Abstract
There is clear evidence that proton-pump inhibitors (PPIs), H2-receptor antagonists (H2RAs), and metformin can reduce serum vitamin B-12 concentrations by inhibiting the absorption of the vitamin. However, it is unclear if the effects of these drugs on serum vitamin B-12 are associated with increased risk of biochemical or functional deficiency (as is indicated by elevated blood concentrations of homocysteine and methylmalonic acid) or clinical deficiency (including megaloblastic anemia and neurologic disorders such as peripheral neuropathy and cognitive dysfunction). This review provides an overview of vitamin B-12 absorption and biochemistry and the mechanisms by which PPIs, H2RAs, and metformin affect these functions. It also summarizes the literature relating the use of these drugs to the risk of vitamin B-12 deficiency. Also discussed is that strategies for assessing vitamin B-12 status and diagnosing vitamin B-12 deficiency have evolved in recent years beyond solely measuring serum total vitamin B-12. Multiple analyte testing, a strategy in which ≥2 of 4 biomarkers of vitamin B-12 status—serum total vitamin B-12, holotranscobalamin, homocysteine, and methylmalonic acid—are measured, increases sensitivity and specificity for diagnosing vitamin B-12 deficiency. It is concluded that randomized controlled trials are now needed that use the strategy of multiple analyte testing to determine if PPIs, H2RAs, and metformin do indeed increase the risk of vitamin B-12 deficiency. Until these studies are conducted, a reasonable recommendation for physicians and their patients who are taking these drugs is to monitor vitamin B-12 status and to provide vitamin B-12 supplements if altered blood biomarkers or clinical signs consistent with low or deficient vitamin B-12 status develop.
Keywords: vitamin B-12, cobalamin, proton-pump inhibitor, H2-receptor antagonist, metformin, holotranscobalamin, homocysteine, methylmalonic acid, multiple analyte testing
Introduction
There is general consensus that gastric acid–lowering drugs, including proton pump inhibitors (PPIs) and H2-receptor antagonists (H2RAs), and the antidiabetes drug metformin can reduce circulating vitamin B-12 concentrations with prolonged use. There is decidedly less consensus on the clinical significance of the reductions in serum vitamin B-12 induced by these drugs. The topic has been the subject of several current reviews (1, 2) including systematic reviews and meta-analyses (3–5), which concluded that these drugs increase the risk of vitamin B-12 deficiency. However, a 2017 expert review and best-practice statement produced by the American Gastrointestinal Association (6) concluded that “Long-term PPI users should not routinely raise their intake of… vitamin B-12… beyond the Recommended Dietary Allowance (RDA)” and that “Long-term PPI users should not routinely screen or monitor… vitamin B-12.” Moreover, a 2014 commentary concluded that “there is no evidence that pathological levels of the biochemical markers of B12 are more common in metformin-treated compared with non–metformin-treated patients, despite lowering B12 in serum or plasma” (7). A 2016 review (8) makes a similar conclusion: “There is almost a current consensus on metformin's potential to lower vitamin B-12 levels. Whether the medication can cause cellular vitamin B-12 deficiency remains controversial.”
The present review provides background and context for the current understanding of the relations of PPIs, H2RAs, and metformin with vitamin B-12 status, as well as practical considerations for clinical practice and future research directions. Much of the lack of consensus around the issue is related to unclear and evolving definitions of vitamin B-12 deficiency, including conflation of biochemical or functional deficiencies (as indicated by changes in blood biomarkers of vitamin B-12 status) with clinical deficiency (including megaloblastic anemia and neurological disorders such as peripheral neuropathy and cognitive dysfunction). Accordingly, this review is presented with a particular emphasis on current thought with regard to the assessment of vitamin B-12 status and the diagnosis of deficiency, which have moved in recent years from sole measurement of serum total vitamin B-12 concentration to the measurement of multiple biomarkers of vitamin B-12 status, also known as “multiple analyte testing.”
Current Status of Knowledge
Basics of vitamin B-12 absorption and metabolism
In-depth reviews of all facets of vitamin B-12 nutrition and metabolic functions are readily available (9, 10). In the context of the present review, important aspects of vitamin B-12 function are related to the complex physiology of its absorption in the digestive tract, and its role as a cofactor in 2 biochemical reactions. Relevant details are summarized here.
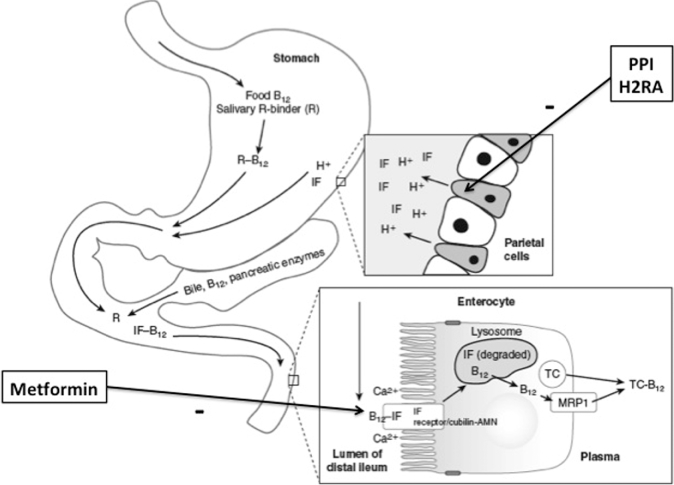
Other than fortified foods (e.g., breakfast cereals) and supplements, foods of animal origin (e.g., liver, beef, chicken, fish, dairy) are the primary sources of dietary vitamin B-12. (Fruit and vegetables do not contain vitamin B-12 unless contaminated with vitamin B-12–producing bacteria.) The digestion of vitamin B-12–containing foods and the ultimate absorption of vitamin B-12 in the small intestine is a uniquely complex process (Figure 1) (11). Food-bound (i.e., protein-bound) vitamin B-12 enters the stomach, along with salivary R binder (a member of a group of vitamin B-12–binding proteins called “haptocorrins” that have the same primary structures but differ with respect to glycosylation and function), where it encounters stomach acid and the proteolytic enzyme pepsin. Stomach acid is produced by the gastric parietal cells, and pepsin is the product of the acid hydrolysis of pepsinogen, which is produced by the gastric chief cells. The acid and pepsin cause the release of vitamin B-12 from food protein, which frees the vitamin B-12 to bind to salivary R binder, which preferentially binds vitamin B-12 in the acid milieu of the stomach. A second vitamin B-12–binding protein, intrinsic factor (IF), is produced by the gastric parietal cells but has low affinity for vitamin B-12 at an acidic pH. The R binder–B12 complex and unbound IF then enter the small intestine where the R binder is hydrolyzed by pancreatic enzymes and vitamin B-12 is released. The higher pH of the small intestine compared with the stomach (∼5–6 compared with ∼1–2, respectively) promotes the binding of vitamin B-12 to IF. The IF–vitamin B-12 complex then travels to the ileum where it binds with the IF–vitamin B-12 receptor complex consisting of 2 proteins, cubilin and amnionless. The binding of IF–vitamin B-12 to the cubilin-amnionless complex is calcium-dependent, possibly due to increased affinity of the receptor for IF–vitamin B-12 induced by calcium. The IF–vitamin B-12 is then internalized into the ileal cell by receptor-mediated endocytosis, the IF is degraded within lysosomes, and free vitamin B-12 is released into the cytoplasm. The vitamin B-12 is then exported into the portal circulation where it is bound to the transport protein, transcobalamin, which delivers the newly absorbed vitamin B-12 to all tissues of the body where it is taken up by receptor-mediated endocytosis. In the blood, a second transport protein, a member of the previously mentioned family of haptocorrins, transports vitamin B-12 solely to the liver.
FIGURE 1.
Vitamin B-12 digestion and absorption: effects of PPIs, H2RAs, and metformin. PPIs and H2RAs inhibit the production of stomach acid by the gastric parietal cells, which is required for the conversion of pepsinogen to pepsin. PPIs work by blocking gastric H+K+-ATPase, which is responsible for pumping H+ ions from within gastric parietal cells into the gastric lumen, where they react with Cl− ions to form hydrochloric acid. H2RAs work by inhibiting the interaction of histamine with the parietal cell histamine H2 receptor. This blocks a cAMP-dependent pathway that promotes H+K+-ATPase function, thus reducing gastric acid production. A lack of gastric acid and pepsin decreases the release of vitamin B-12 from proteins in food and thus reduces its availability for absorption in the ileum. Metformin likely affects vitamin B-12 absorption by interfering with Ca2+, which is required for the IF-mediated absorption of vitamin B-12 via the cubulin-amnionless IF receptor in the ileum. AMN, amnionless; B12, vitamin B-12; H2RA, H2-receptor antagonist; IF, intrinsic factor; MRP1, multidrug resistance protein 1; PPI, proton pump inhibitor; R, salivary R binder or haptocorrin; R-B12, holohaptocorrin; TC, transcobalamin; TC-B12, holotranscobalamin. Adapted from reference 11 with permission.
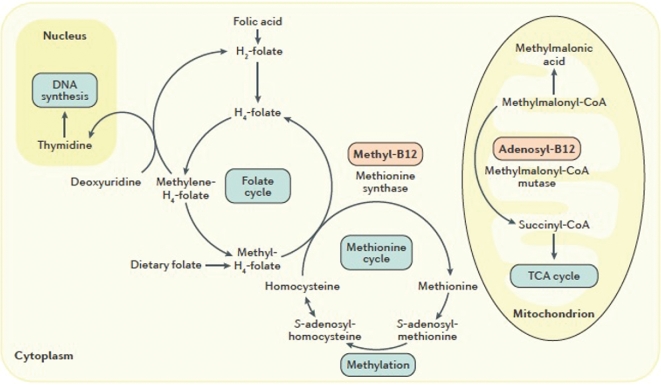
Within cells, vitamin B-12 participates in 2 reactions (Figure 2) (9). In the form of methylcobalamin, it assists in the transfer of methyl groups from the folate derivative 5-methyltetrahydrofolate to the sulfur amino acid homocysteine to produce methionine in a cytosolic reaction catalyzed by the enzyme methionine synthase (N5-methyltetrahydrofolate-homocysteine methyltransferase; EC 2.1.1.13). When vitamin B-12 status is suboptimal or deficient, this reaction is inhibited, and homocysteine accumulates. In the form of 5΄-deoxyadenosylcobalamin, it serves as a cofactor for the enzyme methylmalonyl CoA mutase (EC 5.4.99.2), which catalyzes the mitochondrial conversion of methylmalonyl CoA to succinyl CoA as an intermediate step in the oxidation of odd-chain FAs and the catabolism of ketogenic amino acids. Low or deficient vitamin B-12 status impairs this reaction, causing methylmalonyl CoA to accumulate and be converted via a side-reaction pathway to form methylmalonic acid.
FIGURE 2.
Vitamin B-12 cofactor functions. Vitamin B-12 serves as an essential cofactor for 2 biochemical reactions. In the form of methyl-B12, it assists in folate-dependent conversion of homocysteine to methionine catalyzed by methionine synthase. In the form of adenosyl-B12, it assists in the conversion of methylmalonyl-CoA to succinyl-CoA as an intermediate step in odd-chain FA oxidation and ketogenic amino acid catabolism. When vitamin B-12 is deficient, homocysteine and methylmalonic acid (a side reaction product of methylmalonyl-CoA metabolism) accumulate in the cell and are exported into the blood. Thus, elevations in homocysteine and methylmalonic acid in plasma or serum are functional indicators of vitamin B-12 deficiency. adenosyl-B12, 5΄-deoxyadenosylcobalamin; methyl-B12, methylcobalamin; TCA cycle, tricarboxylic acid cycle. Reproduced from reference 9 with permission.
Assessing vitamin B-12 status and clinical manifestations of deficiency
The concentration of serum total vitamin B-12 is the most commonly used indicator of vitamin B-12 status. The measurement of serum vitamin B-12 actually reflects the total amount of vitamin B-12 bound to 2 distinct transport proteins: transcobalamin and haptocorrin. (Serum transcobalamin was formerly known as “transcobalamin II,” and serum haptocorrin was formerly known as “transcobalamin I and III.”) When vitamin B-12 is bound to these proteins, they are termed “holotranscobalamin” (holoTC) and “holohaptocorrin” (holoHC). Although holoTC delivers vitamin B-12 to all the tissues of the body, holoHC delivers vitamin B-12 only to the liver. In addition, holoTC has a significantly shorter half-life in circulation (<2 h) than holoHC (∼10 d) (10). Thus, a large majority of serum total vitamin B-12 (∼70–80%) is holoHC, whereas the remainder (∼20–30%) is holoTC (10). Because holoTC reflects the portion of circulating vitamin B-12 that is delivered to all tissues, it has been considered as a potentially more sensitive and specific indicator of vitamin B-12 status than serum total vitamin B-12 (12, 13). Although some studies suggest that holoTC is a better biomarker than total vitamin B-12, current thinking is that both total vitamin B-12 and holoTC provide, to some extent, independent information with regard to status (13, 14). In addition to total vitamin B-12 and holoTC, biochemical or functional biomarkers of vitamin B-12 status are plasma total homocysteine and serum methylmalonic acid, both of which become elevated in vitamin B-12 deficiency. Clinical manifestations of deficiency include classical megaloblastic anemia, affecting both the synthesis and form of red and white blood cells, and neurologic disease that can manifest in a variety of ways, including neurodegeneration of the spinal cord (subacute combined degeneration), peripheral neuropathy, and cognitive deficits that may progress or contribute to dementia (9–11).
To summarize, there are 4 blood biomarkers—total vitamin B-12, holoTC, homocysteine, and methylmalonic acid—and several hematologic and neurological outcomes that are indicators of vitamin B-12 status. The difficulty, however, is that none of these indicators alone has ideal sensitivity or specificity for vitamin B-12 deficiency (9, 10). Typical reference ranges and cutoff values for the blood biomarkers are provided in Table 1. However, it must be noted that there is not complete unanimity on these definitions. Moreover, low and high blood concentrations of total vitamin B-12 and holoTC are reasonable indicators of insufficiency and adequacy, but each has a middle concentration range where diagnosis is indeterminate (i.e., ranges in which some individuals are deficient and others are not). Elevated concentrations of homocysteine and methylmalonic acid do indeed occur in vitamin B-12 deficiency, but both homocysteine and methylmalonic acid become elevated as a result of renal insufficiency, whereas homocysteine becomes elevated due to several other conditions, such as folate and vitamin B-6 deficiencies, hypothyroidism, genetic disorders, and by the use of pharmaceuticals that affect homocysteine and one-carbon metabolism (15). Megaloblastic anemia is caused by both vitamin B-12 and folate deficiencies and, along with subacute combined degeneration of the spinal cord, is typically a manifestation of severe deficiency caused by the autoimmune disorder pernicious anemia, in which absorption of vitamin B-12 is abrogated by autoantibodies against IF or the parietal cells that produce IF. Peripheral neuropathies and cognitive deficits may be due to a variety of other conditions, such as diabetes and Alzheimer disease. Concomitant low vitamin B-12 status in these latter conditions may contribute to neurological progression but is likely not the primary cause (16, 17). Thus, diagnosing vitamin B-12 deficiency is not simple.
TABLE 1.
Typical reference ranges and cutoff values for blood biomarkers of vitamin B-12 status in adults1
| Blood biomarker | Typical reference ranges | Typical cutoff values |
|---|---|---|
| Serum total vitamin B-12 | 148–664 pmol/L (200–900 pg/mL) | Deficiency: <148 pmol/L (<200 pg/mL) |
| Insufficiency: 148–221 pmol/L (200–300 pg/mL) | ||
| Serum holoTC | 40–150 pmol/L | Deficiency: <35–40 pmol/L |
| Serum MMA | 50–370 nmol/L | Elevated: >370 nmol/L |
| Plasma total Hcy | 4–10 µmol/L | Elevated: >12–15 µmol/L |
| cB12 | Not available | Adequacy: –0.5 to 1.5 |
| Low: –1.5 to –0.5 | ||
| Possible deficiency: –2.5 to –1.5 | ||
| Probable deficiency: <–2.5 | ||
| Elevated: >1.5 |
cB12, combined vitamin B-12; Hcy, homocysteine; holoTC, holotranscobalamin; MMA, methylmalonic acid.
One strategy that is receiving increasing acceptance is multiple analyte testing. The concept is that the measurement of ≥2 of the 4 blood biomarkers of vitamin B-12 status will provide improved diagnostic sensitivity and specificity over any given biomarker alone. Some strategies provide a sequential, algorithmic approach (14). For example, if an initial measurement of total vitamin B-12 is found to be in the low, but indeterminate, range (e.g., between 148 and 221 pmol/L), then a subsequent measurement of methylmalonic acid is triggered, which, if elevated (e.g., >350 nmol/L), is indicative of vitamin B-12 deficiency. Other approaches include simultaneous measurement of ≥2 biomarkers (e.g., total vitamin B-12 and holoTC, holoTC and methylmalonic acid, etc.) (13). Most recently, a formula has been developed that utilizes all 4 biomarkers simultaneously to produce a value called “combined vitamin B-12” (cB12) (18). Cutoff values of cB12 that correspond to different categories of vitamin B-12 status have been established (19) and are summarized in Table 1. Initial assessments of the utility of cB12 in determining vitamin B-12 status have indicated superior diagnostic capability to any of the 4 biomarkers alone (19–21). However, the measurement of all 4 biomarkers is expensive, and thus the practicality of cB12 in the clinical setting may be limited.
Mechanisms by which PPIs, H2RAs, and metformin affect serum total vitamin B-12 concentrations
PPIs and H2RAs are 2 classes of drugs that are used to treat conditions associated with excess gastric acid production, including dyspepsia, peptic ulcer disease, gastroesophageal reflux disease, Barrett esophagus, and Zollinger-Ellison syndrome. PPIs work by blocking gastric H+K+-ATPase (EC 3.6.3.10), which is responsible for pumping H+ ions from within gastric parietal cells into the gastric lumen, where they react with Cl− ions to form hydrochloric acid (22) (Figure 1). Common examples of PPI drugs include esomeprazole, lansoprazole, and omeprazole. H2RAs work by inhibiting the interaction of histamine with the parietal cell histamine H2 receptor (22) (Figure 1). This blocks a cAMP-dependent pathway that promotes H+K+-ATPase function, thus reducing gastric acid production. Common examples of H2RAs include ranitidine, famotidine, cimetidine, and nizatidine.
The influence of these drugs on vitamin B-12 status is related to the role that gastric acid plays in the digestion of vitamin B-12–containing foods. Gastric acid promotes the conversion of pepsinogen to pepsin, which, as described above, releases vitamin B-12 from food proteins. A lack of gastric acid, due to PPI or H2RA use (or pathophysiologic conditions that affect gastric acid production, such as atrophic gastritis), will reduce the digestive capacity to release vitamin B-12 from foods, and thus reduce the amount of vitamin B-12 that is absorbed in the body. Inhibited vitamin B-12 absorption has been directly shown in empirical studies. Marcuard et al. (23) found that gastric acid output and protein-bound vitamin B-12 absorption were significantly decreased in men (aged 22–50 y, n = 10) after 2 wk of treatment with omeprazole. Serum total vitamin B-12 concentrations were not directly affected, but this was attributed to the short duration of the protocol (2 wk). These findings are consistent with those of Schenk et al. (24), who showed in adults (aged 22–52 y, 5 men and 3 women) that after 9 d of treatment with omeprazole, the absorption of protein-bound vitamin B-12 was significantly decreased, whereas the absorption of unbound vitamin B-12 was not inhibited.
Metformin is used to treat type 2 diabetes, prediabetes, and polycystic ovary syndrome. It works by essentially poisoning mitochondria, which reduces ATP production and increases AMP concentrations (25). The increase in mitochondrial AMP affects signaling pathways within cells that lead to improved insulin sensitivity, promotion of glycolytic pathways, and inhibition of gluconeogenic pathways. The first report of a possible effect of metformin on vitamin B-12 status was by Berchtold et al. (26) in 1969. This was followed shortly by a study by Tomkin et al. (27), who found that 21 (30%) of 71 diabetes patients receiving long-term metformin treatment (mean dose = 1.97 g; mean duration = 4.6 y) exhibited malabsorption of radiolabeled vitamin B-12. In 7 patients who were taken off metformin, vitamin B-12 absorption normalized in 6 patients within 5–28 d. The effect of metformin on vitamin B-12 absorption is thought to be via inhibition of the Ca2+-influenced binding of IF–vitamin B-12 to the cubilin-amnionless receptor complex (Figure 1). Support for this hypothesis comes from Bauman et al. (28), who investigated the effects of metformin and calcium supplements on vitamin B-12 status in individuals with type 2 diabetes (aged 30–60 y). Fourteen patients who were being treated with sulfonylurea were switched to increasing amounts of metformin (850 mg/d for 2 wk, 850 mg 2 times/d for 2 wk, and then 850 mg 3 times/d, if tolerated), whereas 7 subjects were maintained on sulfonylurea and served as nonmetformin controls. After 3 mo, the patients receiving metformin exhibited significant decreases in serum total vitamin B-12 and holoTC, whereas no change was observed in the controls. At 3 mo, the metformin patients were provided with calcium carbonate supplements (1.2 g/d for 1 mo). A significant increase in holoTC, but not total vitamin B-12, was observed after 1 mo of the supplements. Although the sample size of this trial was limited and did not include blinded placebo controls, the findings are consistent with the hypothesis that metformin affects calcium availability for IF–vitamin B-12 receptor-mediated absorption. Random-ized controlled trials of the effects of metformin with and without calcium supplements on vitamin B-12 status are needed to confirm these results.
In summary, there is mechanistic plausibility and empirical evidence that PPIs, H2RAs, and metformin interfere with vitamin B-12 absorption and bioavailability and thus affect circulating vitamin B-12 concentrations. But does this represent an increased risk of biochemical or clinical vitamin B-12 deficiency? With respect to the influence of PPIs, H2RAs, and metformin on vitamin B-12 status, much of the literature has focused on serum total vitamin B-12 concentration. With the exception of metformin's effect on homocysteine, other blood biomarkers of vitamin B-12 status, especially vitamin B-12–related hematologic and neurological outcomes, have rarely been investigated.
Interrelations between PPI and H2RA and indicators of vitamin B-12 status
A recent systematic review and meta-analysis by Jung et al. (3) evaluated the association between long-term (≥10 mo) use of acid-lowering agents (PPIs and H2RAs; exact dosages not specified) and the development of vitamin B-12 deficiency. Four case-control studies (constituting >4200 cases of vitamin B-12 deficiency and >19,000 controls) and 1 observational study were identified that met the inclusion criteria for the analysis. Vitamin B-12 deficiency was defined differently among the studies. One study used medical records to identify cases of pernicious anemia and other vitamin B-12–related anemias, low serum total vitamin B-12, or prescriptions for vitamin B-12 intramuscular injections (29); 2 studies solely depended on medical record evidence of prescriptions for vitamin B-12 intramuscular injections (30, 31); 1 study used serum total vitamin B-12 (<150 pmol/L) as the criterion (32); and only 1 study used a multiple analyte strategy consisting of serum total vitamin B-12 < 96 pmol/L or serum total vitamin B-12 between 96 and 221 pmol/L and elevated methylmalonic acid and elevated homocysteine as the criteria for vitamin B-12 deficiency (33). Four of the 5 studies found a significantly elevated risk of vitamin B-12 deficiency associated with acid-lowering agents, and the overall HR (95% CI) for the studies combined was 1.83 (1.36, 2.46). The 1 study that found no association between vitamin B-12 deficiency and acid-lowering agents used the criterion of serum total vitamin B-12 <150 pmol/L to categorize the cases (32). Compared with the criteria used in the other 4 studies, vitamin B-12 <150 pmol/L may be too liberal of a definition, leading to individuals with adequate vitamin B-12 status misidentified as deficient. Such misclassification may have confounded the ability to detect associations between acid-lowering agents and risk of vitamin B-12 deficiency in that study. Importantly, 2 of the studies included in the Jung meta-analysis evaluated the length of time on acid-lowering agents as a factor; 1 study found that there was no association between short-term (<12 mo) use of acid-lowering agents and vitamin B-12 deficiency (33), and the other found that the association was weaker for shorter durations (<2 y) compared with longer durations (≥2 y) of drug therapy (29).
These findings are consistent with the hypothesis that PPIs and H2RAs increase the risk of vitamin B-12 deficiency with long-term use, but the results are not definitive. The diagnostic criteria for vitamin B-12 deficiency are varied among the studies and not particularly rigorous. None of the studies assessed changes in blood biomarkers of vitamin B-12 status or onset of clinical indications of deficiency, and none of the studies was a randomized controlled trial. An additional case-control study, not included in the meta-analysis, compared serum total vitamin B-12, homocysteine, and mean corpuscular volumes between elderly (>65 y), long-term PPI users (>3 y), and cohabitating partners who were nonusers (34). No differences in total vitamin B-12, homocysteine, or mean corpuscular volume were observed between the groups; and the authors concluded that screening vitamin B-12 status in long-term PPI users is not recommended.
Interrelations between metformin and indicators of vitamin B-12 status
In contrast to PPIs and H2RAs, more studies have addressed the influence of metformin on vitamin B-12 status. In a systematic review, Liu et al. (4) identified 6 randomized controlled trials comparing metformin with placebo or with the antidiabetic drug rosiglitazone in patients with type 2 diabetes or polycystic ovary syndrome. The interventions lasted from 6 to 208 wk. The mean reduction (95% CI) in serum total vitamin B-12 for all 6 trials was –54 pmol/L (–81, –26 pmol/L). In a subgroup analysis, 4 of the 6 studies included doses of metformin <2000 mg/d (35–38), whereas the other 2 studies included doses ≥2000 mg/d (37, 39); the mean decrease in total vitamin B-12 was ∼2-fold greater in the high-dose studies compared with the low-dose studies. Thus, there appears to be a clear effect of metformin on serum total vitamin B-12 that is dose-dependent.
A sizable number of studies have assessed the association between metformin use and plasma total homocysteine concentrations. A recent systematic review and meta-analysis by Zhang et al. (5) identified 13 randomized controlled trials constituting >1300 participants ranging in age from 25 to 61 y with durations of follow-up ranging from 6 to 224 wk. Overall, a neutral effect of metformin (doses ranging from 1000 to 2550 mg/d) on the mean change in plasma homocysteine was calculated, with a mean difference (95% CI) between the groups of 0.40 µmol/L (−0.07, 0.87 µmol/L). However, subgroup analysis found that those who were treated with metformin without folic acid or B-vitamin supplements exhibited a significantly greater increase in plasma homocysteine than the nonmetformin controls (mean difference between the groups: 2.20 µmol/L; 95% CI: 1.37, 2.67 µmol/L). In contrast, metformin users who also took folic acid or B vitamins exhibited a greater decrease in plasma homocysteine than did controls (mean difference between the groups: −0.74 µmol/L; 95% CI: −1.19, −0.30 µmol/L). These findings suggest that B-vitamin supplements, including folic acid and vitamin B-12, are warranted to mitigate metformin-induced increases in plasma homocysteine concentrations. However, these findings do not prove that elevated homocysteine caused by metformin is due to the effects of the drug on vitamin B-12 absorption and status. Metformin may have unknown direct effects on homocysteine metabolism independent of vitamin B-12 but which nonetheless can be mitigated by folic acid and vitamin B-12 supplements.
A few studies have looked at the effect of metformin on multiple vitamin B-12 biomarkers. Leung et al. (40) assessed the effects of 3 mo of metformin therapy (dose not specified) in elderly patients with type 2 diabetes (aged 67–91 y, n = 10). Compared with controls not receiving the drug (n = 10), metformin caused a reduction in serum total vitamin B-12 and the fraction of total vitamin B-12 bound to haptocorrin (holoHC). No effect was observed on holoTC and methylmalonic acid concentrations. Homocysteine was not measured. In women with polycystic ovary syndrome, Greibe et al. (41) compared the effects of metformin therapy (1.5–2.5 g/d; n = 29) with placebo (n = 23) over a 6-mo trial. Similar to the study by Leung et al. (40), metformin was associated with decreases in total vitamin B-12 and holoHC but showed no effect on holoTC. Moreover, the metformin group exhibited a reduction in methylmalonic acid. Obeid et al. (42) compared vitamin B-12 biomarkers in 49 patients with type 2 diabetes receiving metformin (dose and duration of treatment not specified) compared with 43 nonmetformin controls. In this study, total vitamin B-12, holoTC, and methylmalonic acid were lower in the metformin group than in controls, whereas no difference in homocysteine was detected. Moreover, no difference in RBC vitamin B-12 was found between the groups, suggesting that intracellular concentrations of vitamin B-12 are not affected by metformin, at least in this 1 cellular compartment. Considered together, these 3 studies suggest that the effect of metformin on circulating vitamin B-12 concentrations is not sufficient to have a significant effect on functional vitamin B-12 status, as indicated by methylmalonic acid.
One last study to mention is what appears to be the only animal study in which the effect of metformin on vitamin B-12 was investigated. Greibe et al. (43) treated rats with daily subcutaneous injections of metformin (250 mg ⋅ kg−1 ⋅ d−1) or saline for 3 wk and measured changes in plasma vitamin B-12 concentrations. They also compared liver and kidney vitamin B-12 concentrations between the groups at the end of the study, as well as vitamin B-12 absorption by using radiolabeled vitamin B-12. A significant decrease in plasma vitamin B-12 concentrations was observed in the metformin group but not in the saline group. Kidney vitamin B-12 concentration was significantly lower in the metformin group, but the liver concentration was higher. Moreover, there was no effect of metformin on vitamin B-12 absorption. Although rats have some physiologic differences from humans in the absorption, transport, and metabolism of vitamin B-12, these findings raise the alternative hypothesis that the effect of metformin on circulating vitamin B-12 concentrations is not due to the inhibition of absorption but rather due to the redistribution of vitamin B-12 among tissue compartments. This hypothesis remains to be confirmed in other animal models, and remains to be tested in human studies.
In conclusion, there is clear evidence that PPIs, H2RAs, and metformin cause reduced circulating concentrations of vitamin B-12, and that this is likely due to malabsorption of the vitamin related to inhibited stomach acid production and inhibited ileal uptake. However, there is a lack of clear evidence that reduced serum vitamin B-12 induced by these drugs leads to biochemical or functional vitamin B-12 deficiency, as indicated by circulating methylmalonic acid and homocysteine concentrations, or to the hematologic and neurological manifestations of clinical deficiency. These findings justify the recent conclusions by the American Gastrointestinal Association (6) and others (7, 8) (quoted in the Introduction) that in patients taking acid blockers or metformin, routine screening or monitoring of vitamin B-12 status and taking of vitamin B-12 supplements above the RDA are not warranted. However, an important caveat to this conclusion is that this may apply generally to the populations of patients taking acid blockers or metformin, but that specific subgroups or individuals may nonetheless be at risk.
For example, an important consideration is the baseline status of vitamin B-12 in individuals who are prescribed these drugs. Their effects on vitamin B-12 absorption may not be sufficient to drive status to a functionally or clinically deficient state in those who have adequate status to begin with. However, individuals who have low or low-normal vitamin B-12 status may be pushed into a functional or clinical deficiency state after prolonged use. This suggests that prudent clinical practice would be to assess vitamin B-12 status when starting PPIs, H2RAs, or metformin prescriptions and monitoring it on a regular basis (e.g., every few months). Moreover, assessment and monitoring should not be limited to serum total vitamin B-12 but should use a strategy of multiple analyte testing to have greater sensitivity and specificity for detecting deficiency. Practically, this would most likely include measurements of both total vitamin B-12 and methylmalonic acid. HoloTC is not widely available in clinical laboratories and homocysteine is not as specific for vitamin B-12 status as methylmalonic acid. Of course, clinical signs of anemia and neurologic or cognitive deficits would warrant immediate assessment of vitamin B-12 status.
Alternatively, concomitant prophylactic prescription of vitamin B-12 when starting PPIs, H2RAs, or metformin regimens can be considered. Vitamin B-12 supplements are safe, inexpensive, and are not contraindicated when taking these drugs. Oral supplements should be unaffected by PPIs and H2RAs, because supplemental vitamin B-12 is not protein-bound and therefore stomach acid is not required for absorption. Although metformin may affect the absorption of oral supplements, the inhibition is unlikely to result in complete abrogation, and thus supplements are likely to be effective.
In closing, there is a need for more definitive research. With the evolving concept of multiple analyte testing (13, 14, 18–21) randomized placebo-controlled trials are warranted in which cB12—the indicator of vitamin B-12 status that combines all 4 vitamin B-12 biomarkers—is used to determine the influence of PPIs, H2RAs, and metformin on vitamin B-12 status. Extending these trials to clinical endpoints such as anemia, neuropathy, or cognitive change would provide more definitive information, but such studies would be considered unethical.
Acknowledgments
The sole author is responsible for all aspects of the manuscript.
Notes
Published in a supplement to Advances in Nutrition. Presented at the symposium, “Micronutrient Status: Modifying Factors--Drugs, Chronic Disease, Surgery,” held at Columbia University, Institute of Human Nutrition, New York, New York, 17 June 2017. The conference was organized by Columbia University's Institute of Human Nutrition (its contents are solely the responsibility of the authors and do not necessarily represent the official views of Columbia University), and with the aid of an unrestricted grant from Pharmavite, LLC. The Supplement Coordinator for this supplement was Densie Webb. Supplement Coordinator disclosure: Densie Webb was compensated for overseeing the development and publication of the supplement. Airfare and hotel to attend the conference in New York were covered, in addition to payment for work completed. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the author(s) and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Supported by an unrestricted educational grant from PharmaVite LLC.
Author disclosures: JWM, no conflicts of interest.
Abbreviations used:
- cB12
combined vitamin B-12
- holoHC
holohaptocorrin
- holoTC
holotranscobalamin
- H2RA
H2-receptor antagonist
- IF
intrinsic factor
- PPI
proton pump inhibitor
References
- 1. Linder L, Tamboue C, Clements JN. Drug-induced vitamin B12 deficiency: a focus on proton pump inhibitors and histamine-2 antagonists. J Pharm Pract 2017;30(6):639–42. [DOI] [PubMed] [Google Scholar]
- 2. Wilhelm SM, Kale-Pradhan PB. Effects of proton pump inhibitors on vitamin B12. Maturitas 2014;79(1):1–2. [DOI] [PubMed] [Google Scholar]
- 3. Jung SB, Nagaraja V, Kapur A, Eslick GD. Association between vitamin B12 deficiency and long-term use of acid-lowering agents: a systematic review and meta-analysis. Intern Med J 2015;45(4):409–16. [DOI] [PubMed] [Google Scholar]
- 4. Liu Q, Li S, Quan H, Li J. Vitamin B12 status in metformin treated patients: systematic review. PLoS One 2014;9(6):e100379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Q, Li S, Li L, Li Q, Ren K, Sun X, Li J. Metformin treatment and homocysteine: a systematic review and meta-analysis of randomized controlled trials. Nutrients 2016;8(12):798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology 2017;152(4):706–15. [DOI] [PubMed] [Google Scholar]
- 7. Obeid R. Metformin causing vitamin B12 deficiency: a guilty verdict without sufficient evidence. Diabetes Care 2014;37(2):e22–3. [DOI] [PubMed] [Google Scholar]
- 8. Ahmed MA. Metformin and vitamin B12 deficiency: where do we stand? J Pharm Pharm Sci 2016;19(3):382–98. [DOI] [PubMed] [Google Scholar]
- 9. Green R, Allen LH, Bjorke-Monsen AL, Brito A, Gueant JL, Miller JW, Molloy AM, Nexo E, Stabler S, Toh BH, et al. Vitamin B12 deficiency. Nat Rev Dis Primers 2017;3:17040. [DOI] [PubMed] [Google Scholar]
- 10. Green R, Miller JW. Vitamin B12. In: Zempleni J, Suttie J, Gregory J, Stover P, editors. Handbook of vitamins. 5th ed., Boca Raton (FL): CRC Press; 2014. p. 447–89. [Google Scholar]
- 11. Caudill MA, Miller JW, Gregory JF, Shane B. Folate, choline, vitamin B12 and vitamin B6. In: Stipanuk MH, Caudill MA, editors. Biochemical, physiological and molecular aspects of human nutrition. 3rd ed.Maryland Heights (MO): Elsevier; 2013. p. 565–609. [Google Scholar]
- 12. Nexo E, Hoffmann-Lucke E. Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility. Am J Clin Nutr 2011;94(Suppl):359S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller JW, Garrod MG, Rockwood AL, Kushnir MM, Allen LH, Haan MN, Green R. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin Chem 2006;52(2):278–85. [DOI] [PubMed] [Google Scholar]
- 14. Hannibal L, Lysne V, Bjorke-Monsen AL, Behringer S, Grunert SC, Spiekerkoetter U, Jacobsen DW, Blom HJ. Biomarkers and algorithms for the diagnosis of vitamin B12 deficiency. Front Mol Biosci 2016;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Refsum H, Ueland PM, Nygard O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med 1998;49:31–62. [DOI] [PubMed] [Google Scholar]
- 16. Zdilla MJ. Metformin with either histamine H2-receptor antagonists or proton pump inhibitors: a polypharmacy recipe for neuropathy via vitamin B12 depletion. Clin Diabetes 2015;33(2):90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, Smith AD. Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci USA 2013;110(23):9523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fedosov SN. Metabolic signs of vitamin B(12) deficiency in humans: computational model and its implications for diagnostics. Metabolism 2010;59(8):1124–38. [DOI] [PubMed] [Google Scholar]
- 19. Fedosov SN, Brito A, Miller JW, Green R, Allen LH. Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin Chem Lab Med 2015;53(8):1215–25. [DOI] [PubMed] [Google Scholar]
- 20. Fedosov SN. Biochemical markers of vitamin B12 deficiency combined in one diagnostic parameter: the age-dependence and association with cognitive function and blood hemoglobin. Clin Chim Acta 2013;422:47–53. [DOI] [PubMed] [Google Scholar]
- 21. Brito A, Verdugo R, Hertrampf E, Miller JW, Green R, Fedosov SN, Shahab-Ferdows S, Sanchez H, Albala C, Castillo JL, et al. Vitamin B-12 treatment of asymptomatic, deficient, elderly Chileans improves conductivity in myelinated peripheral nerves, but high serum folate impairs vitamin B-12 status response assessed by the combined indicator of vitamin B-12 status. Am J Clin Nutr 2016;103(1):250–7. [DOI] [PubMed] [Google Scholar]
- 22. Olbe L, Carlsson E, Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat Rev Drug Discov 2003;2(2):132–9. [DOI] [PubMed] [Google Scholar]
- 23. Marcuard SP, Albernaz L, Khazanie PG. Omeprazole therapy causes malabsorption of cyanocobalamin (vitamin B12). Ann Intern Med 1994;120(3):211–5. [DOI] [PubMed] [Google Scholar]
- 24. Schenk BE, Festen HP, Kuipers EJ, Klinkenberg-Knol EC, Meuwissen SG. Effect of short- and long-term treatment with omeprazole on the absorption and serum levels of cobalamin. Aliment Pharmacol Ther 1996;10(4):541–5. [DOI] [PubMed] [Google Scholar]
- 25. Pernicova I, Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 2014;10(3):143–56. [DOI] [PubMed] [Google Scholar]
- 26. Berchtold P, Bolli P, Arbenz U, Keiser G. Disturbance of intestinal absorption following metformin therapy observations on the mode of action of biguanides. Diabetologia 1969;5(6):405–12. [DOI] [PubMed] [Google Scholar]
- 27. Tomkin GH, Hadden DR, Weaver JA, Montgomery DA. Vitamin-B12 status of patients on long-term metformin therapy. Br Med J 1971;2(5763):685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 2000;23(9):1227–31. [DOI] [PubMed] [Google Scholar]
- 29. Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013;310(22):2435–42. [DOI] [PubMed] [Google Scholar]
- 30. Mitchell SL, Rockwood K. The association between antiulcer medication and initiation of cobalamin replacement in older persons. J Clin Epidemiol 2001;54(5):531–4. [DOI] [PubMed] [Google Scholar]
- 31. Force RW, Meeker AD, Cady PS, Culbertson VL, Force WS, Kelley CM. Ambulatory care increased vitamin B12 requirement associated with chronic acid suppression therapy. Ann Pharmacother 2003;37(4):490–3. [DOI] [PubMed] [Google Scholar]
- 32. Cotter PE, O'Keeffe ST. Use of proton pump inhibitors is not associated with vitamin B12 deficiency and in older hospital patients: a case control study. Eur Geriatr Med 2011;2:253–5. [Google Scholar]
- 33. Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol 2004;57(4):422–8. [DOI] [PubMed] [Google Scholar]
- 34. den Elzen WP, Groeneveld Y, de Ruijter W, Souverijn JH, le Cessie S, Assendelft WJ, Gussekloo J. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment Pharmacol Ther 2008;27(6):491–7. [DOI] [PubMed] [Google Scholar]
- 35. Wolever TMS, Assiff L, Basu T, Chiasson JL, Boctor M, Gerstein HC, Hunt JA, Josse RG, Lau D, Leiter LA, et al. Miglitol, an alpha-glucosidase inhibitor, prevents the metformin-induced fall in serum folate and vitamin B12 in subjects with type 2 diabetes. Nutr Res 2000;20:1447–56. [Google Scholar]
- 36. Kilicdag EB, Bagis T, Zeyneloglu HB, Tarim E, Aslan E, Haydardedeoglu B, Erkanli S. Homocysteine levels in women with polycystic ovary syndrome treated with metformin versus rosiglitazone: a randomized study. Hum Reprod 2005;20(4):894–9. [DOI] [PubMed] [Google Scholar]
- 37. Carlsen SM, Kjotrod S, Vanky E, Romundstad P. Homocysteine levels are unaffected by metformin treatment in both nonpregnant and pregnant women with polycystic ovary syndrome. Acta Obstet Gynecol Scand 2007;86(2):145–50. [DOI] [PubMed] [Google Scholar]
- 38. Sahin M, Tutuncu NB, Ertugrul D, Tanaci N, Guvener ND. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. J Diabetes Complications 2007;21(2):118–23. [DOI] [PubMed] [Google Scholar]
- 39. de Jager J, Kooy A, Lehert P, Wulffele MG, van der Kolk J, Bets D, Verburg J, Donker AJ, Stehouwer CD. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ 2010;340:c2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leung S, Mattman A, Snyder F, Kassam R, Meneilly G, Nexo E. Metformin induces reductions in plasma cobalamin and haptocorrin bound cobalamin levels in elderly diabetic patients. Clin Biochem 2010;43(9):759–60. [DOI] [PubMed] [Google Scholar]
- 41. Greibe E, Trolle B, Bor MV, Lauszus FF, Nexo E. Metformin lowers serum cobalamin without changing other markers of cobalamin status: a study on women with polycystic ovary syndrome. Nutrients 2013;5(7):2475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Obeid R, Jung J, Falk J, Herrmann W, Geisel J, Friesenhahn-Ochs B, Lammert F, Fassbender K, Kostopoulos P. Serum vitamin B12 not reflecting vitamin B12 status in patients with type 2 diabetes. Biochimie 2013;95(5):1056–61. [DOI] [PubMed] [Google Scholar]
- 43. Greibe E, Miller JW, Foutouhi SH, Green R, Nexo E. Metformin increases liver accumulation of vitamin B12—an experimental study in rats. Biochimie 2013;95(5):1062–5. [DOI] [PubMed] [Google Scholar]