Abstract
Nutritional research and policies have been criticized for relying on observational evidence, using self-report diet assessment methods, and supposedly being unable to present a consensus on what constitutes a healthy diet. In particular, it is often asserted that for progress to occur in nutrition science, large, simple trials, which have worked well in evaluating the efficacy of drugs, need to replace most observational research and small trials in nutrition. However, this idea is infeasible, and is unlikely to advance nutritional sciences or improve policies. This article addresses some commonly held and unfounded “myths” surrounding dietary assessments, effect sizes, and confounding, demonstrating how carefully conducted observational studies can provide reliable and reproducible evidence on diet and health. Also, there is a strong consensus among nutritional researchers and practitioners about the basic elements of a healthy diet. To move forward, we should continue to improve study design and diet assessment methodologies, reduce measurement errors, and leverage new technologies. Advances in the field lie in coalescing evidence from multiple study designs, methodologies, and technologies, and translating what we already know into policy and practice, so we can improve diet quality and enhance health in an equitable and sustainable manner across the world.
Keywords: randomized controlled trials, prospective cohort studies, observational studies, nutrition, epidemiology, policy
Introduction
Nutritional science is often admonished for relying predominantly on observational studies instead of randomized controlled trials (RCTs) of disease endpoints, which are considered the highest standard of scientific evidence in medicine (1, 2). Most recently, Trepanowski and Ioannidis (3) claimed that overreliance on observational study designs in nutritional epidemiology has led to “widespread confusion about optimal nutrition” and a failure to “give reliable answers for a century.” They concluded that “progress in nutrition science may continue to be stunted until most observational research is replaced with randomized study designs.” Although debate and critique are crucial aspects of the scientific process, a blanket dismissal of the entire field of nutritional epidemiology is counterproductive, especially when the criticism is largely based on unfounded assumptions. Many of these erroneous lines of reasoning stem from widely believed “myths” about nutritional science. In this article, we address four such commonly held myths with the aim of clarifying misconceptions about, and enabling a better understanding of, the scientific process of conducting epidemiologic studies on nutrition and health, and translating results into policy.
Debunking myths about nutritional science
Myth 1: human diet cannot be measured reliably
Diet is a complex, multicomponent, interacting exposure that changes over time, making its measurement in free-living populations a challenging task. It is especially challenging when the goal is to study the long-term effects of cumulative dietary intake on the risk of developing chronic diseases over decades; in this scenario, the use of relatively inexpensive but carefully developed self-reported measures, such as validated FFQs (sometimes in combination with objective biomarkers, which are available for only a few nutrients and foods), are the only feasible option (4). A prevailing myth regarding nutritional research is that the diet of free-living human populations cannot be reliably measured using self-report diet assessment methods (5). Several articles have previously addressed this criticism in substantial detail (6–9), which we briefly summarize here.
Self-report measures are often criticized as being unable to accurately quantify absolute energy intake. For instance, single 24-h recalls, the method used by NHANES to measure diet until recently (10), have been critiqued as resulting in energy intake assessments that are not compatible with life (11). First, given the substantial degree of error in quantifying energy intake (or energy expenditure), whether from self-report or objective measures of diet (or physical activity) (12, 13), epidemiologists rarely use these methods to assess absolute energy intake (or expenditure). Instead, weight or change in weight is used as an indicator of energy balance or imbalance over time in free-living populations (7, 14). Self-report measures of diet are used instead to assess food and nutrient intake, with energy intake estimates from such measures utilized only to adjust for potential confounding, control for measurement error, or evaluate dietary composition (13, 15, 16). Second, while usual energy intake should be within the physiologically plausible range, it is perfectly feasible (and compatible with life) for an individual to consume few or no calories on any given day (9). A single 24-h recall, having high within-person variability, is likely to capture extremes of individual intakes; thus, the average of several 24-h recalls is needed to arrive at a more accurate assessment of an individual's usual intake (17), which NHANES has been doing since 2005 (18). Nevertheless, single 24-h recalls can still provide us with useful information about mean intakes at the population level, as well as average population trends in intake over time (8).
Self-report diet assessment methods are also criticized for their reliance on memory, which could lead to systematic errors in reporting. It should be noted that self-report methods have played a key role in public health and medicine, for example, providing the basis for recommendations with regard to smoking and physical activity, detecting adverse effects of medications, and tracing infectious disease outbreaks. In prospective study designs in which diet is assessed before the diagnosis of disease, errors are not associated with disease status, thus they typically lead to attenuation of true associations. In addition, adjustment for energy intake, control for potential determinants of the systematic errors, and various methods to detect and correct for measurement error can be used to further diminish the effects of such errors (8, 19–24). Importantly, methods using self-reported intakes have been extensively validated by comparison with detailed real-time recording of intake and objective biomarkers of intake (13, 25). Lastly, in most etiologic analyses we examine the associations of ranked nutritional exposures, i.e., high compared with low intake, which can provide valuable information about the causal role of dietary factors despite imperfect data on absolute levels of intake.
Memory-based methods are not alone in having errors—objective diet assessment methods have their own sources of random and systematic errors, which should be appropriately accounted for (13). Methods such as direct observation are also unlikely to capture usual intakes, as participants may change their diet because they are being observed. Such methods are also simply not practical in large studies.
Lastly, blanket dismissals of self-report methods are often not accompanied by the offer of suitable alternatives. The novel methods often suggested as replacements, such as camera or mobile technologies, have not yet been validated, and will certainly also have errors. Further, the recommendation that interventions should only assess the effects of prescribing diets is not a viable strategy in nutritional research, not only because it is necessary to measure usual diet outside of intervention studies, but also because adherence can be particularly problematic in nutritional RCTs, as is discussed later in this article.
In conclusion, all diet assessment methods (e.g., 24-h recalls, food records, FFQs, biomarkers, smart-phone apps) have their own strengths and weaknesses, and when applied in appropriate contexts, which leverage their strengths and account for the weaknesses (8), they can yield useful data on the diets of free-living human populations.
Myth 2: most nutritional exposures have tiny effect sizes
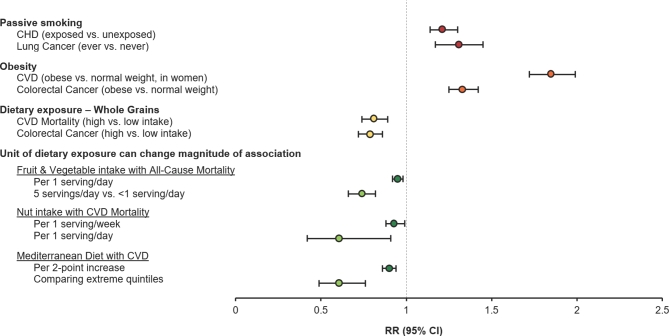
Another commonly held myth in nutrition research is that nutritional risk factors typically have tiny effect sizes, ranging from 0.95 to 1.05, making it difficult for observational research to detect them with any certainty. However, the magnitude of effect depends on the unit of analysis for the exposure or the contrast between groups (26–29) (Figure 1). For instance, in a recent meta-analysis of prospective cohort studies (26), each incremental serving per day of fruit and vegetable consumption was associated with a 5% lower hazard of all-cause mortality (HR: 0.95; 95% CI: 0.92, 0.98). However, when the unit of consumption is increased to 5 servings/d, the amount usually recommended by dietary guidelines (30, 31), the effect size increased to a 26% lower hazard risk of all-cause mortality compared with <1 serving/d (HR: 0.74; 95% CI: 0.66, 0.82). The magnitude of effect also depends on the nutritional status of the underlying study population. For instance, sugar-sweetened beverage (SSB) intake is more strongly associated with weight change in overweight or obese individuals (32). Thus, if the association is measured in a population with a higher prevalence of overweight and obesity, the effect size will be larger than if it is measured in a lean population. Similarly, folate intake—maternal deficiency of which is an established dietary risk factor for neural tube defects in the offspring (33)—is unlikely to be strongly associated with neural tube defect risk in the United States, now that food products have been fortified with folic acid to reduce this risk (33).
FIGURE 1.
Examples of effect estimates from prospective cohort studies. Data from references 26–29 and 41–46. CHD, coronary heart disease; CVD, cardiovascular disease.
Additionally, it is essential not to confuse small but true effect sizes with null findings, and null findings are just as important to document as real effects. This is exemplified by the example of total fat; avoidance was recommended in the 1980s owing to its presumed detrimental effects on cancer and coronary heart disease (CHD) risk (34). It was observational cohort studies that first documented null associations of total fat intake with breast cancer and CHD, questioning this recommendation (35, 36). Today, with evidence from both observational and experimental studies, we know that it is the type of fat consumed, not total fat intake, that is most strongly predictive of cardiovascular risk (37, 38). Similarly, meta-analyses of prospective cohorts have shown that egg consumption (≤1 egg/d) is not associated with increased risk of CHD or stroke in the general population (39, 40). In light of these null findings, the most recent (2015–2020) Dietary Guidelines for Americans (DGA) have removed the previously recommended restrictions on total fat intake (31).
It is often argued that observational studies are only useful for ascertaining effects of high magnitude, such as those of smoking on lung cancer (RR = 10), and that smaller effect sizes can only be validly assessed using randomized study designs. However, some effects that are much lower in magnitude, such as those of passive smoking in relation to heart disease and lung cancer, as well as obesity in relation to cardiovascular disease (CVD) and colorectal cancer, have previously been established as valid and conclusive using observational evidence (41–46) (Figure 1). These magnitudes of effect are comparable to, or even weaker than, many diet-disease associations that have also been documented through observational research. They can, however, still be important, and are similar in magnitude to those of widely used drugs [e.g., a starting dose of statins (47)].
There is thus no justification to cite small effect sizes as a basis to dismiss observational research, because the effect size depends on the exposure contrast, and null findings are important for both etiologic and practical reasons. It should also be noted that randomized trials can reliably discern small effect sizes only if they are adequately powered to do so given predetermined levels of drop out, intervention group–specific adherence, and event rates, and provided the etiologically relevant exposure contrast is being examined (48). Many of these factors can be compromised in nutritional RCTs, as discussed below.
Myth 3: observational studies are so confounded that any results cannot be trusted
Although RCTs of hard endpoints are considered the gold standard of scientific evidence in medicine (1, 2), they are often infeasible to carry out for many nutritional questions owing to practical and ethical considerations (8, 48). Thus, despite the shortcomings, observational research is often better suited to answer nutritional questions in regard to long-term health. In particular, when the outcome in question has a long etiologic period with respect to dietary exposures (i.e., most hard disease endpoints, such as cancer and heart disease), and the nutritional comparison of interest would be practically or ethically infeasible in an interventional setting, observational studies can be an invaluable resource. Long-term prospective cohort studies, in particular, are the strongest observational study design, as their prospective nature makes them less amenable to several potential biases, such as reverse causation, recall bias, and selection bias, commonly found in retrospective or cross-sectional studies (8).
Potential confounding is a major challenge in all types of observational research, and to account for this, researchers use subject matter knowledge to identify, measure, and adjust for all known relevant confounders (8, 49). It is often claimed that confounding is even more problematic in nutritional epidemiology owing to the existence of an intricate network of correlations between various nutritional exposures and other lifestyle and environmental factors (3). Correlation, however, does not necessarily imply confounding. Generally speaking, in order for a variable to be considered a confounder, it must be associated with the exposure of interest in the study population and with the outcome of interest among the non-exposed, and not be caused by either exposure or outcome (50). Thus, numerous correlations between nutritional exposures and other factors do not automatically imply intractable confounding of nutrition-disease associations. The magnitude of confounding by any variable is further limited by the strength of the associations of the variable with both the outcome and the exposure, as well as by the overall prevalence of the confounding variable in the study population (50, 51). Thus, although residual or unmeasured confounding cannot be completely ruled out in observational research, sensitivity analyses can examine the scenarios under which unknown confounders might account for some or all of the observed association between the nutritional exposure and the outcome (52). The most complex source of confounding is often other constituents of the same foods; sometimes we may have more confidence in conclusions about specific foods or food groups than in a specific constituent.
In nutritional epidemiologic research, we rely on many sources of evidence to arrive at informed conclusions about diet-disease associations. When interpreted together with existing short-term experimental evidence (e.g., with changes in blood lipids as the outcome), carefully conducted observational studies can provide valid and reliable effect estimates for dietary exposures as they relate to major diseases. In particular, in scenarios where large-scale, long-term RCTs of incident diseases are infeasible, we use the Bradford Hill criteria (53), namely strength, consistency, temporality, biological gradient (dose-response), plausibility, coherence, and experimental evidence, to arrive at causal interpretations. When satisfied, as has been the case for several diet-disease associations (54–56), timely policy decisions can be made in the absence of RCTs of hard endpoints.
Myth 4: findings from observational studies and RCTs are extremely inconsistent
It is also claimed that there is widespread inconsistency between findings of observational studies and RCTs in nutritional research (1, 2, 57). However, more often than not, when RCTs are able to successfully examine diet-disease relations, their results are remarkably in line with those of prospective observational studies (28, 29, 32, 37, 38, 58–72) (Table 1). Within the broader field of health research, several meta-analyses have confirmed a high level of consistency between the two categories of study design with respect to several clinical and health care interventions (73–75).
TABLE 1.
Examples of effect estimates from prospective cohort studies and RCTs that examine similar diet-disease associations1
| Dietary exposure (refs) | Health outcome | Prospective cohort effect estimate (95% CI) | RCT effect estimate (95% CI) |
|---|---|---|---|
| Total fat (58, 59) | Breast cancer | 0.97 (0.92, 1.03)2,3 (bottom vs. top categories) | 0.91 (0.83, 1.01)4 (low fat vs. control diet) |
| Total fat (60, 61) | CHD | 0.96 (0.91, 1.02)2,3 (bottom vs. top categories) | 0.97 (0.90, 1.06)4 (low fat vs. control diet) |
| Saturated fat (mostly in place of carbohydrate intake) (62, 63) | CHD | 0.93 (0.84, 1.04)2,3 (bottom vs. top categories) | 0.87 (0.74, 1.03)2 (low fat vs. control diet) |
| Replacing saturated fat with polyunsaturated fat (37, 38) | CHD | 0.87 (0.77, 0.97)2 (per 5% of energy replacement) | 0.81 (0.70, 0.95)2 (PUFA replacing SFA vs. control diet) |
| Mediterranean diet (28, 29) | CVD | 0.61 (0.49, 0.76)4 (top vs. bottom categories) | 0.64 (0.53, 0.79)2 (Mediterranean vs. control diet) |
| Mediterranean diet (64, 65) | T2D | 0.80 (0.68, 0.93)2 (top vs. bottom categories) | 0.70 (0.54, 0.92)4 (Mediterranean vs. control diet) |
| Potassium (66, 67) | Hypertension | 0.83 (0.73, 0.95)3,4 (top vs. bottom categories of urinary K excretion) | — |
| SBP, mm Hg | — | −6.22 (−8.82, −3.93)2 (potassium vs. control) | |
| DBP, mm Hg | — | −3.47 (−5.22, −1.73)2 (potassium vs. control) | |
| Dietary Fiber (68–70) | Hypertension | 0.68 (0.51, 0.92)3,4 (top vs. bottom categories) | — |
| SBP, mm Hg | −1.09 (−1.66, −0.52)4 (≥25 g/d vs. <10 g/d) | −1.13 (−2.49, 0.23)2 (fiber supplementation vs. control) | |
| DBP, mm Hg | −1.11 (−1.50, −0.72)4 (≥25 g/d vs. <10 g/d) | −1.26 (−2.04, −0.48)2 (fiber supplementation vs. control) | |
| SSBs (32) | Weight (adults), kg | 0.22 (0.09, 0.34)2 (per serving per day increase) | 0.85 (0.50, 1.20)2 (increasing SSB vs. control) |
| BMI (children), kg/m2 | 0.06 (0.02, 0.10)2 (per serving per day increase) | −0.17 (−0.39, 0.05)2 (reducing SSB vs. control) | |
| DASH (71, 72) | Hypertension | 0.85 (0.73–0.98)4 (top vs. bottom categories) | — |
| SBP, mm Hg | — | −6.74 (−8.25, −5.23)2 (DASH vs. control diet) | |
| DBP, mm Hg | — | −3.54 (−4.29, −2.79)2 (DASH vs. control diet) |
CHD, coronary heart disease; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; RCT, randomized controlled trial; refs, references; SBP, systolic blood pressure; SSB, sugar-sweetened beverage; T2D, type 2 diabetes.
Meta-analysis.
RR inverted for ease of interpretation.
Single study.
Discrepancies between observational studies and RCTs, when they exist, do not necessarily imply bias in the observational studies. Often, the two study designs are answering very different research questions, in different study populations, and hence cannot arrive at the same conclusions. For instance, in studies of vitamin supplementation, observational studies and RCTs may examine different doses, formulations (e.g., natural diet compared with synthetic supplements), durations of intake, timing of intake, and study populations (e.g., general compared with high-risk population), and may differ in focus (e.g., primary compared with secondary prevention). This was exemplified with respect to hormone replacement therapy (HRT) and CHD risk, which has been widely cited as a classic example of discrepancy between observational and randomized designs (76). When observational evidence showing a potential beneficial effect of HRT on CHD risk (77) was contradicted by RCT evidence from the Women's Health Initiative (WHI) indicating a potential increase in CHD risk following HRT (78), a common conclusion was that the former was biased. However, we now know that these discrepancies were likely because of differences in the timing of HRT initiation relative to onset of menopause. The observational studies predominantly included younger women who had initiated HRT within 2–3 y after menopause, while the WHI included older women, many of whom were started on HRT >10 y after menopause (79). Thus, it is likely that HRT initiation at a younger age and soon after menopause is protective of CHD, but initiation at older ages long after menopause may have harmful effects. This suggests that each study design reported valid findings, but for different research questions (76, 80). In addition, observational studies and the WHI trial were highly consistent on the relationships between HRT and risk of stroke, pulmonary embolism, type 2 diabetes, fractures, colorectal cancer, and breast cancer (81).
Thus, inconsistencies between observational and randomized study designs are not as widespread as is commonly believed. When they do exist, it is important to carefully examine the studies for potential sources of discrepancies, instead of castigating one line of evidence in favor of another. As we saw in the case of HRT use, careful examination of both types of studies can offer deep insights into important research questions which may not otherwise be possible.
Are large RCTs the solution?
Trepanowski and Ioannidis (3) propose that “megatrials” with long-term follow-up of many thousands of participants, together with smaller, shorter-term trials answering more focused questions, should replace almost all observational research to ensure progress in nutritional science. However, as mentioned at several points earlier in this article, as well as in several previously published articles (8, 48, 80), large, simple trials are often not the most appropriate or feasible study design for nutritional studies. This is because the drug trial paradigm of pharmaceutical research does not neatly apply to nutritional research (4, 48). Drug trials usually examine the effect of a singular chemical compound, often with known interactions with other drug compounds, on clinical endpoints over short- to medium-term durations in a placebo-controlled, blinded fashion, and often with high compliance and retention. The control group in a drug trial typically has zero exposure, whereas this would not be true in nutrition trials. Nutritional research examines the effect of a complex, dynamic, interacting set of exposures—the human diet—on disease endpoints over several years and even decades. Dietary intervention studies thus often contend with considerable noncompliance and drop out (8, 48, 80). Although unbiased at baseline, bias can be introduced in RCTs after baseline through differential drop-out or extreme crossover of participants across intervention and control groups (8, 48, 80). Noncompliance can be especially problematic, given that blinding of study participants to study arms is usually impossible in nutritional RCTs (except in nutrient supplementation trials) (8, 48, 80). Deciding on the appropriate comparison group is also difficult, as a placebo arm is rarely relevant, diet-disease associations are frequently nonlinear, and ethical considerations often make most control arms infeasible (8, 48, 80).
Ceasing observational nutritional research ignores all of the groundwork laid by such studies based on which well-designed trials have been conducted to answer key questions. Discerning the most important nutritional questions to do trials on requires strong observational evidence, highlighting the etiologically relevant periods during which specified ranges of exposure contrasts might influence disease pathology. Without this information, large RCTs might end up wasting valuable resources and decades of research to effectively answer the “wrong” question (48). Further, slight changes in these aspects of the research question can meaningfully change conclusions, as was exemplified in the case of HRT use and CHD risk discussed above.
Referring to a nutritional example, the inverse associations of antioxidants (e.g., carotenoids and vitamin E) with cancer risk found in observational studies (82, 83) were supposedly later contradicted by RCT evidence that showed a trend toward increased risk for certain cancers with antioxidant supplementation, especially in high-risk populations (84, 85). However, a key difference between these studies might explain these results—in the observational studies, the exposure contrast was within the normal range of intake, while in the RCTs, much higher doses were compared with placebos. Thus, it is possible that, compared with deficient intake, normal levels of antioxidants prevent development of cancer, but excessively high intakes are actually detrimental relative to normal intake, especially in populations already at high risk of developing cancer.
Folate supplementation and CVD provides yet another informative example. Observational (and physiologic) studies showed a link between low folate and risk of CVD (86). Randomized trials in folate-replete populations were null, leading some observers to claim that observational studies were wrong again (87). However, a recent trial in a folate-deficient population in China demonstrated reductions in stroke incidence (88), reconciling the apparent discrepancies and underscoring the need to scrutinize the population characteristics. Additionally, it would be difficult for a large trial to examine associations with mortality in the general population because, if there is evidence of benefit with respect to intermediate disease incidence, the trial would have to be stopped early, before sufficient numbers of deaths may have occurred.
A related problem stemming from large RCTs is obsolescence. It is proposed that major questions to be tested using RCTs should be evaluated for potential for obsolescence. However, this would be difficult to do given that information pertaining to this would, more often than not, only be available after the trial had been initiated; it would be nearly impossible to do without observational research. RCTs, with their intervention groups set at baseline, do not have the kind of flexibility that observational studies have built into their design (48). Long-term prospective cohorts can easily adapt to new information in repeated diet assessments, thereby remaining relevant. This is particularly true of a dynamic exposure such as the human diet, which is constantly evolving over time and across populations.
Another problem is that in long-term dietary intervention trials, compliance usually diminishes over time, ultimately resulting in little or no exposure contrast. This is exemplified by the WHI Trial (61, 89) and Multiple Risk Factor Intervention Trial (90), large RCTs with long follow-up, both of which were largely uninformative regarding the questions they set out to examine owing to inadequate compliance to complex lifestyle interventions (91). In fact, because of these limitations, RCTs were also unable to confirm the long-term deleterious effects of smoking on mortality (92), one of the strongest and most consistent findings in observational epidemiology, and on which both the 1964 Surgeon General's Report on Smoking and Health (93) and many decades of anti-tobacco legislation are based. Lastly, even for pharmaceutical exposures, which tend to be more amenable to randomization than to nutritional exposures, observational designs are often needed where RCTs are not feasible, for instance, to detect adverse events following post-marketing drug use (94, 95).
Contributions of nutritional epidemiology to policies and recommendations
Nutritional research is criticized for an overreliance on observational research, which has supposedly contributed to profound confusion and controversy in nutritional research, with little progress in understanding what constitutes a healthy diet or in stemming disease burden. On the contrary, prospective studies of diet and health outcomes have been remarkably consistent (6, 96–98). Further, calibrating evidence from across observational and short-term experimental studies, there is actually considerable scientific consensus today among nutritional researchers and practitioners about basic elements of a healthy diet (99). These include higher consumption of fruits, vegetables, whole grains, legumes, nuts, low- or nonfat dairy, and seafood; moderate consumption of alcohol; and lower consumption of red and processed meat, sugar-sweetened foods and drinks, and refined grains. These dietary patterns were recently included in the recommendations of the 2015–2020 DGA (31), and this conclusion was also supported by the consensus statement released by a group of nutrition and food systems experts at the Oldways Finding Common Ground conference in 2015 (100).
Nutritional research has also influenced policy applications in the real world, resulting in improved dietary quality and lowered disease burden in the US population. For instance, based on observational and experimental evidence from nutritional studies which showed detrimental effects of trans fats on lipids and CHD risk (56, 101), countries all over the world have banned or indirectly limited trans fats in their food supply systems (102, 103). Similarly, following from strong observational and RCT evidence confirming an increased risk of obesity, diabetes, and CVD associated with higher SSB consumption (17, 32, 104), several parts of the United States removed SSBs from schools and banned their sale in public buildings (105, 106). Mexico instituted a 1 peso/L excise tax on SSBs, which resulted in decreased SSB purchases (107). The extensive review by the 2015 Dietary Guidelines Advisory Committee supported the conclusion that consumption of SSBs and other sweetened foods is associated with increased risk of several diseases (99), resulting in the concomitant 2015–2020 DGA (31), including a new recommendation suggesting limiting added sugar consumption to <10% of the calories consumed per day. This recommendation has bolstered the proposed change to the nutrition facts panel by the FDA to display the total amount of added sugar in food products (108).
Dietary quality has improved as a result of these policy initiatives. In an analysis of repeated cross-sectional dietary data from the nationally representative NHANES, we found steady improvement in dietary quality since 2000, primarily because of reductions in SSB consumption, and almost complete elimination of trans fats in the US diet (109). These improvements are estimated to have prevented >1 million premature deaths and reduced the number of incident cases of diabetes by 12.6% and of CVD by 8.6% (110). This is in line with the reduction in incident diabetes cases recently reported by the CDC (111), as well as a recent investigation which found significant reductions in hospital admissions for myocardial infarction and stroke in New York counties which had introduced trans fat bans relative to those that had not (112).
Thus, there has been a confluence of evidence, both observational and experimental, on the key attributes of a healthy diet, resulting in tangible policy applications and a quantifiable public health impact in the direction of improved health and well-being.
Discussion
Human diet is a multidimensional and time-varying exposure that has behavioral, psychological, social, and cultural components and is set within the larger food environment, which is influenced by macro-level sociopolitical and economic factors. We need to be cognizant of this complexity, and appreciate the inadequacy of a single type of study design or diet assessment technique in capturing dietary effects in their entirety. Observational studies and self-report diet assessment methods do have limitations, and it is crucial that we remain aware of them while continuing to improve study and diet assessment methodologies, reduce measurement errors and minimize their effects, and leverage new technologies. However, these limitations are not a justification for dismissing an entire body of research as inherently and insurmountably flawed. RCTs and objective diet assessment methods come with their own limitations, and if these are downplayed as being of no serious concern, it can lead to misleading conclusions. More funding for well-designed RCTs will indeed bolster the field, but RCTs are not a panacea in nutrition research (48). We need multiple lines of evidence, including carefully conducted prospective cohort studies with repeated measures, to further progress nutritional research. Additionally, while there have been tremendous advances in omics technologies, which have the potential to answer important nutritional questions, we need to manage the unrealistically high expectations from precision nutrition, and integrate it with public health nutrition and global nutrition to have maximum public health impact (113).
By adopting a multitude of designs, approaches, and measurement techniques with complementary sets of strengths and weaknesses, we have been able to triangulate what constitutes a healthy diet, influence policy changes, and reduce disease burden. However, we still face important public health challenges, including an inequitable distribution of the burden of chronic diseases (114, 115), a wide and ever-increasing socioeconomic gap in diet quality (109), a double burden of overnutrition and undernutrition that is still common in many parts of the world (116), and a large and unsustainable impact of the food system on the environment (117). Nutrition research needs to continue playing a leading role in addressing these challenges, with the goal of ensuring a healthful and sustainable future for all.
Acknowledgments
The authors’ responsibilities were as follows—AS and FBH: wrote the first draft of the manuscript; and all authors: revised the manuscript critically for important intellectual content, and read and approved the final manuscript.
Notes
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions of the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor. AS is supported by American Heart Association Grant #16POST29660000. FBH's research is supported by NIH grants HL60712, HL118264, and DK112940.
Author disclosures: EBR received a research grant from the USDA/Blueberry Highbush Council. FBH received research support from the California Walnut Commission and lecture fee from Metagenics. AS, MJS, and WW, no conflicts of interest.
Abbreviations used:
- CHD
coronary heart disease
- CVD
cardiovascular disease
- DGA
dietary guidelines for Americans
- HRT
hormone replacement therapy
- RCT
randomized controlled trial
- SSB
sugar-sweetened beverage
- WHI
Women's Health Initiative
References
- 1. Ioannidis JP. Implausible results in human nutrition research. BMJ 2013;347:f6698. [DOI] [PubMed] [Google Scholar]
- 2. Taubes G. Epidemiology faces its limits. Science 1995;269(5221):164–9. [DOI] [PubMed] [Google Scholar]
- 3. Trepanowski JF, Ioannidis JPA. Perspectives: randomized study designs should predominate in human nutrition research: why and how. Adv Nutr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr 2015;6(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sorensen TI, Speakman JR, Jeansonne M, Allison DB. Energy balance measurement: when something is not better than nothing. Int J Obes (Lond) 2015;39(7):1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hebert JR, Hurley TG, Steck SE, Miller DR, Tabung FK, Peterson KE, Kushi LH, Frongillo EA. Considering the value of dietary assessment data in informing nutrition-related health policy. Adv Nutr 2014;5(4):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Satija A, Yu E, Willett WC, Hu FB. Objective measures are complementary to, rather than a replacement for, self-reported methods. Int J Obes (Lond) 2015;39(7):1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr 2015;6(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gu X, Tucker KL. Reply to E Archer. Am J Clin Nutr 2017;106(3):952–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CDC: National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet].Washington (DC): US Government Printing Office; [updated 2018 April 30]. Available from: https://www.cdc.gov/nchs/nhanes/index.htm. [Google Scholar]
- 11. Archer E, Hand GA, Blair SN. Validity of US nutritional surveillance: National Health and Nutrition Examination Survey caloric energy intake data, 1971–2010. PLoS One 2013;8(10):e76632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr 2012;95(4):989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Willett W. Nutritional epidemiology. 3rd ed New York: Oxford University Press; 2013. [Google Scholar]
- 14. Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation 2012;126(1):126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124(1):17–27. [DOI] [PubMed] [Google Scholar]
- 16. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 17. Huang C, Huang J, Tian Y, Yang X, Gu D. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta-analysis of prospective studies. Atherosclerosis 2014;234(1):11–6. [DOI] [PubMed] [Google Scholar]
- 18. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008;88(2):324–32. [DOI] [PubMed] [Google Scholar]
- 19. Freedman LS, Midthune D, Carroll RJ, Commins JM, Arab L, Baer DJ, Moler JE, Moshfegh AJ, Neuhouser ML, Prentice RL, et al. Application of a new statistical model for measurement error to the evaluation of dietary self-report instruments. Epidemiology 2015;26(6):925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 2011;103(14):1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol 1995;24(2):389–98. [DOI] [PubMed] [Google Scholar]
- 22. Hebert JR, Ebbeling CB, Matthews CE, Hurley TG, Ma Y, Druker S, Clemow L. Systematic errors in middle-aged women's estimates of energy intake: comparing three self-report measures to total energy expenditure from doubly labeled water. Ann Epidemiol 2002;12(8):577–86. [DOI] [PubMed] [Google Scholar]
- 23. Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for measurement error: the case of multiple covariates measured with error. Am J Epidemiol 1990;132(4):734–45. [DOI] [PubMed] [Google Scholar]
- 24. Spiegelman D, Zhao B, Kim J. Correlated errors in biased surrogates: study designs and methods for measurement error correction. Stat Med 2005;24(11):1657–82. [DOI] [PubMed] [Google Scholar]
- 25. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grosso G, Yang J, Marventano S, Micek A, Galvano F, Kales SN. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies. Am J Clin Nutr 2015;101(4):783–93. [DOI] [PubMed] [Google Scholar]
- 28. Martinez-Gonzalez MA, Bes-Rastrollo M. Dietary patterns, Mediterranean diet, and cardiovascular disease. Curr Opin Lipidol 2014;25(1):20–6. [DOI] [PubMed] [Google Scholar]
- 29. Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009;119(8):1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. WHO. Healthy diet: fact sheet no. 394 [Internet]. [updated 2015 Sep 14]. Available from: http://www.who.int/mediacentre/factsheets/fs394/en/.
- 31. US Department of Health and Human Services and US Department of Agriculture. 2015–2020 dietary guidelines for Americans. Washington (DC): US Government Printing Office; 2015. [Google Scholar]
- 32. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98(4):1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pitkin RM. Folate and neural tube defects. Am J Clin Nutr 2007;85(1):285S–8S. [DOI] [PubMed] [Google Scholar]
- 34. US Department of Health and Human Services and US Department of Agriculture. Nutrition and your health: dietary guidelines for Americans. Washington (DC): US Government Printing Office; 1980. [Google Scholar]
- 35. Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337(21):1491–9. [DOI] [PubMed] [Google Scholar]
- 36. Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Hennekens CH, Speizer FE. Dietary fat and the risk of breast cancer. N Engl J Med 1987;316(1):22–8. [DOI] [PubMed] [Google Scholar]
- 37. Jakobsen MU, O'Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89(5):1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010;7(3):e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alexander DD, Miller PE, Vargas AJ, Weed DL, Cohen SS. Meta-analysis of egg consumption and risk of coronary heart disease and stroke. J Am Coll Nutr 2016;35(8):704–16. [DOI] [PubMed] [Google Scholar]
- 40. Rong Y, Chen L, Zhu T, Song Y, Yu M, Shan Z, Sands A, Hu FB, Liu L. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ 2013;346:e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease—a meta-analysis of epidemiologic studies. N Engl J Med 1999;340(12):920–6. [DOI] [PubMed] [Google Scholar]
- 42. Kim CH, Lee Y-CA, Hung RJ, McNallan SR, Cote ML, Lim W-Y, Chang S-C, Kim JH, Ugolini D, Chen Y, et al. Exposure to secondhand tobacco smoke and lung cancer by histological type: a pooled analysis of the International Lung Cancer Consortium (ILCCO). Int J Cancer 2014;135(8):1918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN, Lloyd-Jones DM. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiology 2018;3(4):280–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013;8(1):e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wei H, Gao Z, Liang R, Li Z, Hao H, Liu X. Whole-grain consumption and the risk of all-cause, CVD and cancer mortality: a meta-analysis of prospective cohort studies. Br J Nutr 2016;116(3):514–25. [DOI] [PubMed] [Google Scholar]
- 46. Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013(1):Cd004816 DOI: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hébert JR, Frongillo EA, Adams SA, Turner-McGrievy GM, Hurley TG, Miller DR, Ockene IS. Perspective: randomized controlled trials are not a panacea for diet-related research. Adv Nutr 2016;7(3):423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol 2002;155(2):176–84. [DOI] [PubMed] [Google Scholar]
- 50. Walker AM. Observation and inference: an introduction to the methods of epidemiology. 3rd ed Chestnut Hill (MA): Epidemiology Resources Inc; 1991. [Google Scholar]
- 51. Schlesselman JJ. Assessing effects of confounding variables. Am J Epidemiol 1978;108(1):3–8. [PubMed] [Google Scholar]
- 52. Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med 2013;369(21):2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jacobs DR Jr., Gallaher DD. Whole grain intake and cardiovascular disease: a review. Curr Atheroscler Rep 2004;6(6):415–23. [DOI] [PubMed] [Google Scholar]
- 55. Malik VS, Hu FB. Sweeteners and Risk of Obesity and Type 2 Diabetes: The Role of Sugar-Sweetened Beverages. Curr Diabetes Rep 2012;12(2):195–203. [DOI] [PubMed] [Google Scholar]
- 56. Mozaffarian D, Aro A, Willett WC. Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr 2009;63 Suppl 2:S5–21. [DOI] [PubMed] [Google Scholar]
- 57. Young SS, Karr A. Deming, data and observational studies. Significance 2011;8(3):116–20. [Google Scholar]
- 58. Cao Y, Hou L, Wang W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: a meta-analysis of prospective cohort studies. Int J Cancer 2016;138(8):1894–904. [DOI] [PubMed] [Google Scholar]
- 59. Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative randomized controlled dietary modification trial. JAMA 2006;295(6):629–42. [DOI] [PubMed] [Google Scholar]
- 60. Harcombe Z, Baker JS, Davies B. Evidence from prospective cohort studies does not support current dietary fat guidelines: a systematic review and meta-analysis. Br J Sports Med 2017;51(24):1743–9. [DOI] [PubMed] [Google Scholar]
- 61. Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative randomized controlled dietary modification trial. JAMA 2006;295(6):655–66. [DOI] [PubMed] [Google Scholar]
- 62. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr 2010;91(3):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev 2015;(6):Cd011737 DOI: 10.1002/14651858.CD011737. [DOI] [PubMed] [Google Scholar]
- 64. Esposito K, Chiodini P, Maiorino MI, Bellastella G, Panagiotakos D, Giugliano D. Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine 2014;47(1):107–16. [DOI] [PubMed] [Google Scholar]
- 65. Salas-Salvado J, Bullo M, Estruch R, Ros E, Covas MI, Ibarrola-Jurado N, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med 2014;160(1):1–10. [DOI] [PubMed] [Google Scholar]
- 66. Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ, Joosten MM. Urinary potassium excretion and risk of developing hypertension: the prevention of renal and vascular end-stage disease study. Hypertension 2014;64(4):769–76. [DOI] [PubMed] [Google Scholar]
- 67. Filippini T, Violi F, D'Amico R, Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol 2017;230:127–35. [DOI] [PubMed] [Google Scholar]
- 68. Ascherio A, Hennekens C, Willett WC, Sacks F, Rosner B, Manson J, Witteman J, Stampfer MJ. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension 1996;27(5):1065–72. [DOI] [PubMed] [Google Scholar]
- 69. Ascherio A, Rimm EB, Giovannucci EL, Colditz GA, Rosner B, Willett WC, Sacks F, Stampfer MJ. A prospective study of nutritional factors and hypertension among US men. Circulation 1992;86(5):1475–84. [DOI] [PubMed] [Google Scholar]
- 70. Streppel MT, Arends LR, van't Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med 2005;165(2):150–6. [DOI] [PubMed] [Google Scholar]
- 71. Bai G, Zhang J, Zhao C, Wang Y, Qi Y, Zhang B. Adherence to a healthy lifestyle and a DASH-style diet and risk of hypertension in Chinese individuals. Hypertens Res 2017;40(2):196–202. [DOI] [PubMed] [Google Scholar]
- 72. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis 2014;24(12):1253–61. [DOI] [PubMed] [Google Scholar]
- 73. Anglemyer A, Horvath HT, Bero L. Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials. Cochrane Database Syst Rev 2014;4:MR000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000;342(25):1878–86. [DOI] [PubMed] [Google Scholar]
- 75. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000;342(25):1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vandenbroucke JP. The HRT controversy: observational studies and RCTs fall in line. Lancet 2009;373(9671):1233–5. [DOI] [PubMed] [Google Scholar]
- 77. Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med 1996;335(7):453–61. [DOI] [PubMed] [Google Scholar]
- 78. Writing Group for the Women's Health Initiative I. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288(3):321–33. [DOI] [PubMed] [Google Scholar]
- 79. Bhupathiraju SN, Stampfer MJ. Menopausal hormone therapy and cardiovascular disease: unraveling the role of age and time since menopause onset. Clin Chem 2017;64(5):861–2. [DOI] [PubMed] [Google Scholar]
- 80. Frieden TR. Evidence for health decision making—beyond randomized, controlled trials. N Engl J Med 2017;377(5):465–75. [DOI] [PubMed] [Google Scholar]
- 81. Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, et al. The Women's Health Initiative hormone therapy trials: update and overview of health outcomes during the intervention and post-stopping phases. JAMA 2013;310(13):1353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gallicchio L, Boyd K, Matanoski G, Tao XG, Chen L, Lam TK, Shiels M, Hammond E, Robinson KA, Caulfield LE, et al. Carotenoids and the risk of developing lung cancer: a systematic review. Am J Clin Nutr 2008;88(2):372–83. [DOI] [PubMed] [Google Scholar]
- 83. Li P, Zhang H, Chen J, Shi Y, Cai J, Yang J, Wu Y. Association between dietary antioxidant vitamins intake/blood level and risk of gastric cancer. Int J Cancer 2014;135(6):1444–53. [DOI] [PubMed] [Google Scholar]
- 84. Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, Hercberg S. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer 2010;127(1):172–84. [DOI] [PubMed] [Google Scholar]
- 85. Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 2004;364(9441):1219–28. [DOI] [PubMed] [Google Scholar]
- 86. Debreceni B, Debreceni L. The role of homocysteine-lowering B-vitamins in the primary prevention of cardiovascular disease. Cardiovasc Ther 2014;32(3):130–8. [DOI] [PubMed] [Google Scholar]
- 87. Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA 2006;296(22):2720–6. [DOI] [PubMed] [Google Scholar]
- 88. Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 2015;313(13):1325–35. [DOI] [PubMed] [Google Scholar]
- 89. Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative randomized controlled dietary modification trial. JAMA 2006;295(6):629–42. [DOI] [PubMed] [Google Scholar]
- 90. Multiple Risk Factor Intervention Trial Research Group. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial. JAMA 1982;248(12):1465–77. [PubMed] [Google Scholar]
- 91. Willett WC. The WHI joins MRFIT: a revealing look beneath the covers. Am J Clin Nutr 2010;91(4):829–30. [DOI] [PubMed] [Google Scholar]
- 92. Rose G, Hamilton PJ. A randomised controlled trial of the effect on middle-aged men of advice to stop smoking. J Epidemiol Community Health 1978;32(4):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. US Department of Health Education and Welfare. Smoking and health: report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington (DC): US Government Printing Office; 1964. (PHS publication 1103). [Google Scholar]
- 94. Glasser SP, Salas M, Delzell E. Importance and challenges of studying marketed drugs: what is a phase IV study? Common clinical research designs, registries, and self-reporting systems. J Clin Pharmacol 2007;47(9):1074–86. [DOI] [PubMed] [Google Scholar]
- 95. WHO. The importance of pharmacovigilance: safety monitoring of medicinal products. Geneva (Switzerland); WHO; 2002. [Google Scholar]
- 96. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet 2014;383(9933):1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr 2003;78(3):544S–51S. [DOI] [PubMed] [Google Scholar]
- 98. Hunter DJ, Spiegelman D, Adami H-O, Beeson L, van den Brandt PA, Folsom AR, Fraser GE, Goldbohm RA, Graham S, Howe GR, et al. Cohort studies of fat intake and the risk of breast cancer—a pooled analysis. N Engl J Med 1996;334(6):356–61. [DOI] [PubMed] [Google Scholar]
- 99. US Department of Agriculture and US Department of Health & Human Services. Scientific report of the 2015 Dietary Guidelines Advisory Committee: report to the Secretary of Health & Human Services and the Secretary of Agriculture. Washington (DC): US Government Printing Office; 2015. [Google Scholar]
- 100. Oldways. Oldways Common Ground Consensus Statement on Healthy Eating 2015 [Internet]. Available from: http://oldwayspt.org/common-ground-consensus.
- 101. Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354(15):1601–13. [DOI] [PubMed] [Google Scholar]
- 102. Coombes R. Trans fats: chasing a global ban. BMJ 2011;343:d5567. [DOI] [PubMed] [Google Scholar]
- 103. WHO Regional Office for Europe. Eliminating trans fats in Europe 2015 [Internet]. Available from: http://www.euro.who.int/en/countries/sweden/news/news/2015/09/eliminating-trans-fats-in-europe.
- 104. Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33(11):2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Centers for Disease Control and Prevention. Competitive foods and beverages in US schools: a state policy analysis. Atlanta (GA): US Department of Health and Human Services; 2012. [Google Scholar]
- 106. City of Boston. Mayor Menino issues order to end sugary drink sales on city property [Internet]. Available from: http://www.cityofboston.gov/news/default.aspx?id = 5051.
- 107. Colchero MA, Rivera-Dommarco J, Popkin BM, Ng SW. In Mexico, evidence of sustained consumer response two years after implementing a sugar-sweetened beverage tax. Health Aff (Millwood) 2017;36(3):564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. US Food and Drug Administration. Changes to the nutrition facts label. n.d. [updated 2018 March 5]. Available from: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm385663.htm.
- 109. Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, Willett WC. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med 2014;174(10):1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang DD, Li Y, Chiuve SE, Hu FB, Willett WC. Improvements in US diet helped reduce disease burden and lower premature deaths, 1999–2012; overall diet remains poor. Health Aff (Millwood) 2015;34(11):1916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Centers for Disease Control and Prevention. Diabetes Data and Statistics, US Diabetes Surveillance System: age-adjusted rate of newly diagnosed diabetes per 1,000, adults aged 18–79 years. Atlanta (GA): US Department of Health and Human Services; n.d. [updated 2017 Nov 14]. Available from: https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html. [Google Scholar]
- 112. Brandt EJ, Myerson R, Perraillon MC, Polonsky TS. Hospital admissions for myocardial infarction and stroke before and after the trans-fatty acid restrictions in New York. JAMA Cardiology 2017;2(6):627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang DD, Hu FB. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol 2018;6(5):416–26. [DOI] [PubMed] [Google Scholar]
- 114. Dalstra JAA, Kunst AE, Borrell C, Breeze E, Cambois E, Costa G, Geurts JJM, Lahelma E, Van Oyen H, Rasmussen NK, et al. Socioeconomic differences in the prevalence of common chronic diseases: an overview of eight European countries. Int J Epidemiol 2005;34(2):316–26. [DOI] [PubMed] [Google Scholar]
- 115. National Research Council (US) Panel on Race, Ethnicity, and Health in Later Life. Race/ethnicity, socioeconomic status, and health. In: Bulatao RA, Cohen B, editors. Critical perspectives on racial and ethnic differences in health in late life. Washington (DC): National Academies Press (US); 2004. [PubMed] [Google Scholar]
- 116. WHO. The global burden of disease: 2004 update. Geneva (Switzerland): WHO; 2008. [Google Scholar]
- 117. Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature 2014;515(7528):518–22. [DOI] [PubMed] [Google Scholar]