Abstract
This study quantified mortality associated with serious infections caused by carbapenem-resistant (CRE) and carbapenem-susceptible Enterobacteriaceae (CSE). A systematic literature review was conducted, evaluating outcomes in hospitalized patients with CRE infections from a blood, urinary, pulmonary, or intra-abdominal source. A meta-analysis (MA) calculating odds ratios (ORs) for mortality was performed. Twenty-two studies met the criteria for inclusion in the MA: 12 included mortality data for CRE vs CSE populations. Compared with CSE, CRE was associated with a significantly higher risk of overall mortality (OR, 3.39; 95% confidence interval [CI], 2.35–4.89), as was monotherapy (vs combination therapy) treatment of patients with CRE infections (OR, 2.19; 95% CI, 1.00–4.80). These results document the increased mortality associated with serious CRE infections compared with CSE infections among hospitalized adults. It will be important to reevaluate the mortality in CRE and CSE populations, especially among patients who receive early appropriate therapy, as new antibiotics become available.
Keywords: bacterial drug resistance, carbapenems;, Klebsiella pneumoniae, meta-analysis, mortality
Carbapenems have been considered the last treatment option for infections caused by multidrug-resistant pathogens [1, 2]. However, their use has been compromised due to the increasing prevalence of carbapenem-resistant gram-negative bacteria, which has become a global concern [1, 2]. Although carba penem resistance has historically been limited to hospital-acquired infections caused by Pseudomonas spp. and Acinetobacter spp., it is now becoming more commonplace among infections caused by the Enterobacteriaceae family. Carbapenem-resistant Enterobacteriaceae spp. (CRE) can cause a range of hospital- acquired infection types, with bloodstream infections (BSIs), hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP), intra-abdominal infections (IAIs), and urinary tract infections (UTIs) being the most common [3]. As a testament to the seriousness of this emerging antibiotic-resistant pathogen, the Centers for Disease Control and Prevention published a report in 2013 outlining the top 18 drug-resistant threats to the United States, of which CRE was 1 of 3 pathogens categorized as an urgent threat.
Despite increased appreciation of CRE, the outcomes associated with it have not been well described. There have been a number of attempts in the literature to characterize the outcomes associated with CRE, but the studies have been limited in sample size and scope. There is also a debate as to whether empiric therapy for infections due to CRE should contain 1 or more antibiotics. Because the prevalence of infections due to CRE is expected to increase, an understanding of the evidence on overall and treatment-associated outcomes in patients with these difficult-to-treat infections is required. To evaluate the available evidence in this area, a systematic literature review and meta-analysis (MA) were conducted to investigate the association between CRE and carbapenem-susceptible Enterobacteriaceae (CSE) infections and mortality among hospitalized adult patients.
METHODS
Systematic Literature Review
Systematic searches of MEDLINE (via PubMed), Embase, and Cochrane-indexed databases were conducted to identify English-language articles published between January 1, 1994, and December 1, 2015, using keywords for carbapenem resistance that were paired with terms for the infection types of interest (BSI, HABP/VABP, IAI, and UTI). The search string for this systematic literature review included terms for the broad genus of Gram-negative bacteria included within the Enterobacteriaceae family. This included the term Enterobacter, as well as Klebsiella, Serratia, Escherichia, and Citrobacter, to ensure comprehensive identification of relevant studies (search terms are listed in Supplementary File 1). However, we did not conduct analyses by specific pathogens. The review was conducted per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [4]. A manual check of bibliographies of recent systematic literature reviews and MAs was performed to ensure a comprehensive retrieval of literature of interest.
Study Selection
Abstracts were reviewed and screened manually using the Patients, Interventions, Comparators, Outcomes, Study Design, and Time Period (PICOS-T) inclusion and exclusion criteria (Table 1). Included articles evaluated outcomes in hospitalized patients with BSI, HABP/VABP, IAI, or UTI due to CRE. Full-text articles of the references included during abstract review were obtained, reviewed, and screened by a single investigator using the same inclusion/exclusion criteria applied for abstracts. All excluded studies were confirmed by a senior investigator, with any discrepancies between the 2 investigators resolved by a third investigator.
Table 1.
Inclusion/Exclusion Criteria for Study Selection
| Study Criteria | Patients | Interventions | Comparators | Outcomes | Study Design | Time Frame |
|---|---|---|---|---|---|---|
| Inclusion | Studies evaluating hospitalized adult patients with confirmed or suspected HABP/VABP, cUTIs, bacteremia, or cIAI attributable to CRE pathogens | All antibiotics considered | Any antibiotic | Mortality Infection-related mortality Reinfection Readmission Length of hospital stay Length of ICU stay Any economic outcome Adverse events |
Observational studies (prospective/ retrospective) Randomized trials Nonrandomized trials |
Studies published between January 1, 1994, and December 1, 2015 |
| Exclusion | Studies evaluating patients without CRE infections Studies not reporting separable outcomes for patients with HABP/VABP, cUTIs, bacteremia, or cIAI Studies evaluating pediatric patients Studies of patients with community- acquired infections Studies evaluating patients treated in the outpatient setting |
Treatments other than antibiotics | Studies not reporting clinical or economic outcomes related to CRE infections of interest | Animal, in vitro, or genetic studies; narrative publications, nonsystematic reviews, case studies, case reports, and editorials; comparative studies with <10 patients per treatment group in ≥2 treatment arms or single-arm studies with ≤20 patients | Studies published before 1994 or after December 1, 2015 |
Abbreviations: cIAI, complicated intra-abdominal infection; CRE, carbapenem-resistant Enterobacteriaceae; cUTI, complicated urinary tract infection; HABP/VABP, hospital-acquired and ventilator-associated bacterial pneumonia; ICU, intensive care unit.
Data Extraction
Data extraction was performed on studies included after full-text review using a template developed in Excel (Microsoft Corporation, Redmond, WA) that contained the data elements planned for capture (data elements planned for capture are listed in Supplementary File 2). Study-level data, patient characteristics, treatment details, and outcomes were extracted as available from all included articles by a single investigator and then validated by a second investigator to confirm the completeness and accuracy of extraction. Any discrepancies between the 2 investigators were resolved by a third investigator.
Meta-analyses
Using a random-effects model, frequentist MAs of the odds ratios (ORs) for mortality were conducted by using data extracted or calculated from each study. Heterogeneity, the differences between the results of the compared studies after accounting for sampling error, was quantified using the Cochran Q and the I2 measures [5].
Statistical Analyses
Statistical analyses were conducted using the R 2.15.2 statistical software program (metafor Package, Wolfgang Viechtbauer, GNU General Public License Version 2; http://www. m etafor-project.org/doku.php). Data for overall mortality were analyzed using 30-day or in-hospital mortality; for studies that reported mortality at multiple time points, the latest time point was used (up to mortality at 90 days). Summary ORs for mortality were determined for the following comparisons: CRE vs CSE infections, BSI caused by CRE vs CSE, carbapenem- resistant Klebsiella pneumoniae (CRKP) vs carbapenem- susceptible K. pneumoniae (CSKP) infections, K. pneumoniae carbapenemase (KPC)–producing CRE vs non-KPC- producing CSE infections, and monotherapy vs combination therapy in CRE infections.
RESULTS
Systematic Literature Review
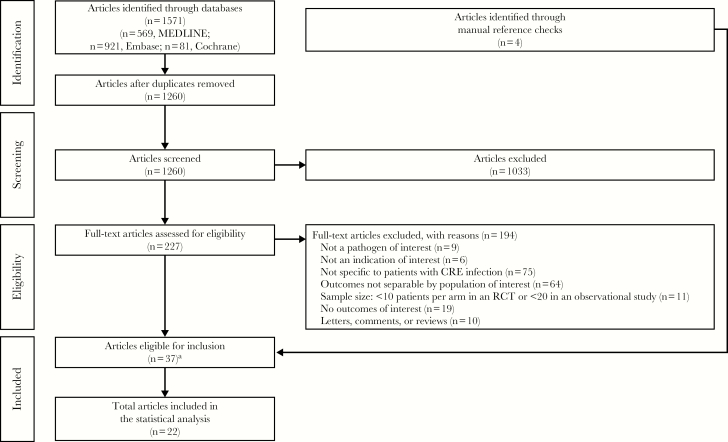
Of the 1260 references identified from the literature searches, 227 were obtained in full text for further review (Figure 1). After review of the 227 full-text citations, 194 articles were rejected and 33 met the criteria for inclusion. The primary reasons for rejection were that the articles were not specific to patients with CRE infections (75/194; 38.7%) or outcomes were not reported separately for the population of interest (64/194; 33.0%). Four additional studies were identified through the manual bibliography review process, resulting in a total of 37 studies that were identified for inclusion in the review and eligible for extraction. Of the 37 identified studies, 22 reported data that met criteria for inclusion in the MA (Table 2) [6–27].
Figure 1.
Systematic literature search and review and study attrition. Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae; RCT, randomized controlled trial. aRepresents 33 articles identified using electronic database search and 4 articles identified using manual reference checks.
Table 2.
Studies Included in the Analyses
| Author, Year | Study Design | Indication | Pathogen | Susceptibility | n/N | Mortality Rate, % |
|---|---|---|---|---|---|---|
| Ben-David, 2012 [7] | Retrospective cohort study | BSI | K. pneumoniae | CRE | 29/42 | 69.0 |
| CSE | 20/85 | 23.5 | ||||
| Bleumin, 2012 [8] | Case-control | Mixed | K. pneumoniae | CRE | 38/43 | 88.4 |
| CSE | 78/148 | 52.7 | ||||
| Brizendine, 2015 [9] | Retrospective cohort study | cUTI | K. pneumoniae | CRE | 4/22 | 18.2 |
| CSE | 1/64 | 1.6 | ||||
| Chang, 2011 [10] | Case-control | BSI | E. coli | CRE | 16/17 | 94.1 |
| CSE | 17/34 | 50.0 | ||||
| Daikos, 2009 [11] | Retrospective cohort study | BSI | K. pneumoniae | CRE | 6/14 | 42.9 |
| CSE | 10/53 | 18.9 | ||||
| Daikos, 2014 [12] | Case-control | BSI | K. pneumoniae | Combination therapy | 28/103 | 27.2 |
| Monotherapy | 32/72 | 44.4 | ||||
| de Oliveira, 2015 [6] | Retrospective cohort study | Mixed | K. pneumoniae | Combination therapy | 32/61 | 52.5 |
| Monotherapy | 21/57 | 36.8 | ||||
| Giacobbe, 2015 [13] | Case-control | BSI | K. pneumoniae | KPC | 73/142 | 51.4 |
| Non-KPC CSE | 112/284 | 39.4 | ||||
| Hussein, 2013 [27] | Case-control | BSI | K. pneumoniae | KPC | 45/103 | 43.7 |
| Non-KPC CSE | 62/214 | 29.0 | ||||
| Kontopidou, 2014 [15] | Retrospective cohort study | Mixed | K. pneumoniae | Combination therapy | 8/43 | 18.6 |
| Monotherapy | 16/64 | 25.0 | ||||
| Mouloudi, 2010 [16] | Case-control | BSI | K. pneumoniae | KPC | 15/19 | 78.9 |
| Non-KPC CSE | 9/22 | 40.9 | ||||
| Ny, 2015 [17] | Case-control | cUTI and Pneumonia | K. pneumoniae | CRE | 14/48 | 29.2 |
| CSE | 7/48 | 14.6 | ||||
| Orsi, 2013 [18] | Case-control | Mixed | K. pneumoniae | KPC | 16/44 | 36.4 |
| Non-KPC CSE | 17/60 | 28.3 | ||||
| Papadimitriou-Olivgeris, 2013 [19] | Case-control | BSI | K. pneumoniae | KPC | 63/164 | 38.4 |
| Non-KPC CSE | 17/62 | 27.4 | ||||
| Patel, 2008 [20] | Case-control | Mixed | K. pneumoniae | KPC | 48/99 | 48.5 |
| Non-KPC CSE | 73/276 | 26.4 | ||||
| Qureshi, 2012 [21] | Case-control | BSI | K. pneumoniae | KPC | 9/19 | 47.4 |
| Non-KPC CSE | 53/272 | 19.5 | ||||
| Qureshi, 2012 [22] | Retrospective cohort study | BSI | K. pneumoniae | Combination therapy | 2/15 | 13.3 |
| Monotherapy | 11/19 | 57.9 | ||||
| Schwaber, 2008 [23] | Case-control | Mixed | K. pneumoniae | CRE | 21/48 | 43.8 |
| CSE | 7/56 | 12.5 | ||||
| Trecarichi, 2015 [24] | Prospective cohort study | BSI | K. pneumoniae | CRE | 6/13 | 46.2 |
| CSE | 3/20 | 15.0 | ||||
| Tumbarello, 2012 [25] | Retrospective cohort study | BSI | K. pneumoniae | Combination therapy | 27/79 | 34.2 |
| Monotherapy | 25/46 | 54.3 | ||||
| Tumbarello, 2015 [14] | Retrospective cohort study | Mixed | K. pneumoniae | Combination therapy | 93/291 | 32.0 |
| Monotherapy | 80/156 | 51.3 | ||||
| Zarkotou, 2011 [26] | Case-control | BSI | K. pneumoniae | Combination therapy | 0/20 | 0.0 |
| Monotherapy | 7/15 | 46.7 |
Abbreviations: BSI, blood stream infection; CRE, carbapenem-resistant Enterobacteriaceae; CSE, carbapenem-susceptible Enterobacteriaceae; cUTI, complicated urinary tract infection.
Mortality Odds Ratios
CRE vs CSE Infections
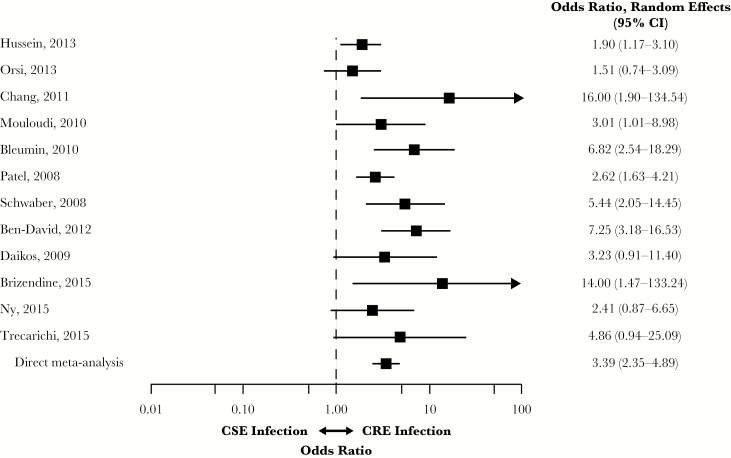
Twelve observational studies examined overall mortality in CRE vs CSE populations; 8 studies were case-control studies, and 4 were retrospective or prospective cohort studies [7–11, 16–18, 20, 23, 24, 27]. Of these studies, half (k = 6) included patients with BSIs, 1 included patients with complicated UTIs (cUTIs), 1 included patients with cUTI or pneumonia, and 4 included patients with a mixture of CRE infections. Most studies (83%; 10/12) evaluated patients with CRE infections attributed to K. pneumoniae pathogens; 1 study each assessed either carbapenem resistance due to Escherichia coli or mixed Enterobacteriaceae pathogens.
Infection with CRE was associated with a significantly higher risk of overall mortality compared with CSE (OR, 3.39; 95% CI, 2.35–4.89) (Figure 2). Heterogeneity was moderate (I2 = 45.5%; Qp = 0.041). The lowest ORs were reported by Orsi et al. and Hussein et al. (OR, 1.51; 95% CI, 0.74–3.09; and OR, 1.90; 95% CI, 1.17–3.10, respectively) [18, 27]; the highest ORs were reported by Chang et al. (OR, 16.00; 95% CI, 1.90–134.54) and Brizendine et al. (OR, 14.00; 95% CI, 1.47–133.24) [9, 10].
Figure 2.
Mortality in patients with CRE vs CSE infections. Abbreviations: CI, confidence interval; CRE, carbapenem-resistant Enterobacteriaceae; CSE, carbapenem- susceptible Enterobacteriaceae.
BSIs Caused by CRE vs CSE
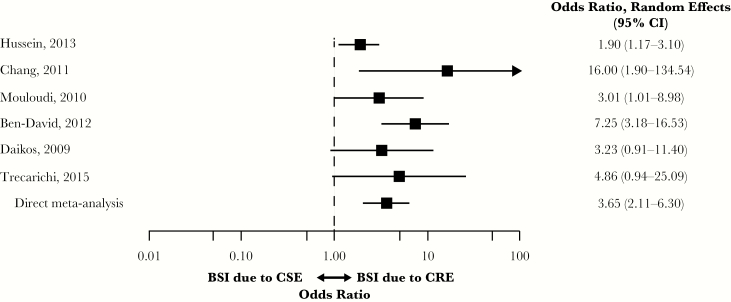
Six studies with mortality data for CRE vs CSE populations with BSIs were included in the MA [7, 10, 11, 16, 24, 27]. In patients with BSIs, CRE was associated with a significantly higher risk of overall mortality compared with CSE (OR, 3.65; 95% CI, 2.11–6.30) (Figure 3). Heterogeneity was moderate (I2 = 39.5%; Qp = 0.063). The lowest OR was reported by Hussein et al. (OR, 1.90; 95% CI, 1.17–3.10) and the highest by Chang et al. (OR, 16.00; 95% CI, 1.90–134.54) [10, 27].
Figure 3.
Mortality in patients with BSIs due to CRE vs CSE. Abbreviations: BSI, blood stream infection; CI, confidence interval; CRE, carbapenem-resistant Enterobacteriaceae; CSE, carbapenem-susceptible Enterobacteriaceae.
CRKP vs CSKP Infections
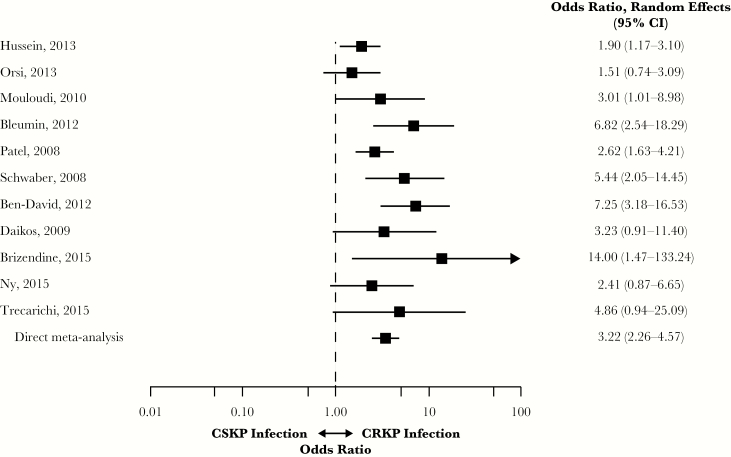
Eleven studies were identified for the analysis of studies of infections caused by CRKP vs CSKP [7–9, 11, 16–18, 20, 23, 24, 27]. Infection with CRKP was associated with a significantly higher risk of overall mortality compared with CSKP (OR, 3.22; 95% CI, 2.26–4.57) (Figure 4). Heterogeneity was moderate (I2 = 42.4%; Qp = 0.057). The lowest ORs were reported by Orsi et al. (OR, 1.51; 95% CI, 0.74–3.09) and Hussein et al. (OR, 1.90; 95% CI, 1.17–3.10) and the highest by Brizendine et al. (OR, 14.00; 95% CI, 1.47–133.24) [9, 18, 27].
Figure 4.
Mortality in patients with infections caused by CRKP vs CSKP. Abbreviations: CI, confidence interval; CRE, carbapenem-resistant Enterobacteriaceae; CSE, carbapenem-susceptible Enterobacteriaceae; CRKP, carbapenem-resistant Klebsiella pneumoniae; CSKP, carbapenem-susceptible K. pneumoniae.
KPC-Producing CRE vs Non-KPC-Producing CSE Infections
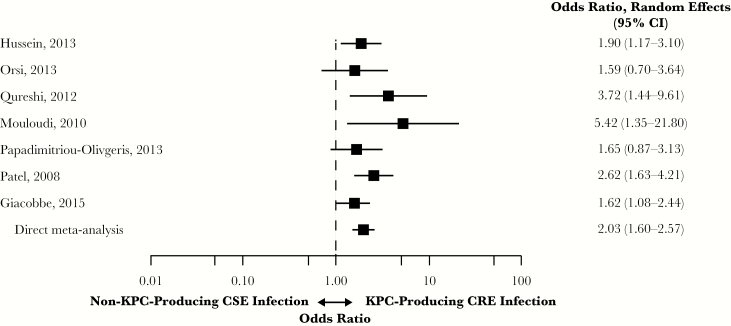
All 7 studies in patients with KPC-producing CRE vs non-KPC-producing CSE infections were case-control [13, 16, 18–21, 27]. Five studies (71%) included patients with BSIs; 2 studies included patients with a mix of infection types. Infection with KPC-producing CRE was associated with a significantly higher risk of overall mortality compared with non-KPC-producing CSE infections (OR, 2.03; 95% CI, 1.60–2.57) (Figure 5). Heterogeneity was low (I2 = 8.9%; Qp = 0.368). The lowest OR was reported by Orsi et al. (OR, 1.59; 95% CI, 0.70–3.64) and the highest by Mouloudi et al. (OR, 5.42; 95% CI, 1.35–21.80) [16, 18].
Figure 5.
Mortality in patients with KPC-producing CRE vs non-KPC-producing CSE infections. Abbreviations: CI, confidence interval; CRE, carbapenem-resistant Enterobacteriaceae; CSE, carbapenem-susceptible Enterobacteriaceae; KPC, Klebsiella pneumoniae carbapenemase.
Monotherapy vs Combination Therapy for CRE Infections
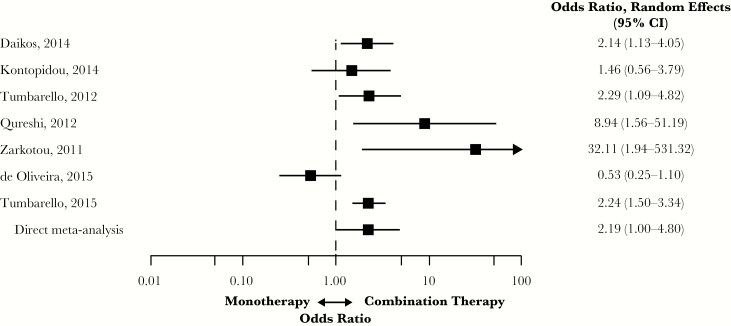
Seven studies included in the analysis compared monotherapy with combination therapy for CRE; 5 were considered retrospective, and 2 were case-control [6, 12, 14, 15, 21, 25, 26]. Four of the studies included patients with BSIs, and 3 studies included patients with a mix of infection types attributed to CRE.
Monotherapy treatment of patients with CRE infections was associated with a significantly higher risk of overall mortality compared with combination therapy (OR, 2.19; 95% CI, 1.00–4.80) (Figure 6). Heterogeneity was high (I2 = 84.2%; Qp = 0.003); de Oliveira et al. reported the lowest OR at 0.53 (95% CI, 0.25–1.10) and Zarkotou et al. the highest at 32.11 (95% CI, 1.94–531.32) [6, 26].
Figure 6.
Mortality in patients with CRE infections treated with monotherapy vs combination therapy. Abbreviations: CI, confidence interval; CRE, carbapenem-resistant Enterobacteriaceae.
DISCUSSION
Two important questions arise for clinicians and public health officials when faced with the emergence of infections due to a new antibiotic-resistant pathogen like CRE. First, there is interest in understanding the incremental clinical and economic burdens associated with infections caused by a resistant pathogen relative to its susceptible counterpart. Knowledge of this is critical to ensure sufficient resource allocation across health care facilities and public agencies to manage and control these newly identified resistant infections. Second, data regarding best treatment practices, preferably from clinical studies, are needed to maximize treatment-related outcomes. The latter is particularly important among patients with infections due to CRE. Beta-lactams have historically been the drugs of choice for the treatment of infections due to Enterobacteriaceae spp. [1]. Resistance to carbapenem has minimized the usefulness of the most commercially available beta-lactams and has led clinicians to use older and, in many cases, more toxic agents to treat infections due to CRE [1].
Overall, the analyses presented here document the increased mortality associated with serious infections caused by CRE relative to CSE infections among adults in the hospital setting. Results show that patients with carbapenem-resistant infections attributable to Enterobacteriaceae pathogens are approximately 3 times more likely to die than patients with infections that are susceptible to carbapenems. This association was consistently found in subgroup analyses of patients with BSI only and in infections caused by K. pneumoniae. Similar results were found in an analysis of patients with KPC-producing infections, where point estimates show that patients are twice as likely to die compared with patients with non-KPC carbapenem- susceptible infections. Analysis of 30-day mortality, limited to BSIs, yielded nearly identical results on mortality risk. These results align with the results of another antibiotic-resistant vs antibiotic-susceptible comparator study, which demonstrated a 2-fold increase in mortality among the CRE group relative to the CSE group [28]. Collectively, these findings highlight the need for expanding therapeutic options, refining infection management, and identifying risk factors for worse outcomes in patients with CRE infections.
The observed differences in mortality may be due to organism-, patient-, and treatment-related factors. Consistent with other infections due to antibiotic-resistant pathogens, patients with CRE tend to have a greater disease severity and more comorbid conditions relative to patients with infections due to CSE [1]. As in most meta-analyses, we relied on the unadjusted bivariate findings across the included studies as data inputs. Although this approach obscures our ability to discern the proportion of CRE-related outcomes attributable to patient-related factors, 5 of the 7 studies in which multivariate analyses were used to adjust for baseline characteristics and conditions indicated that the presence of CRE remained a significant predictor of mortality [7–9, 11, 16, 20, 23]. Thus, overall findings from the meta-analysis and the multivariate findings across the included studies indicate that the differences in mortality between patients with infections due to CRE and CSE are possibly due to treatment- or organism-related factors rather than differences in study population baseline characteristics. However, further studies are needed to clarify the definitive reasons for higher mortality among patients with infections due to CRE vs CSE.
Several treatment-related factors provide a plausible explanation for the greater mortality incurred among patients with infections due to CRE vs CSE. The studies included in this meta-analysis demonstrated that patients with CRE are at an increased risk for delayed administration of a microbiologic active antibiotic [7, 9, 11, 20, 24, 27]. The high rates of delayed appropriate therapy reported across these studies are alarming because numerous investigators across various infection types and pathogens have found an association between delayed treatment and poor outcomes the risk of a poor outcome to treatment delays. In the few CRE vs CSE comparative studies included in this meta-analysis that evaluated the effect of timeliness of therapy on mortality, patients who had delays in receipt of microbiologic active therapy had higher mortality, often independent of carbapenem resistance status [9, 11, 24, 27].
Beyond the complications caused by treatment delays, differences in antibiotic treatment for CRE and CSE infections play a role in observed mortality differences. As stated previously, resistance has minimized the usefulness of most commercially available beta-lactams [2]. Optimal treatment for serious infections due to CRE remains undefined and often involves use of tigecycline, colistin, gentamicin, and amikacin, alone or in combination with each other and carbapenems [1]. In assessing the effect of treatments on mortality outcomes in patients with CRE infections in this meta-analysis, monotherapy led to an increased mortality risk; patients receiving just 1 antimicrobial were twice as likely to die compared with those treated with multiple CRE-active antibiotics. This risk was markedly increased in patients with BSI, where those treated with monotherapy were 3.8 times more likely to die compared with those patients receiving combination therapy. A high level of heterogeneity was noted in monotherapy vs combination therapy analyses, which was likely driven by differences in the treatments received across the monotherapy and combination therapy groups. Even in cases where patients received combination therapy, mortality rates were still 30%–50% in some patient subgroups [14, 15]. In addition, studies found that certain therapies, such as colistin and polymyxin, were associated with increased risk for renal adverse events, including nephrotoxicity. These findings underscore the need for effective antimicrobials with favorable safety profiles because adverse events may increase the risk of death in these patients. Given the high mortality rates in this population, these findings also highlight the need for additional treatment options to achieve more favorable outcomes in CRE infections.
Several points should be addressed when interpreting these findings. Study design limitations included those inherent with any meta-analysis, such as heterogeneity across studies that may originate from differences in study populations, different definitions of carbapenem resistance, and the lack of a multivariate control. Heterogeneity was noted in several meta-analyses. Interestingly, the studies driving the heterogeneity in the CRE vs CSE comparative analyses were mainly those that assessed cUTI, an infection type that is associated with a lower mortality rate relative to others included in this analysis. A targeted analysis taking into account all confounders was not possible, limiting the ability of this meta-analysis to definitively establish the presence of a causal relationship. Because most of the included articles were retrospective, observational cohort studies, treatment selection bias and management decisions with respect to dosage adjustments could not be analyzed, and the potential for publication bias cannot be excluded. The findings of this analysis were primarly due to differences in mortality between CRE and CSE among patients with infections due to Klebsiella spp. Further study is needed is needed to assess the effect of carbapenem resistance among other members of the Enterobacteriaceae family, including Enterobacter spp. This analysis did not evaluate other outcomes (eg, length of hospital stay) or assess cost-effectiveness. Data are needed to evaluate how CRE affects these outcomes. Finally, this analysis was conducted before the availability of newer antibiotics including ceftazidime avibactam and meropenem/vaborbactam.
CONCLUSIONS
Results of the analysis demonstrate the increased mortality associated with serious infections due to CRE relative to CSE infections among adults in the hospital setting. Overall results and results among subgroups of patients with BSIs, infections due to K. pneumoniae, and infections due to KPC-producing pathogens showed that patients with CRE have mortality rates 2- to 3-fold higher than those with infections caused by CSE. Although the exact cause of the higher mortality rates among patients with CRE remains undefined, it is likely driven by delays in receipt of early, appropriate empiric therapy. As new antibiotics and treatment paradigms become available and are more widely used, it will be important to reassess mortality in patients with infections caused by CRE and CSE.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Writing and editorial assistance was provided to the authors by Jennifer L. Venzie, PhD, Todd J. Waldron, PhD, and John E. Fincke, PhD, of Complete Healthcare Communications, LLC (Chadds Ford, PA), a CHC Group company, and was funded by Allergan plc. Neither honoraria nor payments were made for authorship.
Authors’ contributions. All authors contributed to the study concept and developed the study design. A.M., K.F., and T.L. reviewed abstracts and extracted data. All authors analyzed and interpreted the data and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Availability of data and material. Data used for the meta-analysis were extracted from publicly available sources, per the reference list.
Financial support. This work was supported by Allergan plc (Dublin, Ireland). Evidera received funding from Allergan plc (Dublin, Ireland) to conduct the analysis on which this manuscript is based.
Potential conflicts of interest. A. Martin and K. Fahrbach are employees of Evidera. Q. Zhao was an employee of Allergan plc at the time of study conduct and analysis. T. Lodise has received consulting fees or honoraria and support for travel to meetings for this study or other purposes from Allergan plc (Dublin, Ireland). He has also been a consultant for Merck and The Medicines Company and has received payment for lectures, including service on speakers bureaus for Allergan for work not associated with the current study.
References
- 1. Akova M, Daikos GL, Tzouvelekis L, Carmeli Y. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin Microbiol Infect 2012; 18:439–48. [DOI] [PubMed] [Google Scholar]
- 2. Lee CS, Doi Y. Therapy of infections due to carbapenem-resistant gram-negative pathogens. Infect Chemother 2014; 46:149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Healthcare-associated infections Available at: http://www.cdc.gov/hai/organisms/cre/cre-clinicianFAQ.html. Accessed 11 July 2018.
- 4. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Oliveira MS, de Assis DB, Freire MP, et al. Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect 2015; 21:179.e1–7. [DOI] [PubMed] [Google Scholar]
- 7. Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resis tant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 2012; 18:54–60. [DOI] [PubMed] [Google Scholar]
- 8. Bleumin D, Cohen MJ, Moranne O, et al. Carbapenem-resistant Klebsiella pneumoniae is associated with poor outcome in hemodialysis patients. J Infect 2012; 65:318–25. [DOI] [PubMed] [Google Scholar]
- 9. Brizendine KD, Richter SS, Cober ED, van Duin D. Carbapenem-resistant Klebsiella pneumoniae urinary tract infection following solid organ transplantation. Antimicrob Agents Chemother 2015; 59:553–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang HJ, Hsu PC, Yang CC, et al. Risk factors and outcomes of carbapenem- nonsusceptible Escherichia coli bacteremia: a matched case-control study. J Microbiol Immunol Infect 2011; 44:125–30. [DOI] [PubMed] [Google Scholar]
- 11. Daikos GL, Petrikkos P, Psichogiou M, et al. Prospective observational study of the impact of VIM-1 metallo-beta-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 2009; 53:1868–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58:2322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giacobbe DR, Del Bono V, Trecarichi EM, et al. ; ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) Risk factors for bloodstream infections due to colistin-resistant KPC-producing Klebsiella pneumoniae: results from a multicenter case-control-control study. Clin Microbiol Infect 2015; 21:1106.e1–8. [DOI] [PubMed] [Google Scholar]
- 14. Tumbarello M, Trecarichi EM, De Rosa FG, et al. ; ISGRI-SITA (Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva) Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015; 70:2133–43. [DOI] [PubMed] [Google Scholar]
- 15. Kontopidou F, Giamarellou H, Katerelos P, et al. ; Group for the Study of KPC-producing Klebsiella pneumoniae infections in intensive care units Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin Microbiol Infect 2014; 20:O117–23. [DOI] [PubMed] [Google Scholar]
- 16. Mouloudi E, Protonotariou E, Zagorianou A, et al. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol 2010; 31:1250–6. [DOI] [PubMed] [Google Scholar]
- 17. Ny P, Nieberg P, Wong-Beringer A. Impact of carbapenem resistance on epidemiology and outcomes of nonbacteremic Klebsiella pneumoniae infections. Am J Infect Control 2015; 43:1076–80. [DOI] [PubMed] [Google Scholar]
- 18. Orsi GB, Bencardino A, Vena A, et al. Patient risk factors for outer membrane permeability and KPC-producing carbapenem-resistant Klebsiella pneumoniae isolation: results of a double case-control study. Infection 2013; 41:61–7. [DOI] [PubMed] [Google Scholar]
- 19. Papadimitriou-Olivgeris M, Marangos M, Fligou F, et al. KPC-producing Klebsiella pneumoniae enteric colonization acquired during intensive care unit stay: the significance of risk factors for its development and its impact on mortality. Diagn Microbiol Infect Dis 2013; 77:169–73. [DOI] [PubMed] [Google Scholar]
- 20. Patel G, Huprikar S, Factor SH, et al. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–106. [DOI] [PubMed] [Google Scholar]
- 21. Qureshi ZA, Paterson DL, Peleg AY, et al. Clinical characteristics of bacteraemia caused by extended-spectrum β-lactamase-producing Enterobacteriaceae in the era of CTX-M-type and KPC-type β-lactamases. Clin Microbiol Infect 2012; 18:887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, et al. Predictors of carba - penem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 2008; 52:1028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trecarichi EM, Pagano L, Candoni A, et al. ; HeMABIS Registry—SEIFEM Group, Italy Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect 2015; 21:337–43. [DOI] [PubMed] [Google Scholar]
- 25. Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–50. [DOI] [PubMed] [Google Scholar]
- 26. Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–803. [DOI] [PubMed] [Google Scholar]
- 27. Hussein K, Raz-Pasteur A, Finkelstein R, et al. Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae.J Hosp Infect 2013; 83:307–13. [DOI] [PubMed] [Google Scholar]
- 28. Cosgrove SE, Carmeli Y. The impact of antimicrobial resistance on health and economic outcomes. Clin Infect Dis 2003; 36:1433–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.