Abstract
The ability of certain foods to impair or augment the absorption of various vitamins and minerals has been recognized for many years. However, the contribution of botanical dietary supplements (BDSs) to altered micronutrient disposition has received little attention. Almost half of the US population uses some type of dietary supplement on a regular basis, with vitamin and mineral supplements constituting the majority of these products. BDS usage has also risen considerably over the last 2 decades, and a number of clinically relevant herb-drug interactions have been identified during this time. BDSs are formulated as concentrated plant extracts containing a plethora of unique phytochemicals not commonly found in the normal diet. Many of these uncommon phytochemicals can modulate various xenobiotic enzymes and transporters present in both the intestine and liver. Therefore, it is likely that the mechanisms underlying many herb-drug interactions can also affect micronutrient absorption, distribution, metabolism, and excretion. To date, very few prospective studies have attempted to characterize the prevalence and clinical relevance of herb-micronutrient interactions. Current research indicates that certain BDSs can reduce iron, folate, and ascorbate absorption, and others contribute to heavy metal intoxication. Researchers in the field of nutrition may not appreciate many of the idiosyncrasies of BDSs regarding product quality and dosage form performance. Failure to account for these eccentricities can adversely affect the outcome and interpretation of any prospective herb-micronutrient interaction study. This review highlights several clinically relevant herb-micronutrient interactions and describes several common pitfalls that often beset clinical research with BDSs.
Keywords: botanical dietary supplements, herb-micronutrient interactions, phytochemicals, vitamins, trace metals
Introduction
Plants are nature's quintessential organic chemists, thus it is not surprising that certain plant-derived chemicals (phytochemicals), when ingested concomitantly with synthetic drug molecules, can interact through pharmacodynamic and/or pharmacokinetic mechanisms. Drug interactions involving phytochemicals present in various foods and botanical dietary supplements have received significant attention over the last three decades. During that period, a variety of molecular mechanisms have been identified for most food-drug and herb-drug interactions. For a review of these underlying mechanisms, as well as their clinical relevance, see Gurley et al. (1). Even though it is now evident that phytochemicals present in botanical dietary supplements (BDSs) can interact with prescription medications, their effect on micronutrient efficacy/toxicity is less clear. Concurrent ingestion of BDSs with vitamin and/or mineral preparations is quite common, and in fact many dietary supplement preparations are formulated with both botanical and nonbotanical ingredients. However, the degree to which botanicals can alter the disposition of vitamins and minerals remains ambiguous. Although significant in vitro/in vivo disconnects are frequently noted among herb-drug interaction studies (2), there are relatively few such comparisons for herbs and micronutrients. Moreover, although there is a substantial body of prospective clinical herb-drug interaction studies in the medical literature, complementary herb-micronutrient studies are limited. This disparity may stem from the fact that a clinically relevant herb-drug interaction may quickly result in significant morbidity, whereas a herb-micronutrient interaction may not reach clinical significance for several weeks, if at all. The purpose of this review is to examine the few prospective human trials that have assessed the effects of botanicals on micronutrient disposition and gauge their clinical relevance.
Literature searches were performed in April 2017 using the PubMed (2007–2017) and International Pharmaceutical Abstracts (1970–2017) databases. Concept groups for micronutrients (e.g., vitamins, minerals, trace metals), specific botanicals (e.g., ginseng, green tea, milk thistle), and pharmacodynamics or pharmacokinetics (e.g., absorption, distribution, metabolism, excretion) were combined in the search strategies. Two such searches were performed in PubMed—one limited to humans and English language, the other limited to clinical trials. No limits were applied to the International Pharmaceutical Abstracts search. A total of 678 results were retrieved and evaluated. Those most relevant to this review are discussed herein.
Popularity of Dietary Supplements and the Risk for Herb-Micronutrient Interactions
The popularity of dietary supplements in the United States has remained steady since 2000 (Table 1). According to the most recent NHANES data, 52% of US adults take dietary supplements (3). Of these supplement users, 36% take multivitamin and multimineral products and 18% take BDSs (3, 4). The majority of supplement users take only one supplement, but many take multiple supplements, with a small group of ∼10% taking >4 supplements concurrently (3). Considering that there are millions of Americans taking >1 supplement at one time, there are likely to be at least hundreds of thousands of Americans mixing botanical supplements with vitamin and mineral supplements. This could disproportionately impact vulnerable populations, such as the elderly (3, 5). Therefore, the area of herb-micronutrient interactions warrants further study owing to the potential to adversely affect a large portion of the public.
TABLE 1.
Chronology of dietary supplement usage in the United States1
| Study | Time period | US adults taking supplements, % |
|---|---|---|
| NHANES II | 1976–1980 | 34–37 |
| NHANES III | 1988–1994 | 41 |
| NHANES 1999–2000 | 1999–2000 | 52 |
| NHANES 2003–2006 | 2003–2006 | 54 |
| NHANES 2011–2012 | 2011–2012 | 52 |
Data from references 3 and 5.
Botanicals as Sources of Micronutrient Metals
Plants have long been recognized as sources of micronutrients (e.g., vitamins, minerals). BDSs, however, are distinct from most conventional fruits and vegetables. Their phytochemical constituents, especially plant secondary metabolites, are quite diverse and often unique. In addition, certain botanicals can take up metals from the soil and concentrate them within their roots and other plant parts. As a result, many BDSs have biorelevant amounts of nutritive minerals, like iron, copper, and zinc. However, plants grown in polluted soils may also give rise to BDSs contaminated with heavy metals (e.g., arsenic, cadmium, lead, mercury) (6, 7). Durum wheat (7) and St. John's wort (SJW) (8) are recognized examples of plants that bioaccumulate the heavy metals cadmium and lead when grown in contaminated soils. Thus, when processed into dosage forms (e.g., tablets, capsules, soft-gel capsules), some BDSs can serve as clandestine sources of minerals and heavy metals (9–13). In addition, the metal content can vary considerably among BDS brands of the same plant species, and even among lot numbers of the same product (9, 10).
Although many conventional BDSs in the United States contain safe and acceptable amounts of metals (9, 10), others appear to be problematic. Some Ayurvedic and traditional Chinese medicines marketed as BDSs can have undeclared toxic concentrations of lead, mercury, and arsenic (8, 14), whereas the heavy metal bioburden is of lesser consequence from other source countries. In the United States, the risk of heavy metal contamination in BDSs is minimized if manufacturers adhere to the current good manufacturing practices (cGMPs) outlined for the dietary supplement industry (15). These practices call for specific testing and allowable limits on heavy metal concentrations.
In a survey of adult BDS users in the United States, Buettner et al. (16) found that women using Ayurvedic or traditional Chinese medicine herbs, SJW, Ginkgo biloba, echinacea, ginseng (American or Asian), and “other herbs,” including kava, valerian, black cohosh, or nettle, had circulating blood lead concentrations >20% higher than those found in nonusers. They concluded that “among women, including women of reproductive age, specific herbal supplement use is a significant contributor to circulating lead.” These results, however, were taken from adults participating in the NHANES between 1999 and 2004, and may not reflect the current status of BDS use and blood lead concentrations. In 2007, the US Food and Drug Administration introduced its guidance for the dietary supplement industry, outlining the cGMPs, but full implementation was not realized until 2010. Today, BDS manufacturers should have methods in place for quantitating heavy metals in both raw materials and finished products.
Other metals commonly found in BDSs are the result of excipients, such as magnesium stearate, titanium dioxide, or calcium carbonate, that are incorporated into the formulation within allowable limits to facilitate the manufacturing process. In summary, BDSs can be unrecognized sources of metals, in addition to those obtained from the diet and conventional vitamin and mineral dietary supplements, and may contribute to morbidities related to excessive mineral intake.
Botanicals as Modulators of Micronutrient Absorption
Metals
In addition to being sources of micronutrient metals, BDSs can also modulate metal absorption from the gastrointestinal tract. These natural modulators of metal absorption are often polyphenolic phytochemicals present in various BDS formulations. Polyphenolics, a broad category of phytochemicals exhibiting multiple hydroxyl and carbonyl groups within their structure, are commonly found in BDSs as either single entities (e.g., phenolic acids, flavonoids, catechins) or as polymers (e.g., tannins). Inhibitory effects of polyphenolics on iron absorption stem from adjacent hydroxyl and/or carbonyl groups that bind ferric iron to form chelates (17, 18). Such iron chelates are not readily absorbed across the gastrointestinal mucosa.
Phytic acid, green tea catechins, and milk thistle silymarins are among the more widely recognized phytochemical chelators of essential dietary metals commonly found in BDSs. Phytic acid [inositol hexakisphosphate (IP-6)] is a cyclic organic acid that not only serves as a major storage form of phosphorous in plants, but avidly binds metals like calcium, iron, and zinc (Figure 1). Several clinical trials have demonstrated that prolonged ingestion of dietary phytates can lead to both iron and zinc deficiencies (19–21). Purified IP-6 is also available as a BDS, and is frequently touted as an antioxidant. However, although endogenously synthesized IP-6 does exhibit antioxidant properties, exogenously administered IP-6, in the form of BDSs, has poor oral bioavailability (22). Thus, chronic consumption of poorly absorbed phytate-containing BDS formulations may chelate essential metals in the gut lumen and promote various mineral deficiencies.
FIGURE 1.
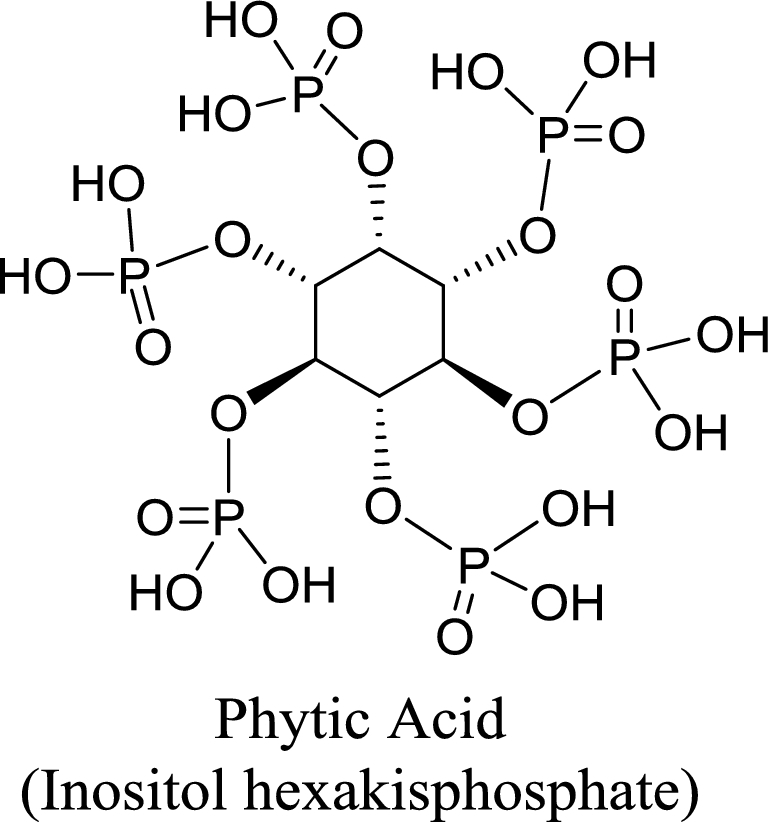
Phytic acid (inositol hexakisphosphate) can complex divalent metal cations in the gut lumen.
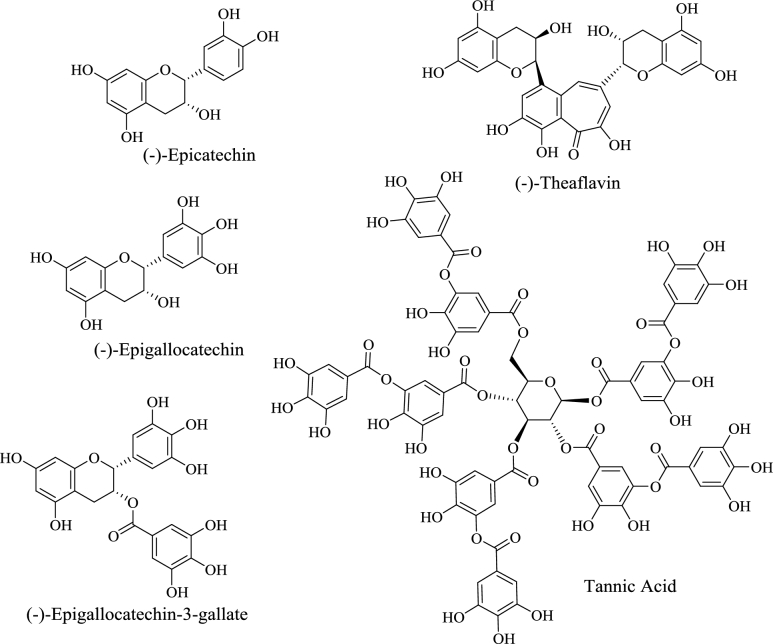
Green tea (Camellia sinensis) catechins are also recognized for their ability to complex iron and prevent its absorption (Figure 2). Whereas animal studies provide some evidence of the iron-chelating properties of green tea (23, 24), few clinical studies have addressed this issue. In a clinical trial involving healthy women, green tea--extract supplementation significantly decreased nonheme iron absorption, as measured by whole-body retention and isotope activity of extrinsically radiolabeled iron (25). Serum iron concentrations were also significantly reduced in obese subjects supplemented with green tea extract (26). In a recent clinical study in which subjects with metabolic syndrome were supplemented for 8 wk with a green tea BDS, plasma iron concentrations were significantly reduced, whereas copper, zinc, and selenium were not affected (27). Green tea supplementation also had no adverse effect on circulating carotenoids or tocopherols. From these few clinical investigations, the potential impact of green tea on iron absorption is concerning, as green tea extract or purified green tea catechins are common components of multi-ingredient BDS marketed for weight loss (28).
FIGURE 2.
Representative polyphenolic phytochemicals commonly found in green and black tea.
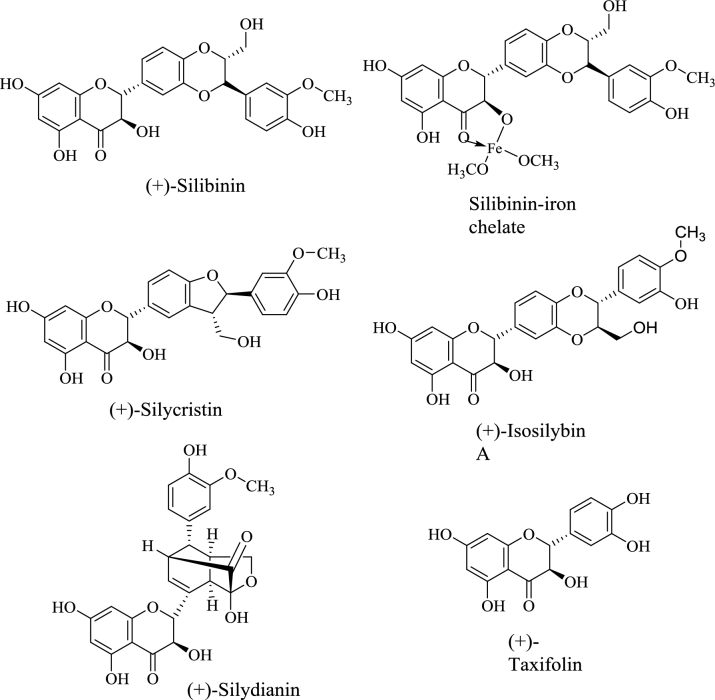
Milk thistle (Silybum marianum), a source of flavanolignans and flavonoids collectively known as silymarin, is commonly used to treat a variety of liver and gallbladder disorders. In 2001, silibinin, a principal component of silymarin, was identified as a natural iron chelator (18) (Figure 3). Since then, several clinical trials have investigated silibinin supplementation as a treatment modality for a variety of iron overload disorders. One of the first described the effects of a silibinin dose-ranging study (360, 720, and 1080 mg/d) conducted in 37 chronic hepatitis C patients (29). After 12 wk of silibinin supplementation, significant reductions in body iron stores were noted, particularly in patients with advanced stages of fibrosis. A small clinical study involving 10 patients with hereditary hemochromatosis demonstrated that ingestion of a silibinin-containing BDS (140 mg), along with a meal containing a known quantity of nonheme iron, significantly reduced dietary iron absorption (30). A larger randomized placebo-controlled study of 119 patients with β-thalassemia evaluated the efficacy of a 420-mg oral dose of silymarin/d combined with subcutaneous desferrioxamine to reduce iron overload (31). After 9 mo, serum iron and total iron-binding capacity were significantly reduced in the silymarin group compared with the placebo. No adverse effects were linked to silymarin supplementation. In addition, those patients receiving silymarin exhibited a significant decrease in serum concentrations of hepcidin and soluble transferrin receptor. The authors concluded that the therapeutic effects of silymarin on a background of desferrioxamine indicate that silymarin alone may be effective in reducing iron body burden (Table 2).
FIGURE 3.
Representative polyphenolic phytochemicals commonly found in milk thistle, including the silibinin-iron complex.
TABLE 2.
Summary of herb-micronutrient interactions and their mechanisms1
| Phytochemical | Micronutrient affected | Effect and interaction mechanism |
|---|---|---|
| Plant polyphenols (tea catechins, phloretin, quercetin) | Iron | Reduced absorption via complexation |
| Folate, ascorbate | Reduced absorption via uptake transporter inhibition | |
| Silymarins | Iron | Reduced absorption via complexation |
| Phytic acid | Calcium, iron, zinc | Reduced absorption via complexation |
| St. John's wort (hyperforin) | Vitamin D3 | Enhanced plasma clearance via induction of CYP3A4 metabolism |
CYP, cytochrome P450.
Taken together, the preponderance of clinical evidence indicates that select polyphenolic phytochemicals can impair dietary metal absorption, particularly iron. Although the examples presented here are limited to phytic acid, green tea catechins, and milk thistle silymarin, the diversity of the polyphenolic phytochemicals available in BDSs is considerable. To what extent chronic BDS usage impacts the incidence and severity of mineral deficiencies, as well as the etiology of their related disorders, remains to be seen. Regardless, the available data suggest that pharmacodynamic herb-micronutrient interactions do exist when select BDSs are ingested concomitantly with dietary metals or mineral supplements.
Vitamins
Clinically relevant pharmacokinetic herb-drug interactions are well documented, and their mechanisms often involve phytochemical-mediated alterations in drug-metabolizing enzymes or transporters (1, 32, 33). Induction of xenobiotic enzymes and efflux transporters often leads to reduced drug efficacy, whereas inhibition of these proteins may promote drug toxicity. In addition, inhibition of drug uptake transporters can markedly reduce drug absorption, thereby reducing efficacy. Green tea catechins are recognized as inhibitors of several drug uptake and efflux transporters (e.g., organic anion transporting polypeptides, organic cation transporters, multidrug and toxin extrusion proteins, P-glycoprotein) (34). Recently, green tea was shown to inhibit the absorption of the antihypertensive drug nadolol, purportedly by inhibiting the intestinal uptake transporter OATP1A2 (35). This green tea–nadolol interaction was believed to underlie the observed loss in hypertension control.
Pharmacokinetic herb-micronutrient interactions are also likely to involve phytochemical-mediated alterations in the activities of enzymes and transporters important in micronutrient disposition. Because uptake transporters of the solute carrier membrane transport protein family (SLC) play a prominent role in the disposition of several vitamins (e.g., folate, ascorbate), chronic exposure to excessive quantities of phytochemicals capable of modulating these carrier proteins may give rise to vitamin deficiencies. The reduced folate carrier SLC19A1 and the proton-coupled folate transporter SLC46A1 are the principal facilitative uptake transporters of folates, although other efflux transporters of the ATP-binding cassette (ABC) family (e.g., multidrug resistance proteins 1–5 and ABCG2) have also been identified in regulating folate transport across the intestinal mucosa (36). To date, very few clinical investigations into the effect of BDS supplementation on vitamin absorption have been reported. Of these, the preponderance has focused on tea catechin polyphenols and folate (37–39). Because reduced folate exposure has been linked to development of neural tube defects, BDS-mediated alterations in folate absorption among pregnant women could prove to be a clinically important herb-micronutrient interaction.
Alemdaroglu et al. (37) reported that daily administration of 900 mg of green or black tea catechin polyphenolics to healthy volunteers (male and female) in a fasted state significantly reduced the bioavailability of coadministered folic acid. In a follow-up study, members of this same research group found that daily administration of a green tea BDS (670 mg) with meals had no significant effect on serum folate concentrations of healthy males, prompting them to conclude that green tea catechins were unlikely to impair folate status (38). This discrepancy between the 2 studies may be explained by the effect of food on catechin absorption. Food has been shown to markedly reduce the absorption of green tea catechins (40); therefore, dosing conditions (fed compared with fasted) may influence the effect of green tea BDS on folate disposition. The influence of food on the green tea–folate interaction may be especially important in pregnant women. A recent survey of pregnant women in Japan noted that a significant association existed between high tea-catechin consumption and low serum folate concentrations, despite regular use of folic acid supplements (39). Unfortunately, the survey did not question whether participants ingested tea catechins with or without food.
Additionally, not all green tea supplements are created equally. Some green tea supplements are decaffeinated; others have varying caffeine content. There is evidence that the caffeine content of tea can bolster catechin polyphenol absorption (41). Is it possible that green tea supplements with high caffeine content have a greater impact on iron and/or folate absorption? One of the most popular marketing categories of BDSs is that of weight loss, and green tea and other natural caffeine sources (e.g., guarana, maté, kola nut) are common ingredients in their formulations. Given the popularity of polyphenol-containing BDSs, their impact on folate and iron absorption is an area that merits more clinical research.
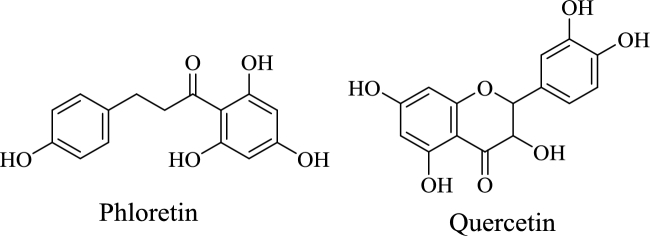
Ascorbate (vitamin C) is absorbed from the lumen of the human intestine by sodium-ascorbate cotransport in enterocytes. This secondary active transport mechanism couples ascorbate uptake to the concentration gradient of sodium ion across the plasma membrane that is maintained by sodium/potassium-ATPase (42). As with the folate transporters, several plant polyphenols, including phloretin and quercetin, have been shown to inhibit both ascorbate transporters in vitro (43–45). Phloretin and quercetin are present in various fruits (e.g., apples, apricots) and vegetables (e.g., radishes, onions) (Figure 4). They are also available as BDSs, where they are found in much greater amounts than in an equivalent mass of raw fruit or vegetable biomass. Theoretically, concomitant ingestion of ascorbic acid with either of these polyphenolic BDSs could impair vitamin C absorption. However, no prospective clinical trials have been conducted to confirm this projection. Vitamin C is present in a variety of citrus fruits that also contain a variety of flavonoids and other plant secondary metabolites, but it is unclear to what extent plant phytochemicals enhance or impair ascorbate absorption. To date, the only clinical study to address this question demonstrated that when stable isotope-labeled vitamin C was used to distinguish absorbed from endogenous vitamin, polyphenol-rich red grape juice significantly reduced ascorbate bioavailability (46). It was estimated that the volume (200 mL) of red grape juice used to administer the isotopically stable vitamin C contained about ∼176 mg of polyphenols. Because many commercially available BDS contain much higher quantities of plant polyphenols than that present in red grape juice, it seems plausible that vitamin C absorption would be adversely affected when concomitantly ingested with select BDS.
FIGURE 4.
Phloretin and quercetin are 2 polyphenolic compounds found in fruits and vegetables.
Another clinical study examined the impact of pycnogenol—a BDS incorporating an aqueous extract of Pinus maritima bark that contains multiple polyphenolic compounds—on vitamin C status in healthy adults (47). No specific quantity of vitamin C was administered as a supplement; rather, vitamin C intake was estimated from food records. After 14 d of pycnogenol supplementation, the investigators failed to note any significant changes in plasma vitamin C concentrations. The authors concluded that pycnogenol had no impact on vitamin C status. However, the polyphenolic content of the BDS was not independently verified. This shortcoming is a common mistake made by investigators unfamiliar with the idiosyncrasies of BDS formulas. Independent verification of phytochemical content is especially important when conducting BDS research, because the actual quantity can vary considerably from that claimed on the product label (32).
The Current Status of Herb-Micronutrient Research: Practices and Precautions
A host of uptake [e.g., SLC and solute carrier organic anion transporter (SLCO) families] and efflux transporters (e.g., the ABC family), as well as xenobiotic metabolizing enzymes [e.g., cytochromes P450 (CYPs), UDP glucuronosyltransferases], play important roles in the absorption, distribution, metabolism, and excretion of other water-soluble (e.g., biotin, niacin, pyridoxine, riboflavin, thiamine) and fat-soluble vitamins (e.g., calciferols, carotenoids, tocopherols) (48–53). A substantial body of in vitro evidence has identified various substrates, inhibitors, and activators of SLC/SLCO and ABC transporters, as well as CYPs and UDP glucuronosyltransferases involved in micronutrient disposition, and among these compounds are certain phytochemicals (32–34, 43, 45). Yet, when it comes to identifying clinically relevant herb-micronutrient interactions, less evidence exists discerning the extent to which these in vitro findings translate to the human in vivo condition. At present, the clinical evidence is limited to the effects of select polyphenolic compounds on the disposition of dietary metals and a few water-soluble vitamins (e.g., folate, ascorbate). In effect, research into clinical herb-micronutrient interactions has lagged far behind that of herb-drug interactions (Table 2).
The current discrepancy between herb-drug and herb-micronutrient interaction research is best illustrated with SJW, a popular botanical noted for its antidepressant activity, which has perhaps the greatest herb-drug interaction potential of any commercially available BDS. Both the antidepressant effect and the drug interaction potential of SJW are linked to the content of the phytochemical hyperforin, a bicyclic polyprenylated acylphloroglucinol found exclusively in Hypericum species (54). Hyperforin is a high-affinity ligand for the human steroid xenobiotic receptor (SXR), an orphan nuclear receptor selectively expressed in the liver and intestine that regulates gene transcription for a variety of xenobiotic metabolizing enzymes and transporters (Figure 5). According to one estimate, hyperforin is the most potent SXR activator discovered to date, even more potent than the drug rifampin (55). It is estimated that >70% of all prescription medications are susceptible to SJW-mediated interactions, resulting in decreased oral bioavailability, enhanced systemic clearance, and reduced drug efficacy. To date, no clinical investigations into potential SJW-micronutrient interactions have been conducted, but other drugs that are SXR ligands have been linked to such interactions. For example, the SXR ligand rifampin can reduce plasma concentrations of biologically active vitamin D3 [1α,25(OH)2D3], presumably via induction of CYP3A4-mediated vitamin D metabolism (56). Rifampin usage has also been linked to vitamin D deficiency and the development of osteomalacia (57). As hyperforin is a more-potent SXR ligand than rifampin, it seems plausible that prolonged SJW usage may also promote vitamin D deficiency and possibly osteomalacia.
FIGURE 5.
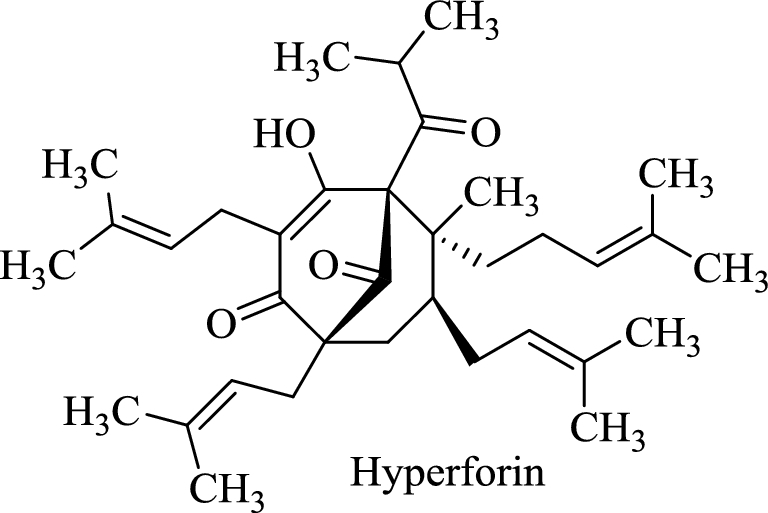
Hyperforin, a high-affinity ligand for human steroid xenobiotic receptor present in St. John's wort.
A wide variety of other nuclear receptors, as well as cross-talk between these proteins, regulate the expression of many xenobiotic metabolizing enzymes in addition to efflux and uptake transporters. Not surprisingly, in addition to hyperforin, a significant number of phytochemicals have been identified as nuclear receptor ligands in vitro (58–60). Besides nuclear receptor activation, other phytochemical-mediated mechanisms for modulating drug disposition have been identified (32, 33, 58), many of which are also likely to affect micronutrients. One principal difference, however, between herb-drug and herb-micronutrient interactions is that the clinical consequences of the latter are often slower to develop and less perceptible to both patients and health care professionals. This is primarily because of differences in the therapeutic ranges of drugs and micronutrients. Herb-mediated interactions involving drugs with narrow therapeutic windows are comparatively more clinically relevant than most herb-micronutrient interactions.
Another aspect of herb-micronutrient interactions that researchers new to this field must be aware of is the capricious nature of BDS formulations and the lack of rigorous FDA oversight. Moreover, the bioavailability of many phytochemicals present in BDS dosage forms is often quite low. This is usually a consequence of poor dosage form performance or extensive first-pass metabolism, or both. However, recognizing that many phytochemicals present in BDSs have poor oral bioavailability, often as a result of poor water solubility, dietary supplement manufacturers have begun to incorporate new formulation technologies (e.g., phytosomes, liposomes, nanoparticles, nanoemulsions) to markedly improve phytochemical absorption (61). One potential unforeseen consequence of these novel phytochemical delivery systems is that botanicals that heretofore had very low interaction potentials may now be rendered more susceptible to interactions involving both drugs and micronutrients. At present, few of these novel BDS formulations have been evaluated for their drug or micronutrient interaction capability.
Although content versus label-claim discrepancies are certainly important, a challenge with more serious consequences is the purposeful adulteration of BDSs with drugs or other botanicals. Although implementation and adaption of dietary supplement cGMPs should remedy such issues, not all manufacturers are sedulous in their adherence to cGMPs. At present, adulteration is common to several categories of BDSs, namely products marketed for body-building supplements, weight loss, sexual performance enhancement, and exercise performance enhancement. Contamination—either accidental or purposeful—with microbes, heavy metal, pesticide residues, or insect parts is another variable that can negatively impact herb-micronutrient interaction studies. Recently, a best practice guideline was published highlighting these and other considerations when conducting herb-drug interaction studies (62). Many, if not all, of these recommendations are applicable to herb-micronutrient interaction studies as well.
Conclusions
Since their introduction in 1994, BDSs have become a staple within the American health care system. Yet, despite their popularity, many of the idiosyncrasies of BDS formulations are unknown or underappreciated by consumers and health care providers alike. Like drugs and micronutrients, phytochemicals within BDS are subject to the same absorption, distribution, metabolism, and excretion pathways that the body employs to deal with any xenobiotic. As such, all 3 are likely to interfere with each other when taken concomitantly, especially if they compete for or modulate a common pathway. For the last 20 y, a substantial research effort has been underway to better understand the interaction risk and interaction mechanisms between BDS and conventional medications, and, during that time, several clinically relevant herb-drug interactions have been identified. A topic just as important, but receiving much less attention, is herb-micronutrient interactions. From the paucity of clinical studies conducted to date, it is apparent that certain classes of phytochemicals can impact the disposition of vitamins and dietary metals. However, the clinical relevance of many of these herb-micronutrient interactions remains to be determined, which means that further investigations into this under-researched area are urgently required.
Acknowledgments
All authors read and approved the final manuscript.
Notes
Published in a supplement to Advances in Nutrition. Presented at the symposium, “Micronutrient Status: Modifying Factors—Drugs, Chronic Disease, Surgery,” held at Columbia University, Institute of Human Nutrition, New York, New York, 17 June 2017. The conference was organized by Columbia University's Institute of Human Nutrition (its contents are solely the responsibility of the authors and do not necessarily represent the official views of Columbia University), and with the aid of an unrestricted grant from Pharmavite, LLC. The Supplement Coordinator for this supplement was Densie Webb. Supplement Coordinator disclosure: Denise Webb was compensated for overseeing the development and publication of the supplement. Airfare and hotel to attend the conference in New York were covered, in addition to payment for work completed. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the author(s) and are not attributable to the sponsors or the publisher, Editor, or Editorial Board of Advances in Nutrition.
Author disclosures: BJG, AT-C, SLT, and EKF, no conflicts of interest.
Abbreviations used:
- ABC
ATP-binding cassette
- BDS
botanical dietary supplement
- cGMP
current good manufacturing practice
- CYP
cytochrome P450
- IP-6
phytic acid
- SJW
St. John's-wort
- SLC
solute carrier protein
- SLCO
solute carrier organic anion transporter
- SXR
steroid xenobiotic receptor
References
- 1. Gurley BJ, Fifer EK, Gardner Z. Phytochemical modulators of human drug metabolism: drug interactions with fruits, vegetables, and botanical supplements. In: Lyubinov AV, editor. Encyclopedia of drug metabolism and interactions. New York: John Wiley & Sons; 2012. [Google Scholar]
- 2. Markowitz JS, von Moltke LL, Donovan JL. Predicting interactions between conventional medications and botanical products on the basis of in vitro investigations. Mol Nutr Food Res 2008;52(7):747–54. [DOI] [PubMed] [Google Scholar]
- 3. Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among adults from 1999–2012. JAMA 2016;316(14):1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz H. Future looks increasingly bright for herbal supplements, market researcher says [ Internet]. [cited 2017 Apr 9] Available from: http://www.nutraingredients-usa.com/Markets/Future-looks-increasingly-bright-for-herbal-supplements-market-researcher-says.
- 5. Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med 2013;173(5):355–61. [DOI] [PubMed] [Google Scholar]
- 6. Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, Thuppil V, Kales SN. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA 2008;300(8):915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vergine M, Aprile A, Sabella E, Genga A, Siciliano M, Rampino P, Lenucci MS, Luvisi A, Bellis L. Cadmium concentration in grains of durum wheat (Triticum turgidum L. subsp. durum). J Agric Food Chem 2017;65(30):6240–6. [DOI] [PubMed] [Google Scholar]
- 8. Mamani MC, Aleixo LM, de Abreu MF, Rath S. Simultaneous determination of cadmium and lead in medicinal plants by anodic stripping voltammetry. J Pharm Biomed Anal 2005;37(4):709–13. [DOI] [PubMed] [Google Scholar]
- 9. Raman P, Patino LC, Nair MG. Evaluation of metal and microbial contamination in botanical supplements. J Agric Food Chem 2004;52(26):7822–7. [DOI] [PubMed] [Google Scholar]
- 10. Grippo AA, Hamilton B, Hannigan R, Gurley BJ. Metal content of ephedra-containing dietary supplements and select botanicals. Am J Health Syst Pharm 2006;63(7):635–44. [DOI] [PubMed] [Google Scholar]
- 11. Kalny P, Wyderska S, Fijalek Z, Wroczynski P. Determination of selected elements in different pharmaceutical forms of some Polish herbal medicinal products. Acta Pol Pharm 2012;69(2):279–83. [PubMed] [Google Scholar]
- 12. Suliburska J, Kaczmarek K. Herbal infusions as a source of calcium, magnesium, iron, zinc and copper in human nutrition. Int J Food Sci Nutr 2012;63(2):194–8. [DOI] [PubMed] [Google Scholar]
- 13. Mihaljev Z, Zivkov-Balos M, Cupic Z, Jaksic S. Levels of some microelements and essential heavy metals in herbal teas in Serbia. Acta Pol Pharm 2014;71(3):385–91. [PubMed] [Google Scholar]
- 14. Ernst E. Toxic heavy metals and undeclared drugs in Asian herbal medicines. Trends Pharmacol Sci 2002;23(3):136–9. [DOI] [PubMed] [Google Scholar]
- 15. US Food & Drug Administration. Guidance for industry: current good manufacturing practice in manufacturing, packaging, labeling, or holding operations for dietary supplements; small entity compliance guide [Internet]. [cited 2017 Aug 8] Available from: https://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/dietarysupplements/ucm238182.htm.
- 16. Buettner C, Mukamal KJ, Gardiner P, Davis RB, Phillips RS, Mittleman MA. Herbal supplement use and blood lead levels of United States adults. J Gen Intern Med 2009;24(11):1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hallberg L, Hulthen L. Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron. Am J Clin Nutr 2000;71(5):1147–60. [DOI] [PubMed] [Google Scholar]
- 18. Borsari M, Gabbi C, Ghelfi F, Grandi R, Saladini M, Severi S, Borella F. Silybin, a new iron-chelating agent. J Inorg Biochem 2001;85(2–3):123–9. [DOI] [PubMed] [Google Scholar]
- 19. Abizari AR, Moretti D, Zimmermann MB, Armar-Klemesu M, Brouwer ID. Whole cowpea meal fortified with NaFeEDTA reduces iron deficiency among Ghanaian school children in a malaria endemic area. J Nutr 2012;142(10):1836–42. [DOI] [PubMed] [Google Scholar]
- 20. Abizari AR, Moretti D, Schuth S, Zimmermann MB, Armar-Klemesu M, Brouwer ID. Phytic acid-to-iron molar ratio rather than polyphenol concentration determines iron bioavailability in whole-cowpea meal among young women. J Nutr 2012;142(11):1950–5. [DOI] [PubMed] [Google Scholar]
- 21. Brnic M, Wegmuller R, Zeder C, Senti G, Hurrell RF. Influence of phytase, EDTA, and polyphenols on zinc absorption in adults from porridges fortified with zinc sulfate or zinc oxide. J Nutr 2014;144(9):1467–73. [DOI] [PubMed] [Google Scholar]
- 22. Grases F, Simonet BM, Vucenik I, Prieto RM, Costa-Bauza A, March JG, Shamsuddin AM. Absorption and excretion of orally administered inositol hexaphosphate (IP(6) or phytate) in humans. Biofactors 2001;15(1):53–61. [DOI] [PubMed] [Google Scholar]
- 23. Mandel SA, Amit T, Kalfon L, Reznichenko L, Weinreb O, Youdim MB. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: special reference to epigallocatechin gallate (EGCG). J Alzheimers Dis 2008;15(2):211–22. [DOI] [PubMed] [Google Scholar]
- 24. Saewong T, Ounjaijean S, Mundee Y, Pattanapanyasat K, Fucharoen S, Porter JB, Srichairatanakool S. Effects of green tea on iron accumulation and oxidative stress in livers of iron-challenged thalassemic mice. Med Chem 2010;6(2):57–64. [DOI] [PubMed] [Google Scholar]
- 25. Samman S, Sandstrom B, Toft MB, Bukhave K, Jensen M, Sorensen SS, Hansen M. Green tea or rosemary extract added to foods reduces nonheme-iron absorption. Am J Clin Nutr 2001;73(3):607–12. [DOI] [PubMed] [Google Scholar]
- 26. Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res 2012;149(3):315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Basu A, Betts NM, Mulugeta A, Tong C, Newman E, Lyons TJ. Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr Res 2013;33(3):180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gurley BJ, Steelman SC, Thomas SL. Multi-ingredient, caffeine-containing dietary supplements: history, safety, and efficacy. Clin Ther 2015;37(2):275–301. [DOI] [PubMed] [Google Scholar]
- 29. Bares JM, Berger J, Nelson JE, Messner DJ, Schildt S, Standish LJ, Kowdley KV. Silybin treatment is associated with reduction in serum ferritin in patients with chronic hepatitis C. J Clin Gastroenterol 2008;42(8):937–44. [DOI] [PubMed] [Google Scholar]
- 30. Hutchinson C, Bomford A, Geissler CA. The iron-chelating potential of silybin in patients with hereditary haemochromatosis. Eur J Clin Nutr 2010;64(10):1239–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moayedi B, Gharagozloo M, Esmaeil N, Maracy MR, Hoorfar H, Jalaeikar M. A randomized double-blind, placebo-controlled study of therapeutic effects of silymarin in beta-thalassemia major patients receiving desferrioxamine. Eur J Haematol 2013;90(3):202–9. [DOI] [PubMed] [Google Scholar]
- 32. Gurley BJ. Pharmacokinetic herb-drug interactions (part 1): origins, mechanisms, and the impact of botanical dietary supplements. Planta Med 2012;78(13):1478–89. [DOI] [PubMed] [Google Scholar]
- 33. Sprouse AA, van Breemen RB. Pharmacokinetic interactions between drugs and botanical dietary supplements. Drug Metab Dispos 2016;44(2):162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knop J, Misaka S, Singer K, Hoier E, Muller F, Glaeser H, Konig J, Fromm MF. Inhibitory effects of green tea and (-)-epigallocatechin gallate on transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-glycoprotein. PLoS One 2015;10(10):e0139370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Misaka S, Yatabe J, Muller F, Takano K, Kawabe K, Glaeser H, Yatabe MS, Onoue S, Werba JP, Watanabe H et al. . Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin Pharmacol Ther 2014;95(4):432–8. [DOI] [PubMed] [Google Scholar]
- 36. Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 2011;31:177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alemdaroglu NC, Dietz U, Wolffram S, Spahn-Langguth H, Langguth P. Influence of green and black tea on folic acid pharmacokinetics in healthy volunteers: potential risk of diminished folic acid bioavailability. Biopharm Drug Dispos 2008;29(6):335–48. [DOI] [PubMed] [Google Scholar]
- 38. Augustin K, Frank J, Augustin S, Langguth P, Ohrvik V, Witthoft CM, Rimbach G, Wolffram S. Green tea extracts lower serum folates in rats at very high dietary concentrations only and do not affect plasma folates in a human pilot study. J Physiol Pharmacol 2009;60(3):103–8. [PubMed] [Google Scholar]
- 39. Shiraishi M, Haruna M, Matsuzaki M, Ota E, Murayama R, Murashima S. Association between the serum folate levels and tea consumption during pregnancy. Biosci Trends 2010;4(5):225–30. [PubMed] [Google Scholar]
- 40. Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res 2005;11(12):4627–33. [DOI] [PubMed] [Google Scholar]
- 41. Nakagawa K, Nakayama K, Nakamura M, Sookwong P, Tsuduki T, Niino H, Kimura F, Miyazawa T. Effects of co-administration of tea epigallocatechin-3-gallate (EGCG) and caffeine on absorption and metabolism of EGCG in humans. Biosci Biotechnol Biochem 2009;73(9):2014–7. [DOI] [PubMed] [Google Scholar]
- 42. Wilson JX. Regulation of vitamin C transport. Annu Rev Nutr 2005;25:105–25. [DOI] [PubMed] [Google Scholar]
- 43. Song J, Kwon O, Chen S, Daruwala R, Eck P, Park JB, Levine M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and glucose. J Biol Chem 2002;277(18):15252–60. [DOI] [PubMed] [Google Scholar]
- 44. Caprile T, Salazar K, Astuya A, Cisternas P, Silva-Alvarez C, Montecinos H, Millan C, de Los Angeles Garcia M, Nualart F. The Na+-dependent L-ascorbic acid transporter SVCT2 expressed in brainstem cells, neurons, and neuroblastoma cells is inhibited by flavonoids. J Neurochem 2009;108(3):563–77. [DOI] [PubMed] [Google Scholar]
- 45. Gess B, Lohmann C, Halfter H, Young P. Sodium-dependent vitamin C transporter 2 (SVCT2) is necessary for the uptake of L-ascorbic acid into Schwann cells. Glia 2010;58(3):287–99. [DOI] [PubMed] [Google Scholar]
- 46. Bates CJ, Jones KS, Bluck LJ. Stable isotope-labelled vitamin C as a probe for vitamin C absorption by human subjects. Br J Nutr 2004;91(5):699–705. [DOI] [PubMed] [Google Scholar]
- 47. Silliman K, Parry J, Kirk LL, Prior RL. Pycnogenol does not impact the antioxidant or vitamin C status of healthy young adults. J Am Diet Assoc 2003;103(1):67–72. [DOI] [PubMed] [Google Scholar]
- 48. Takada T, Suzuki H. Molecular mechanisms of membrane transport of vitamin E. Mol Nutr Food Res 2010;54(5):616–22. [DOI] [PubMed] [Google Scholar]
- 49. Said HM. Recent advances in transport of water-soluble vitamins in organs of the digestive system: a focus on the colon and the pancreas. Am J Physiol Gastrointest Liver Physiol 2013;305(9):G601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hediger MA, Clemencon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med 2013;34(2-3):95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vadlapudi AD, Vadlapatla RK, Mitra AK. Sodium dependent multivitamin transporter (SMVT): a potential target for drug delivery. Curr Drug Targets 2012;13(7):994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 2016;96(1):365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bohn T, Desmarchelier C, Dragsted LO, Nielsen CS, Stahl W, Ruhl R, Keijer J, Borel P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol Nutr Food Res 2017;61(6). doi: 10.1002/mnfr.201600685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beerhues L. Hyperforin. Phytochemistry 2006;67(20):2201–7. [DOI] [PubMed] [Google Scholar]
- 55. Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A 2000;97(13):7500–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Z, Lin YS, Dickmann LJ, Poulton EJ, Eaton DL, Lampe JW, Shen DD, Davis CL, Shuhart MC, Thummel KE. Enhancement of hepatic 4-hydroxylation of 25-hydroxyvitamin D3 through CYP3A4 induction in vitro and in vivo: implications for drug-induced osteomalacia. J Bone Miner Res 2013;28(5):1101–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shah SC, Sharma RK, Hemangini, Chitle AR. Rifampicin induced osteomalacia. Tubercle 1981;62(3):207–9. [DOI] [PubMed] [Google Scholar]
- 58. Shay NF, Banz WJ. Regulation of gene transcription by botanicals: novel regulatory mechanisms. Annu Rev Nutr 2005;25:297–315. [DOI] [PubMed] [Google Scholar]
- 59. Li L, Bonneton F, Chen XY, Laudet V. Botanical compounds and their regulation of nuclear receptor action: the case of traditional Chinese medicine. Mol Cell Endocrinol 2015;401:221–37. [DOI] [PubMed] [Google Scholar]
- 60. Xu C, Huang M, Bi H. PXR- and CAR-mediated herbal effect on human diseases. Biochim Biophys Acta 2016;1859(9):1121–9. [DOI] [PubMed] [Google Scholar]
- 61. Gurley BJ. Emerging technologies for improving phytochemical bioavailability: benefits and risks. Clin Pharmacol Ther 2011;89(6):915–9. [DOI] [PubMed] [Google Scholar]
- 62. Gurley BJ, Markowitz JS, Williams DK, Barone GW. Practical considerations when designing and conducting clinical herb-drug interaction studies. Int J Pharmacokinet 2017;2:57–69. [Google Scholar]