Abstract
A myeloablative regimen that includes total-body irradiation (TBI) before hematopoietic stem cell transplantation results in higher patient survival rates than achieved with regimens without TBI. The TBI protocol, however, varies between institutions. In October 2015, the Japanese Radiation Oncology Study Group initiated a national survey of myeloablative TBI (covering 2010–2014). Among the 186 Japanese institutions performing TBI, 90 (48%) responded. The 82 institutions that had performed myeloablative TBI during this period treated 2698 patients with malignant disease [leukemia (2082 patients, 77.2%), malignant lymphoma (378, 14%)] and 37 with non-malignant disease [severe aplastic anemia (20, 54%), inborn errors of metabolism (5, 14%)]. A linear accelerator was used at all institutions. The institutions were divided into 41 large and 41 small institutions based on the median number of patients. The long source–surface distance technique was the method of choice in the 34 institutions (82.9%) and the moving-couch technique in the 7 (17.1%) in the large institutions. The schedules most routinely used by the participating institutions consisted of 12 Gy/6 fractions/3 days (26 institutions, 63.5%) in the large institutions. The dose rate varied from 5 to 26 cGy/min. The lungs and lenses were routinely shielded in 23 large institutions (56.1%), and only the lungs in 9 large institutions (21.9%). At lung-shielding institutions, the most frequent maximum acceptable total dose for the lungs was 8 Gy (19 institutions, 27.5%). Our results reveal considerable differences in the TBI methods used by Japanese institutions and thus the challenges in designing multicenter randomized trials based on TBI.
Keywords: national survey, myeloablative, total body irradiation, Japanese
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) is widely performed as a curative therapeutic strategy for various hematological diseases. HSCT requires prior myeloablative conditioning, which suppresses the immune system, prevents graft-versus-host disease, and destroys tumor cells in recipients. The conditioning regimens currently in use are chemotherapy alone and a combination of total-body irradiation (TBI) and chemotherapy. The advantages of TBI over chemotherapy are: (i) no sanctuary sites, such as the testicles and central nervous system, are spared; (ii) high, homogeneous radiation doses can be delivered to the whole body; (iii) less possibility of cross-resistance with other antineoplastic agents; (iv) problems related to excretion or detoxification are avoided; (v) dose distribution can be tailored using shielding [1].
In many randomized trials, the therapeutic outcomes of TBI-containing regimens were superior to those without TBI [2, 3]. This has also been demonstrated in national surveys in Japan, such that the use of TBI has rapidly increased [4, 5]. The last national survey of TBI in Japan was performed in 2002, and it revealed considerable variations in the TBI regimens of the various institutions. To update this information, in 2015 the Japanese Radiation Oncology Study Group (JROSG) designed and administered a national survey questionnaire on myeloablative TBI. The results are presented herein.
MATERIALS AND METHODS
The October 2015 survey reviewed patients treated between January 2010 and December 2014. The questionnaire surveyed disease types and the TBI method used at the participating institution, including treatment unit, treatment technique, dose and fractions, and shielded organs. We consulted the Japanese Radiation Oncology (JASTRO) Database Committee about the institutions performing TBI in 2015 and were told that there were 186 Japanese institutions performing TBI at that time. All 186 were invited to participate in our survey. This survey was approved by the Review Board of the University Hospital Medical Information Network (UMIN000018726).
RESULTS
Of the 186 institutions invited to participate in the survey, 90 (48%) responded. Of them, 82 had performed myeloablative TBI during the study period. The number of patients treated at those institutions during this period was as follows: <10 patients in 15 institutions (18.3%), 10–30 patients in 34 institutions (41.5%) and >30 patients in 33 institutions (40.2%). The median number of treated patients was 24. The 82 institutions were divided into large and small institutions based on the number of patients treated; thus, there were 41 large institutions that treated >24 patients and 41small institutions that treated ≤24 patients.
Diagnosis
Of the patients treated with myeloablative TBI, 2698 had malignant disease, including 2082 (77.2%) with leukemia, 378 (14%) with malignant lymphoma, 187 (6.9%) with myelodysplastic syndrome, 22 (0.8%) with neuroblastoma, 11 (0.4%) with multiple myeloma, 5 (0.2%) with myeloid sarcoma, 6 (0.2%) with other diseases, and 7 (0.3%) with unknown diseases (Table 1). The remaining 37 patients undergoing myeloablative TBI had non-malignant disease: 20 patients (54.1%) with severe aplastic anemia, 7 (18.9%) with chronic, active Epstein-Barr virus infection, 5 (13.5%) with inborn errors of metabolism, 2 (5.4%) with myelofibrosis, and 3 (8.1%) with other diseases (Table 2).
Table 1.
Breakdown of the 2698 patients with malignant disease
| Diagnosis | No. of patients (%) |
|---|---|
| Leukemia | 2082 (77.2) |
| Malignant lymphoma | 378 (14) |
| Myelodysplastic syndrome | 187 (6.9) |
| Neuroblastoma | 22 (0.8) |
| Multiple myeloma | 11 (0.4) |
| Myeloid sarcoma | 5 (0.2) |
| Others | 6 (0.2) |
| Unknown | 7 (0.3) |
Table 2.
Breakdown of the 37 patients with non-malignant disease
| Diagnosis | No. of patients (%) |
|---|---|
| Severe aplastic anemia | 20 (54.1) |
| Epstein-Barr virus infection | 7 (18.9) |
| Inborn errors of metabolism | 5 (13.5) |
| Myelofibrosis | 2 (5.4) |
| Others | 3 (8.1) |
Timing of TBI
TBI was administered after conditioning chemotherapy in 38 institutions (46%) and before conditioning chemotherapy in 19 institutions (23%). The timing was undetermined in 18 institutions (22%).
Treatment unit
All of the responding institutions used a linear accelerator (LINAC); none used helical tomotherapy (HT). The beam energies most frequently used in LINAC are shown in Table 3 for the large institutions and in Table 4 for the small institutions.
Table 3.
Methods of total-body irradiation (TBI) in the 41 large institutions
| TBI method | No. of institutions (%) |
|---|---|
| Treatment technique | |
| Long source–surface distance | 34 (82.9) |
| Moving couch | 7 (17.1) |
| Beam energy (megavolts) | |
| 4 | 5 (12.2) |
| 6 | 11 (26.8) |
| 10 | 23 (56.1) |
| 15–20 | 2 (4.9) |
| Patient position | |
| Supine | 29 (70.8) |
| Supine and prone | 7 (17.1) |
| Supine and lateral | 1 (2.4) |
| Lateral | 1 (2.4) |
| Others | 3 (7.3) |
| Beam arrangement | |
| R–L | 25 (61) |
| A–P | 13 (31.7) |
| R–L and A–P | 3 (7.3) |
| Schedule of TBI (dose/fractions/days) | |
| 12 Gy/6 fr/4 days | 1 (2.4) |
| 12 Gy/6 fr/3 days | 26 (63.5) |
| 12 Gy/4 fr/4 days | 7 (17.1) |
| 12 Gy/4 fr/3 days | 1 (2.4) |
| 12 Gy/4 fr/2 days | 5 (12.2) |
| 10 Gy/5 fr/3 days | 1 (2.4) |
| Dose rate (cGy) | |
| 5–9.9 | 13 (31.7) |
| 10–15 | 25 (61) |
| over 15–26 | 3 (7.3) |
| Routinely shielded organs | |
| Lungs + lenses | 23 (56.1) |
| Lungs | 9 (21.9) |
| Lenses | 4 (9.8) |
| Lungs + lenses + kidneys | 1 (2.4) |
| None | 4 (9.8) |
R–L = right–left, A–P = anterior–posterior, Gy = grays, fr = fractions, cGy = centigrays.
Table 4.
Methods of TBI in the 41 small institutions
| TBI method | No. of institutions (%) |
|---|---|
| Treatment technique | |
| Long source–surface distance | 40 (97.6) |
| Moving couch | 1 (2.4) |
| Beam energy (megavolt) | |
| 4 | 1 (2.4) |
| 6 | 9 (21.9) |
| 10 | 30 (73.3) |
| 15–20 | 1 (2.4) |
| Patient position | |
| Supine | 36 (87.9) |
| Supine and lateral | 3 (7.3) |
| Lateral | 1 (2.4) |
| Others | 1 (2.4) |
| Beam arrangement | |
| R–L | 35 (85.3) |
| A–P | 2 (4.9) |
| R–L and A–P | 4 (9.8) |
| Schedule of TBI (dose/fractions/days) | |
| 12 Gy/6 fr/4 days | 1 (2.4) |
| 12 Gy/6 fr/3 days | 26 (63.5) |
| 12 Gy/5 fr/5 days | 1 (2.4) |
| 12 Gy/5 fr/3 days | 1 (2.4) |
| 12 Gy/4 fr/4 days | 4 (9.8) |
| 12 Gy/4 fr/2 days | 4 (9.8) |
| 10 Gy/4 fr/4 days | 1 (2.4) |
| 10 Gy/4 fr/2 days | 3 (7.3) |
| Dose rate (cGy) | |
| 5–9.9 | 20 (48.7) |
| 10–15 | 17 (41.5) |
| >15–26 | 4 (9.8) |
| Routinely shielded organs | |
| Lungs + lenses | 25 (61) |
| Lungs | 12 (29.3) |
| Lenses | 1 (2.4) |
| None | 3 (7.3) |
TBI = total body irradiation, R–L = right–left, A–P = anterior–posterior, fr = fractions, Gy = grays, cGy = centigrays.
Treatment technique
The treatment techniques and patient positions used during TBI are shown in Tables 3 and 4. In the institutions using the long source–surface distance (SSD) technique, the patients were placed in the supine position at 61 institutions and in the supine and prone positions in 3 institutions. Among the 8 institutions using the moving-couch technique, patients were placed in the supine position in 4 institutions and in the supine and prone position in the other 4 institutions.
The beam arrangement is shown in Tables 3 and 4. For all 8 institutions using the moving-couch technique, the beam arrangement was always anterior–posterior.
Two beams were used in each fraction in 69 institutions (84.1%), four beams in 7 institutions (8.5%), one beam in 6 institutions (7.3%), and variable numbers in 1 institution.
Doses and fractions of TBI
The total dose of myeloablative TBI was 6–12 Gy, with the number of fractions ranging from four to six. The treatment duration was 2–5 days. Tables 3 and 4 summarize the detailed results regarding the most frequently used TBI schedules at each institution and provide a comparison with the results of the 1989 survey [4] (Fig. 1).
Fig. 1.
Detailed results for the most frequently used total-body radiation (TBI) schedule at each institution compared with those reported in the 1989 survey.
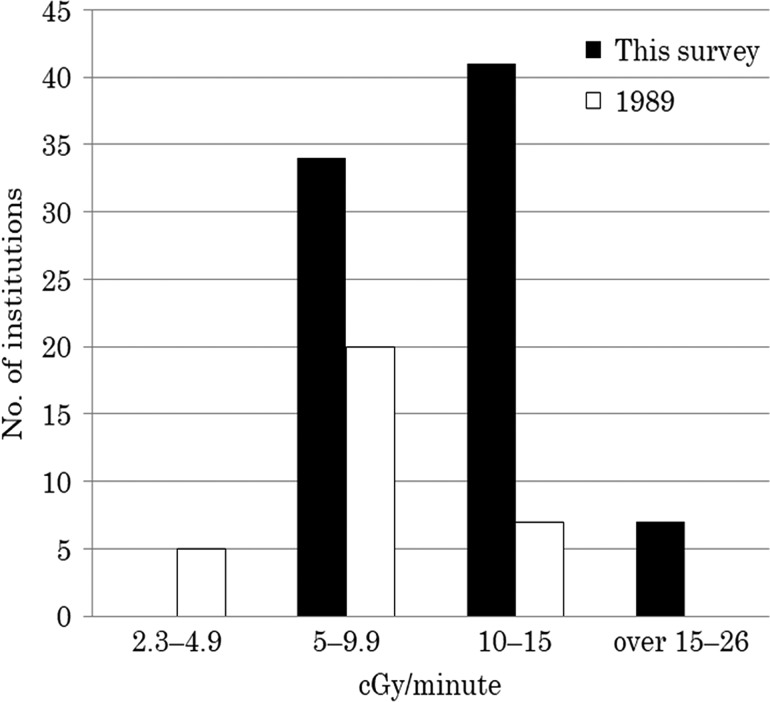
The dose rate in the axis of the beam ranged from 5 to 26 centigray (cGy) per minute and varied between the institutions. The dose rates are detailed in Tables 3 and 4 and compared with those reported in the 1989 survey [4] (Fig. 2).
Fig. 2.
The dose rates compared with those reported in the 1989 survey. cGy = centigrays.
The dose rates in the institutions using the moving-couch technique were expressed as the ratio of the dose to the total duration of irradiation. For the long SSD technique, the dose rates were 5–26 cGy/min, and for the moving-couch technique 5–15.2 cGy/min.
Organ shielding
The organs routinely shielded during TBI are summarized in Tables 3 and 4. None of the institutions routinely shielded the ovaries or testes. Among the institutions practising lung-shielding, the maximum acceptable total dose to the lungs varied widely, ranging from 3.6 to 12 Gy, with 8 Gy as the most frequently administered dose (19 of 69 lung-shielding institutions: 27.5%). The lung shields consisted of lead blocks in 51 (73.9%) of the lung-shielding institutions, acrylic resins in 7 (8.5%), and other materials in 11.
Duration and scheduling of TBI
The duration of a single TBI session, from the time of entry to the time of exit from the radiotherapy room was 60 min in 40 institutions (48.8%), 25–50 min in 32 institutions (39%), and 70–180 min in 10 institutions (12.2%) (Fig. 3). There was no difference in the duration of TBI between institutions using the long-SSD vs the moving-couch technique. The institutions were also queried about how they integrated time-consuming TBI within a tight daily schedule that included other forms of radiotherapy. The freely provided responses included the following: ‘TBI is performed during times when other types of radiotherapy are not scheduled’ (35 institutions, 46.3%); ‘The TBI fraction is limited to once per day’ (6 institutions, 7.3%); and ‘The number of TBI cases is limited’ (17 institutions, 20.7%).
Fig. 3.
Duration of a single TBI session, from the time of entry to the time of exit from the radiotherapy room.
DISCUSSION
In 1987, the first survey of TBI methods was conducted in Europe, and its results indicated that they varied greatly between institutions [6]. Another survey, conducted mainly in Europe in 2014, also revealed the use of a wide variety of TBI methods [1]. The American College of Radiology and the American Society for Radiation Oncology recently issued a guideline for the performance of TBI, but it only addressed general requirements and did not include any methodological recommendations [7]. In Japan, three national surveys on TBI have been conducted thus far: in 1983, 1989 and 2002 (although no report on the results of the 2002 survey has been published). They showed an annual increase in the number of patients undergoing TBI [4, 8] and that the methods varied greatly, including the use of multiple methods within a single Japanese institution [9, 10]. Because of the increasing number of clinical studies on TBI, the actual conditions must be clearly described. As the last national survey in Japan was conducted more than a decade ago, in 2015 the JROSG decided to undertake a new national survey on TBI.
According to the results of that survey, the most common malignant disease treated with TBI between 2010 and 2014 was leukemia. This finding was consistent with the many past reports on leukemia, which described the superior therapeutic outcomes achieved with TBI-containing conditioning regimens performed prior to HSCT, compared with the outcomes that followed conditioning regimens without TBI [3, 5]. The second-most common setting for TBI was malignant lymphoma, as the improved therapeutic outcomes of lymphoma patients treated with TBI-containing conditioning regimens prior to HSCT is well established [11, 12]. Among the non-malignant diseases treated with TBI, the most common was severe aplastic anemia. In the case of HSCT for non-malignant diseases, including severe aplastic anemia, TBI-containing conditioning regimens are associated with a lower toxicity and a lower incidence of graft-versus-host disease [13]. The aim of TBI in patients with non-malignant disease is the achievement of a graft-versus-tumor effect, rather than an anti-tumor effect. In a clinical study on severe aplastic anemia [14], TBI was administered as a single dose of 2 Gy; therefore, in non-malignant diseases, myeloablative TBI is less likely to be given in multiple doses. In the survey described herein, only 37 patients with non-malignant disease underwent TBI.
The order of TBI and conditioning chemotherapy with cyclophosphamide and other drugs varied between the surveyed Japanese institutions, with the order determined on a case-by-case basis in many of them. In many past reports on HSCT, different combinations of TBI and chemotherapy were collectively considered as ‘TBI combined with chemotherapy,’ but no specific order of the components was described.
The treatment unit used in all institutions in this survey was LINAC; HT was not used in any of the responding institutions. In the 2014 mainly European survey on TBI, LINAC was used in 91.1% of the 57 responding institutions, and a cobalt unit in the remaining 8.9%. HT was used only as a substitute for regular LINAC [1]. This usage is similar to the current situation in Japan, where HT has still not been generally adopted.
In the current survey, the most frequent beam energy for LINAC was 10 MV, with no apparent difference between large and small institutions. In the 2014 survey, the beam energy range of the LINAC was 6–25 MV, with 6 MV as the most frequently used beam energy [1]. In the retrospective study of Thomas et al., a beam energy of 15 MV was associated with a higher risk of pulmonary complications than a beam energy of 9 MV [15]. However, there have been no studies specifically comparing the effects of various levels of beam energy on complications.
Regarding treatment techniques, the survey revealed the use of the long-SSD technique in the majority of the institutions. This proportion was largely the same as in the 2002 survey. There was no apparent difference between large and small institutions. Because the moving-couch technique allows irradiation to be administered in a small treatment room, it could be adopted more widely in Japan, where many hospitals have limited space. Nonetheless, according to the survey this technique is still not widely used.
The most common patient position during TBI was supine, and treatment was delivered using two right–left (R–L) beams. Again, there was no apparent difference between large and small institutions. In a previous report, use of the lateral position was associated with a lower rate of pulmonary complications [15]. However, the supine position has a higher reproducibility because the patient thickness is relatively even, and after organ shielding the dose is still distributed evenly.
The total dose range of myeloablative TBI used by the 82 responding institutions was 6–12 Gy, with the most frequent total dose/fraction (fr)/day being 12 Gy/6 fr/3 days, without an apparent difference between large and small institutions. These results were comparable with the most common fractionated-TBI regimen reported in the 1989 and 2002 surveys [4]. Four randomized studies of the total doses and fractions of TBI have been published so far. Clift et al. conducted two randomized studies on patients with myeloid leukemia. In both, disease recurrence decreased in the group in which the total dose was increased from 12.00 to 15.75 Gy, although toxicity to the liver and lungs increased. Ultimately, there was no difference in the overall survival of patients in the two groups [16, 17]. Thomas et al. [18] and Deeg et al. [19] compared single-dose irradiation of 10 Gy with fractionated irradiation of 12 Gy/6 fr. They reported decreased toxicity and improved overall survival in patients in the fractionated dose arm. Girinsky et al. compared single-dose irradiation of 10 Gy and fractionated irradiation of 14.75 Gy in patients with various hematological malignancies. Patients in the fractionated dose arm had less liver toxicity and improved disease-free survival [20]. In the study of Gopal et al. comparing fractionated doses of 10.2 Gy/6 fr/3 days and 12 Gy/4 fr/4 days, the recurrence rate was lower in patients treated with the latter, although there was no difference in the incidence of lung toxicity [21]. Based on these studies, a total dose of 12 Gy is commonly administered. In both the TBI survey conducted mainly in Europe in 2014 and in our survey, the most frequent total dose was 12 Gy, administered to patients in 79–94% of the institutions [1].
There are also reports indicating that toxicity is more strongly associated with the dose rate than with the total dose. According to one report, the risk of interstitial pneumonitis is lower in patients receiving single-dose irradiation of ≤10 cGy/min [22]. In another study, the risk of renal toxicity increased even in patients receiving fractionated irradiation of >20 cGy/min [23]. A randomized study found no association between the dose rate and toxicity [24]. Similarly, in a retrospective multivariate analysis, the number of fractions, not the dose rate, was a significant factor for the risk of interstitial pneumonitis [25]. In our survey, the most varied parameter among the reporting institutions was the dose rate, which ranged from 5 to 26 cGy/min and was therefore higher than that reported in the 1989 survey [4]. There was no apparent difference between large and small institutions.
In our survey, when the moving-couch technique was used, the dose rate was calculated as the ratio of the dose to the total duration of irradiation. Because the moving couch or table transports the patient only once, during which time his or her whole body is irradiated, the point dose rates are high [26, 27]. There are as yet no reports on how to calculate the dose rate in cases in which the moving-couch or moving-table method is used, nor has a potential association between the dose rate and toxicity been investigated. Whether the dose rate for this technique can be assessed in the same manner as for conventional long SSD remains to be determined.
A retrospective study demonstrated that lung shielding reduces pulmonary toxicity [28]. We found that the lungs were routinely shielded in as many as 80.4% of the patients treated in large institutions and 90.3% of those treated in small institutions. This result was comparable with that of the 2014 mainly European survey on TBI [1]. Moreover, the lungs were shielded in all patients treated at the eight institutions using the moving-couch technique. This may be because the total treatment duration is shorter in these patients than in patients treated using the long-SSD technique, and the moving couch allows for more precise placement of the shielding blocks [27]. Among the lung-shielding institutions, the most frequent maximum acceptable total dose for the lungs was 8 Gy, which was comparable with the acceptable total dose determined in the 2014 survey [1]. Another report showed a reduction in the incidence of interstitial pneumonitis, from 11.0% to 2.3%, by reducing the irradiation of the lungs by half, from 12 to 6 Gy, with lung shielding [25]. In another study, there was no difference in lung toxicity between patients receiving 10.2 Gy/6 fr with no lung shielding and those receiving 12 Gy/4 fr with lung shielding [21]. Moreover, in the current survey, 64.6% of institutions shielded the lenses routinely, which was much higher than the 14% reported in the 2014 mainly European study [1]. Reducing the TBI dose to the lenses was shown to contribute to a reduced risk of cataract development [29]. However, shielding the lenses requires caution, because leukemia cells and malignant lymphoma cells frequently invade the eyeballs. Given the concerns about possible disease recurrence due to the protection of tumor cells from irradiation by shielding of the lungs or other organs, no shielding was applied in 9.8% of the institutions in the current survey.
Although our survey had the limitation that only 48% of the TBI-performing institutions responded, the results of the survey demonstrated the considerable differences in the TBI methods practised in institutions in Japan. This variation makes it difficult to design multicenter randomized trials based on TBI regimens. Our results may help in the standardization of TBI regimens, thus facilitating multicenter clinical trials that include TBI.
In addition to questions regarding disease variation and TBI methods, participants in the survey were asked about the time allocated to the administration of TBI, which is a complicated procedure. Moreover, the number of patients undergoing radiotherapy has increased in recent years, such that institutions no longer administer radiation only three times a day, as determined in the 1989 survey [4]. The duration of a single TBI session, from entry to to exit from the radiotherapy room, was ≥60 min in 61% of the institutions. Hence, time constraints are an important issue. Our survey showed that this issue was most commonly addressed by performing TBI during periods when other types of radiotherapy were not scheduled (46.3% of the institutions). This burden could be eased by standardizing the techniques and scheduling of TBI, based on the findings from the current survey.
ACKNOWLEDGEMENTS
We appreciate the support received from all the institutions and researchers involved in this survey.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
REFERENCES
- 1. Giebel S, Miszczyk L, Slosarek K et al. Extreme heterogeneity of myeloablative total body irradiation techniques in clinical practice: a survey of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 2014;120:2760–5. [DOI] [PubMed] [Google Scholar]
- 2. Hartman AR, Williams SF, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant 1998;22:439–43. [DOI] [PubMed] [Google Scholar]
- 3. Gupta T, Kannan S, Dantkale V et al. Cyclophosphamide plus total body irradiation compared with busulfan plus cyclophosphamide as a conditioning regimen prior to hematopoietic stem cell transplantation in patients with leukemia: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther 2011;4:17–9. [DOI] [PubMed] [Google Scholar]
- 4. Inoue T, Mori T, Iino Y et al. National survey of bone marrow transplantation and total body irradiation with special reference to treatment schedule in Japan. J Jpn Soc Ther Radiol Oncol 1989;1:119–26. [Google Scholar]
- 5. Inoue T, Ikeda H, Yamazaki H et al. Role of total body irradiation as based on the comparison of preparation regimens for allogeneic bone marrow transplantation for acute leukemia in first complete remission. Strahlenther Onkol 1993;169:250–5. [PubMed] [Google Scholar]
- 6. Quast U. Total body irradiation—review of treatment techniques in Europe. Radiother Oncol 1987;9:91–106. [DOI] [PubMed] [Google Scholar]
- 7. Wolden SL, Rabinovitch RA, Bittner NH et al. ; American College of Radiology; American Society for Radiation Oncology American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) practice guideline for the performance of total body irradiation (TBI). Am J Clin Oncol 2013;36:97–101. [DOI] [PubMed] [Google Scholar]
- 8. Inoue T. Bone marrow transplantation and total body irradiation. Immunohaematology 1983;5:184–9. [Google Scholar]
- 9. Soejima T, Hirota S, Tsujino K et al. Total body irradiation followed by bone marrow transplantation: comparison of once-daily and twice-daily fractionation regimens. Radiat Med 2007;25:402–6. [DOI] [PubMed] [Google Scholar]
- 10. Ishibashi N, Maebayashi T, Aizawa T et al. Various regimens of total body irradiation for hematopoietic stem cell transplant. Exp Clin Transplant 2016;14:670–5. [PubMed] [Google Scholar]
- 11. Maeng CH, Ko YH, Lim DH et al. Comparison of total body irradiation (TBI) conditioning with non-TBI for autologous stem cell transplantation in newly diagnosed or relapsed mature T- and NK-cell non-Hodgkin lymphoma. Cancer Res Treat 2017;49:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brammer JE, Khouri I, Marin D et al. Stem cell transplantation outcomes in lymphoblastic lymphoma. Leuk Lymphoma 2017;58:366–71. [DOI] [PubMed] [Google Scholar]
- 13. Svenberg P, Remberger M, Svennilson J et al. Allogenic stem cell transplantation for nonmalignant disorders using matched unrelated donors. Biol Blood Marrow Transplant 2004;10:877–82. [DOI] [PubMed] [Google Scholar]
- 14. Anderlini P, Wu J, Gersten I et al. Cyclophosphamide conditioning in patients with severe aplastic anaemia given unrelated marrow transplantation: a phase 1–2 dose de-escalation study. Lancet Haematol 2015;2:e367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomas O, Mahé M, Campion L et al. Long-term complications of total body irradiation in adults. Int J Radiat Oncol Biol Phys 2001;49:125–31. [DOI] [PubMed] [Google Scholar]
- 16. Clift RA, Buckner CD, Appelbaum FR et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood 1991;77:1660–5. [PubMed] [Google Scholar]
- 17. Clift RA, Buckner CD, Appelbaum FR et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood 1990;76:1867–71. [PubMed] [Google Scholar]
- 18. Thomas ED, Clift RA, Hersman J et al. Marrow transplantation for acute nonlymphoblastic leukemic in first remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys 1982;8:817–21. [DOI] [PubMed] [Google Scholar]
- 19. Deeg HJ, Sullivan KM, Buckner CD et al. Marrow transplantation for acute nonlymphoblastic leukemia in first remission: toxicity and long-term follow-up of patients conditioned with single dose or fractionated total body irradiation. Bone Marrow Transplant 1986;1:151–7. [PubMed] [Google Scholar]
- 20. Girinsky T, Benhamou E, Bourhis JH et al. Prospective randomized comparison of single-dose versus hyperfractionated total-body irradiation in patients with hematologic malignancies. J Clin Oncol 2000;18:981–6. [DOI] [PubMed] [Google Scholar]
- 21. Gopal R, Ha CS, Tucker SL et al. Comparison of two total body irradiation fractionation regimens with respect to acute and late pulmonary toxicity. Cancer 2001;92:1949–58. [DOI] [PubMed] [Google Scholar]
- 22. Kim TH, Rybka WB, Lehnert S et al. Interstitial pneumonitis following total body irradiation for bone marrow transplantation using two different dose rates. Int J Radiat Oncol Biol Phys 1985;11:1285–91. [DOI] [PubMed] [Google Scholar]
- 23. Cheng JC, Schultheiss TE, Wong JY. Impact of drug therapy, radiation dose, and dose rate on renal toxicity following bone marrow transplantation. Int J Radiat Oncol Biol Phys 2008;71:1436–43. [DOI] [PubMed] [Google Scholar]
- 24. Ozsahin M, Pène F, Touboul E et al. Total-body irradiation before bone marrow transplantation. Results of two randomized instantaneous dose rates in 157 patients. Cancer 1992;69:2853–65. [DOI] [PubMed] [Google Scholar]
- 25. Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys 2005;63:876–84. [DOI] [PubMed] [Google Scholar]
- 26. Sarfaraz M, Yu C, Chen DJ et al. A translational couch technique for total body irradiation. J Appl Clin Med Phys 2001;2:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Härtl PM, Treutwein M, Hautmann MG et al. Total body irradiation—an attachment free sweeping beam technique. Radiat Oncol 2016;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soule BP, Simone NL, Savani BN et al. Pulmonary function following total body irradiation (with or without lung shielding) and allogeneic peripheral blood stem cell transplant. Bone Marrow Transplant 2007;40:573–8. [DOI] [PubMed] [Google Scholar]
- 29. Ozsahin M, Belkacemi Y, Pene F et al. Total-body irradiation and cataract incidence: a randomized comparison of two instantaneous dose rates. Int J Radiat Oncol Biol Phys 1994;28:343–7. [DOI] [PubMed] [Google Scholar]