Abstract
The male germ line is capable of transmitting a legacy of stress exposure to the next generation of offspring. This transgenerational process manifests by altering offspring affective behaviours, cognition and metabolism. Paternal early life trauma causes hippocampal serotonergic dysregulation in male offspring. We previously showed a transgenerational modification to male offspring anxiety-like behaviours by treatment of adult male breeders with corticosterone (CORT) prior to mating. In the present study, we used offspring from our paternal CORT model and characterised offspring serotonergic function by examining their responses to the 5HT1AR agonist, 8-OH-DPAT, and the selective serotonin reuptake inhibitor, sertraline. We also examined whether post-weaning environmental enrichment, a paradigm well-known to modulate serotonergic signalling in the brain, had the capacity to normalise the anxiety phenotype of male offspring. Finally, we assessed gene expression levels of 5HT1AR and serotonin transporter in the offspring hippocampus to determine whether deficits in gene transcription contributed to the male-only anxiety phenotype. We report that male and female offspring of CORT-treated fathers are hypersensitive to sertraline but have normal hypothermic responses to 8-OH-DPAT. No deficits in htr1a and sert were found in association with paternal CORT treatment, and environmental enrichment did not rescue the anxiety phenotype of male offspring on the elevated-plus maze. These findings indicate that varying forms of paternal stress exert different effects on offspring brain serotonergic function.
Keywords: brain disorders, epigenetic inheritance, environment enrichment, psychiatric disorders, stress, transgenerational epigenetics
Introduction
There is mounting epidemiological and preclinical evidence to indicate that paternal stress influences the mental health status of offspring, even when experienced prior to their conception. Several mouse models have been established to facilitate our understanding of this transgenerational phenomenon [1–4]. In a model of early life trauma involving maternal separation combined with unpredictable maternal stress (MSUS), the progeny of MSUS males exhibit reduced anxiety behaviours [5]. Our group developed a chronic corticosterone (CORT)-supplementation model of stress exposure and discovered transgenerational modifications to offspring anxiety behaviours, particularly in the male offspring [3]. Interestingly, we observed elevated anxiety levels in the F1 male offspring which is in contrast to the anxiolytic outcome in the MSUS model. This suggests that the type of exposure related to stress elicits differentiable transgenerational outcomes on offspring phenotypes, further necessitating careful studies of the underlying molecular pathologies in the offspring brains.
In this study, we sought to identify sex-specific transgenerational responses to paternal CORT-supplementation in the offspring brains. We elected to examine aspects of serotonin signalling since dysregulation of hippocampal serotonergic signalling has long been implicated in anxiety disorders [6, 7]. Allelic variation in the 5HT-1A receptor gene, which dictates differences in expression, is associated with the development of anxiety- and depression-related personality traits [8]. Panic disorder patients have impaired 5-HT1AR function as reflected by their sub-sensitive hypothermic and cortisol responses to the partial 5-HT1AR receptor agonist ipsapirone [9]. Furthermore, two functional imaging studies revealed decreased 5-HT1AR binding in patients with untreated panic disorder [10, 11]. Consistent with those findings, 5-HT1AR knockout mice displayed increased anxiety behaviour manifesting as decreased exploratory behaviour in the open field and reduced open-arm time on the elevated-plus maze [12], similar to the behavioural phenotype of male offspring in our paternal CORT model. In the MSUS model, adult male and female offspring have reduced levels of 5-HT1AR autoradiography binding in the dorsal raphe, CA1 and dentate gyrus regions of the hippocampus [13]. Additionally, offspring have significantly altered fMRI responses to the 5-HT1AR agonist 8-OH-DPAT; especially within the broader cortical regions and amygdala [13]. Interestingly, paternal MSUS exposure was associated with altered 5-HT1AR-evoked fMRI responses in the male offspring only, and was not observed for female offspring.
Accordingly, we examined serotonergic function of the F1 offspring in our paternal CORT model with a combination of acute pharmacological challenges, environmental manipulation, and gene expression profiling. We assessed their 5-HT1AR-mediated hypothermic response to acute 8-OH-DPAT administration, then evaluated their behavioural response to the selective serotonin reuptake inhibitor (SSRI) sertraline in the forced-swim test. We examined whether environmental enrichment, a paradigm we had reported as exerting positive behavioural effects in a range of mouse models of neurological conditions [14–16], could normalise the anxiety phenotype of male offspring on the elevated-plus maze. The light-dark box and novelty-suppressed feeding tests were conducted as supplementary tests of anxiety-related behaviours. Finally, we characterised the gene expression profile of the 5-HT1AR and 5-HTT in the offspring hippocampus, differentiating between the ventral and dorsal aspects of the hippocampus since both are well-established to regulate different phenotypic domains—the ventral hippocampus is involved in emotional processing while the dorsal hippocampus is implicated in cognitive function [17].
Materials and Methods
Animals
Male and female C57Bl/6 mice (8 weeks) were purchased from the Animal Resources Centre (Murdoch, WA) to be used as F0 breeders. All animals were maintained in a temperature and humidity-controlled facility on a 12 h light/dark cycle with food and water ad libitum. After 1 week of habituation, male mice were single-housed and randomly assigned to receiving CORT-supplementation (25 mg/l; Steraloids Inc, RI, USA) for 6 weeks or maintained on untreated water (control group). CORT-supplemented water was fresh prepared and replaced every 3 days. Following treatment, CORT-treated and control males were pair-mated for 5 days. Females were then separated and single-housed until they littered down. At 3 weeks of age, F1 offspring mice were weaned into social groups (4–6 mice/cage) comprised of animals from the same paternal treatment condition but from different dams to avoid litter effects (designated patCORT and control groups). F1 mice were raised undisturbed (except for cage changes) until 10 weeks of age when behavioural testing was conducted. All boxes were provided with sawdust bedding and two tissues for nesting material and changed weekly. All experimental work was approved by the Florey Institute of Neuroscience and Mental Health animal ethics committee and conducted in accordance with NHMRC guidelines.
Environmental Enrichment
Mice allocated to environmental enrichment were group housed in larger cages (measuring 25 × 38 × 25 cm) with raised lids. These cages were supplemented with at least one plastic nesting box, plastic tunnels, toy ladders, objects of various shapes and textures, and shredded paper as additional nesting material. Items in the cages were rearranged periodically and replaced with clean novel objects during the weekly cage change. Our enrichment paradigm excludes the provision of running wheels. This approach to environmental enrichment has been used by us to successfully demonstrate beneficial modulation of physical and behavioural symptoms in a variety of mouse models of neurological conditions [14–16, 18] and gene-edited mouse models of anxiety and depression [19, 20].
8-OH-DPAT-Induced Hypothermia
As previously described [21], basal values were determined just before subcutaneous injection of the 5-HT1A receptor agonist 8-OH-DPAT (0.3 mg/kg) or vehicle (0.9% NaCl, 1 ml/100 g body weight), and body temperature was measured every 30 and 60 min thereafter. The response to 8-OH-DPAT was calculated as the decrease (from baseline) in body temperature during 60-min post injection. All temperatures were measured at ambient temperature (21 ± 1°C).
Acute Antidepressant Effects of Sertraline
Sertraline (Pfizer Inc, CT, USA) was administered at a dose of 25 mg/kg i.p. 30 min prior to the forced-swim test. The FST was conducted as previously described [3]. Each session lasted for 300 s and was videorecorded. Unbiased analysis of total immobility time over the final 240 s was performed using the ForcedSwimScan (CleverSys Inc, VA, USA).
Behavioural Testing
Mice were tested on a series of behavioural tests to assess anxiety with at least 24 h between each test. In all cases, experimenters were blind to treatment and behaviours were monitored by direct observation with automated video-tracking utilised for unbiased analysis of behavioural responses. Mice were acclimatised to the testing rooms for at least 1 h prior to the commencement of any test. Behavioural experimentation was conducted between 0900 and 1300H.
Elevated-Plus Maze
EPM testing was performed as previously reported [3]. Room lighting was ∼175 Lux and lighting within the maze was ∼15 Lux with a curtain separating the maze from the experimenter. Each test session was initiated when a mouse was detected by the Topscan tracking software (CleverSys Inc, VA, USA) to be within the central zone facing a closed arm. The maze was thoroughly wiped down with 80% ethanol after every session to eliminate residual odours. Each session lasted 5 min and the total time spent in the open arms and total distance travelled was measured.
Light-Dark Box
LDB testing (ENV-511, Med Associates, VT, USA) was performed as previously reported [3]. The aversive half of the test arena was illuminated to 700 Lux with white LED lighting. Each test session lasted 10 min and the time spent in each zone was tabulated along with total distance travelled. The arenas were thoroughly cleaned with disinfectant to eliminate odours before commencing the next test session.
Novelty-Suppressed Feeding Test
The NSFT was performed as previously published [22]. Briefly, mice were food deprived for 48 h with 1 h of food access following the first 24 h of food deprivation. Body weights prior to food deprivation and on the day of the test were recorded. The NSFT was conducted in an 80x80x80cm arena lined with 1 cm of saw dust and containing a single food pellet in the centre. Each test session was initiated by placing a mouse in a random corner of the test arena. The number of centre entries (within a 5 cm radius of the food pellet) and the time for each mouse to commence feeding on the pellet were recorded. Mice which did not start feeding within 10 min were excluded from final data analysis. The mouse was immediately removed and placed into a separate cage containing a pre-weighed food pellet for a further 5 min. These pellets were collected and weighed to determine post-test food consumption.
Gene Expression Profiling
A separate cohort of 12-week-old F1 mice, which had only been tested on the EPM and LDB to confirm the F1 behavioural phenotype, were killed by cervical dislocation 24 h after the LDB. Brain dissections were conducted on ice in order to dissociate the hippocampus which was then bisected into the dorsal and ventral halves. These were frozen in dry ice and stored at −80°C until subsequent use. Total RNA was isolated using QIAzol lysis reagent (QIAGEN, VIC, Australia) according to the manufacturers’ instructions. Tissue was disrupted with a Diagenode UCD-300 Bioruptor (Life Research, VIC, Australia) for 6 cycles (30 s on/30 s off) on low setting. RNA concentrations were determined using Nanodrop 2000c spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and only samples with A260/280 ratios ranging 1.7–2.2 were used for cDNA synthesis. RNA quality was verified using Agilent 2100 Bioanalyser Nanochips and all samples were determined to be of high quality with RIN values >9.0. 1000 ng of total RNA was reverse transcribed into cDNA using Superscript VILO cDNA synthesis kit (Invitrogen, Life Technologies, VIC Australia) with a TaKaRa PCR thermal cycler (Takara Shuzo, Tokyo Japan). PCR conditions were as follows: 10 min @ 25°C, 30 min @48°C and 9 min @ 95°C. No enzyme and no template controls were included. cDNA products were diluted 1/10 then stored at −20°C for subsequent use. Semi-quantitative PCR was performed on a ViiA7 real-time PCR system (Applied Biosystems, Carlsbad, CA, USA) using the standard program as specified. 5 μl of cDNA was used in a final reaction volume of 20 μl together with SYBR green (Sigma-Aldrich, Castle Hill, NSW, Australia). Primer sequences and optimal working dilutions were as previous published [15]. Each biological replicate was run in triplicate for the genes of interest and with cyclophilin as the endogenous reference gene. The comparative Ct method (ΔΔCt) was used to determine the relative expression levels of target genes.
Statistical Analysis
All statistical analyses were performed using Graphpad Prism 7.0 (Graphpad software Inc, La Jolla, CA, USA). Male and female data sets were analysed separately due to the many instances of sex-specific effects observed in various paternal stress models. For the 8-OH-DPAT-induced hypothermia experiment, individual data were transformed and expressed as area under curve (AUC) and then analysed by a 2-way ANOVA for potential effects of sex and paternal CORT. Acute SSRI response was analysed by 2-way ANOVA for effects of paternal treatment and drug, followed by post-hoc Tukey’s multiple comparisons test. One-way ANOVA was used to analyse the elevated-plus maze, novelty-suppressed feeding test and forced-swim test data. Where an overall effect was found, post-hoc Bonferroni t-tests were performed to establish between-group differences. qPCR data was analysed by one-way ANOVA with multiple comparisons corrected for False Discovery Rate using the two-stage step-up correction method of Benjamini, Krieger and Yekutieli. All data sets obtained met the requirements for parametric statistical testing. In all analyses, the level of statistical significance was set at α = 0.05.
Results
Experiment 1: Physiological and Behavioural Response to Acute Pharmacological Challenges
Hypothermic Response to Acute 8-OH-DPAT Challenge
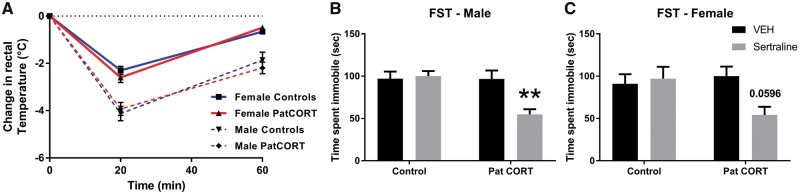
Acute injection with the 5-HT1AR agonist 8-OH-DPAT decreased body temperature in all 4 groups (Fig. 1A). While the AUC analysis of the 60 min post-injection period revealed an overall significant sex effect (F(1, 36) = 54.6, P < 0.001), there was no effect associated with paternal CORT-treatment (F(1, 36) = 0.08, P = 0.79).
Figure 1:
physiological and behavioural responses to acute pharmacological challenges. Offspring 8-OH-DPAT induced hypothermia (A). Male (B) and female (C) acute SSRI response in FST. 2-way ANOVA, post-hoc Tukey’s multiple comparisons test ** P < 0.01
Behavioural Response to Acute Sertraline Challenge in the Forced-Swim Test
For male offspring (Fig. 1B), two-way ANOVA revealed that there were significant overall effects of paternal CORT-treatment (F(1, 29) = 8.854, P = 0.006) and sertraline/vehicle (F(1, 28) = 6.431, P = 0.0171), as well as a significant Pat CORT-treatment × drug interaction (F(1, 28) = 8.71, P = 0.0063). Post-hoc analysis revealed that while this dose of sertraline did not significantly alter forced-swim test response of control offspring (P = 0.9909), it was sufficient to reduce immobility time of PatCORT offspring compare to their respective vehicle-treated counterparts (P < 0.01).
For female offspring (Fig. 1C), there was a significant PatCORT × drug interaction (F(1, 25) = 4.778, P = 0.0384) but neither paternal CORT-treatment (F(1, 25) = 2.003, P = 0.1693) nor sertraline-treatment (F(1, 25) = 2.779, P = 0.108) were significant on their own. Post-hoc analysis confirmed that this dose of sertraline did not modify FST behaviour of control mice (P = 0.981) but there was a trend (P = 0.0596) for sertraline to reduce immobility time of female PatCORT offspring similar to the males.
Experiment 2: Attempting to Modulate Offspring Anxiety through Environmental Enrichment
Elevated-Plus Maze and Light-Dark Box Testing
These initial behavioural results are consistent with paternal stress contributing to altered serotonergic function in their offspring. To broaden our understanding of the behavioural consequences of this pathophysiology, we investigated how PatCORT offspring would respond to environmental enrichment, a paradigm which our group has successfully used as a non-pharmacological approach to modulating serotonergic function and its related behaviours [15, 16].
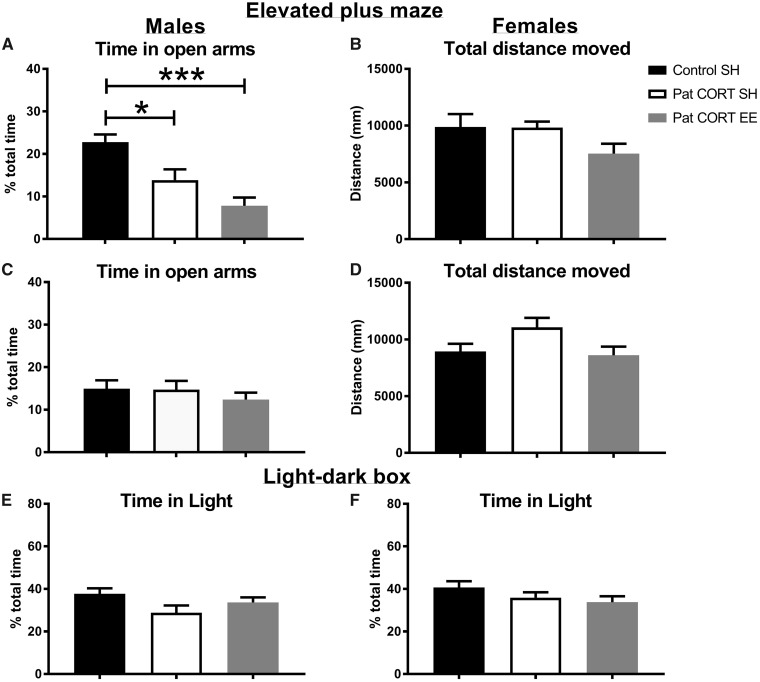
One-way ANOVA revealed an overall effect of treatment on the time that male mice spent in the open arms of the elevated-plus maze (F(2, 32) = 13.74, P < 0.0001) (Fig. 2A). Post-hoc analyses showed that, consistent with our published findings, the PatCORT standard-housed (SH) male offspring spent significantly less time in the open arms of the maze compared to control offspring (P = 0.0210). Environmentally enriched (EE) PatCORT male offspring also spent significantly less time on the open arms compared to the control group (P < 0.001). There was no significant effect of treatment on the total distance travelled by male offspring during the test (F(2, 32) = 2.545, P = 0.0942) (Fig. 2B). One-way ANOVA found no overall effect of treatment on the time female offspring spent on the open arms of the maze (F(2, 36) = 0.554, P = 0.5795) (Fig. 2C). There was also no significant difference in the distance travelled by female offspring (F(2, 37) = 2.974, P = 0.0634) (Fig. 2D).
Figure 2:
offspring performance in tests of anxiety. Male offspring of CORT-treated fathers housed in SH and EE spent significantly less time in the open arms of the maze as compared to the male offspring of control males (A). There were no differences in the overall distance travelled (B). There was no effect of paternal CORT treatment and offspring environmental enrichment on time spent in the open arms of the maze (C) and overall distance travelled by the female offspring (D). Times spent by male (E) and female (F) offspring in the lit half (E) of the light-dark apparatus. n = 11–21 per group. Data presented as mean ± SEM; one-way ANOVA, post-hoc Bonferroni’s t-test * P < 0.05, ***P < 0.001
For the light-dark test, one-way ANOVA reported no significant differences in the time male mice spent in the light half of the LDB (F(2, 49) = 2.492, P = 0.0932; Fig. 2E). There was no significant difference in the distance moved by male offspring during the session (F(2, 49) = 2.47, P = 0.0950). There was also no significant difference in time spent in the light half by female offspring (F(2, 52) = 1.547, P = 0.2225; Fig. 2F) nor in their total distance moved (F(2, 52) = 0.715, P = 0.4939).
Novelty-Suppressed Feeding Test
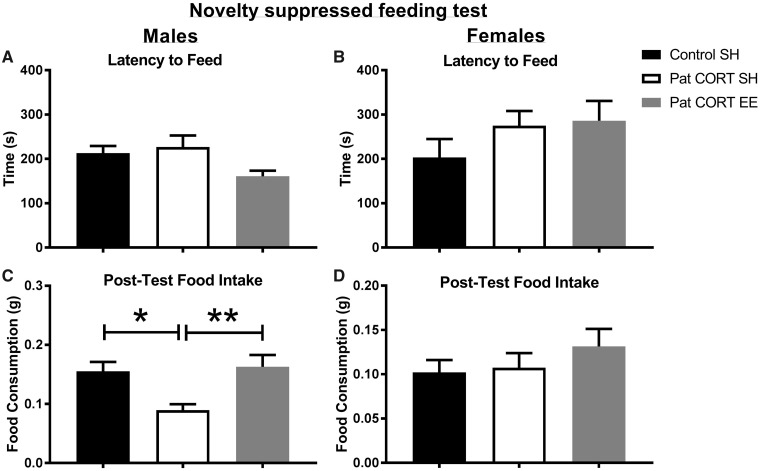
Pre-testing food deprivation resulted in similar weight loss across all groups. On average, males lost 13% and females lost 14% of body weight. One-way ANOVA revealed no significant between-group differences for male (F(2, 22)=1.109, P = 0.3477) and female offspring (F(2, 22)=1.034, P = 0.3723). One-way ANOVA revealed no significant between groups differences in the mean latency to commence feeding for both male (F(2, 22) = 2.811, P = 0.0818) (Fig. 3A) and female offspring (F(2, 22) = 1.243, P = 0.3079) (Fig. 3B).
Figure 3:
effect of paternal CORT and offspring environmental enrichment on offspring behaviour in novelty suppressed feeding test. There were no significant differences between percentage body weight loss amongst the three different treatment groups in both males and females (A). No significant differences were found in the latency to feed, in both male groups (B) and female groups (C). Male offspring of CORT-treated fathers housed in standard housing had a significantly lower food intake post novelty suppressed feeding test, as compared to male offspring of control males, as well as, environmentally enriched male offspring of CORT-treated fathers (D). No differences in post-test food intake were found between the three female treatment groups (E). n = 7–10 mice per treatment group. Data presented as mean ± SEM; one-way ANOVA, post-hoc Bonferroni’s t-test *P < 0.05, **P < 0.01
Interestingly, one-way ANOVA indicated a significant between group difference in post-test food consumption for males (F(2, 21) = 8.214, P = 0.0023) (Fig. 3C). Post-hoc analysis established that male PatCORT SH offspring had a significantly lower food intake compared to the control (P < 0.05) and PatCORT EE groups (P < 0.01). There was no difference in post-test food consumption for females (F(2, 21) = 0.807, P = 0.4596) (Fig. 3D).
Experiment 3: Gene Expression Profiling
Htr1A Expression in Dorsal and Ventral Hippocampus
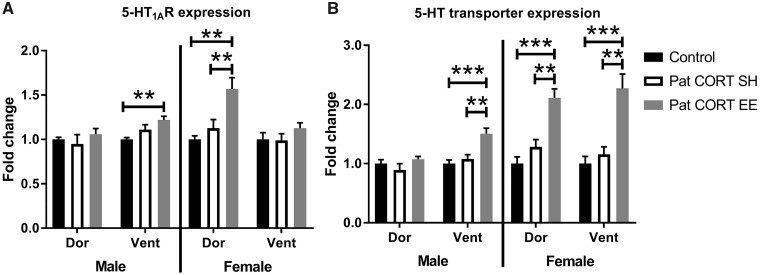
One-way ANOVA of htr1A expression in the dorsal hippocampus of male offspring revealed no significant difference between the groups (F(2, 10) = 0.6136, P = 0.5606) (Fig. 4A). A significant difference was detected for the ventral hippocampus (F(2, 10) = 6.993), P = 0.0126). Post-hoc analysis revealed that htr1A expression for the PatCORT EE offspring was significantly greater compared to the control group (P < 0.01).
Figure 4:
Htr1a and SerT expression patterns in F1 offspring hippocampus. Relative gene expression normalised to the mean fold-change of the respective control group within each region and presented as mean ± SEM. n = 4–6 per group. Data analysed with one-way ANOVA, followed by post-hoc t-tests with FDR corrections **P < 0.01, ***P < 0.001
A significant difference between groups was detected for htr1A expression in the dorsal hippocampus of female offspring (F(2, 11) = 12.57, P = 0.0014; Fig. 4A). Post-hoc analysis conveyed that there was a significant increase in htr1A expression in the dorsal hippocampus of PatCORT EE offspring compared to both the control (P < 0.01) and PatCORT SH groups (P < 0.01). In the ventral hippocampus, no difference in htr1A expression was detected for the female offspring (F(2, 11) = 0.9988, P = 0.3994).
SerT Gene Expression in the Dorsal and Ventral Offspring Hippocampus
No significant difference in SerT gene expression was detected within the dorsal hippocampus (F(2, 10) = 1.571, P = 0.2552; Fig. 4B) of male offspring. In contrast, there was a significant difference for the ventral hippocampus (F(2, 10) = 16.99, P < 0.001). Post-hoc analysis indicated greater SerT expression for the PatCORT EE group compared to the control (P < 0.001) and PatCORT SH groups (P < 0.01).
For female offspring, one-way ANOVA detected an overall difference in SerT expression in the dorsal hippocampus (F(2, 11)=20.6, P < 0.001; Fig. 4B). Post-hoc analysis indicated greater SerT expression for the PatCORT EE group compared to both the control (P < 0.001) and PatCORT SH groups (P < 0.01). Similarly, there were also significant between groups differences detected for the ventral hippocampus (F(2, 11) = 16.74, P < 0.001) with greater SerT expression for the PatCORT EE group compared to the control (P < 0.001) and PatCORT SH groups (P < 0.01).
Discussion
This study confirms that brain serotonergic function is subject to transgenerational influences, especially paternal exposures related to stress. The novel findings that male and female PatCORT offspring display hypersensitivity to sertraline in combination with their unaltered hypothermic responses to 8-OH-DPAT enables some level of distinction between the various serotonin receptors and brain regions which underlie the male-specific anxiety phenotype in the present model of paternal corticosterone treatment. These finding are further evidence that the transgenerational impact of paternal stress on offspring brain function is specific to the type and/or severity of the stress experienced. Unexpectedly, environmental enrichment failed to improve anxiety levels of the male F1 offspring, in spite of increased 5-HT1AR and 5-HTT expression in the hippocampus. EE exerts robust anxiolytic effects across a variety of rodent models of neurological conditions and the absence of a behavioural modification here could reflect an underlying impairment to the capacity for experience-dependent plasticity in the brains of PatCORT offspring.
Sex-specific alterations of offspring anxiety have been reported by our group [3] and others [1, 5, 23] in separate and distinct mouse models of paternal stress. The fact that anxiety behaviour is consistently affected indicates that the transgenerational response to paternal stress is mediated by common molecular systems in all of these mouse models of paternal stress. However, because the outcomes of the behavioural modifications are different (reduced anxiety for male MSUS offspring compared to increased anxiety for male PatCORT offspring), therefore it is likely that the implicated brain regions and signalling pathways are subtly different. Franklin et al. reported reduced 8-OH-DPAT radioligand binding to 5-HT1AR in a range of brain regions of male MSUS offspring such as the hippocampus CA1 subfield, dorsal raphe and lateral periaqueductal grey but not within the cingulate and motor cortices [24]. It is well-accepted that impaired 5HT1AR-mediated signalling drives anxiety behaviour and deficits in social interaction [25–27]. PET studies on unmedicated patients with social anxiety disorders have revealed reduced 5-HT1AR binding in several limbic and paralimbic brain regions including the anterior cingulate cortex and dorsal raphe nuclei, although not in the hippocampus [28, 29]. Based on the collective strength of that evidence, our a priori approach prioritised an examination of hippocampal serotonin function in an attempt to identify the origins of the anxious tendencies of male PatCORT offspring. However, despite having found no differences in htr1a or sert expression within the dorsal and ventral aspects of the hippocampus that correlated with a male-specific anxiety phenotype, it is possible that there are post-transcriptional changes in 5-HT1AR and/or 5-HTT protein levels, ligand binding, and signalling activity. Further studies are required to examine those brain regions identified by other studies. For example, anxiety disorders often encompass altered serotonergic signalling in the medial prefrontal cortex [30, 31]. Variation in the promoter region of the 5-HTT gene and its contribution to stress sensitivity has been widely studied in human cohort studies, non-human primates and rodents. Certain 5-HTT polymorphisms that result in a greater stress sensitivity also lead to altered functional coupling of the medial prefrontal cortex with the amygdala [32, 33].
5-HT1AR autoreceptors in the dorsal raphe nucleus regulate the marked hypothermic response to 8-OH-DPAT (unchanged for PatCORT offspring) which in turn modulates serotonergic turnover in the frontal cortex and hippocampus [34–36]. Dorsal raphe 5-HT1A autoreceptors are also known to regulate SSRI responsivity, specifically to increase sensitivity [37]. That is consistent with human studies which implicate a polymorphism in the htr1a gene promoter with increased susceptibility for depression and variable SSRI treatment response. Additionally, due to their weaker affinity binding, other receptors such as the 5-HT7R, α1/α2-adrenoreceptors and dopamine D2 receptors could partially contribute to the significantly impaired 8-OH-DPAT-evoked fMRI responses detected in the hippocampus of male MSUS offspring [13]. 8-OH-DPAT-induced modification of social behaviour would also be a priority for future investigation [38–40] since social preference (novelty) and social discrimination (memory) are both reportedly impaired in male MSUS offspring [24].
This initial attempt to profile the expression of serotonin receptors in the hippocampus was supported by their strong implication in anxiety pathology, as well as convincing evidence that environmental modulation of serotonergic neurotransmission is a crucial process for the anxiolytic and anti-depressive behavioural benefits of EE [19, 41–45], even in a model of antidepressant-resistant PTSD [46]. The beneficial anxiolytic action of EE is not only observable in rodent models of stress, but in environmentally enriched wild-type mice as they show decreased anxiety-related behaviour on the elevated-plus maze and the light-dark box tests [20, 47, 48]. Thus it was surprising that environmental stimulation, in the form of complex housing configurations, failed to impart any observable anxiolytic effect on male F1 PatCORT offspring. One possibility is that a transgenerational response to paternal exposures related to stress is the development of resistance to the benefits of environmental stimulation, perhaps reflecting an underlying impairment of neuronal and glial plasticity processes. One key cellular event which EE stimulates is increased adult hippocampal neurogenesis [49], and for transgenic mice in which hippocampal neurogenesis is specifically disrupted, the capacity for EE to rescue social defeat-stress induced anxiety and depressive behaviours is abolished [45]. Several studies have demonstrated that the behavioural benefits associated with an up-regulation of hippocampal neurogenesis is regulated by serotonergic neurotransmission [50–52]. However, our laboratory and others have also demonstrated that the anxiolytic effects of EE can manifest in spite of disruptions to 5-HT1AR-mediated signalling [20] or could occur through neurogenesis-independent processes [53, 54]. Findings from this study provide new evidence that anxiety-like behaviours can manifest in the absence of dysregulations of hippocampal serotonin receptor expression; however further investigation regarding the status of adult hippocampal neurogenesis in the PatCORT offspring is necessary. The novel finding of EE having no effect when applied to the F1 generation is an interesting contrast to other transgenerational studies that have implemented EE as an intervention. Particularly, the distinction arises from the fact that upon the implementation of EE in the F0 generation, a study by Gapp et al. (2016) [55] reported a rescue of the F1 phenotype in their model of transgenerational paternal stress. The immediate interpretation of the collective findings of the current study and the study conducted by Gapp et al. [55] suggest that the consequences of transgenerational stress need to be addressed in the paternal generation that is experiencing a certain detrimental lifestyle factor. Hence, it will be of interest to investigate the effects of EE when implemented in the paternal generation in our model.
However, the effectiveness of EE as an intervention in one model of paternal stress does not necessitate its effectiveness in a starkly different model, with differing severity of stress and the age of stress exposure. Gapp et al. [5] have reported that their model of early life trauma affects avoidance behaviours and learning in aversive environments in both the exposed fathers and their offspring. In contrast, our model of chronic adult generalized stress does not cause any behavioural changes in the exposed males and results in a comparatively different offspring phenotype. It is important to be aware of the inconsistencies within the literature concerning approaches to environmental enrichment, and that has a potential to yield different experimental outcomes. Our environmental enrichment paradigm does not incorporate running wheels so as to distinguish between the social and cognitive aspects of enrichment and physical exercise. Numerous studies that implement environmental enrichment, including the EE paradigm implemented by Gapp et al. [55] incorporate running wheels in their protocol. Our lab had recently investigated the transgenerational influence of paternal running [56] and found evidence of an anxiolytic effect on offspring performance in the light-dark box test (but not on the EPM). Hence, the inclusion of running wheels is an important consideration that could be the distinguishing factor between the resulting effect of enrichment. It is likely that environmental enrichment with no incorporation of running wheels, in comparison to pure voluntary wheel running, has varying transgenerational effects that should be a point of future research.
It was interesting to observe that 5-HT1AR expression was up-regulated in the ventral hippocampus of male, but not female, PatCORT offspring. However, this is not the first time sex-specific effects of EE have been reported at a molecular level. For example, 4 weeks of EE reduces levels of hippocampal stress hormone glucocorticoid receptor mRNA levels in male C57Bl/6J mice, but not in females [57]. EE also increases BDNF mRNA levels in the hippocampus of male, but not female mice [63]. The sex-specific nature of EE-induced BDNF up-regulation is also region-specific since it is up-regulated in the frontal cortex of both male and female mice. Refinement of the current study design is required to determine if this sex-specific overexpression of aforementioned serotonergic receptors is a result of the paternal CORT model or is just a reflection of the sex-specific nature of the environmental enrichment paradigm.
One brain region which readily responds to EE is the hypothalamus, through modulation of neurotrophin and hormone secretion, which impacts on metabolism and stress response [58, 59]. Hypothalamic serotonergic neurotransmission is vital for the regulation of feeding, and extracellular 5-HT in the lateral hypothalamus increases during the anticipatory phase prior to feeding and into the initial period of food consumption before a gradual decline after 30 min [60, 61]. Also, hypothalamic levels of 5-HIAA (the 5-HT metabolite) are maintained at low baseline levels, with a single peak during the light-to-dark transition which corresponds with the commencement of feeding behaviour [62]. It was previously reported that EE reduces levels of 5-HIAA in the hypothalamus of male, but not female, mice [63]. While the NSFT results suggest no differences in latency to feed, the suppression of post-NSFT feeding observed in male PatCORT offspring, which is then attenuated for the EE-exposed group, could reflect dysregulation of serotonergic control over appetite or feeding for the male PatCORT offspring. While Franklin et al. [24] found that 8-OH-DPAT binding in the hypothalamus was unaffected in MSUS offspring, the functional profiles of other 5-HT receptors have yet to be established. It would therefore be interesting to explore the expression patterns of the 5-HT1BR and 5-HT2A/C receptors (across a range of paternal stress models) since these are the key serotonergic modulators of appetite and feeding [36, 64, 65]. The appetitive differences observed could also be indicative of potential differences in leptin or ghrelin signalling, with these being the main hormones that regulate appetite [66]. Thus, this novel finding, in the absence of any weight loss difference, uncovers a whole plethora of potential research that can be undertaken in our paternal stress model. Transgenerational modifications to offspring metabolism is well-evident, primarily based on studies of paternal diets and their impact on offspring metabolic and endocrine function [67–69] but this has not been extensively explored in the context of paternal stress. Only a few transgenerational paternal stress models have explored metabolic changes in the offspring. Gapp et al. [5] have reported lower blood glucose and serum insulin levels in MSUS offspring suggestive of insulin hypersensitivity. Post-NSFT food intake measurements are not routinely reported but our observation highlights the importance of such measurements.
Finally, the current study has demonstrated that environmental enrichment of the F1 offspring does not rescue the transgenerational influence of paternal CORT. This was surprising given the abundant literature that delves into the anxiolytic effects of environmental enrichment in various rodent models of stress. From the current study, it can be speculated that a parental modification, that is, increased stress hormone levels, renders the next generation non-responsive to the action of environmental enrichment. Given the absence of beneficial effects of EE as seen by the non-recovery of behavioural phenotypes of the F1 offspring, it would be important for future studies to consider hippocampal neurogenesis in the offspring generation, and continue investigating the receptors or effector molecules that are part of the BDNF signalling cascade. Findings from this study provide evidence that hippocampal serotonin receptor expression does not explain the observed F1 male-specific anxiety phenotype. Nonetheless, further work needs to be performed before we can definitively exclude these receptors from being involved in our paternal CORT model of transgenerational epigenetic inheritance.
Acknowledgements
A.J.H. is an NHMRC Principal Research Fellow. T.R. is an NHMRC Dementia Fellow. This research was supported by an NHMRC Project Grant to A.J.H. and T.Y.P., and the DHB Foundation, Equity Trustees (A.J.H.) This work received support from the Victorian government through the Operational Infrastructure Scheme. Author contributions: T.Y.P. and A.J.H. conceived the study, T.Y.P. designed the study, T.Y.P., A.R., J.G., and T.R. performed the experiments, acquired and analysed the data. T.Y.P., A.R., and T.R. wrote the manuscript. A.J.H. edited and approved the manuscript.
References
- 1. Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, Vizi S, Mansuy IM.. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 2010; 68:408–15. [DOI] [PubMed] [Google Scholar]
- 2. Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL.. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 2013; 33:9003–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Short AK, Fennell KA, Perreau VM, Fox A, O’Bryan MK, Kim JH, Bredy TW, Pang TY, Hannan AJ.. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl Psychiatry 2016; 6:e837.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pang TYC, Short AK, Bredy TW, Hannan AJ.. Transgenerational paternal transmission of acquired traits: stress-induced modification of the sperm regulatory transcriptome and offspring phenotypes. Curr Opin Behav Sci 2017; 14:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM.. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 2014; 17:667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albert PR, Vahid-Ansari F, Luckhart C.. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci 2014; 8:199.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF.. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 1996; 54:129–41. [DOI] [PubMed] [Google Scholar]
- 8. Strobel A, Gutknecht L, Rothe C, Reif A, Mössner R, Zeng Y, Brocke B, Lesch K-P.. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm 2003; 110:1445–53. [DOI] [PubMed] [Google Scholar]
- 9. Lesch KP, Wiesmann M, Hoh A, Müller T, Disselkamp-Tietze J, Osterheider M, Schulte HM.. 5-HT1A receptor-effector system responsivity in panic disorder. Psychopharmacology 1992; 106:111–7. [DOI] [PubMed] [Google Scholar]
- 10. Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ.. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry 2008; 193:229–34. [DOI] [PubMed] [Google Scholar]
- 11. Neumeister A, Bain E, Nugent AC, Carson Re, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC, et al. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci 2004; 24:589–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ramboz S, Oosting R, Amara DA, Kung Hf, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R.. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA 1998; 95:14476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Razoux F, Russig H, Mueggler T, Baltes C, Dikaiou K, Rudin M, Mansuy IM.. Transgenerational disruption of functional 5-HT1AR-induced connectivity in the adult mouse brain by traumatic stress in early life. Mol Psychiatry 2017; 22:519–26. [DOI] [PubMed] [Google Scholar]
- 14. Pang TY, Du X, Catchlove WA, Renoir T, Lawrence AJ, Hannan AJ.. Positive environmental modification of depressive phenotype and abnormal hypothalamic-pituitary-adrenal axis activity in female C57BL/6J mice during abstinence from chronic ethanol consumption. Front Pharmacol 2013; 4:93.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pang TY, Du X, Zajac MS, Howard ML, Hannan AJ.. Altered serotonin receptor expression is associated with depression-related behavior in the R6/1 transgenic mouse model of Huntington's disease. Hum Mol Genet 2009; 18:753–66. [DOI] [PubMed] [Google Scholar]
- 16. Renoir T, Pang Tyc, Mo C, Chan G, Chevarin C, Lanfumey L, Hannan AJ.. Differential effects of early environmental enrichment on emotionality related behaviours in Huntington's disease transgenic mice. J Physiol 2013; 591:41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fanselow MS, Dong HW.. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010; 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du X, Leang L, Mustafa T, Renoir T, Pang TY, Hannan AJ.. Environmental enrichment rescues female-specific hyperactivity of the hypothalamic-pituitary-adrenal axis in a model of Huntington's disease. Transl Psychiatry 2012; 2:e144.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogers J, Li S, Lanfumey L, Hannan AJ, Renoir T.. Environmental enrichment reduces innate anxiety with no effect on depression-like behaviour in mice lacking the serotonin transporter. Behav Brain Res 2017; 332:355–61. [DOI] [PubMed] [Google Scholar]
- 20. Rogers J, Vo U, Buret LS, Pang TY, Meiklejohn H, Zeleznikow-Johnston A, Churilov L, van den Buuse M, Hannan AJ, Renoir T, et al. Dissociating the therapeutic effects of environmental enrichment and exercise in a mouse model of anxiety with cognitive impairment. Transl Psychiatry 2016; 6:e794.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Renoir T, Chevarin C, Lanfumey L, Hannan AJ.. Effect of enhanced voluntary physical exercise on brain levels of monoamines in Huntington disease mice. PLoS Curr 2011; 3:RRN1281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pang TY, Renoir T, Du X, Lawrence AJ, Hannan AJ.. Depression-related behaviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running. Eur J Neurosci 2013; 37:1803–10. [DOI] [PubMed] [Google Scholar]
- 23. Dietz DM, LaPlant Q, Watts EL, Hodes GE, Russo SJ, Feng J, Oosting RS, Vialou V, Nestler EJ.. Paternal transmission of stress-induced pathologies. Biol Psychiatry 2011; 70:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franklin TB, Linder N, Russig H, Thony B, Mansuy IM.. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One 2011; 6:e21842.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL.. Mice lacking the serotonin transporter exhibit 5-HT(1A) receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology 2003; 28:2077–88. [DOI] [PubMed] [Google Scholar]
- 26. Vinkers CH, Oosting RS, van Bogaert MJ, Olivier B, Groenink L.. Early-life blockade of 5-HT(1A) receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biol Psychiatry 2010; 67:309–16. [DOI] [PubMed] [Google Scholar]
- 27. Zanettini C, Carola V, Lo Iacono L, Moles A, Gross C, D'Amato FR.. Postnatal handling reverses social anxiety in serotonin receptor 1A knockout mice. Genes Brain Behav 2010; 9:26–32. [DOI] [PubMed] [Google Scholar]
- 28. Akimova E, Lanzenberger R, Kasper S.. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry 2009; 66:627–35. [DOI] [PubMed] [Google Scholar]
- 29. Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien L-K, Holik A, Attarbaschi T, Mossaheb N, Sacher J, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry 2007; 61:1081–9. [DOI] [PubMed] [Google Scholar]
- 30. Garcia-Garcia AL, Meng Q, Canetta S, Gardier AM, Guiard BP, Kellendonk C, Dranovsky A, Leonardo ED.. Serotonin signaling through prefrontal cortex 5-HT1A receptors during adolescence can determine baseline mood-related behaviors. Cell Rep 2017; 18:1144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stewart A, Maity B, Wunsch AM, Meng F, Wu Q, Wemmie JA, Fisher RA.. Regulator of G-protein signaling 6 (RGS6) promotes anxiety and depression by attenuating serotonin-mediated activation of the 5-HT(1A) receptor-adenylyl cyclase axis. FASEB J 2014; 28:1735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caspi A, Hariri Ar, Holmes A, Uher R, Moffitt TE.. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry 2010; 167:509–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ.. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex 2011; 21:1667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higgins GA, Bradbury AJ, Jones BJ, Oakley NR.. Behavioural and biochemical consequences following activation of 5HT1-like and GABA receptors in the dorsal raphe nucleus of the rat. Neuropharmacology 1988; 27:993–1001. [DOI] [PubMed] [Google Scholar]
- 35. Hillegaart V. Effects of local application of 5-HT and 8-OH-DPAT into the dorsal and median raphe nuclei on motor activity in the rat. Physiol Behav 1990; 48:143–8. [DOI] [PubMed] [Google Scholar]
- 36. Martin KF, Phillips I, Hearson M, Prow MR, Heal DJ.. Characterization of 8-OH-DPAT-induced hypothermia in mice as a 5-HT1A autoreceptor response and its evaluation as a model to selectively identify antidepressants. Br J Pharmacol 1992; 107:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 2010; 65:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC.. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem 2011; 116:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spiacci A Jr, Pobbe RLH, Matthiesen M, Zangrossi H Jr,. 5-HT1A receptors of the rat dorsal raphe lateral wings and dorsomedial subnuclei differentially control anxiety- and panic-related defensive responses. Neuropharmacology 2016;107:471–9. [DOI] [PubMed] [Google Scholar]
- 40. Wang CC, Lin HC, Chan YH, Gean PW, Yang YK, Chen PS.. 5-HT1A-receptor agonist modified amygdala activity and amygdala-associated social behavior in a valproate-induced rat autism model. Int J Neuropsychopharm 2013; 16:2027–39. [DOI] [PubMed] [Google Scholar]
- 41. Nithianantharajah J, Hannan AJ.. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 2006; 7:697–709. [DOI] [PubMed] [Google Scholar]
- 42. Rasmuson S, Olsson T, Henriksson BG, Kelly PAT, Holmes MC, Seckl JR, Mohammed AH.. Environmental enrichment selectively increases 5-HT1A receptor mRNA expression and binding in the rat hippocampus. Brain Res Mol Brain Res 1998; 53:285–90. [DOI] [PubMed] [Google Scholar]
- 43. Chekmareva NY, Sotnikov SV, Diepold RP, Naik RR, Landgraf R, Czibere L.. Environmental manipulations generate bidirectional shifts in both behavior and gene regulation in a crossbred mouse model of extremes in trait anxiety. Front Behav Neurosci 2014; 8:87.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hutchinson KM, McLaughlin KJ, Wright RL, Bryce Ortiz J, Anouti DP, Mika A, Diamond DM, Conrad CD.. Environmental enrichment protects against the effects of chronic stress on cognitive and morphological measures of hippocampal integrity. Neurobiol Learn Mem 2012; 97:250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M.. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry 2010; 15:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hendriksen H, Prins J, Olivier B, Oosting RS.. Environmental enrichment induces behavioral recovery and enhanced hippocampal cell proliferation in an antidepressant-resistant animal model for PTSD. PLoS One 2010; 5:e11943.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huttenrauch M, Salinas G, Wirths O.. Effects of long-term environmental enrichment on anxiety, memory, hippocampal plasticity and overall brain gene expression in C57BL6 mice. Front Mol Neurosci 2016; 9:62.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sale A, Berardi N, Maffei L.. Enrich the environment to empower the brain. Trends Neurosci 2009; 32:233–9. [DOI] [PubMed] [Google Scholar]
- 49. Kempermann G, Gast D, Gage FH.. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 2002; 52:135–43. [DOI] [PubMed] [Google Scholar]
- 50. Ferrés-Coy A, Pilar-Cuellar F, Vidal R, Paz V, Masana M, Cortés R, Carmona MC, Campa L, Pazos Á, Montefeltro A, et al. RNAi-mediated serotonin transporter suppression rapidly increases serotonergic neurotransmission and hippocampal neurogenesis. Transl Psychiatry 2013; 3:e211.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Islam MR, Moriguchi S, Tagashira H, Fukunaga K.. Rivastigmine improves hippocampal neurogenesis and depression-like behaviors via 5-HT1A receptor stimulation in olfactory bulbectomized mice. Neuroscience 2014; 272:116–30. [DOI] [PubMed] [Google Scholar]
- 52. Murata Y, Yanagihara Y, Mori M, Mine K, Enjoji M.. Chronic treatment with tandospirone, a serotonin 1A receptor partial agonist, inhibits psychosocial stress-induced changes in hippocampal neurogenesis and behavior. J Affect Disord 2015; 180:1–9. [DOI] [PubMed] [Google Scholar]
- 53. Mendez-David I, David DJ, Darcet F, Wu MV, Kerdine-Römer S, Gardier AM, Hen R.. Rapid anxiolytic effects of a 5-HT(4) receptor agonist are mediated by a neurogenesis-independent mechanism. Neuropsychopharmacology 2014; 39:1366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nicolas S, Veyssière J, Gandin C, Zsürger N, Pietri M, Heurteaux C, Glaichenhaus N, Petit-Paitel A, Chabry J.. Neurogenesis-independent antidepressant-like effects of enriched environment is dependent on adiponectin. Psychoneuroendocrinology 2015; 57:72–83. [DOI] [PubMed] [Google Scholar]
- 55. Gapp K, Bohacek J, Grossmann J, Brunner AM, Manuella F, Nanni P, Mansuy IM. Potential of environmental enrichment to prevent transgenerational effects of paternal trauma. Neuropsychopharmacology 2016; 41:2749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Short AK, Yeshurun S, Powell R, Perreau VM, Fox A, Kim JH, Pang TY, Hannan AJ.. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl Psychiatry 2017; 7:e1114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin EJ, Choi E, Liu X, Martin A, During MJ.. Environmental enrichment exerts sex-specific effects on emotionality in C57BL/6J mice. Behav Brain Res 2011; 216:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Foglesong GD, Huang W, Liu X, Slater AM, Siu J, Yildiz V, Salton SRJ, Cao L.. Role of hypothalamic VGF in energy balance and metabolic adaption to environmental enrichment in mice. Endocrinology 2016; 157:983–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Skwara AJ, Karwoski TE, Czambel RK, Rubin RT, Rhodes ME.. Influence of environmental enrichment on hypothalamic-pituitary-adrenal (HPA) responses to single-dose nicotine, continuous nicotine by osmotic mini-pumps, and nicotine withdrawal by mecamylamine in male and female rats. Behav Brain Res 2012; 234:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwartz DH, Hernandez L, Hoebel BG.. Serotonin release in lateral and medial hypothalamus during feeding and its anticipation. Brain Res Bull 1990; 25:797–802. [DOI] [PubMed] [Google Scholar]
- 61. Schwartz DH, McClane S, Hernandez L, Hoebel BG.. Feeding increases extracellular serotonin in the lateral hypothalamus of the rat as measured by microdialysis. Brain Res 1989; 479:349–54. [DOI] [PubMed] [Google Scholar]
- 62. Stanley BG, Schwartz DH, Hernandez L, Leibowitz SF, Hoebel BG.. Patterns of extracellular 5-hydroxyindoleacetic acid (5-HIAA) in the paraventricular hypothalamus (PVN): relation to circadian rhythm and deprivation-induced eating behavior. Pharmacol Biochem Behav 1989; 33:257–60. [DOI] [PubMed] [Google Scholar]
- 63. Chourbaji S, Hortnagl H, Molteni R, Riva MA, Gass P, Hellweg R.. The impact of environmental enrichment on sex-specific neurochemical circuitries - effects on brain-derived neurotrophic factor and the serotonergic system. Neuroscience 2012; 220:267–76. [DOI] [PubMed] [Google Scholar]
- 64. Nonogaki K, Nozue K, Oka Y.. Increased hypothalamic 5-HT2A receptor gene expression and effects of pharmacologic 5-HT2A receptor inactivation in obese Ay mice. Biochem Biophys Res Commun 2006; 351:1078–82. [DOI] [PubMed] [Google Scholar]
- 65. Nonogaki K, Ohashi-Nozue K, Oka Y.. A negative feedback system between brain serotonin systems and plasma active ghrelin levels in mice. Biochem Biophys Res Commun 2006; 341:703–7. [DOI] [PubMed] [Google Scholar]
- 66. Klok MD, Jakobsdottir S, Drent ML.. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 2007; 8:21–34. [DOI] [PubMed] [Google Scholar]
- 67. McPherson NO, Lane M, Sandeman L, Owens JA, Fullston T.. An exercise-only intervention in obese fathers restores glucose and insulin regulation in conjunction with the rescue of pancreatic islet cell morphology and MicroRNA expression in male offspring. Nutrients 2017;9:122. [Google Scholar]
- 68. McPherson NO, Owens JA, Fullston T, Lane M.. Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am J Physiol Endocrinol Metab 2015; 308:E805–21. [DOI] [PubMed] [Google Scholar]
- 69. Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ.. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 2010; 467:963–6. [DOI] [PubMed] [Google Scholar]