Abstract
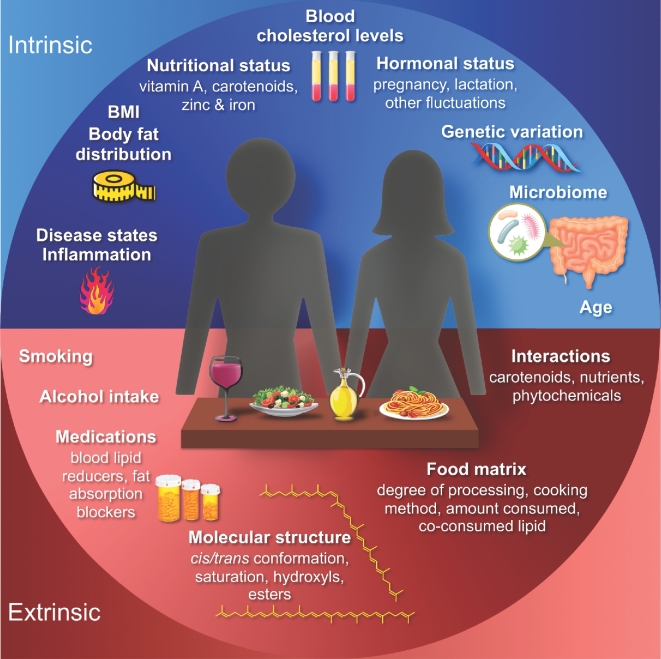
Carotenoids are orange, yellow, and red lipophilic pigments present in many fruit and vegetables, as well as other food groups. Some carotenoids contribute to vitamin A requirements. The consumption and blood concentrations of specific carotenoids have been associated with reduced risks of a number of chronic conditions. However, the interpretation of large, population-based observational and prospective clinical trials is often complicated by the many extrinsic and intrinsic factors that affect the physiologic response to carotenoids. Extrinsic factors affecting carotenoid bioavailability include food-based factors, such as co-consumed lipid, food processing, and molecular structure, as well as environmental factors, such as interactions with prescription drugs, smoking, or alcohol consumption. Intrinsic, physiologic factors associated with blood and tissue carotenoid concentrations include age, body composition, hormonal fluctuations, and variation in genes associated with carotenoid absorption and metabolism. To most effectively investigate carotenoid bioactivity and to utilize blood or tissue carotenoid concentrations as biomarkers of intake, investigators should either experimentally or statistically control for confounding variables affecting the bioavailability, tissue distribution, and metabolism of carotene and xanthophyll species. Although much remains to be investigated, recent advances have highlighted that lipid co-consumption, baseline vitamin A status, smoking, body mass and body fat distribution, and genetics are relevant covariates for interpreting blood serum or plasma carotenoid responses. These and other intrinsic and extrinsic factors are discussed, highlighting remaining gaps in knowledge and opportunities for future research. To provide context, we review the state of knowledge with regard to the prominent health effects of carotenoids.
Keywords: carotenoids, phytochemicals, bioavailability, nutrigenetics, pharmacokinetics
Introduction
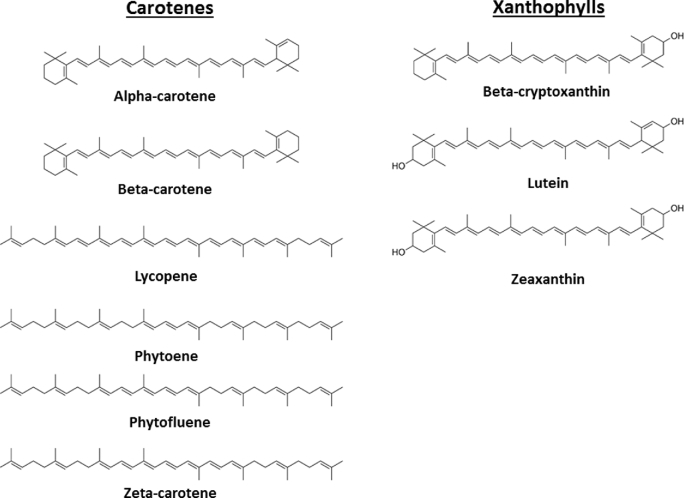
Carotenoids are a diverse class of colorful red, orange, and yellow terpenoid pigments found in fruit, vegetables, eggs, meats, milk, and some fish and crustacean seafoods. Among the >700 carotenoids found in nature, ∼50 are found in the human diet, with approximately half of those being detected in human blood and tissues [reviewed in Krinsky and Johnson (1)]. The major carotenoids in human serum are β-carotene, α-carotene, lycopene, lutein, zeaxanthin, and β-cryptoxanthin (Figure 1) (1). Specific carotenoids can contribute to vitamin A requirements, supporting vision, epithelial cell regeneration, and controlling gene expression via the vitamin A metabolite retinoic acid [reviewed in Tanumihardjo et al. (2)]. Epidemiologic studies have suggested other roles related to the reduction in risk of chronic diseases such as cancers, cardiovascular diseases (CVDs), and age-related macular degeneration (AMD), and improved cognitive and visual functions [reviewed in (3–10)]. Controlled clinical and preclinical trials are required to establish causal relations between carotenoid intake and bioactivity; however, the interpretation of both observational and experimental studies is complicated by the numerous intrinsic and extrinsic variables affecting blood and tissue carotenoid concentrations, and therefore bioactivity, in response to a given carotenoid intake.
FIGURE 1.
Structures of dietary carotenoids discussed in this review.
To accelerate the pace of carotenoid and health research, and to improve the utility of blood or tissue carotenoids concentrations as biomarkers of exposure, the many factors affecting internal carotenoid exposure must be considered. The primary goal of this review is to highlight the recent advances in defining the factors influencing the absorption, distribution, and metabolism of the major circulating carotenoids in healthy adults, in the context of the major potential health impacts of carotenoids.
Current Status of Knowledge
General overview
Carotenoids can be classified into 2 major types: carotenes and xanthophylls. Carotenes, which include β-carotene, α-carotene, and lycopene as well as other less-studied species such as phytoene, phytofluene, zeta-carotene, and neurosporene, are unoxygenated terpenes, whereas xanthophylls, which include lutein, zeaxanthin, and β-cryptoxanthin, are oxygenated (Figure 1) [reviewed in Krinsky and Johnson (1)]. Carotenoid bioavailability varies by cooking and processing of the food as well as the amounts of dietary fat, fiber, and competing compounds in the meal [reviewed in Bohn et al. (11)]. Upon ingestion, carotenoids are released from the food matrix and are emulsified with fat and incorporated into lipid micelles in the small intestine for absorption by intestinal enterocytes. Once thought to be taken up strictly via passive diffusion, carotenoid absorption is facilitated via membrane proteins [reviewed in Bohn et al. (11)].
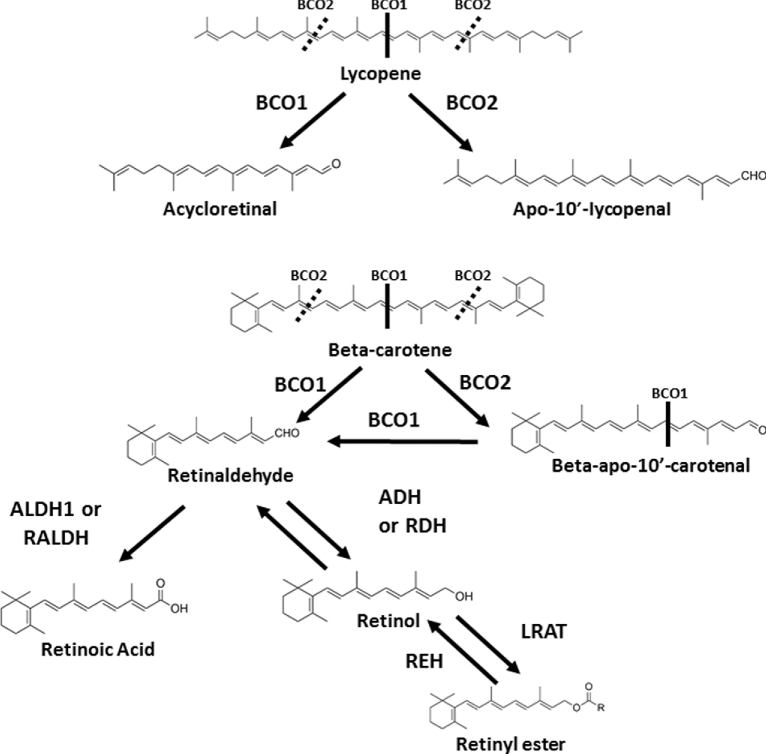
Inside of the enterocyte, carotenoids are packaged into chylomicrons along with lipids and fat-soluble nutrients, which enter the lymphatic system for delivery to the liver [reviewed in Krinsky and Johnson (1)]. En route, some carotenoids may be taken up by peripheral tissues as lipoprotein lipase (LPL) degrades chylomicrons. The resulting chylomicron remnants are taken up by the liver via LDL receptors. Once in the liver, some carotenoids may be stored while the rest are repackaged into lipoproteins and released into the bloodstream. In the circulation, xanthophylls are primarily carried in HDL cholesterol and carotenes in LDL cholesterol (12). Scavenger receptor class B type 1 (SCARB1), expressed on the surface of many different cell types, participates in the transfer of carotenoids between lipoproteins and target tissues, as well as other proteins such as cluster of differentiation 36 (CD36) and NPC1L1 (Niemann-Pick C1–like 1) [reviewed in Bohn et al. (11)]. Figures 2 and 3 summarize the enzymatic metabolic pathways of carotenoids.
FIGURE 2.
Overview of the current understanding of metabolism of major carotenes. α-Carotene can also be metabolized in a similar manner as β-carotene but leads to 1 molecule of retinaldehyde and 1 molecule of α-retinaldehyde upon central cleavage. Not all of the metabolic products are shown. ADH, alcohol dehydrogenase; ALDH1, aldehyde dehydrogenase 1; BCO1, β-carotene-15,15′-oxygenase; BCO2, β-carotene-9,10-oxygenase; CHO, carbohydrate; LRAT, lecithin-retinol acyl transferase; RALDH, retinaldehyde dehydrogenase; RDH, retinol dehydrogenase; REH, retinyl ester hydrolase. Adapted from references 35–38 with permission (but not endorsement).
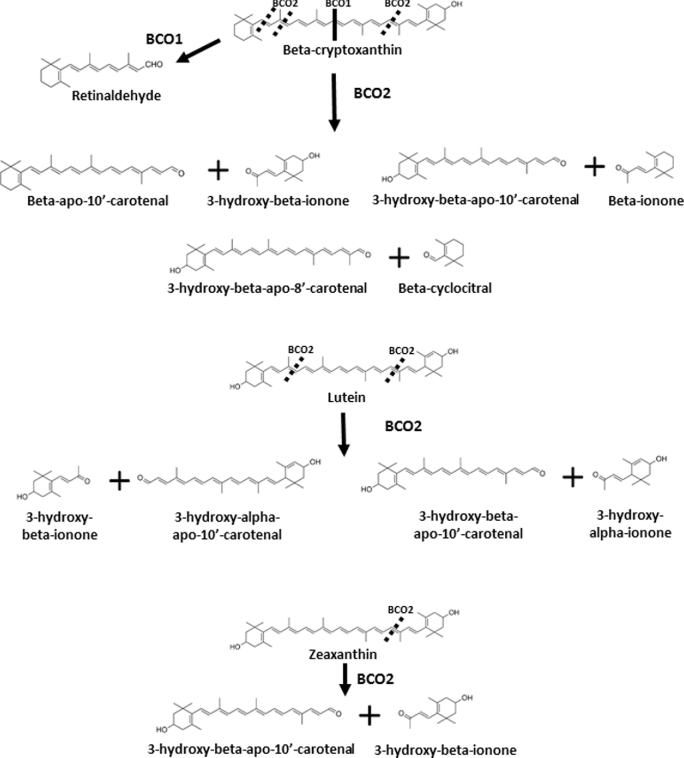
FIGURE 3.
Overview of current understanding of metabolism of major xanthophylls. Not all metabolic products are shown. BCO1, β-carotene-15,15-oxygenase; BCO2, β-carotene-9,10-oxygenase; CHO, carbohydrate. Adapted from reference 36 with permission (but not endorsement).
Structural, dietary, genetic, and physiologic variables affecting carotenoid kinetics and biodistribution
Overview of carotenoid bioavailability, serum or plasma half-life, and metabolism
Knowing the bioavailability and plasma or serum half-life of carotenoids provides a basis for understanding their physiologic relevance as well as the basis for the design and interpretation of interventions. β-Carotene and lycopene are the most extensively studied. For a comprehensive review of β-carotene absorption and bioconversion to vitamin A, see Haskell (13), but key points are highlighted here. First, the bioavailability of pure β-carotene in oil ranges from 9% to 65%, being greater (42–65%) when provided as a microdose (270–540 ng) of 14C-β-carotene (13) and lower (11–35%) when provided as larger doses of unenriched β-carotene (3–40 mg) [reviewed in Haskell (13)]. The bioavailability of β-carotene from vegetables ranges from 5% to 65%, depending on the specific food and its preparation method (13). The absorption of purified 13C-lycopene in oil (10 mg) was 23% and was 34% from tomato paste mixed with water and oil (14, 15), whereas absorption of a larger (38 mg) purified dose was 2.6% (16). The bioavailability of purified 13C-phytoene (a lycopene precursor found in tomatoes; 3.2 mg) in oil was 58% (17). Although there are very limited data on xanthophyll bioavailability, one estimate of percentage of lutein absorption was published to be 55% of a 14C-lutein microdose (125 nmol) in 1 subject (18), which agreed with a previous publication of 45–54% lutein absorption from spinach (19). In general, absorption estimates are variable, and subsequent sections will focus on potential causes for this variability.
Serum or plasma carotenoid half-lives can be used to estimate how long the compound resides in circulation, the dosing interval needed to maintain a particular circulating concentration, and the time required to reach steady state (20). Table 1 summarizes published serum and plasma β-carotene, lycopene, and phytoene half-lives. In general, plasma carotene half-lives range from 1 to 11 d (14, 17, 21–25), although additional tracer studies might refine these estimates. Fewer studies have been performed to determine the half-life of xanthophylls in adults (Table 2). Serum and plasma depletion studies indicate wide variability in xanthophyll half-lives, ranging from 15 to 76 d for lutein and zeaxanthin and 12 to 39 d for β-cryptoxanthin (21, 26, 27), whereas studies with either labeled or unlabeled doses have found the half-life of lutein to be 4.6–14.5 d and of zeaxanthin to be 5.6–12 d (28–32). As with carotenes, causes of this wide variability may be due to differences in study design (e.g., duration, sum of lutein and zeaxanthin compared with analyzing separately) and the subject populations who were analyzed (e.g., age range, healthy compared with diseased, sex). In the serum and plasma depletion studies, lutein had the longest half-life of all carotenoids. Currently, the half-life of β-cryptoxanthin in healthy adults is unknown. Overall, additional studies are needed to better estimate the half-lives of dietary xanthophylls in healthy adults.
TABLE 1.
Serum or plasma carotene half-lives from feeding or depletion studies1
| Carotenoid and intervention/study design | Number and sex of subjects | Age, y | Study duration, wk | Food/agent (carotenoid dose × duration) | Cmax, µmol/L | T 1/2, d | Year (ref) |
|---|---|---|---|---|---|---|---|
| β-Carotene | |||||||
| Postserial-dosing depletion | 9 women | 18–42 | 10 | β-Carotene powder (1.5 mg/d × 4 d) followed by low carotenoid (0.07 mg/d) depletion | 0.56 ± 0.12 | 37 ± 52 | 2001 (21) |
| Serial dosing; half-life calculated from cumulation curve | 4 men/group | 20–40 | 6 | Carrot juice cocktail (6 mg/d × 6 wk) | 1.32 ± 0.52 | Could not calculate | 2002 (22) |
| Carrot juice cocktail (18 mg/d × 6 wk) | 2.36 ± 0.64 | 9.2 ± 2.6 | |||||
| Water-dispersible β-carotene powder (7.2 mg/d × 6 wk) | 4.95 ± 1.15 | 6.4 ± 0.9 | |||||
| Water-dispersible β -carotene powder (21.6 mg/d × 6 wk) | 5.60 ± 0.99 | 11.4 ± 3.7 | |||||
| Postserial-dosing monitoring (self-selected diet period) | 5 men/group | 20–45 | 4 | β-Carotene supplement (12 mg × 6 wk) | 3.6 ± 0.71 | 7–143 | 1992 (23) |
| β-Carotene supplement (30 mg × 6 wk) | 7.9 ± 1.4 | ||||||
| Carrots (30 mg × 6 wk) | 1.4 ± 0.8 | ||||||
| Lycopene | |||||||
| Postserial-dosing monitoring (self-selected diet period) | 5 men/group | 20–45 | 4 | Tomato juice (12 mg lycopene) | 0.03 ± 0.3 | 11–143 | 1992 (23) |
| Postsingle-dose clearance | 5 men/group | 18–45 | 4 | Tomato paste drink | 0.08 ± 0.01 | 1.2 ± 0.43 | 2004 (24) |
| (10–120 mg) | –0.21 ± 0.02 | –2.6 ± 0.6 | |||||
| Postserial-dose clearance | 6 men/group | 18–45 | 4 | Tomato soup (20 mg/d × 8 d) | 0.823 | 6.3 ± 1.72 | 2004 (25) |
| Lycopene tablet (20 mg/d × 8 d) | 0.963 | 5.6 ± 1.32 | |||||
| Postsingle-dose clearance | 4 women, 4 men | 24 ± 12 | 4 | 13C-Lycopene in oil (10 mg × 1 d) | 0.14 ± 0.022 | 6.2 ± 0.32 | 2015 (14) |
| α-Carotene | |||||||
| Postserial-dosing monitoring (self-selected diet period) | 5 men | 20–45 | 4 | Carrots (30 mg × 6 wk) | 1.0 ± 0.4 | 7–143 | 1992 (23) |
| Phytoene | |||||||
| Postsingle-dose clearance | 2 women, 2 men | 28 ± 22 | 4 | 13C-Phytoene in oil (3.2 mg × 1 d) | 0.06 ± 0.0062 | 2.3 ± 0.22 | 2016 (17) |
Values are means ± SDs unless otherwise indicated. Cmax, maximal plasma or serum concentration attained; ref, reference; T1/2, plasma or serum half-life.
Mean ± SE.
Error term not provided.
TABLE 2.
Half-lives of xanthophylls in serum depletion and supplementation studies1
| Intervention/study design and carotenoid | Number of participants (health, sex) | Age, y | Study duration, wk | Food/agent (carotenoid dose × duration) | Cmax, µmol/L | T 1/2, d | Year (ref) |
|---|---|---|---|---|---|---|---|
| Controlled low-carotenoid diet during controlled low-carotenoid diet | 12 (healthy men) | 25–43 | 13 | ≤0.4 mg total carotenoids/d × 13 wk | — | — | 1992 (26) |
| Lutein/zeaxanthin | — | — | — | — | 0.28 ± 0.052 | 33–613 | |
| β-Cryptoxanthin | — | — | — | — | 0.22 ± 0.042 | ≤123 | |
| Serum carotenoid depletion during controlled low-carotenoid diet | 19 (healthy women) | 18–42 | 10 | 0.07 mg total carotenoids/d × 10 wk | — | — | 2001 (21) |
| Lutein | — | — | — | — | L/Z: 0.44 ± 0.042 | 76 ± 172 | |
| Zeaxanthin | — | — | — | — | 38 ± 72 | ||
| β-Cryptoxanthin | — | — | — | — | 0.31 ± 0.052 | 39 ± 42 | |
| Serum carotenoid depletion | 2002 (27) | ||||||
| Lutein | 10 (T1D, both) | 24 ± 6 | 3 | 0.05–0.07 mg/d × 3 wk | 0.153 (control) | ≥153 | |
| 8 (controls, both) | 27 ± 3 | 3 | 0.05–0.07 mg/d × 3 wk | 0.163 (T1D) | ≥153 | ||
| Daily oral lutein supplement4 | 19 (healthy, 50% men) | 20–35 | 9 | Oral lutein supplement: 4.1 or 20.5 mg/d × 6 wk | 4.1 mg/d: 0.4 ± 0.2; 20.5 mg/d: 1.3 ± 0.8 | 4.1 mg/d: 5.5 ± 2.1; 20.5 mg/d: 6.1 ± 1.0 | 2005 (28) |
| Daily oral lutein supplement | 2 (healthy men) | 42, 51 | 52 | Oral lutein supplement: 30 mg/d × 20 wk | 1.85; 3.3 | ∼14.56 | 1997 (29) |
| 13C-Lutein in kale | 7 (healthy, both) | 46 ± 14 | 3 (single dose) | 13C-Lutein in kale, 19 mg | 0.38 ± 0.08 | ∼4.66 | 2005 (30) |
| 14C-Lutein extracted from spinach | 1 (healthy women) | 45 | 9 (single dose) | 14C-Lutein extracted from spinach, 0.071 mg | 0.003 | 9.85 | 2005 (31) |
Values are means ± SDs unless otherwise indicated. Cmax, maximal plasma or serum concentration attained; L/Z, lutein/zeaxanthin; ref, reference; T1/2, plasma or serum half-life; T1D, type 1 diabetes.
Mean ± SE.
Error term not provided.
Supplement contained 8.3% zeaxanthin relative to lutein.
One value is provided for each subject.
Half-life estimated from published data for this publication.
Carotenoid metabolism, enzymatic or nonenzymatic, is a central determinant of circulating and tissue carotenoid concentrations, vitamin A status, and generation of potentially bioactive non–vitamin A metabolites [reviewed in Lobo et al. (33) and Mein et al. (34)]. Carotenoids in humans are believed to be primarily cleaved by 2 enzymes (Figures 2 and 3) (35–38). β-Carotene-15,15′-oxygenase (BCO1; with the aliases BCMO1, CMO1, and CMOI) is a dioxygenase responsible for the central cleavage of provitamin A carotenoids to yield retinal (vitamin A) (37, 39). This enzyme is expressed in a number of tissues including the gastrointestinal tract and liver (40). Upon uptake by the intestinal mucosa, provitamin A carotenoids (β-carotene, α-carotene, and β-cryptoxanthin) are partially converted to retinal by BCO1, reduced to retinol, esterified, and then packaged into chylomicrons along with intact carotenoids and secreted in the lymph for distribution to peripheral tissues and the liver [reviewed in Harrison (41)]. The liver is a major storage site of vitamin A and carotenoids, and hepatic stellate cells are a site of BCO1-facilitated conversion of β-carotene to retinoids (42–44). Carotenoids are found throughout the body (45), with the major portion of lycopene, for example, residing in adipose tissue (46). Retinal is metabolized in tissues by retinol dehydrogenases or alcohol dehydrogenases to the circulating form of vitamin A (i.e., retinol) or by retinal dehydrogenase to the nuclear receptor ligand retinoic acid [reviewed in Mein et al. (33) and Harrison (41)]. Although BCO1 cleaves lycopene in vitro as efficiently as β-carotene (37, 39), lycopene does not accumulate in tomato- or lycopene-fed mice lacking Bco1, suggesting that it may not be metabolized by BCO1 in vivo (47). Similarly, other acyclic carotenes, such as phytoene or phytofluene, do not accumulate in mice lacking Bco1, and therefore may not be BCO1 substrates (47).
A number of current reports have elucidated the role of a second mammalian carotenoid cleavage enzyme, β-carotene-9′,10′-oxygenase (BCO2; also presented in the literature as CMO2, CMO-II, and BCDO2), in carotenoid cleavage. BCO2 cleaves eccentrically at the 9′,10′ position yielding an apo-10′-carotenoid and an ionone (Figure 2) [reviewed in Mein et al. (33)]. This enzyme is expressed in cardiac and skeletal muscle tissue, prostate, endometrial connective tissue, and the pancreas [reviewed in Lietz et al. (48)]. Mice lacking Bco2 accumulate dietary lycopene, lutein, and zeaxanthin (49–51). Isolated ferret BCO2 cleaved cis lycopene isomers (38), a major form of lycopene found in tissues (45). Alternatively, all-trans lycopene was not cleaved in vitro by chicken BCO2. Although the cleavage efficiency for cis lycopene is unknown, chicken BCO2 cleaved α-carotene, β-carotene, and β-cryptoxanthin, as well as lutein and zeaxanthin (36), suggesting that mammalian, but not avian, BCO2 cleaves cis lycopene isomers. Whether BCO2 cleaves phytofluene, ζ-carotene, or phytoene is less clear at this time (47, 50, 51). The role and regulation of xanthophyll metabolism by BCO2 are controversial. Consistent with the in vitro chicken BCO2 results (36), Bco2 knockout mice accumulate xanthophylls (49), and Bco2 expression was inversely associated with lutein and zeaxanthin concentrations in nonhuman primate brain (52). Alternatively, 1 group found that, in vivo, a lack of macular BCO2 activity in the primate eye underlies macular accumulation of lutein and zeaxanthin (53), but the exact structural causes of this inactivation are unclear (53–55).
Extrinsic dietary or environmental variables
Molecular structural variables affecting carotene responses
Carotenoids are found in many isomeric conformations, which may affect bioavailability and metabolism. In foods, lycopene, α-carotene, and β-carotene are primarily present as all-trans isomers, whereas phytoene is present primarily as the 15-cis isomer (56, 57). Phytofluene, ζ-carotene, and neurosporene are also present as cis isomers in plant tissues (57, 58). In human tissues, cis lycopene constitutes 35–79% of total lycopene (45, 59, 60), whereas all-trans β-carotene is generally the major isomer found in human serum and tissues, although there are also measurable amounts of 9- and 13-cis (45, 61). Recent findings suggest that all-trans lycopene is isomerized after absorption (14), with in vitro studies suggesting that lycopene cis isomers are thermodynamically favorable upon dissolution in a nonpolar solvent or oil (58, 62). Alternatively, the prominence of all-trans β-carotene in tissues and serum (61) suggests that it may be the favored form of β-carotene.
The geometric conformation of lycopene has been associated with bioavailability from foods. Tomato food products with greater proportions of cis lycopene result in greater lycopene absorption than all-trans lycopene–rich foods (63, 64). cis Lycopene isomers were also found to be more easily micellarized and taken up than all-trans lycopene in vitro and in a ferret model of lycopene absorption (65, 66). However, all-trans and cis13C-lycopene provided to humans in oil were absorbed at equal rates (14). These disparate findings may suggest that, when dissolved in oil, lycopene's isomeric configuration has less impact on bioavailability than when embedded in a food matrix, or that model systems do not fully recapitulate human lycopene absorption. Inherent challenges in controlling and delivering equimolar amounts of lycopene isomers in a food matrix for bioavailability comparisons warrant complementary studies of purified isomers.
Alternatively, a current in vitro study (66) supports previous in vitro and in vivo findings that all-trans β-carotene is more bioavailable than cis isomers (67–70). As with lycopene, cis β-carotene from foods micellarizes more efficiently (41–45%) than all-trans β-carotene (30–34%), but cellular uptake of all-trans and cis isomers was similar (27–30%) (66). In vitro, all-trans β-carotene absorption was 11% compared with 2–3% for 9-cis and 13-cis β-carotene (66).
The isomeric conformation of β-carotene may also affect its bioconversion to vitamin A. For example, 9-cis and 13-cis β-carotene had 38% and 62% of the bioefficacy (ability to be bioconverted to vitamin A), respectively, of all-trans in gerbils when doses of 141–418 nmol were provided for 7 d (71). A more recent gerbil study found that daily provision of 15 or 30 nmol of 13-cis or 9-cis β-carotene for 21–28 d increased liver retinol stores to be intermediate to, but not different from, an equimolar all-trans β-carotene or vehicle only (72). Together, these data suggest that dose and duration influence the bioefficacy of different β-carotene isomers.
The impact of geometric configuration on the bioavailability and metabolism of other carotenes and xanthophylls has not been investigated thoroughly. For phytoene, the all-trans isomer was relatively enriched in rat tissues compared with what was found in the diet (73). Findings from current studies investigating the bioaccumulation of lutein into neural tissue indicate that uptake and accumulation of circulating trans lutein into the retina and brain are favored over cis isomers (74–76).
Lutein in most fruit and vegetables is in the free form (77), whereas both free and esterified lutein are available in commercial dietary supplements. Earlier studies found esterified and free lutein to be similarly bioavailable (78, 79). More recently, it was found that serum lutein and macular pigment optical density (MPOD) responses were similar between either free or esterified lutein supplementation for 3 mo (80). However, a larger 4-wk supplementation study found greater serum lutein responses from free lutein than from lutein ester supplements (81). These disparate results may be due to different amounts of lipid provided with the intervention, with greater amounts (>20 g) (78, 79) yielding similar bioavailability of free and esterified lutein, whereas lesser amounts (∼5 g) were associated with greater bioavailability of free lutein (81). One randomized, single-dose, crossover study found the bioavailability of esterified and free β-cryptoxanthin to be comparable (82).
Carotenoid mass consumed
Although the mass of carotenoid consumed may seem to be an obvious determinant of the amount of carotenoid found in the blood and tissues, evidence suggests that this relation is complex. The percentage of lycopene absorbed from a single tomato-based beverage providing 10–120 mg lycopene was found to decrease with increasing dose, from 34% of the 10-mg dose to 5% of the 120-mg dose (15). The amount of lipid provided was held constant (5 mL olive oil), which may have limited micellarization and bioavailability of the higher doses (15). Alternatively, doubling a purified tracer β-carotene dose from 20 to 40 mg nearly doubled absorption (83). At this point, one cannot generalize how dose amount will affect carotene absorption because oil amount and delivery matrix are likely modulators.
Lutein supplementation increases blood, tissue, and breast-milk carotenoids in a dose-response manner. In a current 140-d lutein supplementation study, serum lutein and MPOD changed positively, reaching a plateau that was linearly dependent on dose, across doses of 0, 5, 10, or 20 mg/d (84). Lutein supplementation also increased breast milk as well as infant and maternal plasma lutein concentrations in a dose-dependent manner (85). Finally, a recent meta-analysis concluded that lutein and zeaxanthin supplementation increases MPOD in patients with AMD and healthy individuals in a dose-response manner (86).
Co-consumed lipid species and mass
Recent progress has defined the role of co-consumed lipid amount on human carotenoid absorption. Canola oil–containing salad dressing added to raw vegetables increases carotenoid absorption (87, 88), with a linear increase in absorption across 0–32 g oil for α-carotene and lycopene and across 0–8 g for β-carotene (88). Lutein absorption increased linearly across 0–4 g of added oil, with proportionately greater increases in absorption across the range of 8–32 g (88). Consumption of lipid-rich foods, such as avocados or eggs, also increased carotenoid absorption from a meal (89–91).
Lipid source and type have differing effects on carotene and xanthophyll absorption. A recent in vitro study found that unsaturated FAs compared with SFAs promoted micellarization and cellular uptake of β-carotene and lycopene during simulated digestion (92); however, in humans, lipid type (canola oil, soybean oil, or butter) was less impactful on carotene absorption from raw vegetable salads than lipid mass (93). Like carotenes, increasing lipid mass increases xanthophyll absorption (93); however, unlike carotenes, fat source affected lutein absorption, with canola oil promoting greater absorption than butter (93). This is consistent with an earlier study suggesting that co-consuming corn oil leads to greater plasma lutein increases than beef tallow (94). It should be noted that, in the former study, β-cryptoxanthin was better absorbed than lutein and zeaxanthin regardless of fat type and amount (93).
Just as dietary lipids enhance intestinal uptake of carotenoids, the consumption of unabsorbable, fat-soluble compounds may reduce carotenoid absorption (95). For example, an earlier study found that olestra (sucrose polyester) treatment for 16 wk significantly decreased circulating β-carotene, lutein, and zeaxanthin concentrations by 21–29% (96), consistent with another 8-wk study showing even greater decreases (50–85%) in circulating carotenes and xanthophylls (97).
Delivery/food matrix
A number of studies have shown that food matrix is also an important determinant of carotenoid bioavailability. For carotenes, lycopene from fresh red tomatoes is generally less bioavailable than pure lycopene in oil or processed tomato products, and tomato juice lycopene is less bioavailable than from other products, such as tomato sauce and soup (25, 98–100). Lycopene in processed products may be more bioavailable than that in raw tomatoes due to a softer food matrix. Lycopene in tomato juice may be less bioavailable than from other processed products because of the lower lipid content in juice (99). Although cooking, heating, or mechanical or enzymatic processing may also make xanthophylls more accessible by softening the tissue matrix (101), this has been less studied in humans. However, β-cryptoxanthin bioavailability was greater from pasteurized orange juice than from fresh oranges (102).
A recent hypothesis suggests that carotene bioavailability from foods is also affected by the storage form in the plant tissue (103). Papaya carotenes, in which β-carotene is found as “smaller liquid-crystalline deposits” and lycopene as “very small crystalloids,” are more bioavailable than carotenes from tomatoes or carrots, which accumulate carotenes in larger crystals and may be more resistant to micellarization (103).
Similarly, xanthophylls in lipid-rich food matrices seem to have greater relative bioavailability. For example, egg-borne lutein bioavailability is ∼3 times greater than that from spinach (104). Similarly, avocados are a highly bioavailable source of lutein (90, 105). Both avocados and eggs lead to 2–4 times greater increases in serum and retina lutein and zeaxanthin compared with other dietary sources (e.g., spinach) and supplements (105–107). In addition to lipids, dietary fibers (pectin, guar, alginate, cellulose, and wheat bran) were found to significantly reduce the bioavailability of lutein by 40–74% (108) in an acute dose study in 6 healthy women.
Supplements compared with food-borne carotenoids
The bioavailability of lutein and lycopene from foods compared with supplements has been compared. The bioavailability of lutein from supplements was similar to that of spinach and lower than that from eggs (104). However, 2 earlier studies found that a lutein supplement led to greater serum lutein increases than did spinach (19, 109). These results may have differed due to differences in the amount of co-consumed lipid and the cooking method. The bioavailability of supplemental lycopene was greater than from tomato juice and similar to that from tomato soup (25). Therefore, the relative bioavailability of carotenoids from foods may be greater or less than supplements depending on the specific food product. Furthermore, the supplement matrix formulation also affects carotenoid bioavailability (110, 111).
Interactions with other carotenoids, nutrients, and dietary compounds
A series of human and cell culture studies have explored the impact of carotenoid–carotenoid and carotenoid–nutrient interactions on carotenoid absorption. In humans, lutein decreased the absorption of β-carotene by 34% in subjects provided with equal amounts (15 mg) of β-carotene and lutein, whereas lycopene had no effect (112). However, when modeled in vitro, lycopene provided in excess (5 μmol/L) of β-carotene (1 μmol/L) inhibited the absorption of β-carotene, whereas lutein and α-carotene had no effect (113). In contrast, an in vitro study found that co-administration of lutein and β-carotene at equal concentrations (0.45 μmol/L) mutually decreased absorption by more than half (114). At this point, it seems that ratios and concentrations of carotenoids may be important underlying factors in carotenoid-carotenoid interactions for absorption.
Other nutrients and dietary compounds may also inhibit carotenoid absorption. A recent meta-analysis of 41 randomized controlled trials of plant sterol and stanol consumption suggested that these compounds significantly decreased plasma β-carotene, α-carotene, and lycopene by 12–16%, and these reductions were not explained by changes in circulating cholesterol (115). Dietary intake of divalent minerals (e.g., calcium, magnesium, and zinc) may also impede carotenoid bioaccessibility by causing insoluble lipid-soap complex formation and reducing carotenoid solubility (116). However, the effect of calcium on carotenoid bioavailability differed by food source and carotenoid, with supplemental calcium decreasing lycopene bioavailability from tomato paste by 83% (117), whereas there was no effect on absorption of spinach-borne lutein, β-carotene, or β-cryptoxanthin in another study (118).
Medications
Because carotenoids circulate exclusively in lipoproteins (12), the effect of statins, a type of blood cholesterol lowering–drugs, on blood carotenoid concentrations has been investigated. A current randomized, double-blind, placebo-controlled study in middle-aged hypercholesterolemic men found that simvastatin treatment (40 mg/d) for 6 wk significantly reduced total cholesterol and LDL cholesterol and consequently reduced circulating carotenes (β- and α-carotene and lycopene) by 5.2% (P = 0.05) and xanthophylls (lutein, zeaxanthin, and β-cryptoxanthin) by 21% (P < 0.001) (119). However, when adjusted for blood cholesterol concentration, the carotenes were markedly increased with simvastatin treatment (P < 0.01 for all), whereas lutein increased to a lesser degree and β-cryptoxanthin was unchanged. A longer-term, open-label, uncontrolled study of 52 wk involving simvastatin and atorvastatin (n = 104) in Finland reported a short-term reduction in unadjusted plasma β-carotene after 12 wk, but no effect by 52 wk (120). More data are needed in order to generalize the effect of statins on circulating carotenoids.
Drugs that block absorption of lipids from foods also reduce serum concentrations of carotenes. Orlistat, a lipase inhibitor, significantly reduced plasma α- and β-carotene after 4.5 mo of treatment by 45% and 32%, respectively, whereas effects on lycopene were only seen at 3 mo (49% reduction), and xanthophyll concentrations were unchanged (121).
Smoking
Cigarette smokers generally have lower serum carotenoid concentrations than nonsmokers, as a result of lower fruit and vegetable consumption (122–126) and possibly because tobacco smoke can directly degrade carotenoids (127–129). In vitro studies indicate that cigarette smoke can chemically modify carotenoids (127). Both serum and plasma β-carotene have been reported to be lower in smokers in a number of studies (129–135), but smoking is inconsistently associated with reduced blood lycopene concentrations (129, 130, 134–139). Smokers have a greater ratio of serum cis to trans lutein and zeaxanthin than do nonsmokers (129), suggesting that smoking induces in vivo isomerization of xanthophylls (77). Smoking remains a valid variable for continued consideration in population and experimental study design and analysis. The effect of vaporized nicotine inhalation (“vaping”) on carotenoid status is unknown at this time.
Alcohol intake
Alcoholic beverage consumption may affect the rate of hepatic clearance of drugs and phytochemicals. Several epidemiologic studies have shown that alcohol consumption is generally associated with lower serum carotenoids (129, 140–144). However, alcohol intake was positively associated with serum α-carotene and lycopene in healthy, postmenopausal women (145), but not with other major carotenoids. Carotenoid intake is lower in alcohol consumers (146–148), but alcohol intake may also affect carotenoid status through biochemical interactions. Moderate alcohol intake is associated with changes in blood lipoprotein concentrations, whereas chronic excessive alcohol intake is associated with oxidative stress and a decrease in antioxidant enzymes (149–152). Animal studies suggest that alcohol intake changes both vitamin A and carotenoid storage and metabolism (153, 154). The evidence for the specific effect of alcohol consumption on carotenoid status in humans is mixed at this time.
Intrinsic, physiologic variables
Baseline vitamin A status
Recent evidence suggests that provitamin A carotenoid absorption and bioconversion are regulated by vitamin A status (155). Although the absorption of preformed dietary vitamin A is fairly constant in humans, ranging from 77% to 99% in healthy children (156), carotenoid absorption is heterogeneous. For instance, the bioavailability of pure 13C-lycopene in oil had a CV of 73% (14), bioconversion of pure D6-β-carotene to vitamin A had a CV of 44% in healthy adults (157), and absorption of crystalline β-carotene had a CV of 137% in adults (158). One source of this variability may be linked to vitamin A status. Mechanistic studies suggest that vitamin A status regulates SCARB1-mediated uptake of β-carotene, as well as bioconversion of β-carotene to vitamin A in the enterocyte (155). Specifically, retinoic acid negatively regulates carotenoid absorption and bioconversion by binding to retinoic acid receptor (RAR), which heterodimerizes with retinoid X receptor to bind a response element–inducing expression of intestine specific homeobox (ISX). ISX is a homeodomain transcription factor that represses the expression of Bco1 by binding its promoter. In addition, ISX expression is also associated with repressed SCARB1 expression (155). Thus, low vitamin A status reduces retinoic acid availability, increasing expression of SCARB1 to promote intestinal uptake of carotenoids and of BCO1 to produce vitamin A.
One earlier clinical study provides support for this model of product-mediated negative feedback regulation of β-carotene absorption and bioconversion. Specifically, subjects with greater prestudy intakes of vitamin A and β-carotene absorbed less D6-β-carotene and bioconverted it to vitamin A at a lower rate than subjects with lower prestudy vitamin A and β-carotene intakes (158). Thus, it is plausible that the low responders had greater initial vitamin A status than the high responders, resulting in lower β-carotene uptake and conversion to vitamin A. However, prestudy blood β-carotene and vitamin A, or prestudy vitamin A total body stores, were not measured. Evidence of the regulation of β-carotene bioconversion was also indicated in a more recent study in which doubling the amount of deuterium-labeled β-carotene (from 20 to 40 mg) doubled the plasma AUC for β-carotene, a measure of absorption, but only increased the plasma-labeled retinol AUC by 36% (83), suggesting that bioconversion of high β-carotene doses is regulated.
Baseline carotenoid status
For clinical studies of carotenoid absorption, it is common to have a “wash-out” period, generally 2 wk in duration, to decrease circulating carotenoid concentrations [see example in Allen et al. (159)]. This may be important, given that the degree of change in blood carotenoids may be inversely correlated with the initial blood concentrations. This was shown in an earlier study in 56 breast cancer survivors who consumed a high-fruit and -vegetable diet for 3 y, where the changes in blood lycopene, α-carotene, and β-carotene were significantly inversely correlated with the subjects’ baseline blood carotene concentrations (160). A recent analysis of prostate cancer (PCa) patients’ blood carotene responses to a 3-wk tomato juice intervention also showed that baseline blood lycopene, phytoene, and phytofluene concentrations were significantly (P < 0.001) inversely predictive of the change in plasma concentrations of those carotenes (161). The mechanisms by which baseline status of non–provitamin A carotenoids may affect absorption are unknown.
Hormonal status
As metabolic regulators, hormone fluctuations may affect carotenoid status. Limited studies that used carotenoid-controlled diet interventions and prospective cohorts have shown cyclical carotenoid fluctuations correlating with menstrual cycle hormonal fluctuations (162–164). In premenopausal women, lycopene, β-carotene, and lutein fluctuated by ∼5% across the menstrual cycle (164). Although the fluctuation patterns differed slightly between carotenoids, there was a small increase in lutein and β-carotene during the ovulatory phase, and all carotenoids were lowest at menstruation, even when correcting for cyclical fluctuations in blood cholesterol concentrations (164). An earlier, smaller study reported changes in circulating carotenoids over the menstrual cycle, but these were not significant when adjusted for cholesterol (165). Neither study controlled for carotenoid intake. Variations in circulating androgens during the menstrual cycle have been associated with blood carotenes, with testosterone being negatively associated with blood lycopene and β-carotene, and positively associated with lutein (P < 0.05), and luteal progesterone being associated with β-carotene (164). These cyclical effects may be important for minimizing variability in small studies and may shed light on oxidative stress (164) or carotenoid metabolism fluctuations across the menstrual cycle.
One study has suggested that age-related hormonal changes were associated with blood carotenoids. In Italian women aged ≥65 y, circulating β-carotene was inversely associated with estradiol (P = 0.01), independent of other predictors for estradiol [including BMI, testosterone, C-reactive protein (CRP), and lipid intake], whereas other carotenoids assessed had no association (166). The cause of this association is unknown.
Although, to our knowledge, no studies have assessed the influence of testosterone on carotenoid status in men, multiple studies have found that circulating carotenes and xanthophylls are higher in women than in men (130, 133, 167, 168). In addition, a study in castrated (in which the testicular source of endogenous androgen is removed) compared with intact rats indicated that low androgen status promotes liver lycopene accumulation with no impact on serum lycopene (169). A recent controlled-feeding study found that women had greater plasma xanthophyll, but not carotene, responses to fruit and vegetable consumption (168). However, in the Framingham Heart Study cohort, analysis of plasma carotenoids by quintile of carotenoid intake showed similar blood-diet relations for lutein and zeaxanthin in men and women, but an apparent greater response among women for dietary β-cryptoxanthin (170). These findings may suggest a complex relation between sex hormone status and carotenoid biodistribution.
Body composition
Consistent evidence indicates that body composition is associated with carotenoid status. Body fat mass was inversely correlated with plasma carotenoid concentrations in older women but not in younger adults or older men (171). As with carotenes, greater body fat is associated with lower serum xanthophylls (130, 143, 145, 171–174). Furthermore, multiple studies have shown measures of abdominal adiposity (waist circumference, waist:height, and waist:hip) to inversely correlate with blood carotenoids (174–177). Body fat distribution may affect distribution of xanthophylls to tissues, because MPOD is also inversely related to body fat (172, 178–181), with abdominal fat in particular being inversely related to MPOD (180). Consistently, greater BMI is associated with lower circulating carotenoids in both children and in older adults (182–185), although not all studies took carotenoid intake differences into account. These relations may be due to greater fat mass being associated with more oxidative conditions that decrease circulating carotenoid concentrations, or abdominal adipose acting as a sink for circulating carotenoids. Indeed, among various body fat sites, carotenoid accumulation is the greatest in abdominal fat (186). The practical implication of these relations was recently shown, such that the predictive ability of FFQ-estimated carotenoid intake for circulating carotenes was weaker in overweight and obese subjects than in normal-BMI subjects (184). In sum, recent associative studies are consistent with previous findings of inverse associations between BMI and circulating carotenoids.
Inflammatory status and associated disease
Inflammation and oxidative stress are thought to contribute to the pathogenesis of both CVD and type 2 diabetes. Therefore, it may be that carotenoid status differs in patients with these conditions compared with healthy counterparts. Although the directionality of this relation is not clearly understood, a number of studies have shown lower circulating carotenoids in patients with CVD (187). Interestingly, in 1 study, among the major carotenoids, only xanthophylls were significantly lower in patients with coronary artery disease (188). Indeed, circulating xanthophylls are inversely associated with markers of inflammation, including circulating CRP and IL-6 concentrations, and β-cryptoxanthin is specifically inversely associated with circulating fibrinogen, an acute-phase protein that is elevated in inflammation (141, 189, 190). Similarly, serum xanthophyll and carotene concentrations are inversely associated with type 2 diabetes and impaired glucose metabolism (191, 192). In addition to lower serum lutein and zeaxanthin, MPOD is lower in type 2 diabetics than in type 1 diabetics and normal controls (193).
Malabsorption syndromes, including inflammatory bowel disease and celiac disease, as well as pancreatic insufficiency from cystic fibrosis, are associated with lower serum lutein and zeaxanthin concentrations as well as MPOD in adults (194, 195). Patients with chronic cholestasis, which leads to fat malabsorption, have similar serum β-cryptoxanthin concentrations unlike the other carotenoids (196), suggesting that β-cryptoxanthin may be the most efficiently absorbed carotenoid, even in cases of general malabsorption.
Pregnancy and lactation
Pregnancy and lactation induce profound physiologic and metabolic changes, and a limited number of reports indicate that serum and plasma carotenoids differ throughout pregnancy or during lactation. A study in Peruvian women found that they had greater serum α- and β-carotene, lycopene, and lutein and zeaxanthin, but not β-cryptoxanthin, in the third trimester of pregnancy than in the first and second trimesters (197). This agrees with the findings of others (198), although it is generally unclear if this phenomenon is due to a physiologic or a dietary change. A small study (n = 21) followed women over the first 19 d postpartum and found that although plasma xanthophylls decreased over that time, α-carotene, total β-carotene, and lycopene did not change (199). Correspondingly, colostrum collected 4 d postpartum contained greater xanthophylls and carotenes than mature milk collected 19 d postpartum (199). The causes and significance of circulating carotenoid changes during pregnancy and lactation are poorly understood at this time but may be important for maternal and infant nutrition.
Age
A large study in adults (35–74 y of age) found that age was inversely associated with circulating carotenes but positively associated with β-cryptoxanthin and lutein and zeaxanthin concentrations. Advanced age remained a predictor of lycopene and α-carotene concentrations but not of other carotenoids when controlling for blood cholesterol concentrations, BMI, diet, vitamin supplements, sex, smoking, country, and season (134). How age in early life affects circulating carotenoids is not well understood at this time but does not seem to profoundly differ from adults according to NHANES 2001–2002 data (200). The causes of age associations with carotenoid concentrations in the blood are not yet fully understood.
Microbiome
At this time, the impact of the intestinal microbiota on carotenoid absorption has not been thoroughly investigated. The majority of carotenoid absorption is believed to occur in the small intestine (201), whereas the majority of intestinal microbial fermentation occurs in the colon. One study reported that germ-free rats absorbed more α- and β-carotene and had greater liver vitamin A than rats with humanized microbiota (202). Yet, there was no marked degradation of these carotenes when incubated 72 h with either human fecal anaerobic or aerobic bacteria (202), refuting the hypothesis that bacterial carotene metabolism led to a decrease in carotene absorption. Thus, the presence or absence of the microbiome may have an indirect effect on carotenoid absorption. The effects of the gut microbiome on xanthophyll metabolism remain unknown.
Genetic variables associated with blood and tissue carotenoid concentrations
Current advances in human genetics have provided more concrete sources of “host”-associated variables affecting carotenoid absorption and bioavailability (203, 204). Indeed, a recent study in children found blood α- and β-carotene concentrations to be highly heritable (205). Studies have shown variants in genes, such as single nucleotide polymorphisms (SNPs)—a type of single nucleotide variant with a minor allele occurring in ≥1% of the population (206)—and haplotype polymorphisms, to be associated with plasma carotenoids. A haplotype polymorphism refers to a particular combination of SNPs inherited together because of genetic linkage (207). In addition to showing candidate genes involved in carotenoid assimilation, genotype-phenotype studies have shown genetic variation as a determinant of physiologic responses to carotenoids. To date, the most frequent associations with the strongest mechanistic plausibility have been for BCO1 and SCARB1 variants. However, there is an ever-growing number of novel gene-carotenoid relations.
BCO1 variants
A number of studies have tied BCO1 variants with circulating carotene concentrations. An early study of the nutrigenetics of carotenoid status found β-carotene bioconversion efficiency to vary markedly between healthy individuals (208), prompting the hypothesis that polymorphisms in BCO1 could contribute to the “poor converter phenotype” (208). A rare missense mutation in BCO1 reduces BCO1 activity by ≤90% and results in carotene accumulation (209). Specific BCO1 variants have been associated with differences in circulating carotenoids. In a targeted study of 224 BCO1 SNPs in women (n = 2344) of European descent, a number of SNPs were significantly associated with plasma concentrations of α- and β- carotene, β-cryptoxanthin, and lutein and zeaxanthin (210). It is hypothesized that these variants in BCO1 alter BCO1 enzyme activity, either through reduced BCO1 expression or catalytic efficiency, ultimately affecting bioconversion (211). Similarly, BCO1 polymorphisms are also associated with serum and plasma xanthophyll responses (203, 212) and MPOD (204). A genomewide association study (GWAS) in Italian adults also found elevated β-carotene to be associated with the minor allele of rs6564851 (203), an SNP in the ISX-binding regulatory site upstream of the BCO1 gene (213). The minor allele of rs6564851 is hypothesized to reduce BCO1 activity, resulting in higher circulating intact β-carotene (213). For a recently updated list of specific SNP-carotenoid associations, see Bohn et al. (11).
As described above, BCO1 is also indirectly involved in carotenoid absorption. Therefore, the effects of BCO1 variants reducing cleavage activity and retinoic acid generation may increase intestinal carotenoid absorption due to greater SCARB1 expression. Mechanistic studies will continue to shed light on the effects of BCO1 variations on carotene metabolism.
SCARB1 variants associated with blood carotenoid concentrations
Several studies have shown associations between SCARB1 variants and carotene status. SCARB1 is a membrane receptor involved in the uptake of cholesterol, vitamin E, and carotenoids through cell plasma membranes from HDL cholesterol (214), with SCARB1 overexpression inducing from a 1- to 2-fold increase in provitamin A carotenoid uptake compared with controls (215). A genomewide array of 7 million SNPs in African, Hispanic, and European Americans found lycopene concentrations to be decreased in SCARB1 rs1672879, rs701107, and rs838861 minor allele carriers (216), but interestingly, subanalysis showed that these minor alleles were so uncommon in European Americans that they were not predictors of serum lycopene in that population (216). A focused study of 47 SCARB1 SNPs showed a 24% increase in serum lycopene with each SCARB1 rs11057841 minor (T) allele (217). Men homozygous for the SCARB1 rs2706295 T allele had 100% higher α-carotene and 50% higher β-carotene concentrations than those who were homozygous for the C allele (214). Some SCARB1 and CD36 SNP genotypes are associated with lower plasma concentrations of lutein, zeaxanthin, and β-cryptoxanthin (214, 217–219). Several SNPs in SCARB1 are also associated with lower MPOD in women (204). Mechanisms for how these SCARB1 variants affect carotenoid uptake are currently unknown.
Lipid and lipoprotein metabolism-related genes
Given that carotenoids are transported on lipoproteins, proteins involved in their assembly and metabolism are likely to influence carotenoid responses. Variants in hepatic lipase C (LIPC), ATP-binding cassette transporter (ABCA1), microsomal TG transfer protein (MTTP), NPC1L1, LPL, and cholesteryl ester transfer protein (CETP) genes have shown variable associations with carotenoid status (212, 214, 220–223). SNPs in ABC transporters involved in cellular cholesterol efflux are associated with differences in serum and plasma lutein and zeaxanthin responses, postprandial chylomicron lutein response (219, 223, 224), and MPOD (204). SNPs in APOA1 and APOB, the major apolipoproteins in HDL and LDL cholesterol, respectively, and LIPC are associated with postprandial chylomicron lutein response in men (223); however, the authors did not indicate the direction of the associations. SNP genotypes in LIPC are also associated with lower MPOD (204). Genetic polymorphisms in LPL are associated with lutein (direction not indicated) (223) and lower β-cryptoxanthin serum concentrations (225).
Other gene variants associated with carotenoid status
Associations of many other gene variants with blood carotenoids have emerged, although the underlying mechanisms are poorly understood (11). A GWAS in Amish adults showed that the rs7680948 minor allele in SET domain–containing lysine methyltransferase 7 (SETD7) was associated with lycopene (226). SETD7, a histone methyl transferase, is a central transcriptional activator of various genes (226). Another GWAS found 4 SNPs in Slit guidance ligand 3 (SLIT3) and 3 SNPs in the dehydrogenase/reductase 2 (DHRS2) to be associated with lycopene in African Americans (216). SLIT3 is a molecular guidance cue in cellular migration, whereas DHRS2 codes for a NAD/NADP-dependent oxidoreductase that lessens the breakdown of steroids, retinoids, and prostaglandins (216). ELOVL fatty acid elongase 2 (ELOVL2) rs37989709 and rs9468304 were associated with both lycopene and β-carotene blood responses to ingestion of a carotenoid-rich meal (221, 227). ELOVL2 catalyzes the elongation of EPA to docosapentaenoic acid and docosapentaenoic acid to DHA. A combination of 5 SNP genotypes in CD36 present in 29% of the European subjects (n = 312) was associated with 12% greater plasma α-carotene compared with the most frequent CD36 haplotype (215). A number of polymorphisms in other genes with unknown relations to carotenoids have also been identified in association with postprandial chylomicron lutein response. These genes include melanocortin 4 receptor (MC4R) and insulin-induced gene 2 (INSIG2), which both have SNPs associated with obesity (223). Other genes associated with lutein response are ELOVL2 (223, 228) and cordon-bleu WH2 repeat protein like 1 (COBLL1) (223, 228). Polymorphisms in COBLL1 are also associated with lower serum insulin concentrations and lower insulin resistance (229). SNPs in another gene, polycystin 1–like 2 (PKD1L2), located directly upstream from BCO1, have also been associated with differential lutein response (223). Variants in ISX, an upstream regulator of SCARB1 and BCO1, have been associated with differences in postprandial chylomicron lutein response in men (223). In addition, retinoid isomerohydrolase (RPE65), which encodes an enzyme responsible for the conversion of all-trans retinyl esters to 11-cis retinol in the visual cycle, has SNPs associated with serum and retinal xanthophyll status (204, 228). RPE65 shares overall sequence similarities with carotenoid oxygenases (230) and was recently discovered to catalyze the isomerization of lutein to meso-zeaxanthin (231). As more gene-carotenoid relations emerge, experiments to validate and explain the mechanistic drivers of these relations will provide insight into how genetic variation may affect carotene status and health.
Health Aspects of Carotenes
Introduction
Carotene exposures are epidemiologically monitored and studied in the context of a wide number of health conditions, leading to a number of subsequent intervention and mechanistic trials. The following sections summarize the major conditions for which carotenes have been studied. β-Carotene and lycopene are the most abundant carotenes in our diet (232) and our circulation and will be the primary focus of this section.
β-Carotene and other provitamin A active carotenes
Although carotenoids are well-recognized antioxidants [reviewed in Böhm et al. (233)], the primary function of provitamin A carotenoids is for the provision of vitamin A. Almost a century ago, it was shown that yellow carotene pigments from plants, butter fat, and egg yolk are converted to vitamin A. Early findings established the structure of β-carotene, the mechanisms of its cleavage, and the basic functions of vitamin A [for recent review see Tanumihardjo et al. (2)]. For populations who do not consume animal sources of preformed vitamin A, carotenoids are required to meet vitamin A needs.
Vitamin A deficiency is a major worldwide nutrient deficiency, prevalent in poor countries in which an estimated 19 million pregnant women are vitamin A deficient (234). Symptoms include night blindness, xerophthalmia (eye disease that can progress to blindness), and a reduced ability to fight infections. An estimated 10 million preschool-age children and pregnant women develop blindness from xerophthalmia yearly; however, the lives of an estimated 50% of severely deficient children are saved by vitamin A–intervention programs (235).
The primary functions of vitamin A include vision, maintenance of epithelial cells, and reproductive function. Visual function relies largely on the 11-cis retinal metabolite. The visual cycle was originally elucidated by Wald, for which he received the Nobel Prize in 1967 [reviewed in Tanumihardjo et al. (2) and Eroglu and Harrison (236)]. Other vitamin A functions are driven by the metabolites all-trans or 9-cis retinoic acid, which regulate hundreds of metabolic pathways once they are bound to one of several nuclear receptors (RAR and retinoid X receptor). The interested reader is directed to in-depth reviews of the activity of vitamin A and the mechanisms of retinoid signaling (2, 237–239).
The current dietary requirements of healthy individuals for vitamin A were established in 2001 by the Food and Nutrition Board of the National Academy of Sciences (232). Retinol activity equivalents (RAEs) were used to assign differing vitamin A values to preformed vitamin A and provitamin A carotenoids. One RAE was defined as 1 µg of all-trans retinol; however, to account for the inefficient absorption from foods and conversion to vitamin A, 1 RAE = 12 µg β-carotene and 1 RAE = 24 µg α-carotene and β-cryptoxanthin. Although carotene bioavailability and bioefficacy differ with dietary sources and food preparation and differ between individual consumers, the RAEs reflect the best estimates of provitamin A bioefficacy from different foods based on the available data at that time.
Carotenes as antioxidants or pro-oxidants and lung cancer risk
Carotenoids are antioxidants, and substantial in vitro work suggests that carotenes are excellent free radical and singlet oxygen quenchers but may also show some pro-oxidant behaviors [reviewed in Böhm et al. (233)]. One long-standing hypothesis is that these attributes may confer protection against various chronic diseases of aging linked with oxidative stress. However, many in vitro studies are carried out at supraphysiologic concentrations. In addition, other antioxidants are in higher concentrations in human serum and tissues, with serum vitamin C and vitamin E concentrations being ∼30- and 50-fold higher than β-carotene [Appendix tables in (167)] in American men. Thus, the in vivo antioxidant function of carotenes remains unclear.
Early associations between diets high in β-carotene and lower risk of cancers (240) spurred β-carotene supplementation trials in populations at a high risk of lung cancer. The Alpha-Tocopherol and Beta-Carotene Cancer Prevention Trial (ATBC) provided male smokers with daily placebo, 50 mg dl-α-tocopherol acetate, 20 mg β-carotene, or both (241), and the β-Carotene and Retinol Efficacy Trial (CARET) tested 30 mg β-carotene/d and 25,000 IU retinyl palmitate/d in smokers or asbestos-exposed workers (242). Unfortunately, β-carotene supplementation increased lung cancer risk by 16% and 28%, respectively. It is speculated that relatively high daily doses of β-carotene (∼10 times greater than dietary intake), the formation of potentially carcinogenic oxidation products of β-carotene in lung tissue as a result of cigarette smoke, asbestos exposure, or a combination of these may have resulted in procancer effects (243, 244). Remarkably, food-borne β-carotene intake remained inversely associated with lung cancer risk in a recent meta-analysis of 9 studies (4).
The pro–lung cancer effect seems unique to β-carotene supplements. The Age-Related Eye Disease Study 2 (AREDS2) trial found a small increase in lung cancer incidence, mostly in former smokers, with β-carotene supplementation (15 mg/d) compared with no β-carotene (245), whereas lutein (10 mg/d) plus zeaxanthin (2 mg/d) supplementation had no effect. Similarly, although ferrets exposed to cigarette smoke and high β-carotene developed precancerous lung lesions (246), lycopene showed some protection (247).
There are no other reported adverse effects of dietary β-carotene, except for carotenemia, a condition marked by yellowing of the skin due to elevated concentrations of β-carotene (or other carotenoid) accumulation in dermal tissues when consumed in high concentrations (248). This condition is common in young children who frequently consume pureed carrot or winter squash and is reversed over time with decreased carotenoid intake.
β-Carotene and cardiovascular health
Higher intakes of β-carotene–rich fruit and vegetable have generally been associated with lower CVD risk. For example, a series of current studies prospectively followed the relation of serum β-carotene and CVD risk in >1000 Finnish men in the Kuopio Ischemic Heart Disease Risk Factor (KIHC) cohort for ≥15 y and found that low serum β-carotene concentrations were strongly related to the risk of CVD mortality (249), congestive heart failure (250), and sudden cardiac death (251). An analysis of the earlier literature has been previously published [reviewed in Sesso (7)].
Lycopene
Lycopene and risk of PCa
PCa is the most frequently diagnosed cancer in US men and the fifth leading cause of cancer-related deaths worldwide (252). A number of epidemiologic trials carried out over the past 2 decades have evaluated tomato product intake or serum lycopene concentrations and the risk of PCa. Recent systematic reviews and meta-analyses have supported an inverse relation between blood lycopene or lycopene intake with prostate cancer risk or severity (5, 253, 254). For an in-depth review of earlier preclinical and clinical research on lycopene and PCa see Holzapfel et al. (255).
The hypothesis that dietary tomato (the major source of US dietary lycopene) or lycopene may reduce PCa risk is supported by several preclinical animal studies evaluating prostate carcinogenesis and tumor progression. Tomato powder feeding improved survival in a model of chemically driven carcinogenesis (256) and decreased tumor size in a model of tumor progression (257). Another study showed overlapping effects of lycopene and tomato powder feeding on gene expression changes in early carcinogenesis, especially related to androgen metabolism (258). Most recently, lycopene or tomato powder inhibited the progression of genetically driven carcinogenesis in mice (51, 259). Additional tomato and lycopene studies have been conducted [discussed in Tan et al. (51)], providing insight into the scenarios in which these interventions may be effective.
Lycopene metabolites may be biologically important [reviewed in Erdman et al. (260) and Lindshield et al. (261)] and have been identified in tomato products, rodent tissues, and in human blood (262–264). Lycopene metabolites may compete with retinoids as ligands for nuclear receptors and either act as agonists or antagonists for gene expression and affect metabolic pathways (265). Recent findings suggest that BCO2-generated lycopene metabolites contribute to lycopene's anticancer activity (51), such that the BCO2 gene was necessary for maximal anticancer efficacy of lycopene and tomato powder feeding. A recent study showed a lycopene metabolite, apo-10′-lycopenoic acid, could reduce hepatic tumorigenesis, hepatic inflammation, and steatosis (266).
Because of the long latency of PCa, there have not been any long-term clinical lycopene intervention trials with PCa incidence as a primary endpoint. A series of recent phase II trials in men either at high risk of PCa or with early PCa showed lycopene consumption to be associated with stabilized disease (267), less extensive high-grade prostatic intraepithelial neoplasia (a precancerous lesion) (268), or that prostatic lycopene concentrations were inversely predictive of progression to cancer (269). These and other recent small trials, suggest modest impacts of lycopene on PCa progression in high-risk men, warranting larger and longer studies.
Lycopene and CVD
On the basis of epidemiologic studies that have largely supported a reduced risk of CVD with higher consumption of tomato products, >50 human intervention trials with lycopene supplements or tomato-based products have been conducted; however, the majority were statistically underpowered [reviewed in Thies et al. (270)]. A current review of clinical trials concluded that although there is a need for more targeted research, the results support the consumption of a healthy, low-saturated-fat diet containing tomato-based foods as a first-line approach for promoting CVD health (8). They noted that lycopene supplements were impactful for blood pressure management, whereas tomato-based foods were a preferred approach for other CVD risk factors. However, many of the beneficial effects seen in clinical trials were with nonvalidated CVD risk markers, such as lipid peroxidation, DNA oxidative damage, platelet activation, and inflammatory markers [reviewed in Thies et al. (270)]. Some studies found beneficial impacts on blood lipids, CRP, and blood pressure (270). Another recent review concluded that most studies showed beneficial cardioprotective effects in subjects who were “antioxidant deficient” (271).
Safety of lycopene
One systematic risk assessment of lycopene supplements used in placebo-controlled intervention trials has been published (272). For inclusion, the trials must have been carried out for ≥1 wk and with doses >8 mg/d. The only documented side effect of lycopene supplementation in the 16 trials was carotenodermia. Although the authors stated that the absence of any pattern of adverse effects “provides support for a high level of confidence in the safety of this compound,” they also acknowledged that there were no human studies published that focused specifically on the safety of lycopene supplementation (272).
Phytoene, phytofluene, and α-carotene
Three lesser-studied carotenes, phytoene, phytofluene, and α-carotene, may confer some bioactivities. Phytoene and phytofluene are colorless carotenoids found in tomatoes as well as in some other fruit and vegetables [reviewed in Engelmann et al. (273)] but are relatively understudied. Short-term phytofluene, lycopene, or tomato powder supplementation reduced serum testosterone in rats compared to control-fed rats (274), a potential mechanism by which tomato consumption may reduce the risk of PCa (258, 275). Unique from other common dietary carotenoids, phytoene and phytofluene maximally absorb light in the UV-B and -C and UV-A ranges, respectively, and so may contribute to skin protection from UV light exposure. Although the protection does not approach the efficacy of sunscreen [reviewed in Stahl and Sies (276)], it was shown in humans that daily tomato extract consumption for 12 wk conferred greater protection against UV-induced erythema lycopene alone (277). The authors speculated that phytofluene and phytoene in the extract may have contributed to the protection by reactive oxygen species scavenging or UV light filtering (276). Similarly, tomato paste supplementation for 12 wk in women reduced markers of skin damage after UV radiation (278).
Many high β-carotene fruit and vegetables also contain α-carotene; thus, one might expect similar correlations for both carotenoids with chronic disease risk. One recent nested case-control study found that both plasma α- and β-carotene were associated with a lower risk of estrogen receptor–negative breast cancer tumors (279), which was similarly found in a 20-y follow-up of the Nurses’ Health Study (280). A recent systematic review found that blood concentrations of α-carotene, β-carotene, total carotenoids, and retinol were all significantly inversely associated with lung cancer risk or mortality (4).
Health Aspects of Xanthophylls
Lutein and zeaxanthin
Given lutein’s and zeaxanthin's antioxidant and anti-inflammatory properties (281), it is hypothesized that these carotenoids may also have beneficial effects on diseases for which oxidation and inflammation play a role.
Eye diseases
Current evidence supports a role for lutein and zeaxanthin in the development of common age-related diseases [AMD, cataract, diabetic retinopathy (DR)], retinopathy of prematurity (ROP), and retinitis pigmentosa (RP) (282). Lutein and zeaxanthin preferentially accumulate in primate ocular tissue and are the exclusive carotenoids in the retina and lens (283). This selectivity, as well as their known functions as antioxidants (284), anti-inflammatories, and blue light filters (285), supports their role in ocular health. Readers may refer to a current review of lutein and eye diseases (9).
AMD
AMD is a disease affecting the central area of the retina, resulting in an irreversible loss of central vision. AMD is a major cause of blindness in persons aged >40 y in the United States (286). Although the specific pathogenesis of AMD is still unknown, chemical and light-induced oxidative damage to the photoreceptors may be involved. The retina is particularly susceptible to oxidative stress because of its high consumption of oxygen, its high proportion of easily oxidized PUFAs, and its exposure to visible light. A current meta-analysis found that lutein and zeaxanthin intake was inversely related to the risk of advanced AMD (287), and supplementation of patients with AMD improves contrast sensitivity and visual function in a dose-dependent manner (288–290). However, these relations were not present in every study (291–293), perhaps due to between-population variability in dietary intake of lutein and zeaxanthin or amounts reaching the eye. AREDS2 found that participants with low dietary intakes of lutein and zeaxanthin at the start of the study, but who took an AREDS formulation with lutein and zeaxanthin during the study, were ∼25% less likely to develop advanced AMD compared with participants with similar dietary intake who did not take lutein and zeaxanthin (245).
Cataracts
Cataracts are the clouding of the lens leading to a decrease in vision and are the leading cause of blindness in the world (294). Among the carotenoids, only lutein and zeaxanthin are found in the lens (295). A current meta-analysis, which included 1 cohort and 7 cross-sectional studies, reported that there were significant inverse associations between nuclear cataract and blood lutein and zeaxanthin concentrations, with the pooled RRs ranging from 0.63 (95% CI: 0.49, 0.77) for zeaxanthin to 0.73 (95% CI: 0.59, 0.87) for lutein (296).
DR
DR is the most common cause of acquired blindness in individuals between the ages of 20 and 65 y, and rates are increasing with the rise in obesity and type 2 diabetes (297, 298). Given that oxidative stress and inflammation are believed to be involved in DR pathogenesis (297), the protective effects of lutein and zeaxanthin have been investigated. Although low dietary lutein intake is not associated with DR risk (299), MPOD was found significantly differ between healthy controls [0.29 + 0.07 density units (DUs)], diabetics without retinopathy (0.22 + 0.09 DUs), and diabetics with retinopathy (0.14 + 0.05 DUs) (P < 0.001) (300) and was inversely correlated with glycosylated hemoglobin (300). Support that lutein and zeaxanthin may provide a benefit in DR comes from a 3-mo supplementation study of lutein (6 mg/d) and zeaxanthin (0.5 mg/d) in subjects with nonproliferative DR and controls. Specifically, MPOD increased with supplementation, along with visual acuity and contrast sensitivity, and decreased foveal thickness (301).
Retinitis pigmentosa
RP, a group of rare inherited genetic disorders of rod and cone photoreceptors, is a leading cause of inherited blindness (23). Common symptoms include difficulty seeing at night and a loss of peripheral vision. In a randomized, controlled crossover study in RP patients, lutein supplementation (10 mg/d for 12 wk followed by 30 mg/d for 12 wk) had a highly significant effect in preserving visual field and the mean log retinal area was 0.29 higher than when receiving the placebo (P < 0.001) (302), whereas 20 mg of lutein supplementation did not improve central vision in patients with RP or Usher syndrome (303). One possible explanation for this is that only half the patients had significant increases in MPOD (303). In a randomized, controlled, double-masked 4-y-long trial in patients with RP, lutein (12 mg/d) added to vitamin A supplements modestly slowed the midperipheral loss (304). The lutein group lost, on average, 27 decibels/y, whereas the control group lost, on average, 34 decibels/y.
Retinopathy of prematurity
ROP is retinal neovascularization that leads to blindness in premature infants. The developing retina may be susceptible to oxidative damage due to its high proportion of long-chain PUFAs (305), exposure to damaging light, high oxygen fluctuation, and high metabolic activity promoting the production of reactive oxygen species (297). Current trials in term newborns showed that lutein supplementation lowers systemic oxidative stress (306, 307), and lutein along with β-carotene and lycopene supplementation lowered markers of systemic inflammation in preterm infants (308). One study indicated more rapid visual development with lutein, lycopene, and β-carotene supplementation (308). Alternatively, several small supplementation trials did not show a significant effect on ROP incidence (309–311).
Visual function
Increased lutein and zeaxanthin intake may improve visual performance in both healthy adults and in eye disease (312–315). Macular pigment may improve visual function through its light-filtering properties (314). Two placebo-controlled studies found that lutein supplementation alone or with zeaxanthin at 10–20 mg/d for 6–12 mo significantly improved contrast acuity thresholds when ambient illumination was low (316, 317), although the degree of the improvement varied with duration of the supplementation. Under the low-lighting (mesopic) condition, there were significant improvements, suggesting improved vision during night driving. Visual performance in glare conditions was improved in healthy subjects with lutein or lutein and zeaxanthin supplementation (315, 317). Lutein or combined lutein and zeaxanthin supplementation protected against the detrimental effects of long-term computer-display light exposure and improved contrast sensitivity (318), improved chromatic contrast and recovery from photostress (319), and reduced symptoms of visual fatigue associated with visual proofreading tasks in healthy subjects aged 22–45 y (320). In addition to light filtering, lutein and zeaxanthin may improve visual function through biological mechanisms, such as neuronal signaling efficiency in the eye (321).
Cognition
Recent epidemiologic and intervention studies suggest that dietary and serum lutein and zeaxanthin are associated with improved cognitive function during aging (322–325). Lutein and zeaxanthin preferentially accumulate in the human brain, accounting for 46–70% of total carotenoids (74, 75). Macular lutein and zeaxanthin concentrations are significantly correlated with concentrations in matched human and in nonhuman primate brain tissue (326, 327); therefore, MPOD is a biomarker of brain lutein and zeaxanthin. Indeed, several observational studies reported that MPOD and cognitive function were correlated in adult populations (328–332), and brain zeaxanthin concentrations of centenarian decedents were significantly associated with antemortem cognitive function (74). Brain lutein was significantly related to recall and verbal fluency, and although the associations were attenuated with adjustment for covariates, brain lutein was significantly lower in individuals with mild cognitive impairment compared with those with normal cognitive function (133 ± 21 compared with 67 ± 14 pmol/g, respectively; P < 0.05) (74). Last, in a 4-mo randomized, double-blind, placebo-controlled trial, lutein (12 mg/d), DHA (800 mg/d), or both significantly improved verbal fluency by 19–40% over baseline (depending on the intervention) in older women (P < 0.03) (333), whereas the combination significantly improved memory and rate of learning by ∼26% and 10%, respectively (P < 0.03).
Current evidence suggests a role for lutein in adult cognitive health, with emerging evidence showing that lutein accumulates in the brains of infants and children. Brain tissue from 30 infant decedents (334) showed significantly greater xanthophyll concentrations compared with carotenes. As in the adult brain (74), lutein was the major carotenoid (334). However, the relative contribution of lutein to total carotenoids was ∼2 times that of adults (58% compared with 31%, respectively), which may, in part, be due to the prominence of lutein in human milk (335, 336). Antioxidants are essential to the brain because of their high metabolic rate and the high proportion of oxidizable PUFAs, but the human newborn brain has a relative deficiency of endogenous antioxidant enzymes (337). This may be particularly important in the early neonatal period when oxidative stress may lead to pathological conditions. In a randomized, double-blind, placebo-controlled study in healthy term newborns, supplemental lutein significantly increased serum measures of antioxidant activity (306).
Lutein's role in brain function during early life has been a focus of recent research. It is hypothesized that macular lutein may facilitate brain development in early life by improving visual performance and, effectively, environmental inputs into the brain (338). Indeed, environmental enrichment exerts morphological and functional effects at the neuronal level and is accompanied by improvements in cognitive performance (339). A recent exploratory, observational study found that human-milk lutein and choline concentrations were associated with recognition memory in corresponding 6-mo-old infants (340). Indeed, infant brain lutein concentrations are correlated with metabolites involved in neurotransmission, neuronal proliferation and maturation, neurite outgrowth, and synapse formation (341). These beneficial effects of lutein in cognitive health likely persist into childhood. In children between 7 and 10 y of age, MPOD was positively and significantly associated with hippocampus-dependent relational memory and academic performance (342, 343).
Cardiometabolic health
A recent systematic review with meta-analysis found that an increased lutein status (intake or serum concentrations) was associated with a lower risk of coronary heart disease, stroke, and metabolic syndrome, but not with the risk of type 2 diabetes (344). Several biological mechanisms have been proposed for the beneficial effect of lutein on heart health, including vascular changes (345), antioxidant effects (281, 284), and effects on immune response and inflammation (285, 346).
β-Cryptoxanthin
Vitamin A source
β-Cryptoxanthin is the major dietary xanthophyll with provitamin A activity. Accumulating evidence indicates that β-cryptoxanthin is more bioavailable from dietary sources than the other provitamin A carotenoids, including β-carotene (347). Therefore, it may be a comparable source of vitamin A despite only providing 1 molecule of vitamin A upon cleavage.
Antioxidant
Similar to lutein and zeaxanthin, β-cryptoxanthin exhibits in vitro antioxidant functions. As a provitamin A carotenoid, β-cryptoxanthin was shown to have a dose-dependent (0, 1, 4, 10, or 25 μM) protection against H2O2-induced DNA strand breaks in HeLa cells (348). Ferrets given β-cryptoxanthin (∼7.5 or 37.5 μg ⋅ kg body weight−1 ⋅ d−1) and exposed to cigarette smoke showed lower oxidative DNA damage in lung tissue than did ferrets exposed to cigarette smoke only (349). The beneficial effect of β-cryptoxanthin was stronger for the high-dose β-cryptoxanthin than for the low-dose β-cryptoxanthin. To date, to our knowledge, there are no human studies on the antioxidant activity of β-cryptoxanthin.
Lung cancer prevention
Pooled analysis from 7 cohort studies found that, among the carotenoids, only β-cryptoxanthin intake was associated with a lower risk of lung cancer among current smokers (350, 351). β-Cryptoxanthin supplementation in ferrets exposed to cigarette smoke decreased lung inflammation and oxidative damage (349). Studies in mice indicate that β-cryptoxanthin reduced lung tumor multiplicity, and restored sirtuin 1 (SIRT1), p53, and RAR-β, expression in the lung, which were all suppressed by nicotine (352). More recent evidence indicates that β-cryptoxanthin may also inhibit phosphorylation of protein kinase B through downregulation of nicotinic acetylcholine receptor α7 signaling (353). A more extensive discussion of β-cryptoxanthin and lung cancer prevention can be found in Iskandar et al. (353).
Bone health
In vitro studies have shown that β-cryptoxanthin has both stimulatory effects on osteoblastic bone formation and inhibitory effects on osteoclastic bone resorption [reviewed in Burri et al. (354) and Yamaguchi (355)]. These effects are mediated in part via modulation of gene expression, which is not replicated by retinoic acid, suggesting a function independent of vitamin A activity (354). Epidemiologic studies in postmenopausal Japanese women showed that β-cryptoxanthin intake and circulating concentrations are associated with higher bone mineral density (356, 357). However, analysis from the Framingham Osteoporosis Study found no association between β-cryptoxanthin intake and the risk of hip fracture in US women (358). This may be explained by the fact that β-cryptoxanthin intakes in the United States are much lower than in Japan (354), making it difficult to detect differences in the US study.
Safety of xanthophylls
To date, the vast majority of studies evaluating xanthophyll supplementation on health outcomes have been on lutein (272). Doses have ranged from 8 to 40 mg/d, and study durations have ranged from 7 d to 5 y (28, 245, 304, 359, 360). A systematic risk assessment of lutein supplements used in placebo-controlled intervention trials was published in 2006 (272). Only a few of the studies monitored any possible adverse side effects, primarily through self-reporting. However, currently, the AREDS2 has reported no adverse effects, beyond some skin yellowing, with lutein and zeaxanthin supplementation (10 and 2 mg/d, respectively) over 5 y (245). Recently, a case report of bilateral “foveal sparkles” in a woman in her 60s taking a daily 20-mg lutein supplement for 8 y and an unusually high dietary lutein intake (361) indicated that 7 mo after discontinuing the supplement but continuing her dietary habits, the crystals in the right eye resolved, but not those in the left eye. The authors indicated, for these reasons, that chronic consumption of lutein at doses exceeding the AREDS2 of 10 mg/d is not always beneficial.
Conclusions and Future Research Needed
In summary, a number of variables (Figure 4) are associated with carotenoid status, with many emerging from the analysis of very large prospective or cross-sectional studies. Other food-based variables have been discovered through experimentation and should serve as the rationale for the development of dietary interventions. These dietary, physiologic, and genetic determinants of carotenoid responses warrant consideration for study design and interpretation, and for the development of public health and personalized dietary guidance. Furthermore, it will be of continued importance to explore the possible pharmacokinetics and pharmacodynamics of carotenoid metabolites.
FIGURE 4.
Summary of the intrinsic and extrinsic variables affecting physiologic responses to carotenes and xanthophylls.
Nearly all promising carotenoid bioactivity relations shown by epidemiologic analysis should be tested in prospective clinical trials, as feasible, or in appropriate preclinical models. Indeed, the most efficient strides will be made in carotenoid research when studies of carotenoid pharmacokinetics and pharmacodynamics are analyzed in the context of intrinsic and extrinsic modulators of carotenoid exposure. Furthermore, studies should be statistically powered for expected response variability due to these variables. In particular, there is fairly consistent evidence that BMI and fat distribution, as well as age and smoking, may affect circulating carotenoid responses to dietary carotenoids. In addition, carotenoid absorption is likely to be regulated by host vitamin A status.
Additional research should define the bioavailability of carotenoids from different food sources as well as their exposure half-lives in populations of interest. Indeed, the bioavailability of carotenoids differs by source, leading to differing physiologic responses that complicate the interpretation of dietary intake data. Thus, it is important to monitor internal exposures in response to a dietary intervention, and it is essential to control for the intake of both intervention and nonintervention carotenoids consumed in a study. The physiologic importance of interactions with other carotenoids, nutrients, and drugs that may affect absorption and metabolism also deserves continued investigation.
The evidence to date from large-scale GWASs and smaller candidate gene studies indicates that genetics play a role in explaining part of the interindividual variability in responses to carotenoid intake. Research in genetically diverse human populations that defines the contribution of genetics to interindividual variability in carotenoid bioavailability, metabolism, and circulating and tissue concentrations will help to prioritize the role of genetics in interpreting human subject data and in designing personalized nutritional interventions. At the same time, efforts to understand the mechanistic basis for gene polymorphism–carotenoid interactions will expand our understanding of clinically discovered polymorphisms and their impacts on carotenoid exposure and bioactivity.
As the analysis of large data sets continues to become more commonplace, it is expected that the variables discussed herein could gain or lose support, and new variables may emerge. However, continued review and consideration of the modulators of carotenoid exposure should clarify our understanding of the bioactivities of carotenoids in humans.
Acknowledgments
We thank Adam Gillum (USDA, Agricultural Research Service Children's Nutrition Research Center at Baylor College of Medicine, Houston, Texas) for preparing the schematic figure of variables affecting carotenoid status and Sookyoung Jeon (University of Illinois–Urbana/Champaign) for her assistance in preparing the molecular structure figures. This work was presented at the ASN's Carotenoid and Retinoid Interactive Group's (CARIG) Symposium entitled “Moving towards Personalized Nutrition of Dietary Carotenoids: A Review of the Genetic and Nongenetic Factors Impacting Absorption, Metabolism, and Health Impacts” held on 21 April 2017 in Chicago, Illinois. We also thank the International Carotenoid Society and Hagen Schroeter of Mars Symbioscience for partial registration and travel support of the speakers to present at the CARIG symposium. All authors read and approved the final manuscript.
Notes
Supported by the NIH National Center for Complementary and Integrative Health and Office of Dietary Supplements (R00 AT008576; to NEM), and by the USDA–Agricultural Research Service under CRIS 3092-51000-056-03S (to NEM and NH) and CRIS 8050-51000-095-01S (to ESM and EJJ).
Author disclosures: NEM, ESM, NH, JWE, and EJJ, no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the USDA.
Abbreviations used:
- AMD
age-related macular degeneration
- AREDS
Age-Related Eye Disease Study
- BCO1
β-carotene-15,15’-oxygenase
- BCO2
β-carotene-9’,10’-oxygenase
- CD36
cluster of differentiation 36
- COBLL1
cordon-bleu WH2 repeat protein like 1
- CRP
C-reactive protein
- CVD
cardiovascular disease
- DR
diabetic retinopathy
- DU
density unit
- ELOVL2
ELOVL fatty acid elongase 2
- GWAS
genomewide association study
- ISX
intestine specific homeobox
- LIPC
lipase C, hepatic type
- LPL
lipoprotein lipase
- MPOD
macular pigment optical density
- NPC1L1
Niemann-Pick C1–like 1
- PCa
prostate cancer
- RAE
retinol activity equivalent
- RAR
retinoic acid receptor
- ROP
retinopathy of prematurity
- RP
retinitis pigmentosa
- RPE65
retinoid isomerohydrolase
- SCARB1
scavenger receptor class B type 1
- SETD7
SET domain–containing lysine methyltransferase 7
- SIRT1
sirtuin 1
- SNP
single nucleotide polymorphism
References
- 1. Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med 2005;26:459–516. [DOI] [PubMed] [Google Scholar]
- 2. Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—vitamin A review. J Nutr 2016;146(Suppl):1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aune D, Chan DS, Vieira AR, Rosenblatt DAN, Vieira R, Greenwood DC, Norat T. Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 2012;96:356–73. [DOI] [PubMed] [Google Scholar]
- 4. Abar L, Vieira AR, Aune D, Stevens C, Vingeliene S, Navarro Rosenblatt DA, Chan D, Greenwood DC, Norat T. Blood concentrations of carotenoids and retinol and lung cancer risk: an update of the WCRF-AICR systematic review of published prospective studies. Cancer Med 2016;5:2069–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Key TJ, Appleby PN, Travis RC, Albanes D, Alberg AJ, Barricarte A, Black A, Boeing H, Bueno-de-Mesquita HB, Chan JM, et al. Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr 2015;102:1142–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leoncini E, Nedovic D, Panic N, Pastorino R, Edefonti V, Boccia S. Carotenoid intake from natural sources and head and neck cancer: a systematic review and Meta-analysis of epidemiological studies. Cancer Epidemiol Prev Biomark 2015;24:1003–11. [DOI] [PubMed] [Google Scholar]
- 7. Sesso HD. Carotenoids and cardiovascular disease: what research gaps remain? Curr Opin Lipidol 2006;17:11–16. [DOI] [PubMed] [Google Scholar]
- 8. Burton-Freeman BM, Sesso HD. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr 2014;5:457–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chalam KV, Ferguson LR, Li W, Rusovici R, Koushan K. The role of lutein in eye-related disease. Nutrients 2013;5:1823–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson EJ. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev 2014;72:605–12. [DOI] [PubMed] [Google Scholar]
- 11. Bohn T, Desmarchelier C, Dragsted LO, Nielsen CS, Stahl W, Rühl R, Keijer J, Borel P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol Nutr Food Res 2017;61(6):1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am J Clin Nutr 2007;85:762–9. [DOI] [PubMed] [Google Scholar]
- 13. Haskell MJ. The challenge to reach nutritional adequacy for vitamin A: β-carotene bioavailability and conversion—evidence in humans. Am J Clin Nutr 2012;96(Suppl):1193S–203S. [DOI] [PubMed] [Google Scholar]
- 14. Moran NE, Cichon MJ, Riedl KM, Grainger EM, Schwartz SJ, Novotny JA, Erdman JW, Clinton SK. Compartmental and noncompartmental modeling of 13C-lycopene absorption, isomerization, and distribution kinetics in healthy adults. Am J Clin Nutr 2015;102:1436–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diwadkar-Navsariwala V, Novotny JA, Gustin DM, Sosman JA, Rodvold KA, Crowell JA, Stacewicz-Sapuntzakis M, Bowen PE. A physiological pharmacokinetic model describing the disposition of lycopene in healthy men. J Lipid Res 2003;44:1927–39. [DOI] [PubMed] [Google Scholar]
- 16. O'Neill ME, Thurnham DI. Intestinal absorption of β-carotene, lycopene and lutein in men and women following a standard meal: response curves in the triacylglycerol-rich lipoprotein fraction. Br J Nutr 1998;79:149–59. [DOI] [PubMed] [Google Scholar]
- 17. Moran NE, Novotny JA, Cichon MJ, Riedl KM, Rogers RB, Grainger EM, Schwartz SJ, Erdman JW, Clinton SK. Absorption and distribution kinetics of the 13C-labeled tomato carotenoid phytoene in healthy adults. J Nutr 2016;146:368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Moura FF, Ho CC, Getachew G, Hickenbottom S, Clifford AJ. Kinetics of 14C distribution after tracer dose of 14C-lutein in an adult woman. Lipids 2005;40:1069–73. [DOI] [PubMed] [Google Scholar]
- 19. Castenmiller JJ, West CE, Linssen JP, van het Hof KH, Voragen AG. The food matrix of spinach is a limiting factor in determining the bioavailability of beta-carotene and to a lesser extent of lutein in humans. J Nutr 1999;129:349–55. [DOI] [PubMed] [Google Scholar]
- 20. Atkinson AJ. Clinical pharmacokinetics. In: Atkinson AJ Jr., Huang S-M, Lertora JJL, Markey SP, editors, Principles of clinical pharmacology. 3rd ed.San Diego (CA): Elsevier/Academic Press; 2012. p. 13–24. [Google Scholar]
- 21. Burri BJ, Neidlinger TR, Clifford AJ. Serum carotenoid depletion follows first-order kinetics in healthy adult women fed naturally low carotenoid diets. J Nutr 2001;131:2096–100. [DOI] [PubMed] [Google Scholar]
- 22. Thürmann PA, Steffen J, Zwernemann C, Aebischer C-P, Cohn W, Wendt G, Schalch W. Plasma concentration response to drinks containing [beta]-carotene as carrot juice or formulated as a water dispersible powder. Eur J Nutr 2002;41:228–35. [DOI] [PubMed] [Google Scholar]
- 23. Micozzi M, Brown ED, Edwards BK, Bieri J, Taylor PR, Khachik F, Beecher GR, Smith JC. Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am J Clin Nutr 1992;55:1120–5. [DOI] [PubMed] [Google Scholar]
- 24. Gustin DM, Rodvold KA, Sosman JA, Diwadkar-Navsariwala V, Stacewicz-Sapuntzakis M, Viana M, Crowell JA, Murray J, Tiller P, Bowen PE. Single-dose pharmacokinetic study of lycopene delivered in a well-defined food-based lycopene delivery system (tomato paste-oil mixture) in healthy adult male subjects. Cancer Epidemiol Biomarkers Prev 2004;13:850–60. [PubMed] [Google Scholar]
- 25. Cohn W, Thürmann P, Tenter U, Aebischer C, Schierle J, Schalch W. Comparative multiple dose plasma kinetics of lycopene administered in tomato juice, tomato soup or lycopene tablets. Eur J Nutr 2004;43:304–12. [DOI] [PubMed] [Google Scholar]
- 26. Rock CL, Swendseid ME, Jacob RA, McKee RW. Plasma carotenoid levels in human subjects fed a low carotenoid diet. J Nutr 1992;122:96–100. [DOI] [PubMed] [Google Scholar]
- 27. Granado F, Olmedilla B, Blanco I. Serum depletion and bioavailability of lutein in type I diabetic patients. Eur J Nutr 2002;41:47–53. [DOI] [PubMed] [Google Scholar]
- 28. Thürmann PA, Schalch W, Aebischer J-C, Tenter U, Cohn W. Plasma kinetics of lutein, zeaxanthin, and 3-dehydro-lutein after multiple oral doses of a lutein supplement. Am J Clin Nutr 2005;82:88–97. [DOI] [PubMed] [Google Scholar]
- 29. Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res 1997;65:57–62. [DOI] [PubMed] [Google Scholar]
- 30. Novotny JA, Kurilich AC, Britz SJ, Clevidence BA. Plasma appearance of labeled beta-carotene, lutein, and retinol in humans after consumption of isotopically labeled kale. J Lipid Res 2005;46:1896–903. [DOI] [PubMed] [Google Scholar]
- 31. de Moura FF, Ho CC, Getachew G, Hickenbottom S, Clifford AJ. Kinetics of 14C distribution after tracer dose of 14C-lutein in an adult woman. Lipids 2005;40:1069–73. [DOI] [PubMed] [Google Scholar]
- 32. Hartmann D, Thürmann PA, Spitzer V, Schalch W, Manner B, Cohn W. Plasma kinetics of zeaxanthin and 3’-dehydro-lutein after multiple oral doses of synthetic zeaxanthin. Am J Clin Nutr 2004;79:410–7. [DOI] [PubMed] [Google Scholar]
- 33. Lobo GP, Amengual J, Palczewski G, Babino D, von Lintig J. Mammalian carotenoid-oxygenases: key players for carotenoid function and homeostasis. Biochim Biophys Acta 2012;1821:78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mein JR, Lian F, Wang X-D. Biological activity of lycopene metabolites: implications for cancer prevention. Nutr Rev 2008;66:667–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shete V, Quadro L. Mammalian metabolism of β-carotene: gaps in knowledge. Nutrients 2013;5:4849–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seña C, Sun J, Narayanasamy S, Riedl KM, Yuan Y, Curley RW, Schwartz SJ, Harrison EH. Substrate specificity of purified recombinant chicken β-carotene 9′,10′-oxygenase (BCO2). J Biol Chem 2016;291:14609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seña C, Narayanasamy S, Riedl KM, Curley RW, Schwartz SJ, Harrison EH. Substrate specificity of purified recombinant human β-carotene 15,15′-oxygenase (BCO1). J Biol Chem 2013;288:37094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu K-Q, Liu C, Ernst H, Krinsky NI, Russell RM, Wang X-D. The biochemical characterization of ferret carotene-9′, 10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem 2006;281:19327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX. Identification, expression, and substrate specificity of a mammalian β-carotene 15,15′-dioxygenase. J Biol Chem 2001;276:6560–5. [DOI] [PubMed] [Google Scholar]
- 40. Lindqvist A, Andersson S. Cell type-specific expression of β-carotene 15,15′-mono-oxygenase in human tissues. J Histochem Cytochem 2004;52:491–9. [DOI] [PubMed] [Google Scholar]
- 41. Harrison EH. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta 2012;1821:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuura T, Gad MZ, Harrison EH, Ross AC. Lecithin:retinol acyltransferase and retinyl ester hydrolase activities are differentially regulated by retinoids and have distinct distributions between hepatocyte and nonparenchymal cell fractions of rat liver. J Nutr 1997;127:218–24. [DOI] [PubMed] [Google Scholar]
- 43. Shmarakov I, Fleshman MK, D'Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW Jr., von Lintig J, Rubin LP, Harrison EH, et al. Hepatic stellate cells are an important cellular site for β-carotene conversion to retinoid. Arch Biochem Biophys 2010;504:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao D-Y, Katz NB. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med 2002;227:845–51. [DOI] [PubMed] [Google Scholar]
- 45. Stahl W, Schwarz W, Sundquist AR, Sies H. cis-trans isomers of lycopene and β-carotene in human serum and tissues. Arch Biochem Biophys 1992;294:173–7. [DOI] [PubMed] [Google Scholar]
- 46. Moran NE, Erdman JW Jr., Clinton SK. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch Biochem Biophys 2013;539:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ford NA, Elsen AC, Erdman JW Jr. Genetic ablation of carotene oxygenases and consumption of lycopene or tomato powder diets modulate carotenoid and lipid metabolism in mice. Nutr Res 2013;33:733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lietz G, Oxley A, Boesch-Saadatmandi C, Kobayashi D. Importance of β,β-carotene 15,15′-monooxygenase 1 (BCMO1) and β,β-carotene 9′,10′-dioxygenase 2 (BCDO2) in nutrition and health. Mol Nutr Food Res 2012;56:241–50. [DOI] [PubMed] [Google Scholar]
- 49. Amengual J, Lobo GP, Golczak M, Li HNM, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J 2011;25:948–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW. Loss of carotene-9′,10′-monooxygenase expression increases serum and tissue lycopene concentrations in lycopene-fed mice. J Nutr 2010;140:2134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tan H-L, Thomas-Ahner JM, Moran NE, Cooperstone JL, Erdman JW, Young GS, Clinton SK. β-Carotene 9′,10′ oxygenase modulates the anticancer activity of dietary tomato or lycopene on prostate carcinogenesis in the TRAMP model. Cancer Prev Res (Phila) 2017;10:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mohn ES, Erdman JW, Neuringer M, Kuchan MJ, Johnson EJ. Brain xanthophyll content and exploratory gene expression analysis: subspecies differences in rhesus macaque. Genes Nutr 2017;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li B, Vachali PP, Gorusupudi A, Shen Z, Sharifzadeh H, Besch BM, Nelson K, Horvath MM, Frederick JM, Baehr W, et al. Inactivity of human beta-carotene-9’,10’-dioxygenase (BCO2) underlies retinal accumulation of the human macular carotenoid pigment. Proc Natl Acad Sci USA 2014;111:10173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Babino D, Palczewski G, Widjaja-Adhi MAK, Kiser PD, Golczak M, von Lintig J. Characterization of the role of β-carotene 9,10-dioxygenase in macular pigment metabolism. J Biol Chem 2015;290:24844–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim Y-S, Yeom S-J, Oh D-K. Production of β-apo-10′-carotenal from β-carotene by human β-carotene-9′,10′-oxygenase expressed in E. coli. Biotechnol Lett 2011;33:1195–200. [DOI] [PubMed] [Google Scholar]
- 56. Isaacson T, Ronen G, Zamir D, Hirschberg J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell 2002;14:333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Breitenbach J, Sandmann G. ζ-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta 2005;220:785–93. [DOI] [PubMed] [Google Scholar]
- 58. Meléndez-Martínez AJ, Paulino M, Stinco CM, Mapelli-Brahm P, Wang X-D. Study of the time-course of cis/trans (Z/E) isomerization of lycopene, phytoene, and phytofluene from tomato. J Agric Food Chem 2014;62:12399–406. [DOI] [PubMed] [Google Scholar]
- 59. Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, Erdman JW. cis-trans Lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Prev Biomark 1996;5:823–33. [PubMed] [Google Scholar]
- 60. Walfisch Y, Walfisch S, Agbaria R, Levy J, Sharoni Y. Lycopene in serum, skin and adipose tissues after tomato-oleoresin supplementation in patients undergoing haemorrhoidectomy or peri-anal fistulotomy. Br J Nutr 2003;90:759–66. [DOI] [PubMed] [Google Scholar]
- 61. Stahl W, Sundquist AR, Hanusch M, Schwarz W, Sies H. Separation of beta-carotene and lycopene geometrical isomers in biological samples. Clin Chem 1993;39:810–4. [PubMed] [Google Scholar]
- 62. van het Hof KH, Gärtner C, West CE, Tijburg LB. Potential of vegetable processing to increase the delivery of carotenoids to man. Int J Vitam Nutr Res. 1998;68:366–70. [PubMed] [Google Scholar]
- 63. Cooperstone JL, Ralston RA, Riedl KM, Haufe TC, Schweiggert RM, King SA, Timmers CD, Francis DM, Lesinski GB, Clinton SK, et al. Enhanced bioavailability of lycopene when consumed as cis-isomers from tangerine compared to red tomato juice, a randomized, cross-over clinical trial. Mol Nutr Food Res 2015;59:658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Unlu NZ, Bohn T, Francis D, Clinton SK, Schwartz SJ. Carotenoid absorption in humans consuming tomato sauces obtained from tangerine or high-β-carotene varieties of tomatoes. J Agric Food Chem 2007;55:1597–603. [DOI] [PubMed] [Google Scholar]
- 65. Boileau AC, Merchen NR, Wasson K, Atkinson CA, Erdman JW cis-Lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. J Nutr 1999;129:1176–81. [DOI] [PubMed] [Google Scholar]
- 66. Failla ML, Chitchumroonchokchai C, Ishida BK. In vitro micellarization and intestinal cell uptake of cis isomers of lycopene exceed those of all-trans lycopene. J Nutr 2008;138:482–6. [DOI] [PubMed] [Google Scholar]
- 67. Deming DM, Teixeira SR, Erdman JW. All-trans (Beta)-carotene appears to be more bioavailable than 9-cis or 13-cis (Beta)-carotene in gerbils given single oral doses of each isomer. J Nutr 2002;132:2700–8. [DOI] [PubMed] [Google Scholar]
- 68. Erdman JW, Thatcher AJ, Hofmann NE, Lederman JD, et al. All-trans beta-carotene is absorbed preferentially to 9-cis beta-carotene, but the latter accumulates in the tissues of domestic ferrets (Mustela putorius puro). J Nutr 1998;128:2009–13. [DOI] [PubMed] [Google Scholar]
- 69. Johnson EJ, Qin J, Krinsky NI, Russell RM. β-Carotene isomers in human serum, breast milk and buccal mucosa cells after continuous oral doses of all-trans and 9-cis β-carotene. J Nutr 1997;127:1993–9. [DOI] [PubMed] [Google Scholar]
- 70. Stahl W, Schwarz W, Laar J von, Sies H. All-trans β-carotene preferentially accumulates in human chylomicrons and very low density lipoproteins compared with the 9-cis geometrical isomer. J Nutr 1995;125:2128–33. [DOI] [PubMed] [Google Scholar]
- 71. Deming DM, Baker DH, Erdman JW. The relative vitamin A value of 9-cis (Beta)-carotene is less and that of 13-cis (Beta)-carotene may be greater than the accepted 50% that of all-trans (Beta)-carotene in gerbils. J Nutr 2002;132:2709–12. [DOI] [PubMed] [Google Scholar]
- 72. Bresnahan KA, Davis CR, Tanumihardjo SA. Relative vitamin A values of 9-cis- and 13-cis-β-carotene do not differ when fed at physiological levels during vitamin A depletion in Mongolian gerbils (Meriones unguiculatus). Br J Nutr 2014;112:162–9. [DOI] [PubMed] [Google Scholar]
- 73. Werman MJ, Mokady S, Ben-Amotz A. Bioavailability of the isomer mixture of phytoene and phytofluene-rich alga Dunaliella bardawil in rat plasma and tissues. J Nutr Biochem 2002;13:585–91. [DOI] [PubMed] [Google Scholar]
- 74. Johnson EJ, Vishwanathan R, Johnson MA, Hausman DB, Davey A, Scott TM, Green RC, Miller LS, Gearing M, Woodard J, et al. Relationship between serum and brain carotenoids, alpha-tocopherol, and retinol concentrations and cognitive performance in the oldest old from the Georgia Centenarian Study. J Aging Res 2013:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ. Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr 2014;59:659–65. [DOI] [PubMed] [Google Scholar]
- 76. Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci 2005;46:692–702. [DOI] [PubMed] [Google Scholar]
- 77. Perry A, Rasmussen H, Johnson EJ. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J Food Compos Anal 2009:9–15. [Google Scholar]
- 78. Chung H-Y, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr 2004;134:1887–93. [DOI] [PubMed] [Google Scholar]
- 79. Bowen PE, Herbst-Espinosa SM, Hussain EA, Stacewicz-Sapuntzakis M. Esterification does not impair lutein bioavailability in humans. J Nutr 2002;132:3668–73. [DOI] [PubMed] [Google Scholar]
- 80. Yoshizako H, Hara K, Takai Y, Kaidzu S, Obana A, Ohira A. Comparison of macular pigment and serum lutein concentration changes between free lutein and lutein esters supplements in Japanese subjects. Acta Ophthalmol (Copenh) 2016;94:e411–6. [DOI] [PubMed] [Google Scholar]
- 81. Norkus EP, Norkus KL, Dharmarajan TS, Schierle J, Schalch W. Serum lutein response is greater from free lutein than from esterified lutein during 4 weeks of supplementation in healthy adults. J Am Coll Nutr 2010;29:575–85. [DOI] [PubMed] [Google Scholar]
- 82. Breithaupt DE, Weller P, Wolters M, Hahn A. Plasma response to a single dose of dietary beta-cryptoxanthin esters from papaya (Carica papaya L.) or non-esterified beta-cryptoxanthin in adult human subjects: a comparative study. Br J Nutr 2003;90:795–801. [DOI] [PubMed] [Google Scholar]
- 83. Novotny JA, Harrison DJ, Pawlosky R, Flanagan VP, Harrison EH, Kurilich AC. β-Carotene conversion to vitamin a decreases as the dietary dose increases in humans. J Nutr 2010;140:915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bone RA, Landrum JT. Dose-dependent response of serum lutein and macular pigment optical density to supplementation with lutein esters. Arch Biochem Biophys 2010;504:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sherry CL, Oliver JS, Renzi LM, Marriage BJ. Lutein supplementation increases breast milk and plasma lutein concentrations in lactating women and infant plasma concentrations but does not affect other carotenoids. J Nutr 2014;144:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ma L, Liu R, Du JH, Liu T, Wu SS, Liu XH. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients 2016;8(7):E426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brown MJ, Ferruzzi MG, Nguyen ML, Cooper DA, Eldridge AL, Schwartz SJ, White WS. Carotenoid bioavailability is higher from salads ingested with full-fat than with fat-reduced salad dressings as measured with electrochemical detection. Am J Clin Nutr 2004;80:396–403. [DOI] [PubMed] [Google Scholar]
- 88. White WS, Zhou Y, Crane A, Dixon P, Quadt F, Flendrig LM. Modeling the dose effects of soybean oil in salad dressing on carotenoid and fat-soluble vitamin bioavailability in salad vegetables. Am J Clin Nutr 2017;106:1041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kim JE, Gordon SL, Ferruzzi MG, Campbell WW. Effects of egg consumption on carotenoid absorption from co-consumed, raw vegetables. Am J Clin Nutr 2015;102:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr 2005;135:431–6. [DOI] [PubMed] [Google Scholar]
- 91. Kopec RE, Cooperstone JL, Schweiggert RM, Young GS, Harrison EH, Francis DM, Clinton SK, Schwartz SJ. Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high–β-carotene tomato sauce and from carrots. J Nutr 2014;144:1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Failla ML, Chitchumronchokchai C, Ferruzzi MG, Goltz SR, Campbell WW. Unsaturated fatty acids promote bioaccessibility and basolateral secretion of carotenoids and α-tocopherol by Caco-2 cells. Food Funct 2014;5:1101. [DOI] [PubMed] [Google Scholar]
- 93. Goltz SR, Campbell WW, Chitchumroonchokchai C, Failla ML, Ferruzzi MG. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol Nutr Food Res 2012;56:866–77. [DOI] [PubMed] [Google Scholar]
- 94. Handelman GJ, Nightingale ZD, Lichtenstein AH, Schaefer EJ, Blumberg JB. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am J Clin Nutr 1999;70:247–51. [DOI] [PubMed] [Google Scholar]
- 95. van Het Hof KH West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr 2000;130:503–6. [DOI] [PubMed] [Google Scholar]
- 96. Koonsvitsky BP, Berry DA, Jones MB, Lin PYT, Cooper DA, Jones DY, Jackson JE. Olestra affects serum concentrations of α-tocopherol and carotenoids but not vitamin D or vitamin K status in free-living subjects. J Nutr 1997;127(Suppl):1636S–45S. [PubMed] [Google Scholar]
- 97. Schlagheck TG, Riccardi KA, Zorich NL, Torri SA, Dugan LD, Peters JC. Olestra dose response on fat-soluble and water-soluble nutrients in humans. J Nutr 1997;127(Suppl):1646S–65S. [DOI] [PubMed] [Google Scholar]
- 98. Tang G, Ferreira ALA, Grusak MA, Qin J, Dolnikowski GG, Russell RM, Krinsky NI. Bioavailability of synthetic and biosynthetic deuterated lycopene in humans. J Nutr Biochem 2005;16:229–35. [DOI] [PubMed] [Google Scholar]
- 99. Grainger EM, Hadley CW, Moran NE, Riedl KM, Gong MC, Pohar K, Schwartz SJ, Clinton SK. A comparison of plasma and prostate lycopene in response to typical servings of tomato soup, sauce or juice in men before prostatectomy. Br J Nutr 2015;114:596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gärtner C, Stahl W, Sies H. Lycopene is more bioavailable from tomato paste than from fresh tomatoes. Am J Clin Nutr 1997;66:116–22. [DOI] [PubMed] [Google Scholar]
- 101. Yonekura L, Nagao A. Intestinal absorption of dietary carotenoids. Mol Nutr Food Res 2007;51:107–15. [DOI] [PubMed] [Google Scholar]
- 102. Aschoff JK, Rolke CL, Breusing N, Bosy-Westphal A, Högel J, Carle R, Schweiggert RM. Bioavailability of β-cryptoxanthin is greater from pasteurized orange juice than from fresh oranges—a randomized cross-over study. Mol Nutr Food Res 2015;59:1896–904. [DOI] [PubMed] [Google Scholar]
- 103. Schweiggert RM, Kopec RE, Villalobos-Gutierrez MG, Högel J, Quesada S, Esquivel P, Schwartz SJ, Carle R. Carotenoids are more bioavailable from papaya than from tomato and carrot in humans: a randomised cross-over study. Br J Nutr 2014;111:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chung H-Y, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr 2004;134:1887–93. [DOI] [PubMed] [Google Scholar]
- 105. Scott TM, Rasmussen HM, Vishwanathan R, Chen O, Johnson EJ. Avocado consumption increases macular pigment density in older adults: a rando mized, controlled trial. Nutrients 2017;9(9):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vishwanathan R, Goodrow-Kotyla EF, Wooten BR, Wilson TA, Nicolosi RJ. Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins. Am J Clin Nutr 2009;90:1272–9. [DOI] [PubMed] [Google Scholar]
- 107. Wenzel AJ, Gerweck C, Barbato D, Nicolosi RJ, Handelman GJ, Curran-Celentano J. A 12-wk egg intervention increases serum zeaxanthin and macular pigment optical density in women. J Nutr 2006;136:2568–73. [DOI] [PubMed] [Google Scholar]
- 108. Riedl J, Linseisen J, Hoffmann J, Wolfram G. Some dietary fibers reduce the absorption of carotenoids in women. J Nutr 1999;129:2170–6. [DOI] [PubMed] [Google Scholar]
- 109. van het Hof KH, Brouwer IA, West CE, Haddeman E, Steegers-Theunissen RP, van Dusseldorp M, Weststrate JA, Eskes TK, Hautvast JG. Bioavailability of lutein from vegetables is 5 times higher than that of beta-carotene. Am J Clin Nutr 1999;70:261–8. [DOI] [PubMed] [Google Scholar]
- 110. Marriage BJ, Williams JA, Choe YS, Maki KC, Vurma M, DeMichele SJ. Mono- and diglycerides improve lutein absorption in healthy adults: a randomised, double-blind, cross-over, single-dose study. Br J Nutr 2017;118:813–21. [DOI] [PubMed] [Google Scholar]
- 111. Evans M, Beck M, Elliott J, Etheve S, Roberts R, Schalch W. Effects of formulation on the bioavailability of lutein and zeaxanthin: a randomized, double-blind, cross-over, comparative, single-dose study in healthy subjects. Eur J Nutr 2013;52:1381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. van den Berg H, van Vliet T. Effect of simultaneous, single oral doses of beta-carotene with lutein or lycopene on the beta-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am J Clin Nutr 1998;68:82–9. [DOI] [PubMed] [Google Scholar]
- 113. During A, Hussain MM, Morel DW, Harrison EH. Carotenoid uptake and secretion by CaCo-2 cells β-carotene isomer selectivity and carotenoid interactions. J Lipid Res 2002;43:1086–95. [DOI] [PubMed] [Google Scholar]
- 114. O'Sullivan L, Aisling SA, O'Brien NM. Investigation of β-carotene and lutein transport in Caco-2 cells: carotenoid-carotenoid interactions and transport inhibition by ezetimibe. Int J Vitam Nutr Res 2009;79:337–47. [DOI] [PubMed] [Google Scholar]
- 115. Baumgartner S, Ras RT, Trautwein EA, Mensink RP, Plat J. Plasma fat-soluble vitamin and carotenoid concentrations after plant sterol and plant stan ol consumption: a meta-analysis of randomized controlled trials. Eur J Nutr 2016;56:909–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Corte-Real J, Iddir M, Soukoulis C, Richling E, Hoffmann L, Bohn T. Effect of divalent minerals on the bioaccessibility of pure carotenoids and on physical properties of gastro-intestinal fluids. Food Chem 2016;197(Part A):546–53. [DOI] [PubMed] [Google Scholar]
- 117. Borel P, Desmarchelier C, Dumont U, Halimi C, Lairon D, Page D, Sébédio JL, Buisson C, Buffière C, Rémond D. Dietary calcium impairs tomato lycopene bioavailability in healthy humans. Br J Nut r 2017;116:2091–6. [DOI] [PubMed] [Google Scholar]
- 118. Corte-Real J, Guignard C, Gantenbein M, Weber B, Burgard K, Hoffmann L, Richling E, Bohn T. No influence of supplemental dietary calcium intake on the bioavailability of spinach carotenoids in humans. Br J Nutr 2017;117:1560–9. [DOI] [PubMed] [Google Scholar]
- 119. Rydén M, Leanderson P, Kastbom K-O, Jonasson L. Effects of simvastatin on carotenoid status in plasma. Nutr Metab Cardiovasc Dis 2012;22:66–71. [DOI] [PubMed] [Google Scholar]
- 120. Vasankari T, Ahotupa M, Viikari J, Nuotio I, Strandberg T, Vanhanen H, Gylling H, Miettinen T, Tikkanen MJ. Effect of 12-month statin therapy on antioxidant potential of LDL and serum antioxidant vitamin concentrations. Ann Med 2004;36:618–22. [DOI] [PubMed] [Google Scholar]
- 121. Sundl I, Pail E, Mellitzer K, Toplak H, Winklhofer-Roob BM. Effects of orlistat therapy on plasma concentrations of oxygenated and hydrocarbon carotenoids. Lipids 2006;41:113–8. [DOI] [PubMed] [Google Scholar]
- 122. Dallongeville J, Marécaux N, Fruchart JC, Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr 1998;128:1450–7. [DOI] [PubMed] [Google Scholar]
- 123. Wei W, Kim Y, Boudreau N. Association of smoking with serum and dietary levels of antioxidants in adults: NHANES III, 1988-1994. Am J Public Health 2001;91:258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ma J, Hampl JS, Betts NM. Antioxidant intakes and smoking status: data from the continuing survey of food intakes by individuals 1994-1996. Am J Clin Nutr 2000;71:774–80. [DOI] [PubMed] [Google Scholar]
- 125. Phillips EL, Arnett DK, Himes JH, McGovern PG, Blackburn H, Luepker RV. Differences and trends in antioxidant dietary intake in smokers and non-smokers, 1980-1992: the Minnesota Heart Survey. Ann Epidemiol 2000;10:417–23. [DOI] [PubMed] [Google Scholar]
- 126. Järvinen R, Knekt P, Seppänen R, Reunanen A, Heliövaara M, Maatela J, Aromaa A. Antioxidant vitamins in the diet: relationships with other personal characteristics in Finland. J Epidemiol Community Health 1994;48:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hurst JS, Contreras JE, Siems WG, Van Kuijk FJGM. Oxidation of carotenoids by heat and tobacco smoke. BioFactors 2004;20:23–35. [DOI] [PubMed] [Google Scholar]
- 128. Handelman GJ, Packer L, Cross CE. Destruction of tocopherols, carotenoids, and retinol in human plasma by cigarette smoke. Am J Clin Nutr 1996;63:559–65. [DOI] [PubMed] [Google Scholar]
- 129. Gabriel HE, Liu Z, Crott JW, Choi S-W, Song BC, Mason JB, Johnson EJ. A comparison of carotenoids, retinoids, and tocopherols in the serum and buccal mucosa of chronic cigarette smokers versus nonsmokers. Cancer Epidemiol Biomarkers Prev 2006;15:993–9. [DOI] [PubMed] [Google Scholar]
- 130. Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr 1996;126:129–37. [DOI] [PubMed] [Google Scholar]
- 131. Michaud DS, Giovannucci EL, Ascherio A, Rimm EB, Forman MR, Sampson L, Willett WC. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol Prev Biomark 1998;7:283–90. [PubMed] [Google Scholar]
- 132. Ritenbaugh C, Peng YM, Aickin M, Graver E, Branch M, Alberts DS. New carotenoid values for foods improve relationship of food frequency questionnaire intake estimates to plasma values. Cancer Epidemiol Prev Biomark 1996;5:907–12. [PubMed] [Google Scholar]
- 133. Tucker KL, Chen H, Vogel S, Wilson PWF, Schaefer EJ, Lammi-Keefe CJ. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. J Nutr 1999;129:438–45. [DOI] [PubMed] [Google Scholar]
- 134. Stuetz W, Weber D, Dollé MET, Jansen E, Grubeck-Loebenstein B, Fiegl S, Toussaint O, Bernhardt J, Gonos ES, Franceschi C, et al. Plasma carotenoids, tocopherols, and retinol in the age-stratified (35-74 years) general population: a cross-sectional study in six European countries. Nutrients 2016;8:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Maggio M, de Vita F, Lauretani F, Bandinelli S, Semba RD, Bartali B, Cherubini A, Cappola AR, Ceda GP, Ferrucci L. Relationship between carotenoids, retinol, and estradiol levels in older women. Nutrients 2015;7:6506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ascherio A, Stampfer MJ, Colditz GA, Rimm EB, Litin L, Willett WC. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among american men and women. J Nutr 1992;122:1792–801. [DOI] [PubMed] [Google Scholar]
- 137. Buiatti E, Muñoz N, Kato I, Vivas J, Muggli R, Plummer M, Benz M, Franceschi S, Oliver W. Determinants of plasma anti-oxidant vitamin levels in a population at high risk for stomach cancer. Int J Cancer 1996;65:317–22. [DOI] [PubMed] [Google Scholar]
- 138. Forman MR, Beecher GR, Lanza E, Reichman ME, Graubard BI, Campbell WS, Marr T, Yong LC, Judd JT, Taylor PR. Effect of alcohol consumption on plasma carotenoid concentrations in premenopausal women: a controlled dietary study. Am J Clin Nutr 1995;62:131–5. [DOI] [PubMed] [Google Scholar]
- 139. Mayne ST, Cartmel B, Silva F, Kim CS, Fallon BG, Briskin K, Zheng T, Baum M, Shor-Posner G, Goodwin WJ. Plasma lycopene concentrations in humans are determined by lycopene intake, plasma cholesterol concentrations and selected demographic factors. J Nutr 1999;129:849–54. [DOI] [PubMed] [Google Scholar]
- 140. Rock CL, Thornquist MD, Neuhouser ML, Kristal AR, Neumark-Sztainer D, Cooper DA, Patterson RE, Cheskin LJ. Diet and lifestyle correlates of lutein in the blood and diet. J Nutr 2002;132(Suppl):525S–30S. [DOI] [PubMed] [Google Scholar]
- 141. Gruber M, Chappell R, Millen A, LaRowe T, Moeller SM, Iannaccone A, Kritchevsky SB, Mares J. Correlates of serum lutein + zeaxanthin: findings from the Third National Health and Nutrition Examination Survey. J Nutr 2004;134:2387–94. [DOI] [PubMed] [Google Scholar]
- 142. Kitamura Y, Tanaka K, Kiyohara C, Hirohata T, Tomita Y, Ishibashi M, Kido K. Relationship of alcohol use, physical activity and dietary habits with serum carotenoids, retinol and alpha-tocopherol among male Japanese smokers. Int J Epidemiol 1997;26:307–14. [DOI] [PubMed] [Google Scholar]
- 143. Woodside JV, Young IS, Gilchrist SECM, Vioque J, Chakravarthy U, de Jong PTVM, Rahu M, Seland J, Soubrane G, Tomazzoli L, et al. Factors associated with serum/plasma concentrations of vitamins A, C, E and carotenoids in older people throughout Europe: the EUREYE study. Eur J Nutr 2013;52:1493–501. [DOI] [PubMed] [Google Scholar]
- 144. Ito Y, Sasaki R, Suzuki S, Aoki K. Relationship between serum xanthophyll levels and the consumption of cigarettes, alcohol or foods in healthy inhabitants of Japan. Int J Epidemiol 1991;20:615–20. [DOI] [PubMed] [Google Scholar]
- 145. Wawrzyniak A, Hamułka J, Friberg E, Wolk A. Dietary, anthropometric, and lifestyle correlates of serum carotenoids in postmenopausal women. Eur J Nutr 2013;52:1919–26. [DOI] [PubMed] [Google Scholar]
- 146. Herbeth B, Samara A, Stathopoulou M, Siest G, Visvikis-Siest S. Alcohol consumption, beverage preference, and diet in middle-aged men from the STANISLAS study. J Nutr Metab 2012;2012:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kesse E, Clavel-Chapelon F, Slimani N, van Liere M, E3N Group. Do eating habits differ according to alcohol consumption? Results of a study of the French cohort of the European Prospective Investigation into Cancer and Nutrition (E3N-EPIC). Am J Clin Nutr 2001;74:322–7. [DOI] [PubMed] [Google Scholar]
- 148. Ruf T, Nagel G, Altenburg H-P, Miller AB, Thorand B. Food and nutrient intake, anthropometric measurements and smoking according to alcohol consumption in the EPIC Heidelberg study. Ann Nutr Metab 2005;49:16–25. [DOI] [PubMed] [Google Scholar]
- 149. Walsh K, Alexander G. Alcoholic liver disease. Postgrad Med J 2000;76:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zima T, Fialová L, Mestek O, Janebová M, Crkovská J, Malbohan I, Stípek S, Mikulíková L, Popov P. Oxidative stress, metabolism of ethanol and alcohol-related diseases. J Biomed Sci 2001;8:59–70. [DOI] [PubMed] [Google Scholar]
- 151. Koch OR, Pani G, Borrello S, Colavitti R, Cravero A, Farrè S, Galeotti T. Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol Aspects Med 2004;25:191–8. [DOI] [PubMed] [Google Scholar]
- 152. Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology 2006;43:S63–74. [DOI] [PubMed] [Google Scholar]
- 153. Chung J, Veeramachaneni S, Liu C, Mernitz H, Russell RM, Wang X-D. Vitamin E supplementation does not prevent ethanol-reduced hepatic retinoic acid levels in rats. Nutr Res 2009;29:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Grummer MA, Erdman JW. Effect of chronic alcohol consumption and moderate fat diet on vitamin A status in rats fed either vitamin A or beta-carotene. J Nutr 1983;113:350–64. [DOI] [PubMed] [Google Scholar]
- 155. Widjaja-Adhi MAK, Lobo GP, Golczak M, Von Lintig J. A genetic dissection of intestinal fat-soluble vitamin and carotenoid absorption. Hum Mol Genet 2015;24:3206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Lietz G, Furr HC, Gannon BM, Green MH, Haskell M, Lopez-Teros V, Novotny JA, Palmer AC, Russell RM, Tanumihardjo SA, et al. Current capabilities and limitations of stable isotope techniques and applied mathematical equations in determining whole-body vitamin A status. Food Nutr Bull 2016;37:S87–103. [DOI] [PubMed] [Google Scholar]
- 157. Green MH, Ford JL, Oxley A, Green JB, Park H, Berry P, Boddy AV, Lietz G. Plasma retinol kinetics and β-carotene bioefficacy are quantified by model-based compartmental analysis in healthy young adults with low vitamin A stores. J Nutr 2016;146:2129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hickenbottom SJ, Follett JR, Lin Y, Dueker SR, Burri BJ, Neidlinger TR, Clifford AJ. Variability in conversion of β-carotene to vitamin A in men as measured by using a double-tracer study design. Am J Clin Nutr 2002;75:900–7. [DOI] [PubMed] [Google Scholar]
- 159. Allen CM, Schwartz SJ, Craft NE, Giovannucci EL, de Groft VL, Clinton SK. Changes in plasma and oral mucosal lycopene isomer concentrations in healthy adults consuming standard servings of processed tomato products. Nutr Cancer 2003;47:48–56. [DOI] [PubMed] [Google Scholar]
- 160. McEligot AJ, Rock CL, Flatt SW, Newman V, Faerber S, Pierce JP. Plasma carotenoids are biomarkers of long-term high vegetable intake in women with breast cancer. J Nutr 1999;129:2258–63. [DOI] [PubMed] [Google Scholar]
- 161. Moran NE, Thomas-Ahner JM, Fleming JL, McElroy JP, Grainger EM, Riedl KM, Schwartz SJ, Clinton SK. SNPs in lipid and carotenoid metabolism and absorption genes impact carotenoid responses to a tomato-soy juice intervention. FASEB J 2016;30Supplement:34.6. [Google Scholar]
- 162. Forman MR, Beecher GR, Muesing R, Lanza E, Olson B, Campbell WS, McAdam P, Raymond E, Schulman JD, Graubard BI. The fluctuation of plasma carotenoid concentrations by phase of the menstrual cycle: a controlled diet study. Am J Clin Nutr 1996;64:559–65. [DOI] [PubMed] [Google Scholar]
- 163. Forman MR, Johnson EJ, Lanza E, Graubard BI, Beecher GR, Muesing R. Effect of menstrual cycle phase on the concentration of individual carotenoids in lipoproteins of premenopausal women: a controlled dietary study. Am J Clin Nutr 1998;67:81–7. [DOI] [PubMed] [Google Scholar]
- 164. Mumford SL, Browne RW, Schliep KC, Schmelzer J, Plowden TC, Michels KA, Sjaarda LA, Zarek SM, Perkins NJ, Messer LC, et al. Serum antioxidants are associated with serum reproductive hormones and ovulation among healthy women. J Nutr 2016;146:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Rock CL, Demitrack MA, Rosenwald EN, Brown MB. Carotenoids and menstrual cycle phase in young women. Cancer Epidemiol Prev Biomark 1995;4:283–8. [PubMed] [Google Scholar]
- 166. Maggio M, Ceda GP, Lauretani F, Bandinelli S, Ruggiero C, Guralnik JM, Jeffrey Metter E, Ling SM, Paolisso G, Valenti G, et al. Relationship between higher estradiol levels and 9-year mortality in older women: the Invecchiare In Chianti Study. J Am Geriatr Soc 2009;57:1810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for vitamin C, vitamin E, selenium and carotenoids. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 168. Couillard C, Lemieux S, Vohl M-C, Couture P, Lamarche B. Carotenoids as biomarkers of fruit and vegetable intake in men and women. Br J Nutr 2016;116:1206–15. [DOI] [PubMed] [Google Scholar]
- 169. Boileau TWM, Clinton SK, Erdman JW. Tissue lycopene concentrations and isomer patterns are affected by androgen status and dietary lycopene concentration in male F344 rats. J Nutr 2000;130:1613–8. [DOI] [PubMed] [Google Scholar]
- 170. Tucker KL, Chen H, Vogel S, Wilson PW, Schaefer EJ, Lammi-Keefe CJ. Carotenoid intakes, assessed by dietary questionnaire, are associated with plasma carotenoid concentrations in an elderly population. J Nutr 1999;129:438–45. [DOI] [PubMed] [Google Scholar]
- 171. Yeum KJ, Booth SL, Roubenoff R, Russell RM. Plasma carotenoid concentrations are inversely correlated with fat mass in older women. J Nutr Health Aging 1998;2:79–83. [PubMed] [Google Scholar]
- 172. Broekmans WMR, Berendschot TTJM, Klöpping-Ketelaars IAA, de Vries AJ, Goldbohm RA, Tijburg LBM, Kardinaal AFM, van Poppel G. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am J Clin Nutr 2002;76:595–603. [DOI] [PubMed] [Google Scholar]
- 173. Johnson EJ, Hammond BR, Yeum KJ, Qin J, Wang XD, Castaneda C, Snodderly DM, Russell RM. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr 2000;71:1555–62. [DOI] [PubMed] [Google Scholar]
- 174. Kabat GC, Heo M, Ochs-Balcom HM, LeBoff MS, Mossavar-Rahmani Y, Adams-Campbell LL, Nassir R, Ard J, Zaslavsky O, Rohan TE. Longitudinal association of measures of adiposity with serum antioxidant concentrations in postmenopausal women. Eur J Clin Nutr 2016;70:47–53. [DOI] [PubMed] [Google Scholar]
- 175. Burrows TL, Hutchesson MJ, Rollo ME, Boggess MM, Guest M, Collins CE. Fruit and vegetable intake assessed by food frequency questionnaire and plasma carotenoids: a validation study in adults. Nutrients 2015;7:3240–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Suzuki K, Inoue T, Hioki R, Ochiai J, Kusuhara Y, Ichino N, Osakabe K, Hamajima N, Ito Y. Association of abdominal obesity with decreased serum levels of carotenoids in a healthy Japanese population. Clin Nutr 2006;25:780–9. [DOI] [PubMed] [Google Scholar]
- 177. Wallström P, Wirfält E, Lahmann PH, Gullberg B, Janzon L, Berglund G. Serum concentrations of beta-carotene and alpha-tocopherol are associated with diet, smoking, and general and central adiposity. Am J Clin Nutr 2001;73:777–85. [DOI] [PubMed] [Google Scholar]
- 178. Hammond BR Jr., Ciulla TA, Snodderly DM. Macular pigment density is reduced in obese subjects. Invest Ophthalmol Vis Sci 2002;43:47–50. [PubMed] [Google Scholar]
- 179. Bovier ER, Lewis RD, Hammond BR Jr. The relationship between lutein and zeaxanthin status and body fat. Nutrients 2013;5:750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Mares JA, LaRowe TL, Snodderly DM, Moeller SM, Gruber MJ, Klein ML, Wooten BR, Johnson EJ, Chappell RJ; CAREDS Macular Pigment Study Group and Investigators. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the carotenoids in Age-related Eye Disease Study, an ancillary study of the Women's Health Initiative. Am J Clin Nutr 2006;84:1107–22. [DOI] [PubMed] [Google Scholar]
- 181. Nolan J, O'Donovan O, Kavanagh H, Stack J, Harrison M, Muldoon A, Mellerio J, Beatty S. Macular pigment and percentage of body fat. Invest Ophthalmol Vis Sci 2004;45:3940–50. [DOI] [PubMed] [Google Scholar]
- 182. Gunanti IR, Marks GC, Al-Mamun A, Long KZ. Low serum concentrations of carotenoids and vitamin E are associated with high adiposity in Mexican-American children. J Nutr 2014;144:489–95. [DOI] [PubMed] [Google Scholar]
- 183. Strauss RS. Comparison of serum concentrations of α-tocopherol and β-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). J Pediatr 1999;134:160–5. [DOI] [PubMed] [Google Scholar]
- 184. Vioque J, Weinbrenner T, Asensio L, Castelló A, Young IS, Fletcher A. Plasma concentrations of carotenoids and vitamin C are better correlated with dietary intake in normal weight than overweight and obese elderly subjects. Br J Nutr 2007;97:977–86. [DOI] [PubMed] [Google Scholar]
- 185. Andersen LF, Jacobs DR, Gross MD, Schreiner PJ, Williams OD, Lee D-H. Longitudinal associations between body mass index and serum carotenoids: the CARDIA study. Br J Nutr 2006;95:358–65. [DOI] [PubMed] [Google Scholar]
- 186. Chung H-Y, Ferreira ALA, Epstein S, Paiva SAR, Castaneda-Sceppa C, Johnson EJ. Site-specific concentrations of carotenoids in adipose tissue: relations with dietary and serum carotenoid concentrations in healthy adults. Am J Clin Nutr 2009;90:533–9. [DOI] [PubMed] [Google Scholar]
- 187. Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr 2006;83:1265–71. [DOI] [PubMed] [Google Scholar]
- 188. Lidebjer C, Leanderson P, Ernerudh J, Jonasson L. Low plasma levels of oxygenated carotenoids in patients with coronary artery disease. Nutr Metab Cardiovasc Dis 2007;17:448–56. [DOI] [PubMed] [Google Scholar]
- 189. Kritchevsky SB, Bush AJ, Pahor M, Gross MD. Serum carotenoids and markers of inflammation in nonsmokers. Am J Epidemiol 2000;152:1065–71. [DOI] [PubMed] [Google Scholar]
- 190. Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, Guralnik J, Fried LP. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol 2006;163:18–26. [DOI] [PubMed] [Google Scholar]
- 191. Coyne T, Ibiebele TI, Baade PD, Dobson A, McClintock C, Dunn S, Leonard D, Shaw J. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr 2005;82:685–93. [DOI] [PubMed] [Google Scholar]
- 192. Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Yano M. High-serum carotenoids associated with lower risk for developing type 2 diabetes among Japanese subjects: Mikkabi cohort study. BMJ Open Diabetes Res Care 2015;3:e000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Scanlon G, Connell P, Ratzlaff M, Foerg B, McCartney D, Murphy A, O'Connor K, Loughman J. Macular pigment optical density is lower in type 2 diabetes, compared with type 1 diabetes and normal controls. Retina 2015;35:1808–16. [DOI] [PubMed] [Google Scholar]
- 194. Ward MS, Zhao DY, Bernstein PS. Macular and serum carotenoid concentrations in patients with malabsorption syndromes. J Ocul Biol Dis Infor 2008;1:12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Schupp C, Olano-Martin E, Gerth C, Morrissey BM, Cross CE, Werner JS. Lutein, zeaxanthin, macular pigment, and visual function in adult cystic fibrosis patients. Am J Clin Nutr 2004;79:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Floreani A, Baragiotta A, Martines D, Naccarato R, D'Odorico A. Plasma antioxidant levels in chronic cholestatic liver diseases. Aliment Pharmacol Ther 2000;14:353–8. [DOI] [PubMed] [Google Scholar]
- 197. Horton DK, Adetona O, Aguilar-Villalobos M, Cassidy BE, Pfeiffer CM, Schleicher RL, Caldwell KL, Needham LL, Rathbun SL, Vena JE, et al. Changes in the concentrations of biochemical indicators of diet and nutritional status of pregnant women across pregnancy trimesters in Trujillo, Peru, 2004–2005. Nutr J 2013;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Herrera E, Ortega H, Alvino G, Giovannini N, Amusquivar E, Cetin I. Relationship between plasma fatty acid profile and antioxidant vitamins during normal pregnancy. Eur J Clin Nutr 2004;58:1231–8. [DOI] [PubMed] [Google Scholar]
- 199. Schweigert FJ, Bathe K, Chen F, Büscher U, Dudenhausen JW. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur J Nutr 2004;43:39–44. [DOI] [PubMed] [Google Scholar]
- 200. CDC. 2008 National report on biochemical indicators of diet and nutrition—vitamins A and E a nd xarotenoids [Internet]. Accessed 6/29/2017. Available from: https://www.cdc.gov/nutritionreport/99-02/part_2a.html. [Google Scholar]
- 201. Reboul E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. Nutrients 2013;5:3563–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Grolier P, Borel P, Duszka C, Lory S, Alexandre-Gouabau MC, Azais-Braesco V, Nugon-Baudon L. The bioavailability of α- and β-carotene is affected by gut microflora in the rat. Br J Nutr 1998;80:199–204. [PubMed] [Google Scholar]
- 203. Ferrucci L, Perry JRB, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, et al. Common variation in the β-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet 2009;84:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo RP Jr., Truitt B, Klein ML, et al. Genetic determinants of macular pigments in women of the carotenoids in age-related eye disease study. Invest Ophthalmol Vis Sci 2013;54:2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Farook VS, Reddivari L, Mummidi S, Puppala S, Arya R, Lopez-Alvarenga JC, Fowler SP, Chittoor G, Resendez RG, Kumar BM, et al. Genetics of serum carotenoid concentrations and their correlation with obesity-related traits in Mexican American children. Am J Clin Nutr 2017;106:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Frazer KA, Murray SS, Schork NJ, Topol EJ. Human genetic variation and its contribution to complex traits. Nat Rev Genet 2009;10:241–51. [DOI] [PubMed] [Google Scholar]
- 207. International HapMap Consortium. A haplotype map of the human genome. Nature 2005;437:1299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Leung WC, Hessel S, Méplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding β-carotene 15,15′-monoxygenase alter β-carotene metabolism in female volunteers. FASEB J 2009;23:1041–53. [DOI] [PubMed] [Google Scholar]
- 209. Lindqvist A, Sharvill J, Sharvill DE, Andersson S. Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr 2007;137:2346–50. [DOI] [PubMed] [Google Scholar]
- 210. Hendrickson SJ, Hazra A, Chen C, Eliassen AH, Kraft P, Rosner BA, Willett WC. β-Carotene 15,15’-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent. Am J Clin Nutr 2012;96:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the carotene 15,15’-monoxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr 2012;142(Suppl):161S–5S. [DOI] [PubMed] [Google Scholar]
- 212. Borel P. Genetic variations involved in interindividual variability in carotenoid status. Mol Nutr Food Res 2012;56:228–40. [DOI] [PubMed] [Google Scholar]
- 213. Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem 2013;288:9017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Borel P, Moussa M, Reboul E, Lyan B, Defoort C, Vincent-Baudry S, Maillot M, Gastaldi M, Darmon M, Portugal H, et al. Human plasma levels of vitamin E and carotenoids are associated with genetic polymorphisms in genes involved in lipid metabolism. J Nutr 2007;137:2653–9. [DOI] [PubMed] [Google Scholar]
- 215. Borel P, Lietz G, Goncalves A, de Edelenyi FS, Lecompte S, Curtis P, Goumidi L, Caslake MJ, Miles EA, Packard C, et al. CD36 and SR-BI are involved in cellular uptake of provitamin a carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J Nutr 2013;143:448–56. [DOI] [PubMed] [Google Scholar]
- 216. Zubair N, Kooperberg C, Liu J, Di C, Peters U, Neuhouser ML. Genetic variation predicts serum lycopene concentrations in a multiethnic population of postmenopausal women. J Nutr 2015;145:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. McKay GJ, Loane E, Nolan JM, Patterson CC, Meyers KJ, Mares JA, Yonova-Doing E, Hammond CJ, Beatty S, Silvestri G. Investigation of genetic variation in scavenger receptor class B, member 1 (SCARB1) and association with serum carotenoids. Ophthalmology 2013;120:1632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Borel P, de Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand J-M, Meunier N, Drouault-Holowacz S, Bieuvelet S. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann Med 2011;43:47–59. [DOI] [PubMed] [Google Scholar]
- 219. Meyers KJ, Mares JA, Igo RP, Truitt B, Liu Z, Millen AE, Klein M, Johnson EJ, Engelman CD, Karki CK, et al. Genetic evidence for role of carotenoids in age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS). Invest Ophthalmol Vis Sci 2014;55:587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Merle BM, Maubaret C, Korobelnik J-F, Delyfer M-N, Rougier M-B, Lambert J-C, Amouyel P, Malet F, Goff ML, Dartigues J-F, et al. Association of HDL-related loci with age-related macular degeneration and plasma lutein and zeaxanthin: the Alienor Study. PLoS One 2013;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Borel P, Desmarchelier C, Nowicki M, Bott R. Lycopene bioavailability is associated with a combination of genetic variants. Free Radic Biol Med. 2015;83:238–44. [DOI] [PubMed] [Google Scholar]
- 222. Borel P, Moussa M, Reboul E, Lyan B, Defoort C, Vincent-Baudry S, Maillot M, Gastaldi M, Darmon M, Portugal H, et al. Human fasting plasma concentrations of vitamin E and carotenoids, and their association with genetic variants in apo C-III, cholesteryl ester transfer protein, hepatic lipase, intestinal fatty acid binding protein and microsomal triacylglycerol transfer protein. Br J Nutr 2008;101:680–7. [DOI] [PubMed] [Google Scholar]
- 223. Borel P, Desmarchelier C, Nowicki M, Bott R, Morange S, Lesavre N. Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am J Clin Nutr 2014;100:168–75. [DOI] [PubMed] [Google Scholar]
- 224. Herron KL, McGrane MM, Waters D, Lofgren IE, Clark RM, Ordovas JM, Fernandez ML. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J Nutr 2006;136:1161–5. [DOI] [PubMed] [Google Scholar]
- 225. Herbeth B, Gueguen S, Leroy P, Siest G, Visvikis-Siest S. The lipoprotein lipase serine 447 stop polymorphism is associated with altered serum carotenoid concentrations in the Stanislas Family Study. J Am Coll Nutr 2007;26:655–62. [DOI] [PubMed] [Google Scholar]
- 226. D'Adamo CR, D'Urso A, Ryan KA, Yerges-Armstrong LM, Semba RD, Steinle NI, Mitchell BD, Shuldiner AR, McArdle PF. A common variant in the SETD7 gene predicts serum lycopene concentrations. Nutrients 2016;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Borel P, Desmarchelier C, Nowicki M, Bott R. A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary β-carotene bioavailability in healthy men. J Nutr 2015;145:1740–7. [DOI] [PubMed] [Google Scholar]
- 228. Yonova-Doing E, Hysi PG, Venturini C, Williams KM, Nag A, Beatty S, Liew SHM, Gilbert CE, Hammond CJ. Candidate gene study of macular response to supplemental lutein and zeaxanthin. Exp Eye Res 2013;115:172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Mancina RM, Burza MA, Maglio C, Pirazzi C, Sentinelli F, Incani M, Montalcini T, Pujia A, Congiu T, Loche S, et al. The COBLL1 C allele is associated with lower serum insulin levels and lower insulin resistance in overweight and obese children. Diabetes Metab Res Rev 2013;29:413–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, Voolstra O, Vogt K. Towards a better understanding of carotenoid metabolism in animals. Biochim Biophys Acta Mol Basis Dis 2005;1740:122–31. [DOI] [PubMed] [Google Scholar]
- 231. Shyam R, Gorusupudi A, Nelson K, Horvath MP, Bernstein PS. RPE65 has an additional function as the lutein to meso-zeaxanthin isomerase in the vertebrate eye. Proc Natl Acad Sci USA 2017;114:10882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2014. [PubMed] [Google Scholar]
- 233. Böhm F, Edge R, Truscott G. Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: potential effects for human health. Mol Nutr Food Res 2012;56:205–16. [DOI] [PubMed] [Google Scholar]
- 234. Christian P, West KP. Nutrition: vitamin A supplementation—maternal and neonatal survival. Nat Rev Endocrinol 2011;7:190–2. [DOI] [PubMed] [Google Scholar]
- 235. Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr 2012;96(Suppl):1204S–6S. [DOI] [PubMed] [Google Scholar]
- 236. Eroglu A, Harrison EH. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids Thematic Review Series: fat-soluble vitamins: vitamin A. J Lipid Res 2013;54:1719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Tanoury ZA, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. Thematic Review Series: fat-soluble vitamins: vitamin A. J Lipid Res 2013;54:1761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 2011;343:d5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Wright CB, Redmond TM, Nickerson JM. A history of the classical visual cycle. Prog Mol Biol Transl Sci. 2015;134:433–48. [DOI] [PubMed] [Google Scholar]
- 240. Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature 1981;290:201–8. [DOI] [PubMed] [Google Scholar]
- 241. Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst 1996;88:1560–70. [DOI] [PubMed] [Google Scholar]
- 242. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst 1996;88:1550–9. [DOI] [PubMed] [Google Scholar]
- 243. Olson JA. Benefits and liabilities of vitamin A and carotenoids. J Nutr 1996;126(Suppl):1208S–12S. [DOI] [PubMed] [Google Scholar]
- 244. Holick CN, Michaud DS, Stolzenberg-Solomon R, Mayne ST, Pietinen P, Taylor PR, Virtamo J, Albanes D. Dietary carotenoids, serum β-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol 2002;156:536–47. [DOI] [PubMed] [Google Scholar]
- 245. Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:2005–15. [DOI] [PubMed] [Google Scholar]
- 246. Liu C, Russell RM, Wang X-D. Exposing ferrets to cigarette smoke and a pharmacological dose of beta-carotene supplementation enhance in vitro retinoic acid catabolism in lungs via induction of cytochrome P450 enzymes. J Nutr 2003;133:173–9. [DOI] [PubMed] [Google Scholar]
- 247. Liu C, Lian F, Smith DE, Russell RM, Wang X-D. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res 2003;63:3138–44. [PubMed] [Google Scholar]
- 248. Schwartz R, Grzbowski J. Carotenemia: overview, pathophysiology, etiology. Medscape [Internet]. 2017[cited 2017 Dec 12]. Available from: https://emedicine.medscape.com/article/1104368-overview. [Google Scholar]
- 249. Karppi J, Laukkanen JA, Mäkikallio TH, Ronkainen K, Kurl S. Low β-carotene concentrations increase the risk of cardiovascular disease mortality among Finnish men with risk factors. Nutr Metab Cardiovasc Dis 2012;22:921–8. [DOI] [PubMed] [Google Scholar]
- 250. Karppi J, Kurl S, Mäkikallio TH, Ronkainen K, Laukkanen JA. Serum β-carotene concentrations and the risk of congestive heart failure in men: a population-based study. Int J Cardiol 2013;168:1841–6. [DOI] [PubMed] [Google Scholar]
- 251. Karppi J, Laukkanen JA, Mäkikallio TH, Ronkainen K, Kurl S. Serum β-carotene and the risk of sudden cardiac death in men: a population-based follow-up study. Atherosclerosis 2013;226:172–7. [DOI] [PubMed] [Google Scholar]
- 252. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- 253. Rowles JL, Ranard KM, Smith JW, An R, Erdman JW. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis 2017;20:361–77. [DOI] [PubMed] [Google Scholar]
- 254. Chen P, Zhang W, Wang X, Zhao K, Negi DS, Zhuo L, Qi M, Wang X, Zhang X. Lycopene and risk of prostate cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Holzapfel N, Holzapfel B, Champ S, Feldthusen J, Clements J, Hutmacher D. The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. Int J Mol Sci 2013;14:14620–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Boileau TW-M, Liao Z, Kim S, Lemeshow S, Erdman JW, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst 2003;95:1578–86. [DOI] [PubMed] [Google Scholar]
- 257. Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW. Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res 2007;67:836–43. [DOI] [PubMed] [Google Scholar]
- 258. Wan L, Tan H-L, Thomas-Ahner JM, Pearl DK, Erdman JW, Moran NE, Clinton SK. Dietary tomato and lycopene impact androgen signaling- and carcinogenesis-related gene expression during early TRAMP prostate carcinogenesis. Cancer Prev Res (Phila) 2014;7:1228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Zuniga KE, Clinton SK, Erdman JW. The interactions of dietary tomato powder and soy germ on prostate carcinogenesis in the TRAMP model. Cancer Prev Res (Phila) 2013;6:548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Erdman JW, Ford NA, Lindshield BL. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys 2009;483:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Lindshield BL, Canene-Adams K, Erdman JW. Lycopenoids: are lycopene metabolites bioactive? Arch Biochem Biophys 2007;458:136–40. [DOI] [PubMed] [Google Scholar]
- 262. Gajic M, Zaripheh S, Sun F, Erdman JW. Apo-8’-lycopenal and apo-12’-lycopenal are metabolic products of lycopene in rat liver. J Nutr 2006;136:1552–7. [DOI] [PubMed] [Google Scholar]
- 263. Kopec RE, Riedl KM, Harrison EH, Curley RW, Hruszkewycz DP, Clinton SK, Schwartz SJ. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem 2010;58:3290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Tan H-L, Moran NE, Cichon MJ, Riedl KM, Schwartz SJ, Erdman JW, Pearl DK, Thomas-Ahner JM, Clinton SK. β-Carotene-9′,10′-oxygenase status modulates the impact of dietary tomato and lycopene on hepatic nuclear receptor–, stress-, and metabolism-related gene expression in mice. J Nutr 2014;144:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Aydemir G, Kasiri Y, Birta E, Béke G, Garcia AL, Bartók E-M, Rühl R. Lycopene-derived bioactive retinoic acid receptors/retinoid-X receptors-activating metabolites may be relevant for lycopene's anti-cancer potential. Mol Nutr Food Res 2013;57:739–47. [DOI] [PubMed] [Google Scholar]
- 266. Ip BC, Liu C, Lichtenstein AH, von Lintig J, Wang X-D. Lycopene and apo-10’-lycopenoic acid have differential mechanisms of protection against hepatic steatosis in β-carotene-9’,10’-oxygenase knockout male mice. J Nutr 2015;145:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, Forman JD, Cher ML, Powell I, Pontes JE, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer 2007;59:1–7. [DOI] [PubMed] [Google Scholar]
- 268. Gann PH, Deaton RJ, Rueter EE, van Breemen RB, Nonn L, Macias V, Han M, Ananthanarayanan V. A phase II randomized trial of lycopene-rich tomato extract among men with high-grade prostatic intraepithelial neoplasia. Nutr Cancer 2015;67:1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Mariani S, Lionetto L, Cavallari M, Tubaro A, Rasio D, De Nunzio C Hong GM, Borro M, Simmaco M. Low prostate concentration of lycopene is associated with development of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. Int J Mol Sci 2014;15:1433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Thies F, Mills LM, Moir S, Masson LF. Cardiovascular benefits of lycopene: fantasy or reality? Proc Nutr Soc 2016;1–8. [DOI] [PubMed] [Google Scholar]
- 271. Müller L, Caris-Veyrat C, Lowe G, Böhm V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—a critical review. Crit Rev Food Sci Nutr 2016;56:1868–79. [DOI] [PubMed] [Google Scholar]
- 272. Shao A, Hathcock JN. Risk assessment for the carotenoids lutein and lycopene. Regul Toxicol Pharmacol. 2006;45:289–98. [DOI] [PubMed] [Google Scholar]
- 273. Engelmann NJ, Clinton SK, Erdman JW. Nutritional aspects of phytoene and phytofluene, carotenoid precursors to lycopene. Adv Nutr 2011;2:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Campbell JK, Stroud CK, Nakamura MT, Lila MA, Erdman JW. Serum testosterone is reduced following short-term phytofluene, lycopene, or tomato powder consumption in F344 rats. J Nutr 2006;136:2813–9. [DOI] [PubMed] [Google Scholar]
- 275. Ford NA, Moran NE, Smith JW, Clinton SK, Erdman JW. An interaction between carotene-15,15’-monooxygenase expression and consumption of a tomato or lycopene-containing diet impacts serum and testicular testosterone. Int J Cancer 2012;131:E143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr 2012;96(Suppl):1179S–84S. [DOI] [PubMed] [Google Scholar]
- 277. Aust O, Stahl W, Sies H, Tronnier H, Heinrich U. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int J Vitam Nutr Res 2005;75:54–60. [DOI] [PubMed] [Google Scholar]
- 278. Rizwan M, Rodriguez-Blanco I, Harbottle A, Birch-Machin MA, Watson REB, Rhodes LE. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: a randomized controlled trial. Br J Dermatol 2011;164:154–62. [DOI] [PubMed] [Google Scholar]
- 279. Bakker MF, Peeters PH, Klaasen VM, Bueno-de-Mesquita HB, Jansen EH, Ros MM, Travier N, Olsen A, Tjønneland A, Overvad K, et al. Plasma carotenoids, vitamin C, tocopherols, and retinol and the risk of breast cancer in the European prospective investigation into cancer and nutrition cohort. Am J Clin Nutr 2016;103:454–64. [DOI] [PubMed] [Google Scholar]
- 280. Eliassen AH, Liao X, Rosner B, Tamimi RM, Tworoger SS, Hankinson SE. Plasma carotenoids and risk of breast cancer over 20 y of follow-up. Am J Clin Nutr 2015;101:1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Sujak A, Gabrielska J, Grudziński W, Borc R, Mazurek P, Gruszecki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch Biochem Biophys 1999;371:301–7. [DOI] [PubMed] [Google Scholar]
- 282. Mares J. Lutein and zeaxanthin isomers in eye health and disease. Annu Rev Nutr 2016;36:571–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res 2001;72:215–23. [DOI] [PubMed] [Google Scholar]
- 284. Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci 1997;38:1802–11. [PubMed] [Google Scholar]
- 285. Kijlstra A, Tian Y, Kelly ER, Berendschot TTJM. Lutein: more than just a filter for blue light. Prog Retin Eye Res 2012;31:303–15. [DOI] [PubMed] [Google Scholar]
- 286. Klein R, Chou C-F, Klein BEK, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol 2011;129:75–80. [DOI] [PubMed] [Google Scholar]
- 287. Ma L, Dou H-L, Wu Y-Q, Huang Y-M, Huang Y-B, Xu X-R, Zou Z-Y, Lin X-M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: a systematic review and meta-analysis. Br J Nutr 2012;107:350–9. [DOI] [PubMed] [Google Scholar]
- 288. Wolf-Schnurrbusch UEK, Zinkernagel MS, Munk MR, Ebneter A, Wolf S. Oral lutein supplementation enhances macular pigment density and contrast sensitivity but not in combination with polyunsaturated fatty acids. Invest Ophthalmol Vis Sci 2015;56:8069–74. [DOI] [PubMed] [Google Scholar]
- 289. Huang Y-M, Dou H-L, Huang F-F, Xu X-R, Zou Z-Y, Lin X-M. Effect of supplemental lutein and zeaxanthin on serum, macular pigmentation, and visual performance in patients with early age-related macular degeneration. BioMed Res Int 2015;2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Liu R, Wang T, Zhang B, Qin L, Wu C, Li Q, Ma L. Lutein and zeaxanthin supplementation and association with visual function in age-related macular degeneration. Invest Ophthalmol Vis Sci 2014;56:252–8. [DOI] [PubMed] [Google Scholar]
- 291. Cho E, Hankinson SE, Rosner B, Willett WC, Colditz GA. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am J Clin Nutr 2008;87:1837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. LaRowe TL, Mares JA, Snodderly DM, Klein ML, Wooten BR, Chappell R; CAREDS Macular Pigment Study Group. Macular pigment density and age-related maculopathy in the Carotenoids in Age-Related Eye Disease Study: an ancillary study of the Women's Health Initiative. Ophthalmology 2008;115:876–83, e1. [DOI] [PubMed] [Google Scholar]
- 293. Robman L, Vu H, Hodge A, Tikellis G, Dimitrov P, McCarty C, Guymer R. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can J Ophthalmol 2007;42:720–6. [DOI] [PubMed] [Google Scholar]
- 294. Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012;96:614–8. [DOI] [PubMed] [Google Scholar]
- 295. Yeum KJ, Taylor A, Tang G, Russell RM. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Invest Ophthalmol Vis Sci 1995;36:2756–61. [PubMed] [Google Scholar]
- 296. Liu X-H, Yu R-B, Liu R, Hao Z-X, Han C-C, Zhu Z-H, Ma L. Association between lutein and zeaxanthin status and the risk of cataract: a meta-analysis. Nutrients 2014;6:452–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Gong X, Rubin LP. Role of macular xanthophylls in prevention of common neovascular retinopathies: retinopathy of prematurity and diabetic retinopathy. Arch Biochem Biophys 2015;572:40–8. [DOI] [PubMed] [Google Scholar]
- 298. Ruta LM, Magliano DJ, Lemesurier R, Taylor HR, Zimmet PZ, Shaw JE. Prevalence of diabetic retinopathy in type 2 diabetes in developing and developed countries. Diabet Med 2013;30:387–98. [DOI] [PubMed] [Google Scholar]
- 299. Sahli MW, Mares JA, Meyers KJ, Klein R, Brady WE, Klein BEK, Ochs-Balcom HM, Donahue RP, Millen AE. Dietary intake of lutein and diabetic retinopathy in the Atherosclerosis Risk in Communities Study (ARIC). Ophthalmic Epidemiol 2016;23:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Lima VC, Rosen RB, Maia M, Prata TS, Dorairaj S, Farah ME, Sallum J. Macular pigment optical density measured by dual-wavelength autofluorescence imaging in diabetic and nondiabetic patients: a comparative study. Invest Ophthalmol Vis Sci 2010;51:5840–5. [DOI] [PubMed] [Google Scholar]
- 301. Hu B-J, Hu Y-N, Lin S, Ma W-J, Li X-R. Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol 2011;4:303–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Bahrami H, Melia M, Dagnelie G. Lutein supplementation in retinitis pigmentosa: PC-based vision assessment in a randomized double-masked placebo-controlled clinical trial [NCT00029289]. BMC Ophthalmol 2006;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Aleman TS, Duncan JL, Bieber ML, de Castro E, Marks DA, Gardner LM, Steinberg JD, Cideciyan AV, Maguire MG, Jacobson SG. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci 2001;42:1873–81. [PubMed] [Google Scholar]
- 304. Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Brockhurst RJ, Hayes KC, Johnson EJ, Anderson EJ, Johnson CA, Gaudio AR, et al. Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol 2010;128:403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Acar N, Berdeaux O, Grégoire S, Cabaret S, Martine L, Gain P, Thuret G, Creuzot-Garcher CP, Bron AM, Bretillon L. Lipid composition of the human eye: are red blood cells a good mirror of retinal and optic nerve fatty acids? PloS One 2012;7:e35102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Perrone S, Longini M, Marzocchi B, Picardi A, Bellieni CV, Proietti F, Rodriguez A, Turrisi G, Buonocore G. Effects of lutein on oxidative stress in the term newborn: a pilot study. Neonatology 2010;97:36–40. [DOI] [PubMed] [Google Scholar]
- 307. Perrone S, Tei M, Longini M, Santacroce A, Turrisi G, Proietti F, Felici C, Picardi A, Bazzini F, Vasarri P, et al. Lipid and protein oxidation in newborn infants after lutein administration. Oxid Med C ell Longev 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Rubin LP, Chan GM, Barrett-Reis BM, Fulton AB, Hansen RM, Ashmeade TL, Oliver JS, Mackey AD, Dimmit RA, Hartmann EE, et al. Effect of carotenoid supplementation on plasma carotenoids, inflammation and visual development in preterm infants. J Perinatol 2012;32:418–24. [DOI] [PubMed] [Google Scholar]
- 309. Manzoni P, Guardione R, Bonetti P, Priolo C, Maestri A, Mansoldo C, Mostert M, Anselmetti G, Sardei D, Bellettato M, et al. Lutein and zeaxanthin supplementation in preterm very low-birth-weight neonates in neonatal intensive care units: a multicenter randomized controlled trial. Am J Perinatol 2013;30:025–32. [DOI] [PubMed] [Google Scholar]
- 310. Dani C, Lori I, Favelli F, Frosini S, Messner H, Wanker P, Marini SD, Oretti C, Boldrini A, Massimiliano C, et al. Lutein and zeaxanthin supplementation in preterm infants to prevent retinopathy of prematurity: a randomized controlled study. J Matern Fetal Neonatal Med 2012;25:523–7. [DOI] [PubMed] [Google Scholar]
- 311. Romagnoli C, Giannantonio C, Cota F, Papacci P, Vento G, Valente E, Purcaro V, Costa S. A prospective, randomized, double blind study comparing lutein to placebo for reducing occurrence and severity of retinopathy of prematurity. J Matern Fetal Neonatal Med 2011;24(Suppl 1):147–50. [DOI] [PubMed] [Google Scholar]
- 312. Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216–30. [DOI] [PubMed] [Google Scholar]
- 313. Olmedilla B, Granado F, Blanco I, Vaquero M. Lutein, but not alpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition 2003;19:21–4. [DOI] [PubMed] [Google Scholar]
- 314. Stringham JM, Hammond BR. The glare hypothesis of macular pigment function. Optom Vis Sci 2007;84:859–64. [DOI] [PubMed] [Google Scholar]
- 315. Stringham JM, Hammond BR. Macular pigment and visual performance under glare conditions. Optom Vis Sci 2008;85:82–8. [DOI] [PubMed] [Google Scholar]
- 316. Kvansakul J, Rodriguez-Carmona M, Edgar DF, Barker FM, Köpcke W, Schalch W, Barbur JL. Supplementation with the carotenoids lutein or zeaxanthin improves human visual performance. Ophthalmic Physiol Opt 2006;26:362–71. [DOI] [PubMed] [Google Scholar]
- 317. Yao Y, Qiu Q, Wu X-W, Cai Z, Xu S, Liang X. Lutein supplementation improves visual performance in Chinese drivers: 1-year randomized, double-blind, placebo-controlled study. Nutrition 2013;29:958–64. [DOI] [PubMed] [Google Scholar]
- 318. Ma L, Lin X-M, Zou Z-Y, Xu X-R, Li Y, Xu R. A 12-week lutein supplementation improves visual function in Chinese people with long-term computer display light exposure. Br J Nutr 2009;102:186–90. [DOI] [PubMed] [Google Scholar]
- 319. Hammond BR, Fletcher LM, Roos F, Wittwer J, Schalch W. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on photostress recovery, glare disability, and chromatic contrast. Invest Ophthalmol Vis Sci 2014;55:8583–9. [DOI] [PubMed] [Google Scholar]
- 320. Yagi A, Fujimoto K, Michihiro K, Goh B, Tsi D, Nagai H. The effect of lutein supplementation on visual fatigue: a psychophysiological analysis. Appl Ergon 2009;40:1047–54. [DOI] [PubMed] [Google Scholar]
- 321. Stringham JM, Hammond BR Jr. Dietary lutein and zeaxanthin: possible effects on visual function. Nutr Rev 2005;63:59–64. [DOI] [PubMed] [Google Scholar]
- 322. Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol 2005;57:713–20. [DOI] [PubMed] [Google Scholar]
- 323. Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006;67:1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Feart C, Letenneur L, Helmer C, Samieri C, Schalch W, Etheve S, Delcourt C, Dartigues J-F, Barberger-Gateau P. Plasma carotenoids are inversely associated with dementia risk in an elderly French cohort. J Gerontol A Biol Sci Med Sci 2016;71:683–8. [DOI] [PubMed] [Google Scholar]
- 325. Nooyens ACJ, Milder IEJ, van Gelder BM, Bueno-de-Mesquita HB, van Boxtel MPJ, Verschuren WMM. Diet and cognitive decline at middle age: the role of antioxidants. Br J Nutr 2015;113:1410–7. [DOI] [PubMed] [Google Scholar]
- 326. Vishwanathan R, Neuringer M, Snodderly DM, Schalch W, Johnson EJ. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr Neurosci 2013;16:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Vishwanathan R, Schalch W, Johnson EJ. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutr Neurosci 2016;19:95–101. [DOI] [PubMed] [Google Scholar]
- 328. Vishwanathan R, Iannaccone A, Scott TM, Kritchevsky SB, Jennings BJ, Carboni G, Forma G, Satterfield S, Harris T, Johnson KC, et al. Macular pigment optical density is related to cognitive function in the elderly. Age Aging 2013;43:271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Renzi LM, Dengler MJ, Puente A, Miller LS, Hammond BR. Relationships between macular pigment optical density and cognitive function in unimpaired and mildly cognitively impaired older adults. Neurobiol Aging 2014;35:1695–9. [DOI] [PubMed] [Google Scholar]
- 330. Feeney J, Finucane C, Savva GM, Cronin H, Beatty S, Nolan JM, Kenny RA. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol Aging 2013;34:2449–56. [DOI] [PubMed] [Google Scholar]
- 331. Kelly D, Coen RF, Akuffo KO, Beatty S, Dennison J, Moran R, Stack J, Howard AN, Mulcahy R, Nolan JM. Cognitive function and its relationship with macular pigment optical density and serum concentrations of its constituent carotenoids. J Alzheimers Dis 2015;48:261–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Bovier ER, Renzi LM, Hammond BR. A double-blind, placebo-controlled study on the effects of lutein and zeaxanthin on neural processing speed and efficiency. PloS One 2014;9:e108178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Johnson EJ, McDonald K, Caldarella SM, Chung H-Y, Troen AM, Snodderly DM. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci 2008;11:75–83. [DOI] [PubMed] [Google Scholar]
- 334. Vishwanathan R, Sen S, Kuchan MJ, Johnson EJ. Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr 2014;59:659–65. [DOI] [PubMed] [Google Scholar]
- 335. Khachik F, Spangler CJ, Smith JC Jr., Canfield LM, Steck A, Pfander H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem 1997;69:1873–81. [DOI] [PubMed] [Google Scholar]
- 336. Lietz G, Mulokozi G, Henry JCK, Tomkins AM. Xanthophyll and hydrocarbon carotenoid patterns differ in plasma and breast milk of women supplemented with red palm oil during pregnancy and lactation. J Nutr 2006;136:1821–7. [DOI] [PubMed] [Google Scholar]
- 337. Perrone S, Tataranno LM, Stazzoni G, Ramenghi L, Buonocore G. Brain susceptibility to oxidative stress in the perinatal period. J Matern Fetal Neonatal Med 2015;28(Suppl 1):2291–5. [DOI] [PubMed] [Google Scholar]
- 338. Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. J Neurosci 2008;28:12176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Sale A, Berardi N, Maffei L. Enrich the environment to empower the brain. Trends Neurosci 2009;32:233–9. [DOI] [PubMed] [Google Scholar]
- 340. Cheatham CL, Sheppard KW. Synergistic effects of human milk nutrients in the support of infant recognition memory: an observational study. Nutrients 2015;7:9079–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Lieblein-Boff JC, Johnson EJ, Kennedy AD, Lai C-S, Kuchan MJ. Exploratory metabolomic analyses reveal compounds correlated with lutein concentration in frontal cortex, hippocampus, and occipital cortex of human infant brain. PloS One 2015;10:e0136904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Barnett SM, Khan NA, Walk AM, Raine LB, Moulton C, Cohen NJ, Kramer AF, Hammond Jr BR, Renzi-Hammond L, Hillman CH. Macular pigment optical density is positively associated with academic performance among preadolescent children. Nutr Neurosci 2017, DOI: 10.1080/1028415X.2017.1329976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Hassevoort KM, Khazoum SE, Walker JA, Barnett SM, Raine LB, Hammond BR, Renzi-Hammond LM, Kramer AF, Khan NA, Hillman CH, et al. Macular carotenoids, aerobic fitness, and central adiposity are associated differentially with hippocampal-dependent relational memory in preadolescent children. J Pediatr 2017;183:108–14, e1. [DOI] [PubMed] [Google Scholar]
- 344. Leermakers ET, Darweesh SK, Baena CP, Moreira EM, Melo van Lent D, Tielemans MJ, Muka T, Vitezova A, Chowdhury R, Bramer WM, et al. The effects of lutein on cardiometabolic health across the life course: a systematic review and meta-analysis. Am J Clin Nutr 2016;103:481–94. [DOI] [PubMed] [Google Scholar]
- 345. Zou Z-Y, Xu X-R, Lin X-M, Zhang H-B, Xiao X, Ouyang L, Huang Y-M, Wang X, Liu Y-Q. Effects of lutein and lycopene on carotid intima-media thickness in Chinese subjects with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Br J Nutr 2014;111:474–80. [DOI] [PubMed] [Google Scholar]
- 346. Xu X-R, Zou Z-Y, Xiao X, Huang Y-M, Wang X, Lin X-M. Effects of lutein supplement on serum inflammatory cytokines, ApoE and lipid profiles in early atherosclerosis population. J Atheroscler Thromb 2013;20:170–7. [DOI] [PubMed] [Google Scholar]
- 347. Burri BJ. Beta-cryptoxanthin as a source of vitamin A. J Sci Food Agric 2015;95:1786–94. [DOI] [PubMed] [Google Scholar]
- 348. Lorenzo Y, Azqueta A, Luna L, Bonilla F, Domínguez G, Collins AR. The carotenoid β-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis 2009;30:308–14. [DOI] [PubMed] [Google Scholar]
- 349. Liu C, Bronson RT, Russell RM, Wang X-D. β-Cryptoxanthin supplementation prevents cigarette smoke-induced lung inflammation, oxidative damage, and squamous metaplasia in ferrets. Cancer Prev Res (Phila) 2011;4:1255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Männistö S, Smith-Warner SA, Spiegelman D, Albanes D, Anderson K, van den Brandt PA, Cerhan JR, Colditz G, Feskanich D, Freudenheim JL, et al. Dietary carotenoids and risk of lung cancer in a pooled analysis of seven cohort studies. Cancer Epidemiol Prev Biomark 2004;13:40–8. [DOI] [PubMed] [Google Scholar]
- 351. Min K, Min J. Serum carotenoid levels and risk of lung cancer death in US adults. Cancer Sci 2014;105:736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Iskandar AR, Liu C, Smith DE, Hu K-Q, Choi S-W, Ausman LM, Wang X-D. β-Cryptoxanthin restores nicotine-teduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev Res (Phila) 2013;6:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Iskandar AR, Miao B, Li X, Hu K-Q, Liu C, Wang X-D. β-Cryptoxanthin reduced lung tumor multiplicity and inhibited lung cancer cell motility by downregulating nicotinic acetylcholine receptor α7 signaling. Cancer Prev Res (Phila) 2016;9:875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Burri BJ, La Frano MR, Zhu C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr Rev 2016;74:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Yamaguchi M. Role of carotenoid β-cryptoxanthin in bone homeostasis. J Biomed Sci 2012;19:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Ando F, Yano M. Bone mineral density in post-menopausal female subjects is associated with serum antioxidant carotenoids. Osteoporos Int 2008;19:211–9. [DOI] [PubMed] [Google Scholar]
- 357. Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Ando F, Shimokata H, Yano M. Dietary patterns of antioxidant vitamin and carotenoid intake associated with bone mineral density: findings from post-menopausal Japanese female subjects. Osteoporos Int 2011;22:143–52. [DOI] [PubMed] [Google Scholar]
- 358. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res 2009;24:1086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Roodenburg AJ, Leenen R, van het Hof KH, Weststrate JA, Tijburg LB. Amount of fat in the diet affects bioavailability of lutein esters but not of α-carotene, β-carotene, and vitamin E in humans. Am J Clin Nutr 2000;71:1187–93. [DOI] [PubMed] [Google Scholar]
- 360. Dagnelie G, Zorge IS, McDonald TM. Lutein improves visual function in some patients with retinal degeneration: a pilot study via the Internet. Optometry 2000;71:147–64. [PubMed] [Google Scholar]
- 361. Choi RY, Chortkoff SC, Gorusupudi, Bernstein PS. Crystalline maculo pathy associated with high-dose lutein supplementation. JAMA Ophthalmol 2016;134:1445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]