Abstract
Flattening filter–free (FFF) photon beams minimize the intrafraction motion of tumors, and this feature is useful in pulmonary malignancies, such as non-small-cell lung cancer (NSCLC). However, the radiobiological effects of such beams on NSCLC cells, which are often treated with stereotactic body radiotherapy (SBRT), have not been investigated sufficiently. Although cell motility may be promoted by photon beams with a low dose, the relationship between cell motility and the dose rate of photon beams has not been evaluated. The purpose of this study was to evaluate the radiobiological effects of FFF photon beams on cell survival and motility in NSCLC. A human lung cancer cell line (A549) was irradiated with conventional flattening filter (FF) and FFF photon beams at dose rates of 300 (FF), 500 and 2000 MU/min (FFF). While cell survival was estimated using the colony formation assay, cell motility was evaluated using the Boyden chamber and Matrigel invasion assays. FFF photon beams with a high dose rate neither affected the survival of A549 cells nor caused any significant difference in their motility. On the other hand, high-dose irradiation reduced cell survival and motility regardless of the dose rate. Photon beams with a high dose rate used for radiation therapy are suitable for SBRT from the standpoint of both cell survival and motility, in addition to their physical characteristics.
Keywords: flattening filter–free, SBRT, cell survival, cell motility
INTRODUCTION
Flattening filter–free (FFF) photon beams allow for approximately a 2–6-fold increase in the instantaneous dose of X-ray pulse delivered compared with conventional flattening filter (FF) photon beams, thereby making the administration of radiation at high dose rates (>2000 MU/min) feasible [1]. This advantage of FFF photon beams with high dose rate is that they shorten the beam-on time by approximately 10–30% [1] and improve the clinical outcomes [2]. These photon beams also help in minimizing the intrafraction motion of tumors such as lung cancer [3]. This is especially relevant when using a large dose per fraction, such as in SBRT [4–6].
The physical characteristics of FFF photon beams have been reported earlier [6, 7]. On the other hand, not much has been reported about their radiobiological effects. Lohse et al. changed the dose per pulse by removing the flattening filter and found that cell survival decreased with the subsequent increase in the dose rate [8]. However, no significant difference was observed in cell survival after irradiation with photon beams at high dose rates [9–11]. Thus, the effect of the dose rate of radiation on cell survival has remained unclear. In addition, pulmonary malignancies such as non-small-cell lung cancer (NSCLC) are treated by high-dose-rate photon beams in the clinic [12]. However, the dose-rate effects of the high doses of FFF photon beams on NSCLC treated using SBRT have not been sufficiently investigated. The results of the past clinical trials would be questionable if there were significant differences in the radiobiological effects of FFF photon beams with high dose rates, such as in the survival fraction (SF).
There are some reports that cell motility, which is related to metastasis after treatment, is promoted by photon irradiation [13, 14]. Nevertheless, little attention has been given to the relationship between cell motility and the dose-rate effect of the photon beams used. In addition to local tumor control, assessment of metastasis plays a major role in the treatment outcomes following radiation therapy. Assessment of the influence photon beams with high dose rates have on cell motility has made us realize the need for further consideration of their radiobiological effects.
The purpose of this study was to investigate the radiobiological effects of photon beams with high dose rates on cell motility and SF in NSCLC.
MATERIALS AND METHODS
Cell culture
A549 human lung adenocarcinoma cells were cultured in DMEM medium (Sigma-Aldrich, St Louis, MO, USA) with 10% fetal bovine serum (FBS) (Gibco, Gaithersburg, MD, USA) at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Irradiation
Cells were irradiated using 6 MV FF and 7 MV FFF photon beams of the linear accelerator ARTISTETM (Siemens, Erlangen, Germany). Irradiation doses of 0.5, 2, 4 and 8 Gy were delivered to the bottom of the dish at dose rates of 300 (FF), 500 and 2000 MU/min (FFF). A small field size of 5 cm × 5 cm was used. The build-up was attained by adding 5 cm of water-equivalent phantom, and a 10-cm-thick layer of tough water was added below the dish. The culture dish with cells between the two tough water was scanned using computed tomography (BrightSpeed; GE Medical Systems, Milwaukee, WI, USA). The irradiation dose delivered to cells was calculated by a treatment planning system (Xio; Elekta, Stockholm, Sweden) using a convolution/superposition algorithm with heterogeneity correction.
Colony formation assay
Immediately after irradiation, cells were washed in phosphate-buffered saline (Invitrogen, Carlsbad, CA, USA), harvested with 0.025% trypsin-EDTA (Invitrogen) and seeded into culture dishes. Fourteen days after culturing, these cells were fixed with 10% formalin and stained with a 0.04% crystal violet solution. After staining, the number of colonies with >50 cells per colony was counted to identify surviving cells. The SF was calculated and fitted to the equation of the linear–quadratic model expressed as
where D indicates the radiation dose, α the incidence of double-strand breaks by one particle and β the incidence of double-strand breaks by two particles.
Boyden chamber assay
A 48-well micro chemotaxis chamber (Neuro Probe, Gaithersburg, MD, USA) with a polycarbonate filter containing 8-μm pores (Neuro Probe) was used to assess chemotaxis. The bottom of the filter was precoated with 10 μg/ml of collagen type I–C (Nitta Gelatin Inc., Osaka, Japan). The irradiated cells were trypsinized, washed twice with serum-free medium and suspended in serum-free medium supplemented with 0.1% BSA. The cell suspension (50 μl at 1 × 106 cells/ml) was added to the upper well, and medium containing 10% FBS was placed in the bottom chamber. Cells were incubated on the membranes for 3 h at 37°C in 5% CO2 atmosphere. After 3 h, cells that had not migrated were scraped off with a cotton swab, and the filter membrane was removed with a blade. Cells that had migrated to the bottom side of the membrane were fixed with 10% formalin and stained with hematoxylin. Cell migration was quantitated by counting the number of migrated cells using a microscope at ×20 magnification.
Matrigel invasion assay
Invasion of cancer cells was measured by counting the number of cells that had migrated through Transwell® inserts with 8-μm pores (Corning Inc., Corning, NY, USA) coated with Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA). Sorted or unsorted cells were exposed to various doses of irradiation. The cells were trypsinized and washed twice with serum-free medium, followed by the addition of 100 μl of cell suspension (1 × 106 cells/ml) to the upper well. DMEM supplemented with 10% FBS (700 μl) as a chemoattractant was added to the lower well. The cells were then incubated for 24 h at 37°C in an atmosphere of 5% CO2. The number of cells that had invaded the lower surface of the Matrigel-coated membrane was counted in all fields using a microscope at ×200 magnification.
Statistics
The results were expressed as averages with standard deviations. The statistical significance was tested using the Student’s t-test. A P-value of <0.05 was considered statistically significant.
RESULTS
Cell survival at different doses of radiation
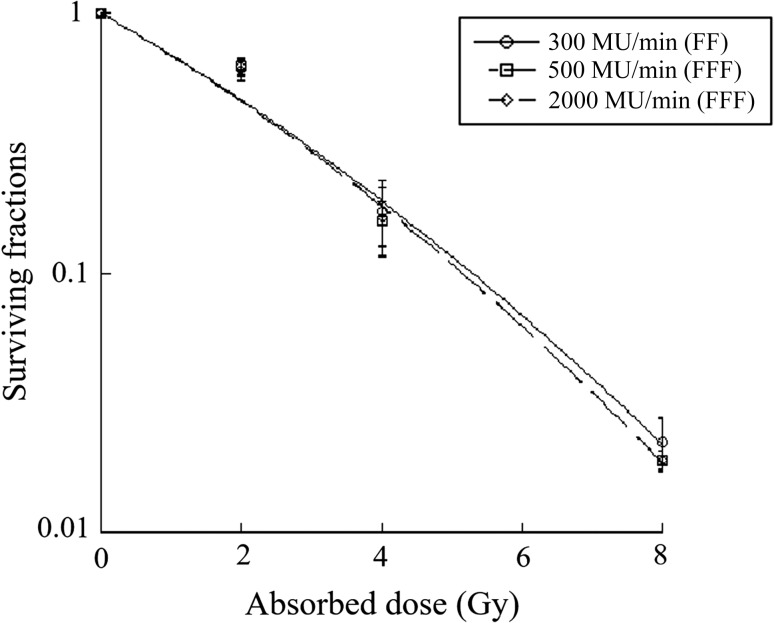
The cell SF was determined using a colony formation assay. A549 cells were irradiated with photon beams at three dose rates (300, 500 and 2000 MU/min). The curve representing the results of linear–quadratic fits to the data is shown in Fig 1. No statistical differences were seen between the SFs following irradiation with doses of 2, 4 or 8 Gy.
Fig. 1.
Surviving fractions of A549 cells following irradiation at different doses. Each point represents the mean ± S.D. The curve represents the results of the linear–quadratic fit to the data. A549 cells exposed to different doses of radiation showed no statistical differences in their surviving fractions (P > 0.05).
The effect of radiation on cell migration
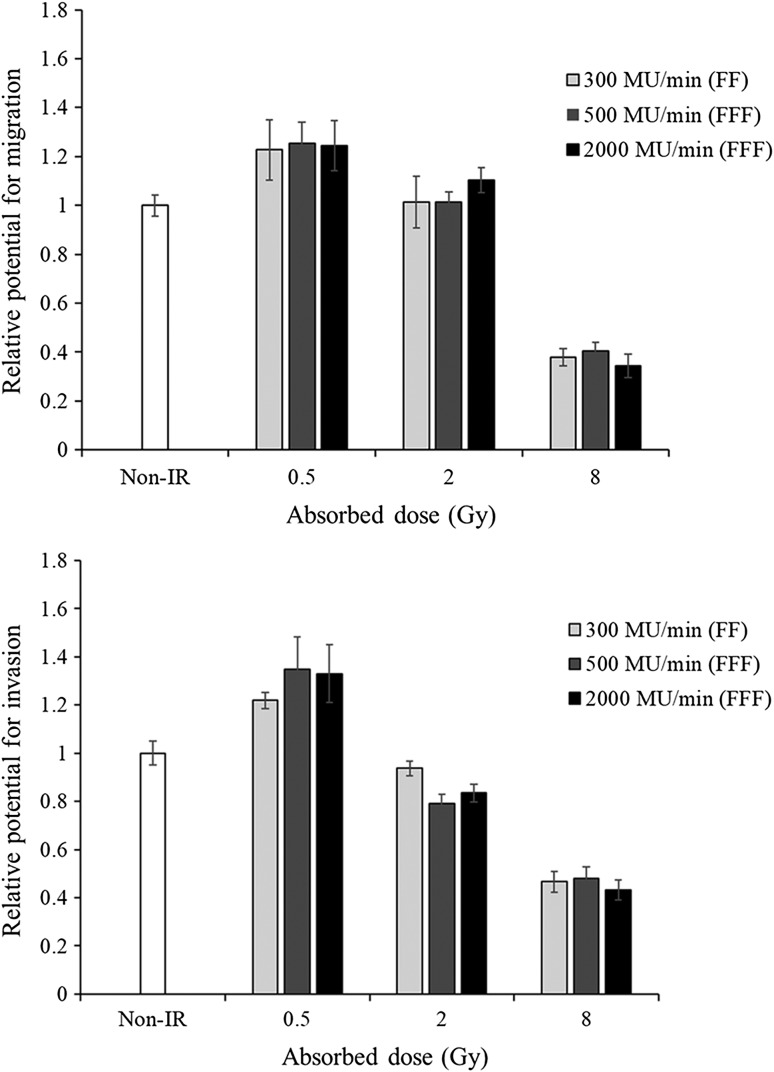
The effect of high-dose radiation on cell migration was evaluated in A549 cells using the Boyden chamber assay. No significant differences were seen in the migration of cells exposed to radiation at different dose rates. However, the migration of irradiated (8 Gy) vs non-irradiated cells showed significant differences at all dose rates (Fig. 2). The results were normalized to non-irradiated cells. Each bar represents the mean ± S.D.
Fig. 2.
The effects of radiation on the migration and invasive potential of A549 cells (upper: migration, lower: invasion). A549 cells were irradiated with photon beams of 0.5, 2 and 8 Gy at dose rates of 300 (FF), 500 and 2000 MU/min (FFF). Cells exposed to radiation at different dose rates (300, 500 and 2000 MU/min) did not show any significant differences in their capacity for migration or invasion.
The effect of radiation on cell invasion
The invasive capability of A549 cells exposed to high-dose photon beams was assessed using the Matrigel invasion assay. Cells exposed to radiation at different dose rates (300, 500 and 2000 MU/min) did not show any significant differences in their invasive capacity. However, the invasive capability of irradiated vs non-irradiated cells showed significant differences (P < 0.05) for all radiation conditions (Fig. 2). The results were normalized to non-irradiated cells. Each bar represents the mean ± S.D.
DISCUSSION
The colony formation assay in this study showed that dose rate has no effect on the SF of NSCLC (A549) cells irradiated with high-dose photon beams. Our findings were consistent with those reported previously [9–11]. Ling et al. have shown that it is the overall beam-on-time and not the dose rates that influence cell survival following external beam radiotherapy using conventional linacs [14]. Sublethal damage repair (SLDR) is induced within several minutes to hours after irradiation of DNA by photon beams. The cytotoxic effects of radiation decrease with increase in the dose-delivery time [15, 16]. However, the dose-delivery time at the dose rate of linacs was very short for SLDR in this study. Therefore, it is necessary to evaluate the effects of dose-delivery time instead of dose rates in radiation therapy.
It therefore appears that, as seen with other cancers, dose rates do not affect the SF of irradiated NSCLC cells. Our findings also suggest that the results of past clinical trials could still be valid regardless of the dose rates used in the clinic. Compared with FF beams, the FFF photon beams provide better clinical outcomes since they provide an effective irradiation method with a reduced dose-delivery time.
Cell motility showed no significant change in response to the different dose rates of FF and FFF photon beams in this study. Increases in migration and invasive capacity in response to low-dose irradiation by conventional photon beams have been reported previously [13, 17]. We have demonstrated that the migration and invasive ability of cancer cells can be increased with low-dose (0.5 Gy) photon beams, even if the photon beams are at a high dose rate. In addition to local control of the tumor, assessment of metastasis plays a major role in the treatment outcome. Consequently, it seems reasonable to presume that photon beams with high dose rates promote cell motility in the same manner as conventional photon beams. Further studies are needed to investigate the involvement of various proteins in the invasive capability of cancer cells. The expression of these proteins in normal vs cancer cells should be evaluated using in vivo studies.
A major finding of this study is that dose rates do not affect cell survival and motility. Moreover, while cell migration and invasion are promoted by low-dose photon beams, a high dose (of ~8 Gy) can suppress motility of A549 lung cancer cells. It is therefore believed that a large dose of ~8 Gy can kill cancer cells and strongly suppress their metastatic potential. In fact, SBRT uses a large dose per fraction in the clinic because it has the potential to suppress cell motility. Furthermore, FFF photon beams with high dose rates can minimize the intrafraction motion [1, 18] and are thus effective for tumors such as lung cancers, which are susceptible to changes in position due to breathing. In other words, irradiation with a large dose per fraction, as in the case of SBRT, can potentially suppress cell survival and motility while reducing the treatment time in the clinic. However, it remains a challenge to determine the changes in the expression of proteins involved in cell motility in response to the different dose rates used in radiation therapy. Additionally, normal and cancer cells should be compared for their responses to photon beams with high dose rates.
In conclusion, no significant differences were observed in the survival and motility of A549 cells irradiated with different dose rates, even when using high-dose-rate photon beams. The photon beams with high dose rates used in SBRT for lung cancer could suppress cell survival and motility.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING
This work was supported by a Grant-in Aid for Scientific Research (C) [grant number JP18K07632].
REFERENCES
- 1. Prendergast BM, Fiveash JB, Popple RA et al. Flattening filter–free linacs improves treatment delivery efficiency in stereotactic body radiation therapy. Med Phys 2013;14:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kragl G, af Wetterstedt S, Knausl B et al. Dosimetric characteristics of 6 and 10 MV unflattened photon beams. Radiat Oncol 2009;93:141–6. [DOI] [PubMed] [Google Scholar]
- 3. Purdie TG, Bissonnette JP, Franks K et al. Cone-beam computed tomography for on-line image guidance of lung stereotactic radiotherapy: localization, verification, and intrafraction tumor position. Radiat Oncol 2007;68:243–52. [DOI] [PubMed] [Google Scholar]
- 4. Palma DA, Sorenson JV, Verbakel WF et al. Lung density changes after stereotactic radiotherapy: a quantitative analysis in 50 patients. Int J Radiat Oncol Biol Phys 2010;81:974–8. [DOI] [PubMed] [Google Scholar]
- 5. Allibhai Z, Taremi M, Bezjak A et al. The impact of tumor size on outcomes after streotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;87:1064–70. [DOI] [PubMed] [Google Scholar]
- 6. Ueki N, Matsuo Y, Shibuya K et al. Differences in the dose–volume metrics with heterogeneity correction status and its influence on local control in stereotactic body radiation therapy for lung cancer. J Radiat Res 2013;54:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dzierma Y, Licht N, Nuesken F et al. Beam properties and stability of a flattening-filter free 7 MV beam—an overview. Med Phys 2012;39:2595–602. [DOI] [PubMed] [Google Scholar]
- 8. Lohse I, Lang S, Hrbacek J et al. Effect of high dose per pulse flattening filter–free beams on cancer cell survival. Radiot Oncol 2011;101:226–32. [DOI] [PubMed] [Google Scholar]
- 9. Verbakel WF, van den Berg J, Slotman BJ et al. Comparable cell survival between high dose rate flattening filter free and conventional dose rate irradiation. Acta Oncol 2012;52:652–7. [DOI] [PubMed] [Google Scholar]
- 10. Sorensen BS, Vestergaard A, Overgaard J et al. Dependence of cell survival on instantaneous dose rate of a linear accelerator. Radiat Oncol 2011;101:223–5. [DOI] [PubMed] [Google Scholar]
- 11. King RB, Hyland WB, Cole AJ et al. An in vitro study of the radiobiological effects of flattening filter free radiotherapy treatments. Phys Med Biol 2013;58:83–94. [DOI] [PubMed] [Google Scholar]
- 12. Navarria P, Ascolese AM, Mancosu P et al. Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC). Radiat Oncol 2013;107:414–8. [DOI] [PubMed] [Google Scholar]
- 13. Wild-Bode C, Michael Weller M, Rimner A et al. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 2001;61:2744–50. [PubMed] [Google Scholar]
- 14. Ling CC, Gerweck LE, Zaider M et al. Dose-rate effects in external beam radiotherapy redux. Radiat Oncol 2010;95:261–8. [DOI] [PubMed] [Google Scholar]
- 15. Matsuya Y, Ohtsubo Y, Tsutsumi K et al. Quantitative estimation of DNA damage by photon irradiation based on the microdosimetric-kinetic model. J Radiat Res 2014;55:484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuya Y, Tsutsumi K, Sasaki K et al. Evaluation of the cell survival curve under radiation exposure based on the kinetics of lesions in radiation to dose-delivery time. J Radiat Res 2015;56:90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahashi Y, Teshima T, Kawaguchi N et al. Heavy ion irradiation inhibits in vitro angiogenesis even at sublethal dose. Cancer Res 2003;63:4253–7. [PubMed] [Google Scholar]
- 18. Prendergast BM, Dobelbower MC, Bonner JA et al. Stereotactic body radiation therapy (SBRT) for lung malignancies: preliminary toxicity results using a flattening filter–free linear accelerator operating at 2400 monitor units per minute. Radiat Oncol 2013;8:273. [DOI] [PMC free article] [PubMed] [Google Scholar]