascular mechanisms of action. Int J Vasc Med 2010;2010:834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ohnishi A, Branch RA, Jackson K, Hamilton R, Biaggioni I, Deray G, Jackson EK. Chronic caffeine administration exacerbates renovascular, but not genetic, hypertension in rats. J Clin Invest 1986;78:1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Massey LK, Sutton RA. Acute caffeine effects on urine composition and calcium kidney stone risk in calcium stone formers. J Urol 2004;172:555–8. [DOI] [PubMed] [Google Scholar]
- 68. Tiselius HG, Larsson L. Biochemical evaluation of patients with urolithiasis. Eur Urol 1981;7:31–4. [DOI] [PubMed] [Google Scholar]
- 69. Escribano J, Balaguer A, Pagone F, Feliu A, Roque IF. Pharmacological interventions for preventing complications in idiopathic hypercalciuria. Cochrane Database Syst Rev 2009;CD004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tracy CR, Pearle MS. Update on the medical management of stone disease. Curr Opin Urol 2009;19:200–4. [DOI] [PubMed] [Google Scholar]
- 71. Caprioli G, Cortese M, Maggi F, Minnetti C, Odello L, Sagratini G, Vittori S. Quantification of caffeine, trigonelline and nicotinic acid in espresso coffee: the influence of espresso machines and coffee cultivars. Int J Food Sci Nutr 2014;65:465–9. [DOI] [PubMed] [Google Scholar]
- 72. Pino-Garcia R, Gonzalez-SanJose ML, Rivero-Perez MD, Muniz P. Influence of the degree of roasting on the antioxidant capacity and genoprotective effect of instant coffee: contribution of the melanoidin fraction. J Agric Food Chem 2012;60:10530–9. [DOI] [PubMed] [Google Scholar]
- 73. Arai K, Terashima H, Aizawa S, Taga A, Yamamoto A, Tsutsumiuchi K, Kodama S. Simultaneous determination of trigonelline, caffeine, chlorogenic acid and their related compounds in instant coffee samples by HPLC using an acidic mobile phase containing octanesulfonate. Anal Sci 2015;31:831–5. [DOI] [PubMed] [Google Scholar]
- 74. McCusker RR, Fuehrlein B, Goldberger BA, Gold MS, Cone EJ. Caffeine content of decaffeinated coffee. J Anal Toxicol 2006;30:611–3. [DOI] [PubMed] [Google Scholar]
- 75. McCusker RR, Goldberger BA, Cone EJ. Caffeine content of energy drinks, carbonated sodas, and other beverages. J Anal Toxicol 2006;30:112–4. [DOI] [PubMed] [Google Scholar]
- 76. Ferraro PM, Taylor EN, Gambaro G, Curhan GC. Caffeine intake and the risk of kidney stones. Am J Clin Nutr 2014;100:1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]


