Abstract
Cardiac activity can induce dose–volume evaluation errors for cardiac structures. The purpose of this study was to quantify the variation in dose–volume parameters for the heart, pericardium and left ventricular myocardium (LVM) throughout the cardiac circle. The heart, pericardium and LVM of 22 patients were contoured on 20 phases of electrocardiography-gated 4D computed tomography (4DCT) images acquired during breath-hold. Radiotherapy plans were designed on 0% phase of the 4DCT images, and the dose distributions of the plans were imported into MIM Maestro and deformed to each phase to generate distributions for all phases. Variations in dose–volume parameters for the heart, pericardium and LVM were compared among different phases. The rates of variation in Dmean for the heart and pericardium were 3.33 ± 1.04% and 2.66 ± 1.15%, respectively. The mean values of the maximum difference in V5, V10, V20, V30 and V40 were all <2% for the heart and pericardium and were not statistically significant (P > 0.05). The rate of variation in Dmean for the LVM reached 87.05 ± 38.34%, and the maximum differences in V5, V10, V20, V30 and V40 were 13.76 ± 4.46%, 13.64 ± 4.33%, 12.84 ± 4.55%, 11.62 ± 4.85% and 3.63 ± 2.56%, respectively; all differences were statistically significant (P < 0.05). Variations in dose–volume parameters were more significant in the LVM than in the heart and pericardium (P < 0.05). The dose–volume parameters for the LVM were significantly influenced by cardiac activity, whereas those for the heart and pericardium were not; therefore, individual dosimetric evaluation and limitation must be performed for the LVM.
Keywords: thoracic radiotherapy, cardiac activity, dose–volume parameters, variation
INTRODUCTION
Radiation therapy is an important treatment in thoracic cancer, such as lung cancer, breast cancer and esophageal cancer, and can offer an irreplaceable advantage [1–4]. However, patient survival time and quality of life can be seriously impacted by radiation-induced heart disease (RIHD), with some studies showing the long-term complications of radiotherapy partially offsetting its benefit [5–7]. RIHD is a common complication in thoracic tumor radiotherapy, and includes pericarditis, conduction artery disease, myocardial disease, valvular disease, and conduction abnormalities [8–10].
The conventional predictors of RIHD are dose–volume parameters. Therefore, the accurate calculation of dose–volume parameters can help protect the heart and prevent RIHD. According to some studies, during thoracic radiotherapy, the position of the heart and its substructures can change due to respiratory movement and cardiac activity [11, 12]. These variations can lead to errors in the calculations of the dose–volume parameters of the heart and substructures based on traditional static 3D computed tomography (3DCT). One study reported that the cardiac dose is associated with the risk of cardiac late effects [13]. Thus, errors in dose–volume parameters caused by cardiac activity will reduce their predictive efficiency for RIHD. However, few studies have quantified the dosimetry errors caused by cardiac activity. Therefore, it is important to quantify the dose–volume parameter variations for cardiac structures caused by cardiac activity, which is the focus of this study.
Due to the randomness of 3DCT scans, uncertainty in the range of variation of dose–volume parameters will be introduced if the 3DCT dose is compared with the 4DCT dose. To obtain the precise variation for dose–volume parameters, the dose–volume parameters should be compared between each phase of 4DCT images to provide more significant results for guiding clinical practice. To acquire complete images of the heart in the cardiac cycle, this study used electrocardiography (ECG)-gated 4DCT [14–16]. In addition, breath-hold was used in this study, which can reduce the influence of respiratory movement on the heart and substructures. Several studies have shown that the heart dose in patients who received breath-hold treatments was lower than in those who received free-breathing treatments [17–19]. For example, Hayden et al. found that the average heart V30 was reduced from 7.1% to 2.4% and that the average mean heart dose was reduced from 6.9 Gy to 3.9 Gy with breath-hold treatment compared with free-breathing treatment [19]. These allow us to effectively evaluate dose variations in the heart and substructures during the cardiac cycle.
In the present study, we wanted to determine the variation rules for dosimetric parameters caused by cardiac geometrical variations. Therefore, we quantified variations in dose–volume parameters for the heart, pericardium and left ventricular myocardium (LVM) in the cardiac cycle based on breath-hold ECG-gated 4DCT.
MATERIALS AND METHODS
Patients
ECG-gated 4DCT images of 22 patients in breath-hold, which were collected from March 2015 to November 2016, were retrospectively analyzed in this study. Among the 22 patients, 12 patients were male and 10 patients were female. The patients ranged in age between 35 and 67 years, and the median age was 58 years. The tumor types evaluated in this study were thoracic esophageal carcinomas. This study was approved by the Research Ethics Board of the Shandong Cancer Hospital, and written informed consent was obtained from all of the patients.
4DCT image acquisition
All 22 patients underwent 4DCT scans using a Siemens Dual-source CT instrument (Siemens SOMATOM Definition; Munich, Germany). All of the CT images were reconstructed via the 5% cardiac cycle. Twenty cardiac cycle images were reconstructed (from 0% to 95% in 5% intervals). Images were reconstructed at a 0.75-mm slice thickness in 0.5-mm increments. All of the images were imported into MIM Maestro (MIM) 6.6.9 to delineate and analyze the heart, pericardium and LVM.
Delineation of the heart, pericardium and LVM
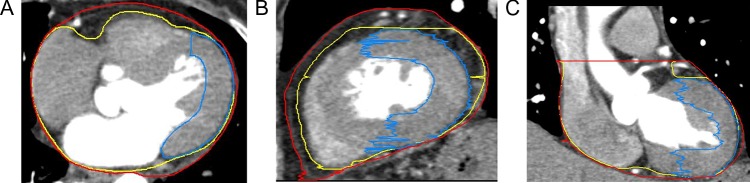
The heart, pericardium and LVM were contoured on all phases of ECG-gated 4DCT images. The upper boundary of the heart was the top of the left atrium, and the lower boundary was the apex cordis. The upper boundary of the pericardium was the same as that for the heart, and the lower boundary was defined as a loss of visual confirmation of the pericardium structure. The LVM was from the top of the left ventricle to the apex cordis, not including the interventricular septum, and the boundary between the LVM and interventricular septum was the left anterior descending coronary artery. The window width/window level was (400/40) HU (Fig. 1). Deformable registration technology was first used to contour structures automatically, and the intensity-based free-form deformable registration algorithm provided by MIM was used. Manual modification was then performed. All of the delineations were performed by the same oncologist and were assessed and confirmed by a second oncologist to ensure minimal delineation errors.
Fig. 1.
Delineation of the heart, pericardium, and left ventricular myocardium (shown in yellow, red and blue, respectively).
Design plans
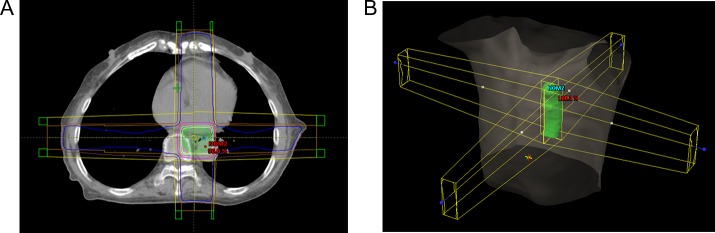
The radiotherapy plans were designed on the 0% phase of 4DCT images. All radiotherapy plans in this study were classic 3D conformal radiation therapy (3D-CRT) plans with four radiation beams, which were set at angles of 0°, 90°, 180° and 270°; the energy of each radiation beam was 6 MV, with a total dose for the planning target volume (PTV) of 60 Gy (Fig. 2). The radiotherapy plans for this study were designed using Eclipse 13.5 (Varian Medical Systems, USA), with the ‘Acuros XB’ calculation model for the volume dose and the algorithm ‘Acuros External Beam (Version 13.5.35)’. The calculation grid size was 0.25 cm. The dose distribution met the requirement that the PTV received 95% of the prescribed dose. The dose distributions of the plans were imported into MIM and deformed to 20 phases of 4DCT images. A 4D dose–volume histogram (DVH) was rebuilt, which shows the dose distributions of the heart, pericardium and LVM in all phases. The intensity-based free-form deformable registration algorithm of MIM was used for dose reconstruction.
Fig. 2.
A sample image of the treatment plan. (A) Dose distribution of the treatment plan. (B) Setting of the radiation beams.
Quantitative analysis
The dose–volume parameters, including mean dose (Dmean), V5, V10, V20, V30 and V40 of different phases for the heart, pericardium and LVM were analyzed and compared. The rate of variation in volume or Dmean was calculated using the equation:
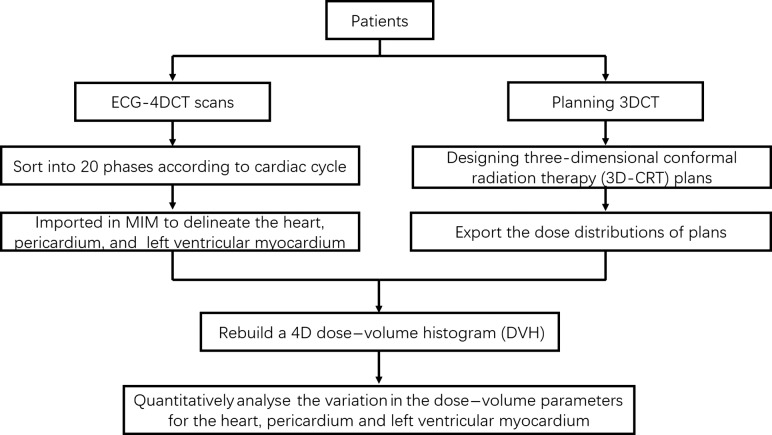
The differences in V5, V10, V20, V30, V40 were defined as the maximum value minus their minimum value. To determine whether the safety of the heart could be ensured in the cardiac cycle by contouring the pericardium as an organ at risk (OAR), the volume of the heart contour in all phases beyond the pericardium contour in a certain phase was estimated, the extreme phases with the maximum and minimum pericardium volume were defined as the reference frames, and then the relevant dose errors were analyzed (Fig. 3).
Fig. 3.
Workflow of the study.
Statistical analysis
All of the data were analyzed using SPSS v19.0 software (SPSS Inc., Chicago, IL). To compare two groups of data, the independent samples t-test was used when the data had a normal distribution and the variance was homogenous; otherwise, the Mann–Whitney U-test was used. In this study, parameters such as V5 and V10 for the heart; V5, V10, V20 and V30 for the pericardium; and V5 for the LVM were normally distributed with homogenous variances and analyzed with the independent samples t-test. Dmean, V20, V30 and V40 for the heart; Dmean and V40 for the pericardium, and Dmean, V10, V20, V30 and V40 for the LVM were analyzed using the Mann–Whitney U-test. The differences were considered statistically significant when P < 0.05.
RESULTS
The rate of variation in volume for the heart, pericardium and LVM
The rate of variation in volume was 16.49 ± 3.85%, 12.62 ± 3.94% and 24.23 ± 11.35% for the heart, pericardium and LVM, respectively.
The variation of Dmean for the heart, pericardium and LVM
Dmean for both the heart and pericardium did not differ significantly between the various phases of the cardiac cycle (P > 0.05), whereas Dmean for the LVM did differ significantly (P < 0.05). The rate of variation in Dmean for the heart (maximum: 5.84%) was higher than that for the pericardium (maximum: 5.86%), and the mean value of the rate of variation in Dmean for the LVM was 26.14-fold and 32.73-fold higher than that for the heart and pericardium, respectively. The maximum rate of variation in Dmean reached 163.52% for the LVM (Table 1).
Table 1.
Mean dose variation of the heart, pericardium and left ventricular myocardium
| Maximum Dmean (Gy) | Minimum Dmean (Gy) | Rate of change (%) | P | |
|---|---|---|---|---|
| Heart | 23.16 ± 2.55 | 22.42 ± 2.44 | 3.33 ± 1.04 | 0.156 |
| Pericardium | 23.11 ± 2.20 | 22.51 ± 2.15 | 2.66 ± 1.15 | 0.241 |
| LVM | 11.54 ± 3.79 | 6.70 ± 3.67 | 87.05 ± 38.34 | 0.000 |
Dmean = mean dose variation, LVM = left ventricular myocardium.
The variations in V5, V10, V20, V30 and V40 for the heart, pericardium and LVM
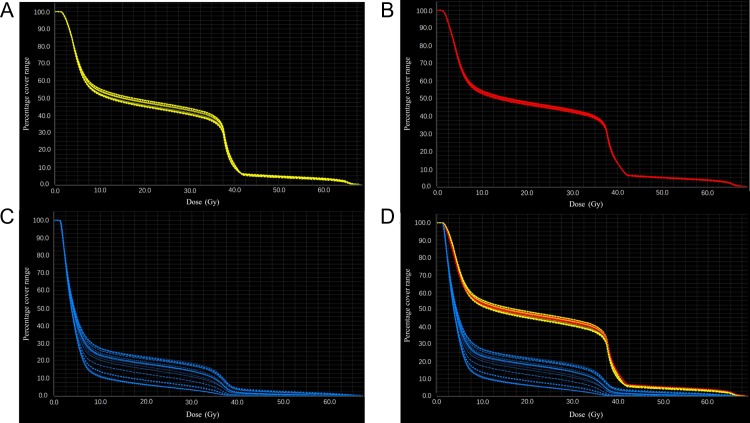
As shown in Table 2 and Fig. 4, the mean values of the maximum difference in V5, V10, V20, V30 and V40 over the cardiac cycle were all <2% for the heart and <1.5% for the pericardium; the values were not statistically significant (P > 0.05). However, for the LVM, the maximum differences of V5, V10, V20, V30 and V40 were 13.76 ± 4.46%, 13.64 ± 4.33%, 12.84 ± 4.55%, 11.62 ± 4.85% and 3.63 ± 2.56%, respectively. The differences in those parameters were significantly different (P < 0.05) and significantly larger than those of the heart and pericardium (P < 0.05).
Table 2.
Variations in the V5, V10, V20, V30 and V40 of the heart, pericardium and left ventricular myocardium
| Maximum | Minimum | Difference | P | ||
|---|---|---|---|---|---|
| V5 (%) | Heart | 71.36 ± 6.70 | 69.61 ± 6.52 | 1.75 ± 0.69 | 0.385 |
| Pericardium | 70.05 ± 5.85 | 68.65 ± 5.68 | 1.40 ± 0.59 | 0.425 | |
| LVM | 40.19 ± 11.79 | 26.42 ± 15.05 | 13.76 ± 4.46 | 0.002 | |
| V10 (%) | Heart | 58.82 ± 6.44 | 56.95 ± 6.19 | 1.87 ± 0.74 | 0.330 |
| Pericardium | 58.05 ± 5.48 | 56.70 ± 5.19 | 1.35 ± 0.60 | 0.405 | |
| LVM | 28.92 ± 9.64 | 15.28 ± 11.55 | 13.64 ± 4.33 | 0.000 | |
| V20 (%) | Heart | 52.13 ± 6.29 | 50.18 ± 6.09 | 1.94 ± 0.77 | 0.146 |
| Pericardium | 51.44 ± 5.23 | 50.09 ± 4.98 | 1.34 ± 0.66 | 0.388 | |
| LVM | 23.24 ± 9.03 | 10.40 ± 9.15 | 12.84 ± 4.55 | 0.000 | |
| V30 (%) | Heart | 46.59 ± 6.13 | 44.62 ± 5.95 | 1.97 ± 0.78 | 0.152 |
| Pericardium | 45.98 ± 5.06 | 44.65 ± 4.83 | 1.34 ± 0.71 | 0.375 | |
| LVM | 18.22 ± 8.32 | 6.60 ± 7.19 | 11.62 ± 4.85 | 0.000 | |
| V40 (%) | Heart | 13.26 ± 6.16 | 11.87 ± 6.20 | 1.39 ± 0.42 | 0.110 |
| Pericardium | 14.28 ± 6.49 | 13.37 ± 6.41 | 0.91 ± 0.23 | 0.336 | |
| LVM | 4.53 ± 3.86 | 0.90 ± 2.03 | 3.63 ± 2.56 | 0.000 |
LVM = left ventricular myocardium.
Fig. 4.
(A) DVH distribution in the heart at different phases. (B) DVH distribution in the pericardium at different phases. (C) DVH distribution in the left ventricular myocardium at different phases. (D) Complexity of the DVH distribution in the heart, pericardium, and left ventricular myocardium at different phases. DVH = dose–volume histogram.
Comparison of Dmean between the heart and pericardium
The volume of the heart contours in the various phases beyond the pericardium contour in a certain phase ranged from 0.40 ± 0.32% to 3.94 ± 3.18% in the cardiac cycle. The largest volume reached 13.77%, which might contribute to dose variations. The largest difference in Dmean between the heart and pericardium was 2.08 Gy.
DISCUSSION
In the present study, the variations in dose–volume parameters for the heart, pericardium and LVM caused by cardiac activity were quantified, and then we analyzed the feasibility of protecting the heart or LVM by contouring the pericardium as an OAR.
The occurrence of RIHD is related to the dose administered to the heart, and a previous study showed that the rates of major coronary events increased linearly with the mean dose to the heart at a rate of 7.4% per gray [20]. Hong et al. thought the dose constraints for the heart could be added for left-breast patients, because the D5 of the heart still reached 14.8 ± 12.3 Gy despite the use of intensity-modulated tangential beam irradiation [21]. The dose–volume parameters are important indexes for evaluating the dose to the heart and its substructures, which are considered to be the major factors predicting RIHD. However, the dose–volume parameters used in RIHD prediction are variable and cannot be easily standardized [6]. Kataria et al. suggested that respiratory movements and cardiac activity were major sources of discrepancy between the estimated and delivered radiation doses for cardiac OARs, which could lead to inadequate cardiac protection [11]. However, the variation range in the dose, caused by respiratory movement or cardiac activity, remains unclear. To address this question, ECG-gated 4DCT based on breath-hold was used in this study to analyze the impacts of cardiac activity on the heart and substructures. Shim et al. found that the mean value of the heart D5 was reduced from 15.33 Gy to 5.26 Gy and that V18 was reduced from 4.50 to 1.83 with breath-hold treatment compared with free-breathing treatment for breast cancer radiotherapy [18]. In addition, we employed MIM, a commercial software package used in clinical practice, and the algorithm of deformation image registration has been proven to have high accuracy [22]. Based on these approaches, the impacts of cardiac activity on dose–volume parameters can be more accurately assessed.
Thoracic esophageal carcinomas were evaluated in the present study because the variation in the position of thoracic esophageal tumors is smaller than that of other thoracic tumors, such as pulmonary or breast carcinoma. In addition, the beam we utilized and the dose that the heart and substructures received were relatively fixed, which allowed convenient analysis of the variations in dose–volume parameters for the heart and substructures over the cardiac cycle.
Although the heart and pericardium exhibited larger volumetric variation, the dose–volume parameters Dmean, V5, V10, V20, V30 and V40 of the heart and pericardium did not significantly differ between the various phases of the cardiac cycle. The mean values of the maximum difference in V5, V10, V20, V30 and V40 were <2%, indicating that the impacts of cardiac activity on dose–volume parameters of the heart and pericardium were not as large as predicted. The geometrical variations in the heart and pericardium caused by cardiac activity did not lead to notable variations in the dose–volume parameters, which might be attributed to the large volumes of the heart and pericardium. The dose errors caused by delineation errors of the heart and pericardium were also included. Our findings indicate that cardiac activity is not a major factor influencing the evaluation errors of dose–volume parameters for the heart and pericardium and that the evaluation errors might be related to respiratory movements. Moreover, the method of evaluating the dose for the heart and pericardium based on traditional static 3DCT shows high accuracy when patients undergo breath-hold radiotherapy.
The variations in Dmean, V5, V10, V20, V30 and V40 for the pericardium were smaller than those for the heart, which indicated that the impacts of cardiac activity on the dose–volume parameters of the pericardium were smaller than those of the heart. Based on these findings, methods for evaluating and limiting the cardiac dose by contouring the pericardium as an OAR in clinical practice might reduce the impacts of cardiac activity on dose–volume parameters.
Taylor et al. indicated that part of the heart could receive >20 Gy during the treatment of left-sided breast cancer patients, the average maximum point dose to the heart was 30.7 ± 10.8 Gy, and the cardiac structures that received the highest doses were the apex of the left ventricle and the left anterior descending of the coronary artery [23]. Therefore, the precise description and dose limitation for cardiac substructures (such as the LVM) is particularly important. Torres et al. proposed that it would be beneficial for dose limitation to delineate the left ventricular wall and thus reduce the long-term cardiac events produced in breast cancer radiotherapy [24]. These results show that dose limitation for the left ventricle is important for reducing RIHD. Therefore, accurate dose calculation for the LVM, a major part of the left ventricle, is very important. Indeed, it is necessary to analyze the variation in dose–volume parameters for the LVM.
In this study, we found the LVM volumetric variation had led to a significant dose variation by delineating the LVM according to a uniform standard on each phase of the 4DCT images. The maximum rate of variation in Dmean for the LVM was 163.52% in the cardiac cycle, and the mean values of the rate of variation in Dmean were 26.14-fold and 32.73-fold higher for the LVM than for the heart and pericardium, respectively. The differences in Dmean, V5, V10, V20, V30 and V40 for the LVM were statistically significant. These differences might be caused by significant volumetric variation (the volume variation of the LVM is 24.23%, which is 46.94% and 92.00% larger than those of the heart and pericardium, respectively); in addition, because the LVM is shaped like a hollow half sphere, its movement leads to irregular morphological variation (such as variation in the thickness and curvature), which could have a large effect on the dose variation. This result means that some blindness in selecting dose–volume parameter limits could be associated with variations in dose–volume parameters for the LVM due to cardiac activity. Under this scenario, the prediction efficiency of the dose–volume parameters would be reduced, potentially increasing the probability of RIHD, which could be deadly for some patients. When using traditional evaluation methods and treatments, these non-negligible variations could lead to excessive exposure of the LVM, which would seriously impact the prognosis of patients and must be given more attention. The rate of variation in Dmean for LVM was more significant than those for heart and pericardium, and their variation rules also differed from those of the heart and pericardium. These findings indicate that caution should be taken when protecting the LVM by contouring the heart or pericardium as an OAR (which is typically performed in clinical practice) because this approach can introduce large errors. Therefore, we recommend that the dose–volume parameters of the LVM should be evaluated separately by contouring the LVM as an OAR.
Although evaluating the cardiac dose by contouring the pericardium as an OAR for those patients in breath-hold treatment had high accuracy, it did not cover all phases of the heart because of cardiac activity. The largest volume of the heart in the various phases beyond the pericardium was 13.77% when the pericardium contour was used, which might result in dose disparities. Could this contour method effectively protect the heart during the cardiac cycle if analyzed from the dose perspective? The largest difference between the Dmean values of the heart and pericardium was 2.08 Gy. This finding indicates that when applying the pericardium contour as an OAR to protect the heart, although a pericardium contour with a large range can ensure the safety of the heart during radiotherapy, the heart can still receive additional irradiation due to cardiac activity. Therefore, the methods of cardiac contouring used in clinical practice do not always ensure heart safety in radiotherapy.
Compared with previous studies that analyzed the geometrical variations in the heart and substructures in the cardiac cycle, the strengths of the present study include the quantification of dose–volume parameter variations for the heart and substructures in the cardiac cycle, which may provide more guiding significance for clinical practice. However, this study also had limitations. For example, the variations in the dose–volume parameters caused by cardiac activity alone were quantified; however, the combined impact of cardiac activity and respiratory movements was not evaluated; this will be analyzed in the future.
Based on the results of this study, cardiac activity is not a major factor contributing to evaluation errors for heart and pericardium dose–volume parameters. However, it might have a larger impact on the evaluation of the dose–volume parameters of the LVM. Therefore, it is important to evaluate the dose–volume parameters of the LVM separately for thoracic tumor radiotherapy. Because the dose–volume parameters of the heart and pericardium showed some differences over the cardiac cycle, and the dose–volume parameter variations in the LVM caused by cardiac activity were different from those of the heart and pericardium, therefore, protecting the heart or LVM should be performed with caution when contouring the pericardium as an OAR, although this is the conventional method in thoracic tumor radiotherapy.
CONFLICT OF INTEREST
The authors state that there is no conflict of interest in this study.
FUNDING
This study was supported by the National Natural Science Foundation of China (81301936).
REFERENCES
- 1. Leong T, Everitt C, Yuen K et al. A prospective study to evaluate the impact of FDG-PET on CT-based radiotherapy treatment planning for oesophageal cancer. Radiother Oncol 2006;78:254–61. [DOI] [PubMed] [Google Scholar]
- 2. Duane F, Aznar MC, Bartlett F et al. A cardiac contouring atlas for radiotherapy. Radiother Oncol 2017;122:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chandran S, Vaughan R, Jacob A et al. A novel endoscopic marker for radiological localization and image-guided radiotherapy in esophageal and gastric cancers (with video). Gastrointest Endosc 2016;83:309–17. [DOI] [PubMed] [Google Scholar]
- 4. Chui CS, Hong L, Mc Cormick B. Intensity-modulated radiotherapy technique for three-field breast treatment. Int J Radiat Oncol Biol Phys 2005;62:1217–23. [DOI] [PubMed] [Google Scholar]
- 5. Evans ES, Prosnitz RG, Yu X et al. Impact of patient-specific factors, irradiated left ventricular volume, and treatment set-up errors on the development of myocardial perfusion defects after radiation therapy for left-sided breast cancer. Int J Radiat Oncol Biol Phys 2006;66:1125–34. [DOI] [PubMed] [Google Scholar]
- 6. Gagliardi G, Constine LS, Moiseenko V et al. Radiation dose–volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:s77–85. [DOI] [PubMed] [Google Scholar]
- 7. Nolan MT, Russell DJ, Marwick TH. Long-term risk of heart failure and myocardial dysfunction after thoracic radiotherapy: a systematic review. Can J Cardiol 2016;32:908–20. [DOI] [PubMed] [Google Scholar]
- 8. Stewart JR, Fajardo LF, Gillette SM et al. Radiation injury to the heart. Int J Radiat Oncol Biol Phys 1995;31:1205–11. [DOI] [PubMed] [Google Scholar]
- 9. Kole TP, Aghayere O, Kwah J et al. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:1580–6. [DOI] [PubMed] [Google Scholar]
- 10. Benoff LJ, Schweitzer P. Radiation therapy-induced cardiac injury. Am Heart J 1995;129:1193–6. [DOI] [PubMed] [Google Scholar]
- 11. Kataria T, Bisht SS, Gupta D et al. Quantification of coronary artery motion and internal risk volume from ECG gated radiotherapy planning scans. Radiother Oncol 2016;121:59–63. [DOI] [PubMed] [Google Scholar]
- 12. Qi XS, Hu A, Wang K et al. Respiration induced heart motion and indications of gated delivery for left-sided breast irradiation. Int J Radiat Oncol Biol Phys 2012;82:1605–11. [DOI] [PubMed] [Google Scholar]
- 13. Hahn E, Jiang H, Ng A et al. Late cardiac toxicity after mediastinal radiotherapy for Hodgkin lymphoma: contributions of coronary artery and whole heart dose–volume variables to risk prediction. Int J Radiat Oncol Biol Phys 2017;98:1116–23. [DOI] [PubMed] [Google Scholar]
- 14. Hugo GD, Rosu M. Advances in 4D radiation therapy for managing respiration: part I – 4D imaging. Z Med Phys 2012;22:258–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Funabashi N, Komiyama N, Kato H et al. Retrospective ECG-gated left ventriculography using multislice CT following left ventricular bolus injection and evaluation of its utility and motion artifact at every cardiac phase. Int J Cardiol 2006;113:132–8. [DOI] [PubMed] [Google Scholar]
- 16. Shah C, Badiyan S, Berry S et al. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother Oncol 2014;112:9–16. [DOI] [PubMed] [Google Scholar]
- 17. Mast ME, Van Kempen-Harteveld L, Heijenbrok MW et al. Left-sided breast cancer radiotherapy with and without breath-hold: does IMRT reduce the cardiac dose even further? Radiother Oncol 2013;108:248–53. [DOI] [PubMed] [Google Scholar]
- 18. Shim JG, Kim JK, Park W et al. Dose–volume analysis of lung and heart according to respiration in breast cancer patients treated with breast conserving surgery. J Breast Cancer 2012;15:105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayden AJ, Rains M, Tiver K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J Med Imaging Radiat Oncol 2012;56:464–72. [DOI] [PubMed] [Google Scholar]
- 20. Darby SC, Ewertz M, Mcgale P et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 21. Hong L, Hunt M, Chui C et al. Intensity modulated tangential beam irradiation of the intact breast. Int J Radiat Oncol Biol Phys 1999;44:1155–64. [DOI] [PubMed] [Google Scholar]
- 22. Piper J. Evaluation of an intensity-based free-form deformable registration algorithm. Med Phys 2007;34:2353–4. [Google Scholar]
- 23. Taylor CW, Povall JM, Mcgale P et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys 2008;72:501–7. [DOI] [PubMed] [Google Scholar]
- 24. Folgar-Torres A, Alvarado-Astudillo A, Feltes N et al. Anterior left ventricular territory: true oar in left breast radiotherapy. Rep Pract Oncol Radiother 2013;18:s166–7. [Google Scholar]