Abstract
Extensive research has been focused on radiation-induced brain injury. Animal and human studies have shown that flavonoids have remarkable toxicological profiles. This study aims to investigate the neuroprotective effects of quercetin in an experimental radiation-induced brain injury. A total of 32 adult male Wistar-Albino rats were randomly divided into four groups (control, quercetin, radiation, and radiation+quercetin groups, with eight rats in each group). Doses (50 mg/kg) of quercetin were administered to the animals in the quercetin and radiation+quercetin groups; radiation and radiation+quercetin groups were exposed to a dose of 20 Gy to the cranium region. Tissue samples, and biochemical levels of tissue injury markers in the four groups were compared. In all measured parameters of oxidative stress, administration of quercetin significantly demonstrated favorable effects. Both plasma and tissue levels of malondialdehyde and total antioxidant status significantly changed in favor of antioxidant activity. Histopathological evaluation of the tissues also demonstrated a significant decrease in cellular degeneration and infiltration parameters after quercetin administration. Quercetin demonstrated significant neuroprotection after radiation-induced brain injury. Further studies of neurological outcomes under different experimental settings are required in order to achieve conclusive results.
Keywords: radiation, brain injury, quercetin, neuroprotection
INTRODUCTION
The efficacy of radiotherapy in brain metastases, primary central nervous system tumors and various tumors is known, and radiotherapy has been used for a long time. With increasing doses of radiotherapy, the rate of tumor control increases. At the same time, the risk of normal brain tissues being affected is also increased, which in turn results in an increased risk of complications. Therefore, tumor control, in a sense, is dependent on the radiotherapy tolerance of normal brain tissue [1, 2]. Brain injury due to radiation is a continuous and dynamic process. Based on the time-course of the clinical picture, it can be classified into three phases, namely: acute, early delayed, and late delayed [3]. The mechanisms of the radiation-induced brain injury corresponding to these clinical findings are not fully understood. Theories about after-irradiation ischemia due to direct destruction of brain parenchymal cells and damage to the vascular system, have been developed [4, 5].
Recent research on radiation-induced brain injury has benefited from using animal models. In particular, rats have been used to elicit a variety of pathological changes (e.g. vascular lesions, edema, necrosis and demyelination) [4]. At the molecular, cellular and tissue levels, neuroinflammation, epigenetic and histopathological changes, apoptosis, impaired cell proliferation and differentiation, and other radiation-induced phenomena can be observed. In radiation damage, radiation dose, fractionation and volume play an important role.
The basic effect of ionizing radiation occurs in two ways, either by causing cell death or by the mechanism of indirect action. The actual injury is caused by the indirect mechanism. Ionized radiation generates reactive oxygen species (ROS) that are created by the ionization of water in the environment. The resulting ROS lead to the oxidation of macromolecules such as proteins, DNA and lipids, and mediate the damaging effect of ionizing radiation in biological systems. As a result, lipid peroxidation and protein oxidation products increase [6–8]. The antioxidant system is a protective mechanism that fights against oxidants; it consists of many antioxidants that are derived from exogenous/endogenous sources. Antioxidant drugs or agents that neutralize ROS have been reported to reduce ionizing radiation–induced injury [2, 7, 9, 10]. Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a flavonoid found in many vegetables and fruits. Animal and human studies have shown that it has a remarkable toxicological profile [11, 12]. In addition to its effects on cardiovascular diseases, cancers, infections, inflammatory processes, gastrointestinal tract function, diabetes, and nervous system disorders, its neuroprotective properties have recently been reported as well [13, 14].
Various in vitro studies in experimental animals and humans have provided supportive evidence for neuroprotective effects of quercetin against both neurotoxic chemicals as well as in various models of neuronal damage and neurodegenerative diseases [13–25]. Although many hypotheses have been developed, the precise mechanisms of these protective effects have not been fully explored. While its neuroprotective properties are known, literature on the role of quercetin in ionizing radiation–induced brain injury is still in its infancy. To our knowledge, this study is the first one to investigate the neuroprotective effects of quercetin against brain injury after ionizing radiation.
MATERIAL AND METHODS
Chemicals
Quercetin was purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Animals and experimental protocol
After the study was approved by the Animal Experiments Local Ethical Committee of Zonguldak (Turkey), Bulent Ecevit University (BEUN) School of Medicine, 2017-10-06/04, 32 adult male Wistar-Albino rats weighing 300–350 g were randomly divided into four groups. All of the rats included in the study were obtained from BEUN Experimental Animals Research Unit, and all of them were fed with standard rat pellets and housed in temperature- and humidity-controlled (23 ± 1°C and 55% relative humidity) rooms that were lit on a daily schedule (12:12 h light/dark) until the day of the experiment. During the experiment, the care of the laboratory animals was in accordance with international guidelines.
The control group was given only physiological saline (PS) (n = 8); group QUER was given Quercetin 50 mg/kg body weight (BW) daily in distilled water and 0.25 ml PS for 15 days (n = 8); group RAD was given only irradiation (n = 8); and group RAD+QUER was given Quercetin 50 mg/kg BW daily in distilled water and 0.25 ml PS for 15 days and then irradiated (n = 8). At the end of 15 days, the animals of group RAD and group RAD+QUER were exposed to a dose of 20 Gy to the cranium region. All rats were decapitated at 7 days after exposure to radiation.
Irradiation
Simulation of a rat was done with a 1 mm slice computerized tomography scan, and the dose calculation was performed with the Eclipse treatment planning system version 8.9 (Varian Medical Systems, Palo Alto, CA, USA). Anaesthetized (90 mg/kg ketamine and 10 mg/kg xylazine i.p.) rats in the prone position were subjected to cranium irradiation with a single dose of 20 Gy of photons using a 6 MV linear accelerator (Clinac, Varian Medical Systems, Palo Alto, CA, USA) at a dose rate of ~1 Gy/min, with the source–axis distance technique, with 1.0 cm of bolus material on the surface. Animals were returned to their home cages following irradiation. Control animals were anaesthetized but were not exposed to radiation.
Chemical analysis
Tissue samples were homogenized with phosphate-buffered saline (pH 7.4) using a glass Teflon homogenizer (Ultra Turrax IKA T18 Basic) after cutting the tissues into small pieces with scissors (for 2 min at 5000 rpm). The homogenate was then centrifuged at 5000 g for 15 min. The supernatant was used for the analysis. Serum and tissue levels of total antioxidant status (TAS) and malondialdehyde (MDA) were measured by colorimetric method using TAS and a MDA kit (Oxford Biomedical Research, Oxford, USA) in accordance with the manufacturer’s protocol.
Histopathological evaluation
Brain samples taken from rats were fixed in 10% formalin for 12 h and then embedded in paraffin. Five-micron-thick sections were taken from the tissues and stained with hematoxylin and eosin stain. The white matter of the brain tissue was examined in the four groups. Seven parameters were evaluated: hypertrophy in astrocytes, microglial reaction, inflammatory reaction, vascular telengiectasis, endothelial enlargement, edema, and axonal damage. The damage severity score in the tissue and cells was determined. Each criterion was scored from 0 to 3 (0 = normal; 1 = mild damage; 2 = moderate damage; 3 = severe damage), similar to the study of Takahashi et al. [26].
Statistical analysis
Statistical analyses were performed in SPSS 19.0 package software. The descriptive statistics of the measurement variables, consisting of median, minimum and maximum values, are provided. The Shapiro–Wilk test was used for the normal distribution test, the Kruskal–Wallis test was used for comparison of the four independent groups, and the Mann–Whitney U-test with Bonferroni correction was used for subgroup comparisons. Q1, Q3, median, minimum and maximum values are presented with box plots. We considered the 95% confidence interval, and a P value <0.05 was considered statistically significant in all statistical comparisons in the study.
RESULTS
The values for MDA and TAS in plasma and tissues are presented in Table 1.
Table 1.
Values of MDA and TAS in plasma and tissue
| Control | QUER | RAD | RAD+QUER | |
|---|---|---|---|---|
| MDA plasma (μmol/l) | 3.96 (2.97–5.03) | 4.59 (3.12–6.21) | 7.77 (6.23–8.34)a | 4.44 (3.84–6.28)b |
| TAS plasma (mmol/l Troloxequivalent) | 0.35 (0.32–0.38) | 0.36 (0.33–0.38) | 0.30 (0.29–0.32)a | 0.36 (0.34–0.38)b |
| MDA tissue (nmol/g wet tissue) | 54.15 (44.60–70.40) | 52.80 (44.90–66.30) | 95.95 (88.60–110.70)a | 58.95 (50.50–63.30)b |
| TAS tissue (μmol Troloxequivalents/g) | 19.85 (15.70–22.70) | 20.15 (17.40–24.60) | 15.10 (13.50–16.30)a | 23.05 (21.80–25.40)b |
aShows significant differences between Control and RAD groups (P < 0.05).
bShows significant differences between RAD and RAD+QUER groups (P < 0.05).
Plasma changes
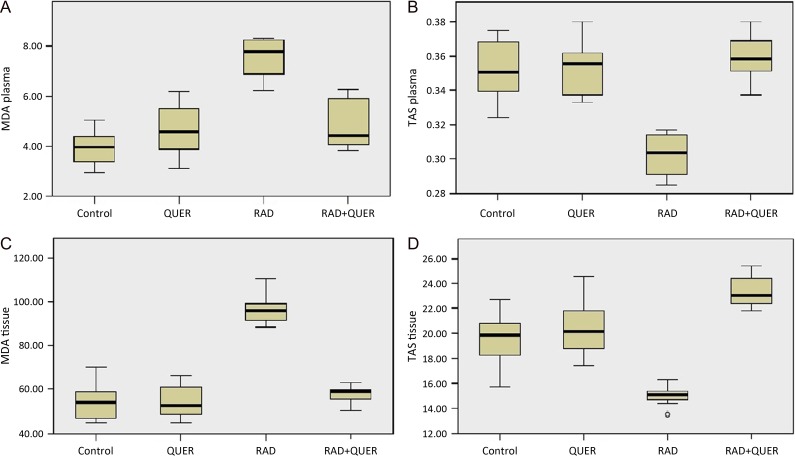
The median plasma MDA level of the control group was 3.96 μmol/l (2.97–5.03) and was significantly increased to 7.77 μmol/l (6.23–8.34) in the RAD group (P < 0.001). In the RAD+QUER group, this value decreased significantly to 4.44 μmol/l (3.84–6.28) (P = 0.031), (Fig. 1A).
Fig. 1.
Q1, Q3, median, minimum and maximum values are presented with box plot. (A) Levels of plasma MDA (μmol/l) (B) Levels of plasma TAS (mmol/lTroloxequivalent) (C) Levels of tissue MDA (nmol/g wet tissue) (D) Levels of tissue TAS (μmol Troloxequivalents/g) in groups.
The median plasma TAS level of the control group was 0.35 mmol/l Trolox equivalent (0.32–0.38) and was significantly decreased to 0.30 mmol/l Trolox equivalent (0.29–0.32) in the RAD group (P = 0.009). In the RAD+QUER group, this value increased significantly to 0.36 mmol/LTrolox equivalent (0.34–0.38) (P = 0.001), (Fig. 1B).
Tissue changes
The median tissue MDA level of the control group was 54.15 nmol/g wet tissue (44.60–70.40), and the median tissue MDA level was significantly increased in the RAD group to 95.95 nmol/g wet tissue (88.60–110.70) (P < 0.001). In the RAD+QUER group, this value significantly decreased to 58.95 nmol/g wet tissue (50.50–63.30) (P = 0.033), (Fig. 1C).
The median tissue TAS level of the control group was 19.85 μmol Trolox equivalents/g (15.70–22.70) and was significantly decreased in the RAD group, at 15.10 μmol Trolox equivalents/g (13.50–16.30) (P < 0.001). In the RAD+QUER group, this value was significantly increased to 23.05 μmol Trolox equivalents/g (21.80–25.40) (P < 0.001), (Fig. 1D).
Histopathological evaluation
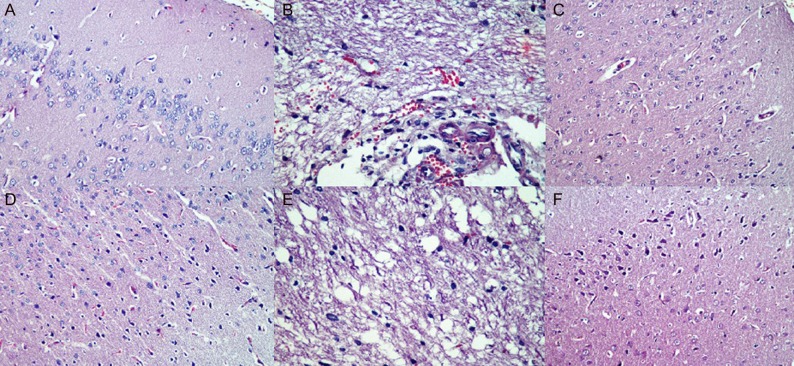
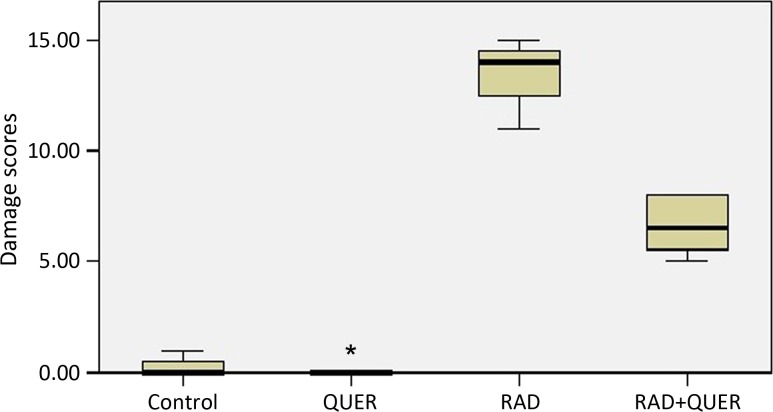
Control and QUER groups had normal histomorphologic structure in the brain cortex. In the RAD group, astrocytes showed diffuse hypertrophy, numerical increase and light clusters in nuclear chromatin. The microglial cell reaction was diffuse but relatively light. The inflammatory cell reaction was diffuse; in particular, the lymphocyte response was observed. Vascular dilatation, congestion and swelling degeneration in endothelial cells were widely observed. Edema was more prominent, especially around veins, and Rosenthal fibrils were frequently distinguished (Fig. 2A–E). In the RAD+QUER group, hypertrophy in astrocytes became less frequent. Chromatin clusters had disappeared. The microglial cell reaction diluted. The inflammatory cell reaction was milder. Vascular dilatation and endothelial damage were not significant. Edema was milder (Fig. 2F). A significant change in damage scores between the groups was detected (P < 0.05), (Fig. 3).
Fig. 2.
Effects of radiation on brain cortex in RAD group. (A) Astrocytes showed diffuse hypertrophy (H&E, ×40). (B) Vascular dilatation, congestion and endothelial cell enlargement (H&E, ×40). (C) Microglial infiltration (H&E, ×20). (D) Inflammatory reaction and congestion (H&E, ×20). (E) Edema and prominence of Rosenthal fibers (H&E, ×40). (F) In RAD+QUER group: hypertrophy in astrocytes became less frequent, chromatin clusters had disappeared, the microglial cell reaction diluted, the inflammatory cell reaction was milder, vascular dilatation and endothelial damage were not significant, and edema was milder.
Fig. 3.
Levels of damage scores in groups.
DISCUSSION
Radiotherapy is a widely used method in the treatment of central nervous system tumors. The goal is to apply minimal damage to healthy tissues while delivering an effective dose to the target lesion. Despite protective measures, side effects cannot be prevented completely. The mechanism of tissue damage caused by radiation is not fully understood [27]. In this study, for the first time, biochemical and histopathologic changes were observed by creating a cerebral tissue injury model with single-dose radiation.
There are many studies on neural damage and radiation doses, and no consensus has been formed [1, 2, 4, 5, 7, 8, 10]. During radiotherapy, ionizing radiation has been shown to interact with biological systems to produce excess ROS, leading to significant cell damage in terms of DNA, proteins, and membrane lipids. Production of excessive oxygen radicals shifts the balance between pro-oxidant and antioxidant systems towards the pro-oxidant system. ROS also reduces the intracellular concentration of antioxidants. ROS production is considered to be an important cause of radiation-induced tissue damage [8, 18, 27, 28]. The increase in lipid peroxidation is accompanied by an increase in free radical compounds in neuronal cells. Neural membranes in the brain are affected more rapidly by free radicals, especially because they are rich in unsaturated fatty acids, and a higher lipid peroxidation is observed. Therefore, with the increase in the formation of free radicals, both the structure and function of neurons are affected [2, 6, 27].
Publications suggesting that radiation-induced brain injury can be reduced by the use of agents that inhibit the action of ROS have been reported [2, 7, 10]. Specific evidence exists on the neuroprotective effects of quercetin [13–16, 21]. Studies show that quercetin can exert neuroprotection and antagonize oxidative stress [18, 19]. Oxidative stress occurs when ROS accumulate in cells, from either excessive production or insufficient neutralization, causing damage to proteins, lipids and DNA. Mitochondria are a major contributor of cellular ROS; ROS produced in the mitochondria can also target the electron transport chain, resulting in a cycle in which ROS production increases, followed by ATP depletion and ultimately cell death [29, 30]. Based on these premises, the identification of novel compounds that can counteract oxidative stress, and potentially become therapeutics, has drawn considerable interest from academic scholars recently. Natural compounds have received much attention in this regard, and polyphenols such as quercetin have been investigated.
There are also publications advocating the effect of the pro-oxidant properties of quercetin in cell defense, as well as its antioxidant properties [31]. In addition, the effects of polyphenols on neuroinflammation have also been studied. It has been suggested that some isoflavones reduce microglial activation and cause the subsequent release of proinflammatory factors, and polyphenols may have beneficial anti-inflammatory properties [32]. Quercetin has been shown to reduce lipopolysaccharide-induced nitric oxide release from a mouse neuroglia cell line [33]. In addition, quercetin also inhibits cytokine production by astrocytes [34].The cellular/molecular mechanisms for the anti-inflammatory effects of quercetin are not known, but a possible pathway may be related to induction of PON2 which has anti-inflammatory activity in addition to its antioxidant activity [30].
MDA, which is created as a result of the reaction between polyunsaturated fatty acids and free radicals, is an end product of MDA lipid peroxidation. It changes the membrane properties by causing cross-connection among lipids, proteins, and nucleic acids. So, MDA is one of the best-known products of lipid oxidation and it can be used as a marker of cell membrane injury [35]. TAS is used to assess the antioxidant status of biological samples, and it can evaluate the antioxidant response against the free radicals. In serum samples, TAS measures mainly the antioxidant activity of albumin and uric acid. It also measures the antioxidant activity of ascorbic acid, α-tocopherol and bilirubin [36]. In this study, oxidative and antioxidant activities induced by radiation in the brain were evaluated by MDA and TAS measurements in brain tissue and blood. A significant increase was observed in the RAD group, whereas the MDA values of the blood and the tissue were low in the control group (P < 0.001). There was a significant decrease in both parameters in the RAD+QUER group (P < 0.001). A significant decrease was observed in the RAD group, whereas the TAS values of blood and tissue were high in the control group (P < 0.001). A significant increase was observed in both parameters in the RAD+QUER group (P < 0.001). Musik et al. reported a decrease in TAS in selenium-administered rats. They recommended measuring TAS rather than determining changes in individual antioxidants [37].
In addition, histopathological evaluation of brain tissue was performed. Hypertrophy, microglial reaction, inflammatory reaction, vascular telengiectasis, endothelial enlargement, edema and axonal damage in astrocytes were evaluated. While the brain tissues were normal in the control and QUER groups, the RAD group showed diffuse hypertrophy, and numerical increase and mild clustering of nuclear chromatin in the astrocytes. The microglial cell reaction was diffuse but relatively mild. The inflammatory cell reaction was diffuse, and the lymphocyte response was especially observed. Vascular dilatation, congestion and swelling degeneration in endothelial cells were widely observed. Edema was more prominent, particularly around the veins, and Rosenthal fibrils were frequently distinguished. In the RAD+QUER group, mild hypertrophy in astrocytes was generally observed. Chromatin clusters had disappeared. The microglial cell reaction became less frequent. The inflammatory cell reaction was milder. Vascular dilatation and endothelial damage were not significant. Edema was milder. In our study, congestion of the lumen of the capillaries with erythrocytes, and a decrease in the frequency of neuronal degeneration in the cerebral cortex also characterized the RAD group. The most distinctive histological changes after irradiation were vascular telengiectasis, swelling degeneration in endothelial cells, and edema. After radiation, based on these observations, it can be said that vascular systems may be responsible for radiation-induced brain damage. This observation needs to be further investigated at a molecular and genetic level. These degenerative changes in the brain cortex in group RAD were significantly reduced in the RAD+QUER group. These damages may also vary with radiation volume and exposure time [4]. Due to increased permeability of small vessels, these damages cause edema in the brain tissue and migration of the inflammatory cells.
In response to RAD, astrocytes undergo proliferation, exhibit hypertrophic nuclei, and show increased expression of glial fibrillary acidic protein. Conditioned medium from irradiated microglial cells has been shown to induce astrogliosis, which might contribute to radiation-induced edema. However, the exact role of astrocytes in the overall pathogenesis of late radiation-induced brain injury is still unclear, but they are likely to have a contribution by interacting with both vascular and other parenchymal elements in the brain [38].
Biochemical and histopathological results show that oxidative stress is increased in the neural tissue damage induced by radiation. Quercetin treatment reduces this effect. Based on these findings, radiation-induced tissue injury can be reduced by lipid peroxidation of quercetin and its positive effects on the antioxidant system.
A limitation of our study is that we analyzed only certain biochemical and histopathological parameters. We found that quercetin exhibits protective and therapeutic effects on radiation-induced brain injury in rats; however, additional experimental and clinical studies are required to confirm our findings before quercetin treatment for radiation-induced brain injury can be used clinically.
CONCLUSIONS
Quercetin demonstrated significant neuroprotection after radiation-induced brain injury. Further studies of neurological outcomes under different experimental settings are required in order to achieve conclusive results.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Mildenberger M, Beach TG, McGeer EG et al. An animal model of prophylactic cranial irradiation: histologic effects at acute, early and delayed stages. Int J Radiat Oncol Biol Phys 1990;18:1051–60. [DOI] [PubMed] [Google Scholar]
- 2. Cihan YB, Arsav V, Göcen E. Royal jelly in the prevention of radiation-induced brain damages. J Neurol Sci 2011;28:475–86. [Google Scholar]
- 3. Behin A, Delattre JY. Complications of radiation therapy on the brain and spinal cord. Semin Neurol 2004;24:405–17. [DOI] [PubMed] [Google Scholar]
- 4. Yang L, Yang J, Li G et al. Pathophysiological responses in rat and mouse models of radiation-induced brain injury. Mol Neurobiol 2017;54:1022–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hopewell JW. Late radiation damage to the central nervous system: a radiobiological interpretation. Neuropathol Appl Neurobiol 1979;5:329–43. [DOI] [PubMed] [Google Scholar]
- 6. Gorman AM, McGowan A, O’Neill CO et al. Oxidative stress and apoptosis in neurodegeneration. J Neurol Sci 1996;139:45–52. [DOI] [PubMed] [Google Scholar]
- 7. Sezen O, Ertekin MV, Demircan B et al. Vitamin E and L-carnitine, separately or in combination, in the prevention of radiation-induced brain and retinal damages. Neurosurg Rev 2008;31:205–13. [DOI] [PubMed] [Google Scholar]
- 8. Steen RG, Spence D, Wu S et al. Effect of therapeutic ionizing radiation on the human brain. Ann Neurol 2001;50:787–95. [DOI] [PubMed] [Google Scholar]
- 9. Kondziolka D, Somaza S, Martinez AJ et al. Radioprotective effects of the 21-aminosteroid U-74389G for stereotactic radiosurgery. Neurosurgery 1997;41:203–8. [DOI] [PubMed] [Google Scholar]
- 10. Erol FS, Topsakal C, Ozveren MF et al. Protective effects of melatonin and vitamin E in brain in damage due to gamma radiation: an experimental study. Neurosurg Rev 2004;27:65–9. [DOI] [PubMed] [Google Scholar]
- 11. Harwood M, Danielewska-Nikiel B, Borzelleca JF et al. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol 2007;45:2179–205. [DOI] [PubMed] [Google Scholar]
- 12. Russo M, Spagnuolo C, Tedesco I et al. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem Pharmacol 2012;83:6–15. [DOI] [PubMed] [Google Scholar]
- 13. Ossola B, Kääriäinen TM, Männistö PT. The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf 2009;8:397–409. [DOI] [PubMed] [Google Scholar]
- 14. Dajas F. Life or death: neuroprotective and anticancer effects of quercetin. J Ethnopharmacol 2012;143:383–96. [DOI] [PubMed] [Google Scholar]
- 15. Magalingam KB, Radhakrishnan A, Ramdas P et al. Quercetin glycosides induced neuroprotection by changes in the gene expression in a cellular model of Parkinson’s disease. J Mol Neurosci 2015;55:609–17. [DOI] [PubMed] [Google Scholar]
- 16. Waseem M, Parvez S. Neuroprotective activities of curcumin and quercetin with potential relevance to mitochondrial dysfunction induced by oxaliplatin. Protoplasma 2016;253:417–30. [DOI] [PubMed] [Google Scholar]
- 17. Ansari MA, Abdul HM, Joshi G et al. Protective effect of quercetin in primary neurons against Abeta(1–42): relevance to Alzheimer’sdisease. J Nutr Biochem 2009;20:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishisaka A, Ichikawa S, Sakakibara H et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic Biol Med 2011;51:1329–36. [DOI] [PubMed] [Google Scholar]
- 19. Das S, Mandal AK, Ghosh A et al. Nanoparticulated quercetin in combating age related cerebral oxidative injury. Curr Aging Sci 2008;1:169–74. [DOI] [PubMed] [Google Scholar]
- 20. Xia SF, Xie ZX, Qiao Y et al. Differential effects of quercetin on hippocampus-dependent learning and memory in mice fed with different diets related with oxidative stress. Physiol Behav 2015;138:325–31. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Yi B, Ma J et al. Quercetin promotes neuronal and behavioral recovery by suppressing inflammatory response and apoptosis in a rat model of intracerebral hemorrhage. Neurochem Res 2015;40:195–203. [DOI] [PubMed] [Google Scholar]
- 22. Arikan S, Ersan I, Karaca T et al. Quercetin protects the retina by reducing apoptosis due to ischemia-reperfusion injury in a rat model. Arq Bras Oftalmol 2015;78:100–4. [DOI] [PubMed] [Google Scholar]
- 23. Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR et al. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015;93:134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Denny Joseph KM, Muralidhara. Enhanced neuroprotective effect of fish oil in combination with quercetin against 3-nitropropionic acid induced oxidative stress in rat brain. Prog Neuropsychopharmacol Biol Psychiatry 2013;40:83–92. [DOI] [PubMed] [Google Scholar]
- 25. Denny Joseph KM, Muralidhara. Combined oral supplementation of fish oil and quercetin enhances neuroprotection in a chronic rotenone rat model: relevance to Parkinson’s disease. Neurochem Res 2015;40:894–905. [DOI] [PubMed] [Google Scholar]
- 26. Takahashi S, Sun XZ, Kubota Y et al. Histological and elemental changes in the rat brain after local irradiation with carbon ion beams. J Radiat Res 2002;43:143–52. [DOI] [PubMed] [Google Scholar]
- 27. Durak MA, Parlakpinar H, Polat A et al. Protective and therapeutic effects of molsidomine on radiation induced neural injury in rats. Biotech Histochem 2017;92:68–77. [DOI] [PubMed] [Google Scholar]
- 28. Panagiotakos G, Alshamy G, Chan B et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One 2007;2:e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ott M, Gogvadze V, Orrenius S et al. Mitochondria, oxidative stress and cell death. Apoptosis 2007;12:913–22. [DOI] [PubMed] [Google Scholar]
- 30. Costa LG, Garrick JM, Roquè PJ et al. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev 2016;2016:2986796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys 2008;476:107–12. [DOI] [PubMed] [Google Scholar]
- 32. Chinta SJ, Ganesan A, Reis-Rodrigues P et al. Anti-inflammatory role of the isoflavone diadzein in lipopolysaccharide-stimulated microglia: implications for Parkinson’s disease. Neurotoxic Res 2013;23:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen JC, Ho FM, Chao PDL et al. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol 2005;521:9–20. [DOI] [PubMed] [Google Scholar]
- 34. Sharma V, Mishra M, Ghosh S et al. Modulation of interleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: implications in neuroprotection. Brain Res Bull 2007;73:55–63. [DOI] [PubMed] [Google Scholar]
- 35. Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr 1993;57:715S–24S. [DOI] [PubMed] [Google Scholar]
- 36. Marrocco I, Altieri F, Peluso I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid Med Cell Longev 2017;2017:6501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Musik I, Kiełczykowska M, Kocot J. Oxidant balance in brain of rats receiving different compounds of selenium. Biometals 2013;26:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greene-Schloesser D, Robbins ME, Peiffer AM et al. Radiation-induced brain injury: a review. Front Oncol 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]