Abstract
Disturbed metabolism as a consequence of obesity and diabetes may cause cardiac diseases (recently highlighted in the cardiovascular research spotlight issue on metabolic cardiomyopathies).1 In turn, the metabolism of the heart may also be disturbed in genetic and acquired forms of hypertrophic cardiac disease. Herein, we provide an overview of recent insights on metabolic changes in genetic hypertrophic cardiomyopathy and discuss several therapies, which may be explored to target disturbed metabolism and prevent onset of cardiac hypertrophy.
This article is part of the Mini Review Series from the Varenna 2017 meeting of the Working Group of Myocardial Function of the European Society of Cardiology.
Keywords: Hypertrophic cardiomyopathy, Mutations , Metabolism
1. HCM: inefficient sarcomere contraction as primary defect
Hypertrophic cardiomyopathy (HCM) is the most frequent inherited cardiomyopathy with a recently reported prevalence of 1:200.2 HCM has an extremely wide phenotypic variation. Its diverse appearance on cardiac imaging has only been recognized fully in the past decade since cardiac magnetic resonance has been introduced as the gold standard imaging assessment for diagnostic characterization and follow-up of these patients. The same genetic signature can translate into extreme cardiac morphological findings extending from an almost normal appearance or localized (segmental) hypertrophy to significant hypertrophy affecting predominantly the septum and/or the lateral wall and/or the apex.3 Aside from the diastolic abnormalities of the hypertrophic phenotype per se, additional pathophysiological consequences accompany the HCM heart such as outflow tract obstruction where the mitral valve becomes involved in the acceleration of flow seen in the obstructed outflow tract. The latter may also result in mitral regurgitation, all of which contribute to the symptoms experienced by these patients. After the first identification of a sarcomeric gene mutation in 1989,4,5 more than 1400 mutations have been identified, mostly in genes encoding sarcomeric proteins.6 Most mutations (∼90%) are found in the thick filament proteins myosin heavy chain (MyHC, MYH7 gene) and cardiac myosin binding protein C (cMyBP-C, MYBPC3 gene), and the thin filament protein troponin T (cTnT, TNNT2 gene). Initial studies on mutation-induced changes in sarcomere function revealed an increase in myofilament Ca2+-sensitivity as opposed to a decreased myofilament Ca2+-sensitivity in dilated cardiomyopathy (DCM).7–11 The opposite effects on myofilament Ca2+-sensitivity appears to be a consistent observation for thin filament mutations causing HCM and DCM, respectively (see also ‘Complex road from genotype to phenotype in dilated cardiomyopathies’ in the current issue),8,11 while the increase in myofilament Ca2+-sensitivity appears to be mostly secondary to disease progression in HCM with thick filament mutations.12 Rather than an increase in myofilament Ca2+-sensitivity, a common cellular phenotype induced by HCM sarcomere mutations is an inefficient sarcomere contraction, which is attributed to diverse changes in sarcomere properties: (i) an increase in myofilament Ca2+-sensitivity, which coincides with an increase in adenosine triphosphatase (ATPase) activity indicating increased adenosine triphosphate (ATP) utilization at the sarcomeres13; (ii) a blunted length-dependent activation, which will cause a less efficient sarcomere response to increased stress14; (iii) increased kinetics of activation and relaxation, which underlie increased tension cost, i.e. increased ATP utilization for force development at the sarcomere level15–19; and (iv) reduced super relaxed state of the cross-bridges, which will increase ATP utilization at low diastolic cytosolic calcium levels.20 Evidence for a mutation-induced reduction in the efficiency of cardiac performance in asymptomatic mutation carriers comes from imaging studies combining [11C]-acetate positron emission tomography and cardiovascular magnetic resonance imaging to assess myocardial external efficiency (MEE, i.e. the amount of oxygen used for cardiac work).18,21,22 These studies revealed reduced MEE in mutation carriers, indicating that inefficient cardiac contractility precedes the development of hypertrophy. Thus, recent ex vivo and in vivo analyses support the paradigm proposed by Ashrafian et al.23 that energy depletion causes HCM.
2. Linking inefficient sarcomere contraction with metabolic changes
Energy depletion induced by increased ATP utilization for sarcomere contraction is expected to impair cellular mechanisms regulating Ca2+ homeostasis and metabolism. Increased diastolic Ca2+ levels have been reported in HCM mouse models and human HCM patient samples,19,24 indicating impaired Ca2+ handling. In addition, a reduced PCr (phosphocreatine)/ATP ratio was observed in HCM with and without hypertrophy indicating deficits in cardiac energetics at an early stage of HCM.25 In the healthy heart, creatine kinase (CK) catalyses the transfer of phosphate from PCr to adenosine diphosphate (ADP), thereby regenerating ATP, while preventing accumulation of cytosolic ADP (Figure 1). Reducing PCr or experimental inhibition of CK activity has been causally linked to the development of heart failure, as it elevates left ventricle end-diastolic pressure (i.e. diastolic dysfunction), reduces contractility and increases mortality in rats.26–28 The consequence of low PCr and/or a reduced CK activity is that cytosolic levels of ADP will increase. Increases in (ADP) of >50% have been reported in HCM mouse models.16,29 Selectively increasing ADP levels without altering cytosolic ATP levels has been shown to limit myocardial relaxation in rats.30 High ADP levels impair relaxation of wild-type rat hearts via ADP-mediated defects in sarcomere function.31 Moreover, ADP increased myofilament Ca2+-sensitivity in human HCM samples.32 Thus, enhanced Ca2+-sensitivity is caused directly by the mutation and indirectly via increased ADP levels (Figure 2). These studies support the idea that energy depletion results in elevations of ADP, thereby causes diastolic dysfunction.
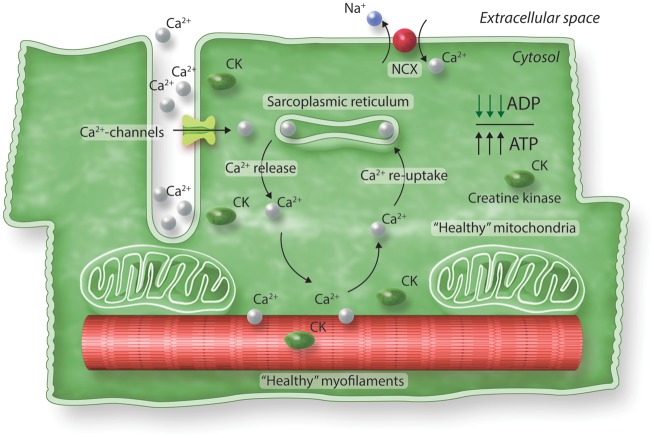
Figure 1.
Excitation-contraction coupling in a healthy heart. Contraction is initiated upon Ca2+ entry in the muscle cell, which activates Ca2+ release from the SR. Ca2+ binds to the myofilaments, which causes contraction. To relax Ca2+ detaches from myofilaments and is pumped back into the SR. A small fraction of Ca2+ is removed out of the cell via the Na+-Ca2+ exchanger (NCX). Mitochondria take care of sufficient ATP needed for proper contraction and relaxation of cardiomyocytes. In the healthy heart, CK catalyses the transfer of phosphate from phosphocreatine to ADP, thereby regenerating ATP, while preventing accumulation of cytosolic ADP.
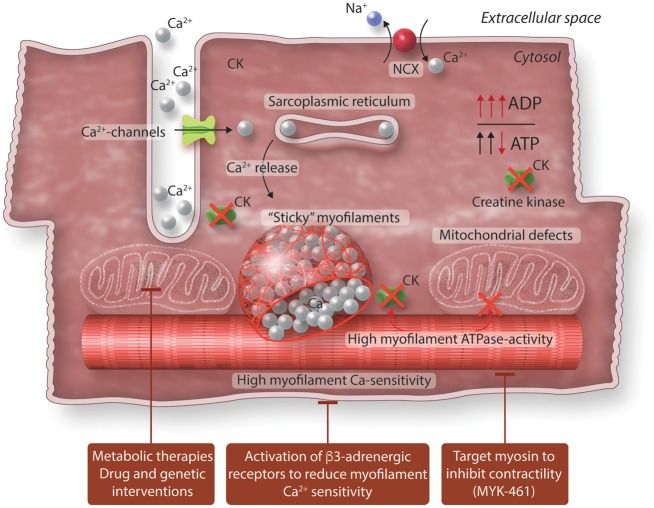
Figure 2.
Excitation-contraction coupling in diseased heart and possible targets for therapy. Mutation-induced changes in myofilament properties increase ATP utilization. Cellular metabolism changes as a consequences of mutation-induced and ADP-mediated increases in myofilament Ca2+-sensitivity, impaired mitochondrial function and reduced creatine kinase activity. Different therapies may target impaired metabolism in HCM.
3. Mitochondrial dysfunction
Impaired sarcomere energetics also provokes mitochondrial dysfunction, increase reactive oxygen species (ROS) and lead to altered ion homeostasis and lethal arrhythmias.33 Increased binding of Ca2+ to the myofilaments (via increased Ca2+-sensitivity) will reduce the Krebs cycle activity. At the same time, high ATP utilization increases ADP, which will reduce the levels of NADH and NADPH, thereby triggers oxidative stress. The composition of intracellular metabolic substrates is essential to regulate ATP production and limit production of ROS by the mitochondria. In mitochondria, ADP accelerates ATP production via oxidation of NADH to NAD+. At the same time Ca2+ stimulates the Krebs cycle (conversion of NAD+ to NADH) to match the ADP-mediated reduction in NADH, thereby maintaining the NADH/NAD+ redox state.34,35 The mutation-induced increase in myofilament Ca2+-sensitivity will enhance ATP utilization and increase ADP levels. The increase in ADP will increase oxidation of both NADH and NADPH and perturb the NADH/NAD+ balance.36 As NADPH is needed to detoxify ROS, the ADP-mediated NADPH oxidation will reduce the mitochondrial capacity to lower ROS. Moreover, as more Ca2+ will be bound to the sarcomeres due to the increased Ca2+-sensitivity, less Ca2+ will be available to stimulate the mitochondrial Krebs cycle and regenerate NADH. Through these mechanisms, impaired sarcomere energetics may thus provoke mitochondrial dysfunction and increase ROS.
4. Vascular endothelial dysfunction and rarefaction
While inefficient sarcomere contraction and relaxation will increase energy demand of the heart, pathogenic vascular remodelling may disrupt energy supply. HCM patients have abnormal myocardial perfusion reserve, which is more pronounced in the endocardium vs. mid and epicardial layers. Reduced cardiac perfusion has been reported in HCM patients, which was most severe in patients with a sarcomere mutation.37,38 No microvascular dysfunction was observed in asymptomatic mutation carriers.21 The observation of reduced coronary flow reserve in HCM patients with normal coronary angiograms led to the concept of microvascular (endothelial) dysfunction as secondary pathomechanism in HCM development.39,40 Blunted coronary flow in response to adenosine (i.e. endothelial dysfunction) has been observed in hypertrophied and non-hypertrophied regions of the heart.40 These studies suggest that mutation-induced cardiac contractile dysfunction precedes and possibly causes vascular (endothelial) dysfunction, which subsequently initiates remodelling (hypertrophy) of the heart. The inability of the capillary network to match the hypertrophic and disarrayed myocardium increases proportionately with the measured wall thickness on cardiac imaging, i.e. the most hypertrophic segments have the poorest perfusion reserve.41,42 Histological analysis revealed reduced capillary density (i.e. rarefaction) in septal tissue samples from patients with obstructive HCM.43 A significant proportion of patients with HCM progress to develop myocardial replacement fibrosis, typically located within the area of maximal wall hypertrophy. The presence of fibrosis appears to predict those phenotypes that later progress onto heart failure44 or are more likely to develop malignant ventricular arrhythmias.45
5. Changes in substrate metabolism in hypertrophied muscle
The healthy heart has a wide substrate versatility because it is able to metabolize fatty acids, carbohydrates, lactate, ketone bodies, and specific amino acids.46 In normal condition, cardiomyocytes generate more than two-thirds of the ATP by the oxidation of fatty acids and the remainder one-third by the oxidation of other substrates such as glucose. Interestingly, though, the oxidation of glucose is more energy efficient than that of fatty acids (ATP/O ratio = 3.17 for glucose vs. ±2 to 2.5 for fatty acids). In the case of acute increases in cardiac load, rapid supply of ATP is guaranteed by several mechanisms: increase in coronary flow and in oxygen extraction from the arterial coronary blood, and a metabolic shift from fatty acid oxidation to glucose oxidation (the Randle cycle). This ‘glucose-fatty acid cycle’ is a homeostatic mechanism that controls fuel selection and adapts substrate supply and demands in normal tissues and in the blood.47 This shift from fatty acid oxidation to increased glucose metabolism is common in end-stage heart failure.48 As a consequence, fatty acids and their derivatives accumulate into cells, causing lipotoxicity, 49 while glucose oxidation increases. This shift occurs mostly in mitochondria (‘aerobic glycolysis’ by oxidation of pyruvate) in order to guarantee more energy for the energy depleted failing heart. However, in failing hearts, a large part of glucose is converted to lactate through anaerobic glycolysis, which is less energy efficient. In the heart, it is possible that the latter process is the result of relative hypoxia caused by a reduced capillary density in combination with a higher workload of the hypertrophied heart. Recent findings indicate a central role for dihydrolipoyl succinyltransferase (DLST), the E2 subcomponent of the α-ketoglutarate dehydrogenase complex, a rate-controlling tricarboxylic acid cycle enzyme, in cardiac oxidative metabolism and hypertrophy. Its decrease in the diseased heart parallels a reduction of oxidative metabolism, whereas its cardiac overexpression improves oxidative metabolism and protects against cardiac hypertrophy and dysfunction.50
6. Atrial fibrillation
A high incidence of atrial fibrillation (AF) is observed in HCM, which worsens ventricular function. HCM patients with paroxysmal AF show reduced exercise capacity and is associated with markedly increased risk of death by stroke and heart failure.51,52 Moreover, AF is associated with advanced disease progression in HCM patients.52 AF may be caused by atrial dilatation in response to diastolic ventricular dysfunction. However, it may also involve a direct effect of the mutant protein on atrial myocyte function. A study in zebrafish harbouring an atrial-specific myosin light chain (MYL4) mutation, which was associated with early-onset AF in human, showed disrupted sarcomere structure, atrial enlargement and AF-like electrical abnormalities.53 However, not all HCM sarcomere mutations are expressed in atrial cardiomyocytes. In a recent clinical study, no significant correlations were found between genotype and onset or severity of AF in a HCM cohort with mutations in MYBPC3, MYH7 and ‘other genotypes’ (including thin filament gene mutations TNNT2, TNNI3, TPM1, and MYL2 and Z-line).54 Based on the latter study, the authors proposed that intrinsic atrial myopathy may be caused by rare (atrial-specific) mutations. If sarcomere mutations directly alter functional and structural properties of atrial cardiomyocytes warrants further experimental studies.
7. Non-myocyte compartment of the hypertrophied heart
The pathophysiology of HCM is not limited to sarcomere defects within cardiomyocytes but is also characterized by structural alterations in cardiomyocytes and the non-myocyte compartment of the heart. In a healthy heart, ∼70% of the cardiomyocyte volume consists of myofibrils. This fraction is reduced in manifest human HCM, and largely explains the decreased cardiomyocyte maximal force generation capacity observed in HCM biopsies.55 Cardiomyocytes solely account for 25–35% of all heart cells, while the non-myocyte populations are predominant and consist mostly of endothelial cells and cardiac fibroblasts.56 Studies in HCM mice identified the pro-fibrotic transforming growth factor beta (TGF-β), most likely released from cardiac fibroblasts, as the main determinant of non-myocyte proliferation and myocardial fibrosis observed in HCM.57 Since cardiac fibroblasts are responsible for extracellular matrix maintenance, and thus bridge biomechanical forces to and from cardiomyocytes, it has been speculated that the high basal myocardial activation observed in HCM cardiomyocytes (i.e. exacerbated biomechanical forces) is transmitted to the non-myocyte population, leading to increased expression of pro-fibrotic TGF-β.58 This is supported by ex vivo culture studies of both cardiac fibroblasts and cardiomyocytes that showed increased expression of TGF-β following repetitive stretch procedures.59,60 Early manifestation of myocardial fibrosis is a hallmark of HCM and correlates well with the degree of hypertrophy, diastolic dysfunction and energy consumption,44,61 indicating that targeting the extracellular matrix via TGF-β may represent a way to modify disease progression.
8. Therapies
8.1 Targeting metabolism
On the basis of the consideration that inhibition of mitochondrial fatty acid oxidation leads to cardiac hypertrophy, a study in rats has recently shown that the restoration of fatty acid metabolism confers beneficial effects on the hypertrophic heart.62 CD36-deficient (Cluster of differentiation 36, a major sarcolemmal fatty acid transporter) spontaneously hypertensive rats with established hypertrophy were treated with Tricaprylin, a triglyceride of caprylic acid, that stimulates fatty acid oxidation and maintains the cellular redox status. This treatment decreased cardiomyocyte cross-sectional area and reduced interstitial fibrosis, along decreased expression of BNP, calcineurin A and oxidative stress biomarkers. Cardiac function and energetics were also influenced by substrate availability. In fact, fenofibrate treatment in the absence of the appropriate metabolic substrate resulted in the mobilization of endogenous triglycerides and caused an imbalance of the cellular redox status, leading to enhanced free radical production and adverse cardiac changes. Conversely, medium-chain triglycerides have the capacity to bypass CD36 and serve as substrate for fatty acid oxidation,63 maintaining the intracellular redox status. Perhexiline is a metabolic drug which shifts metabolism away from the preferred fatty acids toward carbohydrates, and would thereby increase ATP supply. Perhexiline treatment enhanced glycolysis and protected against catecholamine-induced cardiac damage in a mouse model of peripartum cardiomyopathy.64
Metabolic remodelling appears to be reversible as regression of left ventricular hypertrophy is preceded by improved cardiac energy metabolism, as indicated in a mouse study of aortic constriction surgery followed by debanding.65 Debanding—unloading of the hyperpertrophic heart—significantly reduced left ventricular mass and wall thickness, along with profound changes in transcripts and proteins of cardiac substrate metabolism. However, debanding did not normalize the transcripts of proteins regulating glucose and fatty acid metabolism. This paradox is likely explained by the fact that cardiac energy metabolism is regulated at multiple levels, including many post-translational modifications. These data agree with the only partial reversal of depressed metabolic gene expression in the failing heart after implantation of a left ventricular assist device.66 Likewise, aortic valve replacement surgery in patients with aortic valve stenosis increased MEE, but MEE was not corrected to control values 4 months after surgery.67 Although only partial correction of MEE was observed, the improvement of MEE closely correlated with increased exercise capacity.67 These studies involve a hemodynamic, non-genetic overload of the heart, and may not translate to genetic forms of HCM. However, therapy targeting metabolism may be effective in HCM. Perhexiline treatment of HCM mice harbouring a MYBPC3 mutation improved some features of the HCM phenotype (reduced cardiac mass), which was associated with metabolic changes.68 Treatment of symptomatic HCM patients with improved exercise capacity.69 The therapeutic benefit of perhexiline may be the resultant of its multiple pleiotropic actions.70 Far from inducing a simple shift from fatty acid to glucose oxidation, perhexiline may cause complex rebalancing of carbon and nucleotide phosphate fluxes to increase metabolic flexibility and to maintain cardiac output.71 The benefit of metabolic drug therapy may depend on the ability of the heart to shift from mitochondrial lipid to glucose oxidation. As described above, hypertrophied hearts shift their metabolism from fatty acids to glucose utilization and glycolytic metabolism in an attempt to optimize energetic status.72 Mitochondrial oxidative metabolism decreases, while glycolysis as an alternate source of ATP production increases. Accordingly, in vivo imaging studies in advanced HCM patients suggest that metabolism shifted to the lower oxygen consuming glucose metabolism.22 Though initially adaptive, in the long run the (chronic) metabolic shift is detrimental for the heart as increased glycolysis increases pyruvate and lactate. The latter is accompanied by accumulation of H+ in the cytosol, which eventually leads to elevated calcium (i.e. impaired relaxation).72 While several pathways are activated in the severe (hypertrophic) stage of disease as compensatory mechanism, paradoxically, chronic stimulation of these pathways is detrimental. Likewise, chronic metabolic therapy may be harmful for the heart. Based on positive effects of exercise in cardiac disease, which is intermittent by its very nature, one may consider if intermittent metabolic drug-therapy, as opposed to chronic drug-treatment, represents a more effective and novel approach to treat cardiomyopathy.
Noteworthy, combined proteomics and metabolomics analysis revealed impaired energy generating pathways in mice with very high creatine levels that subsequently develop cardiac hypertrophy and dysfunction. Overall, these studies indicate that either low or very high levels of creatine perturb cardiac performance, and suggests that there is a therapeutic window of optimizing the cardiac energy balance in the heart.73
In conclusion, the hypertrophied and failing heart shows several metabolic changes. Improving the efficiency of energy generation in the hypertrophied heart can be exploited in order to optimize specific therapies. Metabolic alterations are (partially) reversible and their early identification may represent a therapeutic option (Figure 2).
8.2 Stimulation of β3-adrenergic receptors
Activation of β3-adrenergic receptors (β3AR) may be a way to modify altered energetic status of the HCM heart. β3AR are expressed in human cardiac myocytes and endothelial cells.74,75 They differ from the other two βAR isotypes in a number of ways; (i) in cardiac muscle, they exert effects that are antipathetic to those of β1-2AR on contractility (i.e. they act as “endogenous β1-2AR blockers”)74; (ii) β3AR expression increases in cardiac myocytes from diseased including failing, hearts74; (iii) β3AR lack consensus sequences for phosphorylation by GRK2 or protein kinase A (PKA) in their C-terminal tail, which attenuates or suppresses their desensitization, depending on the cell context.76 These characteristics make β3AR attractive targets in the context of heart failure, a condition with prevailing hyperadrenergism, when β1-2AR usually are desensitized/downregulated. Reduced β1AR signalling has also been observed in human HCM evident from reduced PKA-mediated phosphorylation of sarcomeric target proteins.12,14 Decreased PKA-mediated phosphorylation of troponin I (TnI) causes increased myofilament Ca2+-sensitivity, which will further exacerbate the energetic defect in HCM. In human cardiac muscle, β3AR couple through G-alpha-i to activation of the constitutive nitric oxide synthase (NOS),77 endothelial NOS and neuronal NOS (nNOS), both expressed in cardiac myocytes.78 β3AR expression and activity correlates with tonic increases in cGMP.77 Downstream activation of cGMP-dependent kinase (PKG)-I-alpha is expected to phosphorylate a number of targets functionally relevant to both excitation-contraction coupling and cardiac muscle remodelling. PKG modulates phospholamban phosphorylation to increase Ca2+ reuptake in the sarcoplasmic reticulum (SR),79 resulting in improved diastolic relaxation as well as increased SR load. PKG phosphorylates TnI to decrease myofilament Ca2+-sensitivity (Figure 2).80 PKG also modulates the phosphorylation of titin on specific residues, with putative improvements in myocyte elastic properties.81 nNOS also modulates PKA-mediated phospholamban phosphorylation and improves Ca2+ reuptake in the SR through cGMP-independent effects on protein phosphatase.82
These effects should directly improve relaxation and decrease myofilament Ca2+-sensitivity, with expected beneficial effects on energetics in HCM. In addition, β3AR uniquely exert antioxidant properties in hypertrophic cardiac muscle.83,84 This may counteract the adverse pro-oxidant consequences of increased ADP and decreased Ca2+ uptake by mitochondria. In addition, activation of the β3AR/NOS/cGMP pathway attenuates hypertrophic remodelling in several mouse models of neurohormonal or hemodynamic overload.78,83,85 Fibrosis is also decreased, through β3AR modulation of paracrine signalling from cardiac myocytes to fibroblasts, e.g. secondary to β3AR/nNOS anti-oxidant effects.83 Coronary perfusion is also expected to be improved, as β3AR expressed in human coronary microvascular endothelial cells are coupled to both nitric oxide and EDHF-dependent relaxations,75 as well as pro-angiogenic effects.86 Finally, systemic activation of β3AR in beige/brown fat may add indirect metabolic effects through increased lipolysis and improved systemic insulin sensitivity.87 Direct effects on cardiac metabolism, i.e. on the selection of energetic fuels (lipids versus glucose), particularly in the stressed or failing heart, are currently being studied.
8.3 Targeting myosin
An alternative way to modify cardiac contraction is the use of small molecules which directly target myosin. Omecamtiv mercabil (OM), a myosin activator is currently tested in clinical trials in patients with systolic heart failure.88 While a myosin activator may increase cardiac contractile performance, it may come at the expense of increased cardiac oxygen consumption as the compound may also increase myosin ATPase activity.89 Interestingly, a recent study showed that OM increases contractility at [Ca2+], which are close to values at systole under basal conditions, while it decreased force at high (maximal) Ca2+ activation.90 The latter study showed that the effect of OM depends on the concentration of both OM and intracellular Ca2+ levels, and the authors indicated that OM may be used to increase contractility and enhance function of a failing heart, while it may be used to reduce contractility in diastolic failure as observed in HCM dependent on its activating and inhibitory actions, respectively. A myosin inhibitor (mevacamten, also known as MYK-461), which was shown to reduce contractility,91 and most likely reduces oxygen consumption of the hypertrophied heart, suppressed HCM in a mouse models with MYH7 mutations.92 MYK-461 is currently tested in HCM by Myocardia. The use of myosin activators and inhibitors is an attractive novel approach to correct cardiac dysfunction, thereby influence metabolism.
8.4 Genetic interventions
Recently, a novel role was identified for microRNA-146a in regulating cardiac metabolism via suppression of oxidative metabolism.50 MicroRNA-146a targets a key component of the α-ketoglutarate dehydrogenase complex named DLST. Overexpression of DLST or inhibition of microRNA-146a blunted the hypertrophic response upon pressure overload in mice, which coincided with partial maintenance of oxidative metabolism. Increased miRNA-146a has been linked with reduced cardiac erbB4 signalling, which is central in regulating glucose metabolism.93 While inhibition of microRNA-146a may directly improve metabolism of cardiac muscle, energy supply may be improved via modulation of cardiac perfusion. MiRNAs may thus represent targets to improve metabolism and energy supply of the hypertrophied heart. In addition, mitochondrial-derived noncoding RNAs that are likely involved in metabolic processes have recently been found in patients with myocardial infarction and may be useful biomarkers of cardiac diseases and/or prognostic markers.94
9. Conclusion
Studies in mice and human have indicated that metabolic changes in development of HCM may represent an attractive therapeutic target. Recent studies in HCM mouse models and human cardiac biopsies emphasized that, although the final clinical HCM phenotype may be independent of genotype, the initial mutation-induced defects in sarcomere function11,15,16 and subsequent changes in signalling pathways95 may significantly differ based on the affected gene and even based on the specific mutation. This emphasizes the need to study the early mutation-induced changes in mitochondrial and metabolic pathways, which will aid in the development of patient-tailored (mutation-tailored) preventive therapies.
Conflict of interest: T.T. filed and licensed patents on cardiac non-coding RNAs. T.T. is founder of Cardior Pharmaceuticals. M.M. filed and licensed patents on non-coding RNAs as biomarkers. And all others have none to declare.
Funding
J.V. is supported by the Netherlands Cardiovascular Research Initiative, an initiative with support of the Dutch Heart Foundation, CVON2014-40 DOSIS. S.H. has received funding from the European Union Commission’s Seventh Framework programme under grant agreement N° 305507 (HOMAGE), N° 602904 (FIBROTARGETS) and FP7-Health-2013-Innovations-1 N° 602156 (HECATOS). We acknowledge the support from the Netherlands Cardiovascular Research Initiative, an initiative with support of the Dutch Heart Foundation, CVON2011-ARENA, CVON2016-Early HFPEF, 2015-10, and CVON ShePREDICTS, grant 2017-21. This research is co-financed as a PPP-allowance Research and Innovation by the Ministry of Economic Affairs within Top Sector Life sciences & Health. This research was co-funded by the C3 project ‘Vision Core Leuven’ of the Leuven University. C.G.T. is supported by a Federico II University “Ricerca di Ateneo” grant. T.T. received funding from the European Research Area Network on Cardiovascular Diseases (ERA-CVD), Project EXPERT. J.-L.B. funded by grants from the Fonds National de la Recherche Scientifique (FNRS; PDR T.0144.13), European Union (UE LSHM-CT-05-018833, “beta3lvh”), and the Federation Wallonie-Bruxelles (Action de Recherche Concertée ARC11-16/035). Funding of A.F.L.-M. and I.F.-P.: Project DOCnet (NORTE-01-0145-FEDER-000003), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF), and the project NETDIAMOND (POCI-01-0145-FEDER-016385), supported by European Structural and Investment Funds, Lisbon’s Regional Operational Program 2020 and Portuguese funds from the Portuguese Foundation for Science and Technology.
References
- 1. Maack C, Murphy E.. Metabolic cardiomyopathies—fighting the next epidemic. Cardiovasc Res 2017;113:367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Semsarian C, Ingles J, Maron MS, Maron BJ.. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015;65:1249–1254. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, Spirito P, Ten Cate FJ, Wigle ED, Vogel RA, Abrams J, Bates ER, Brodie BR, Danias PG, Gregoratos G, Hlatky MA, Hochman JS, Kaul S, Lichtenberg RC, Lindner JR, O’rourke RA, Pohost GM, Schofield RS, Tracy CM, Winters WL, Klein WW, Priori SG, Alonso-Garcia A, Blomström-Lundqvist C, De Backer G, Deckers J, Flather M, Hradec J, Oto A, Parkhomenko A, Silber S, Torbicki A; Task Force on Clinical Expert Consensus Documents. American College of Cardiology; Committee for Practice Guidelines. European Society of Cardiology. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003;42:1687–1713. [DOI] [PubMed] [Google Scholar]
- 4. Jarcho JA, McKenna W, Pare JA, Solomon SD, Holcombe RF, Dickie S, Levi T, Donis-Keller H, Seidman JG, Seidman CE.. Mapping a gene for familial hypertrophic cardiomyopathy to chromosome 14q1. N Engl J Med 1989;321:1372–1378. [DOI] [PubMed] [Google Scholar]
- 5. Geisterfer-Lowrance AA, Kass S, Tanigawa G, Vosberg HP, McKenna W, Seidman CE, Seidman JG.. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 1990;62:999–1006. [DOI] [PubMed] [Google Scholar]
- 6. Ho CY, Charron P, Richard P, Girolami F, Van Spaendonck-Zwarts KY, Pinto Y.. Genetic advances in sarcomeric cardiomyopathies: state of the art. Cardiovasc Res 2015;105:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bottinelli R, Coviello DA, Redwood CS, Pellegrino MA, Maron BJ, Spirito P, Watkins H, Reggiani C.. A mutant tropomyosin that causes hypertrophic cardiomyopathy is expressed in vivo and associated with an increased calcium sensitivity. Circ Res 1998;82:106–115. [DOI] [PubMed] [Google Scholar]
- 8. Morimoto S, Yanaga F, Minakami R, Ohtsuki I.. Ca2+-sensitizing effects of the mutations at Ile-79 and Arg-92 of troponin T in hypertrophic cardiomyopathy. Am J Physiol 1998;275:C200–C207. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi-Yanaga F, Morimoto S, Harada K, Minakami R, Shiraishi F, Ohta M, Lu QW, Sasaguri T, Ohtsuki I.. Functional consequences of the mutations in human cardiac troponin I gene found in familial hypertrophic cardiomyopathy. J Mol Cell Cardiol 2001;33:2095–2107. [DOI] [PubMed] [Google Scholar]
- 10. Morimoto S, Lu QW, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, Sasaguri T, Ohtsuki I.. Ca(2+)-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci USA 2002;99:913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson P, Griffiths PJ, Watkins H, Redwood CS.. Dilated and hypertrophic cardiomyopathy mutations in troponin and alpha-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ Res 2007;101:1266–1273. [DOI] [PubMed] [Google Scholar]
- 12. van Dijk SJ, Paalberends ER, Najafi A, Michels M, Sadayappan S, Carrier L, Boontje NM, Kuster DW, van Slegtenhorst M, Dooijes D, dos Remedios C, ten Cate FJ, Stienen GJ, van der Velden J.. Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ Heart Fail 2012;5:36–46. [DOI] [PubMed] [Google Scholar]
- 13. Miller T, Szczesna D, Housmans PR, Zhao J, de Freitas F, Gomes AV, Culbreath L, McCue J, Wang Y, Xu Y, Kerrick WG, Potter JD.. Abnormal contractile function in transgenic mice expressing a familial hypertrophic cardiomyopathy-linked troponin T (I79N) mutation. J Biol Chem 2001;276:3743–3755. [DOI] [PubMed] [Google Scholar]
- 14. Sequeira V, Wijnker PJ, Nijenkamp LL, Kuster DW, Najafi A, Witjas-Paalberends ER, Regan JA, Boontje N, Ten Cate FJ, Germans T, Carrier L, Sadayappan S, van Slegtenhorst MA, Zaremba R, Foster DB, Murphy AM, Poggesi C, Dos Remedios C, Stienen GJ, Ho CY, Michels M, van der Velden J.. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ Res 2013;112:1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Witjas-Paalberends ER, Ferrara C, Scellini B, Piroddi N, Montag J, Tesi C, Stienen GJ, Michels M, Ho CY, Kraft T, Poggesi C, van der Velden J.. Faster cross-bridge detachment and increased tension cost in human hypertrophic cardiomyopathy with the R403Q MYH7 mutation. J Physiol 2014;592:3257–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spindler M, Saupe KW, Christe ME, Sweeney HL, Seidman CE, Seidman JG, Ingwall JS.. Diastolic dysfunction and altered energetics in the alphaMHC403/+ mouse model of familial hypertrophic cardiomyopathy. J Clin Invest 1998;101:1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chandra M, Tschirgi ML, Tardiff JC.. Increase in tension-dependent ATP consumption induced by cardiac troponin T mutation. Am J Physiol Heart Circ Physiol 2005;289:H2112–H2119. [DOI] [PubMed] [Google Scholar]
- 18. Witjas-Paalberends ER, Güçlü A, Germans T, Knaapen P, Harms HJ, Vermeer AM, Christiaans I, Wilde AA, Dos Remedios C, Lammertsma AA, van Rossum AC, Stienen GJ, van Slegtenhorst M, Schinkel AF, Michels M, Ho CY, Poggesi C, van der Velden J.. Gene-specific increase in energetic cost of contraction in hypertrophic cardiomyopathy caused by thick filament mutations. Cardiovasc Res 2014;103:248–257. [DOI] [PubMed] [Google Scholar]
- 19. Ferrantini C, Coppini R, Pioner JM, Gentile F, Tosi B, Mazzoni L, Scellini B, Piroddi N, Laurino A, Santini L, Spinelli V, Sacconi L, De Tombe P, Moore R, Tardiff J, Mugelli A, Olivotto I, Cerbai E, Tesi C, Poggesi C.. Pathogenesis of hypertrophic cardiomyopathy is mutation rather than disease specific: a comparison of the cardiac troponin T E163R and R92Q mouse models. J Am Heart Assoc 2017;6:e005407.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McNamara JW, Li A, Lal S, Bos JM, Harris SP, van der Velden J, Ackerman MJ, Cooke R, dos Remedios CG.. MYBPC3 mutations are associated with a reduced super-relaxed state in patients with hypertrophic cardiomyopathy. PLoS One 2017;12:e0180064.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Timmer SA, Germans T, Brouwer WP, Lubberink M, van der Velden J, Wilde AA, Christiaans I, Lammertsma AA, Knaapen P, van Rossum AC.. Carriers of the hypertrophic cardiomyopathy MYBPC3 mutation are characterized by reduced myocardial efficiency in the absence of hypertrophy and microvascular dysfunction. Eur J Heart Fail 2011;13:1283–1289. [DOI] [PubMed] [Google Scholar]
- 22. Güçlü A, Knaapen P, Harms HJ, Parbhudayal RY, Michels M, Lammertsma AA, van Rossum AC, Germans T, van der Velden J.. Disease stage-dependent changes in cardiac contractile performance and oxygen utilization underlie reduced myocardial efficiency in human inherited hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2017;10:e005604.. [DOI] [PubMed] [Google Scholar]
- 23. Ashrafian H, Redwood C, Blair E, Watkins H.. Hypertrophic cardiomyopathy: a paradigm for myocardial energy depletion. Trends Genet 2003;19:263–268. [DOI] [PubMed] [Google Scholar]
- 24. Coppini R, Ferrantini C, Yao L, Fan P, Del Lungo M, Stillitano F, Sartiani L, Tosi B, Suffredini S, Tesi C, Yacoub M, Olivotto I, Belardinelli L, Poggesi C, Cerbai E, Mugelli A.. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 2013;127:575–584. [DOI] [PubMed] [Google Scholar]
- 25. Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, McKenna WJ, Ostman-Smith I, Clarke K, Watkins H.. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol 2003;41:1776–1782. [DOI] [PubMed] [Google Scholar]
- 26. Hamman BL, Bittl JA, Jacobus WE, Allen PD, Spencer RS, Tian R, Ingwall JS.. Inhibition of the creatine kinase reaction decreases the contractile reserve of isolated rat hearts. Am J Physiol 1995;269:H1030–H1036. [DOI] [PubMed] [Google Scholar]
- 27. Tian R, Nascimben L, Ingwall JS, Lorell BH.. Failure to maintain a low ADP concentration impairs diastolic function in hypertrophied rat hearts. Circulation 1997;96:1313–1319. [DOI] [PubMed] [Google Scholar]
- 28. Horn M, Remkes H, Strömer H, Dienesch C, Neubauer S.. Chronic phosphocreatine depletion by the creatine analogue β-guanidinopropionate is associated with increased mortality and loss of ATP in rats after myocardial infarction. Circulation 2001;104:1844–1849. [DOI] [PubMed] [Google Scholar]
- 29. He H, Javadpour MM, Latif F, Tardiff JC, Ingwall JS.. R-92L and R-92W mutations in cardiac troponin T lead to distinct energetic phenotypes in intact mouse hearts. Biophys J 2007;93:1834–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian R, Christe ME, Spindler M, Hopkins JC, Halow JM, Camacho SA, Ingwall JS.. Role of MgADP in the development of diastolic dysfunction in the intact beating rat heart. J Clin Invest 1997;99:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sequeira V, Najafi A, McConnell M, Fowler ED, Bollen IAE, Wüst RCI, Dos Remedios CG, Helmes M, White E, Stienen GJ, Tardiff JC, Kuster DW, van der Velden J.. Synergistic role of ADP and Ca2+ in diastolic myocardial stiffness. J Physiol 2015;593:3899–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sequeira V, Najafi A, Wijnker PJ, Dos Remedios CG, Michels M, Kuster DW, van der Velden J.. ADP-stimulated contraction: a predictor of thin-filament activation in cardiac disease. Proc Natl Acad Sci USA 2015;112:E7003–E7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wijnker PJ, Sequeira V, Kuster DW, van der Velden J.. Hypertrophic cardiomyopathy: a vicious cycle triggered by sarcomere mutations and secondary disease hits. Antiox Redox Signal 2018; doi:10.1089/ars.2017.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol 2002;34:1259–1271. Review. [DOI] [PubMed] [Google Scholar]
- 35. Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B.. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res 2006;99:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nickel AG, von Hardenberg A, Hohl M, Löffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, Puhl SL, Wagner M, Bogeski I, Cortassa S, Kappl R, Pasieka B, Lafontaine M, Lancaster CR, Blacker TS, Hall AR, Duchen MR, Kästner L, Lipp P, Zeller T, Müller C, Knopp A, Laufs U, Böhm M, Hoth M, Maack C.. Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure. Cell Metab 2015;22:472–484. [DOI] [PubMed] [Google Scholar]
- 37. Olivotto I, Girolami F, Sciagra R, Ackerman MJ, Sotgia B, Bos JM, Nistri S, Sgalambro A, Grifoni C, Torricelli F, Camici PG, Cecchi F.. Microvascular function is selectively impaired in patients with hypertrophic cardiomyopathy and sarcomere myofilament gene mutations. J Am Coll Cardiol 2011;58:839–848. [DOI] [PubMed] [Google Scholar]
- 38. Camici PG, Olivotto I, Rimoldi OE.. The coronary circulation and blood flow in left ventricular hypertrophy. J Mol Cell Cardiol 2012;52:857–864. [DOI] [PubMed] [Google Scholar]
- 39. Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, Watkins H.. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation 2007;115:2418–2425. [DOI] [PubMed] [Google Scholar]
- 40. Olivotto I, d'Amati G, Basso C, Van Rossum A, Patten M, Emdin M, Pinto Y, Tomberli B, Camici PG, Michels M.. Defining phenotypes and disease progression in sarcomeric cardiomyopathies: contemporary role of clinical investigations. Cardiovasc Res 2015;105:409–423. [DOI] [PubMed] [Google Scholar]
- 41. Ismail TF, Hsu L-Y, Greve AM, Gonçalves C, Jabbour A, Gulati A, Hewins B, Mistry N, Wage R, Roughton M, Ferreira PF, Gatehouse P, Firmin D, O’Hanlon R, Pennell DJ, Prasad SK, Arai AE.. Coronary microvascular ischemia in hypertrophic cardiomyopathy—a pixel-wise quantitative cardiovascular magnetic resonance perfusion study. J Cardiovasc Magn Reson 2014;16:49.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raphael CE, Cooper R, Parker KH, Collinson J, Vassiliou V, Pennell DJ, de Silva R, Hsu LY, Greve AM, Nijjer S, Broyd C, Ali A, Keegan J, Francis DP, Davies JE, Hughes AD, Arai A, Frenneaux M, Stables RH, Di Mario C, Prasad SK.. Mechanisms of myocardial ischemia in hypertrophic cardiomyopathy: insights from wave intensity analysis and magnetic resonance. J Am Coll Cardiol 2016;68:1651–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Güçlü A, Happé C, Eren S, Korkmaz IH, Niessen HW, Klein P, van Slegtenhorst M, Schinkel AF, Michels M, van Rossum AC, Germans T, van der Velden J.. Left ventricular outflow tract gradient is associated with reduced capillary density in hypertrophic cardiomyopathy irrespective of genotype. Eur J Clin Invest 2015;45:1252–1259. [DOI] [PubMed] [Google Scholar]
- 44. O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK.. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol 2010;56:867–874. [DOI] [PubMed] [Google Scholar]
- 45. Dawson DK, Hawlisch K, Prescott G, Roussin I, Di Pietro E, Deac M, Wong J, Frenneaux MP, Pennell DJ, Prasad SK.. Prognostic role of CMR in patients presenting with ventricular arrhythmias. JACC Cardiovasc Imaging 2013;6:335–344. [DOI] [PubMed] [Google Scholar]
- 46. Heggermont WA, Papageorgiou AP, Heymans S, van Bilsen M.. Metabolic support for the heart: complementary therapy for heart failure? Eur J Heart Fail 2016;18:1420–1429. [DOI] [PubMed] [Google Scholar]
- 47. Hue L, Taegtmeyer H.. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 2009;297:E578–E591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lionetti V, Stanley WC, Recchia FA.. Modulating fatty acid oxidation in heart failure. Cardiovasc Res 2011;90:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H.. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 2004;18:1692–1700. [DOI] [PubMed] [Google Scholar]
- 50. Heggermont WA, Papageorgiou AP, Quaegebeur A, Deckx S, Carai P, Verhesen W, Eelen G, Schoors S, van Leeuwen R, Alekseev S, Elzenaar I, Vinckier S, Pokreisz P, Walravens AS, Gijsbers R, Van Den Haute C, Nickel A, Schroen B, van Bilsen M, Janssens S, Maack C, Pinto Y, Carmeliet P, Heymans S.. Inhibition of microRNA-146a and overexpression of its target dihydrolipoyl succinyltransferase protect against pressure overload-Induced cardiac hypertrophy and dysfunction. Circulation 2017;136:747–761. [DOI] [PubMed] [Google Scholar]
- 51. Azarbal F, Singh M, Finocchiaro G, Le VV, Schnittger I, Wang P, Myers J, Ashley E, Perez M.. Exercise capacity and paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. Heart 2014;100:624–630. [DOI] [PubMed] [Google Scholar]
- 52. Finocchiaro G, Haddad F, Knowles JW, Caleshu C, Pavlovic A, Homburger J, Shmargad Y, Sinagra G, Magavern E, Wong M, Perez M, Schnittger I, Myers J, Froelicher V, Ashley EA.. Cardiopulmonary responses and prognosis in hypertrophic cardiomyopathy: a potential role for comprehensive noninvasive hemodynamic assessment. JACC Heart Fail 2015;3:408–418. [DOI] [PubMed] [Google Scholar]
- 53. Orr N, Arnaout R, Gula LJ, Spears DA, Leong-Sit P, Li Q, Tarhuni W, Reischauer S, Chauhan VS, Borkovich M, Uppal S, Adler A, Coughlin SR, Stainier DY, Gollob MH.. A mutation in the atrial-specific myosin light chain gene (MYL4) causes familial atrial fibrillation. Nat Commun 2016;7:11303.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bongini C, Ferrantini C, Girolami F, Coppini R, Arretini A, Targetti M, Bardi S, Castelli G, Torricelli F, Cecchi F, Ackerman MJ, Padeletti L, Poggesi C, Olivotto I.. Impact of genotype on the occurrence of atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol 2016;117:1151–1159. [DOI] [PubMed] [Google Scholar]
- 55. Witjas-Paalberends ER, Piroddi N, Stam K, van Dijk SJ, Sequeira V, Ferrara C, Scellini B, Hazebroek M, ten Cate FJ, van Slegtenhorst M, Dos Remedios CG, Niessen HW, Tesi C, Stienen GJ, Heymans S, Michels M, Poggesi C, van der Velden J.. Mutations in MYH7 reduce the force generating capacity of sarcomeres in human familial hypertrophic cardiomyopathy. Cardiovasc Res 2013;99:432–441. [DOI] [PubMed] [Google Scholar]
- 56. Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD.. Revisiting cardiac cellular composition. Circ Res 2016;118:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG.. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires tgf-β. J Clin Invest 2010;120:3520–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Teekakirikul P, Padera RF, Seidman JG, Seidman CE.. Hypertrophic cardiomyopathy: translating cellular cross talk into therapeutics. J Cell Biol 2012;199:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Wamel AJ, Ruwhof C, van der Valk-Kokshoorn LJ, Schrier PI, van der Laarse A.. Stretch-induced paracrine hypertrophic stimuli increase TGF-β1 expression in cardiomyocytes. Mol Cell Biochem 2002;236:147–153. [DOI] [PubMed] [Google Scholar]
- 60. Ruwhof C, van Wamel AE, Egas JM, van der Laarse A.. Cyclic stretch induces the release of growth promoting factors from cultured neonatal cardiomyocytes and cardiac fibroblasts. Mol Cell Biochem 2000;208:89–98. [DOI] [PubMed] [Google Scholar]
- 61. Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG, Díez J, Seidman CE.. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med 2010;363:552–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saifudeen I, Subhadra L, Konnottil R, Renuka Nair R.. Metabolic modulation by medium-chain triglycerides reduces oxidative stress and ameliorates CD36-mediated cardiac remodeling in spontaneously hypertensive rat in the initial and established stages of hypertrophy. J Cardiac Fail 2017;23:240–251. [DOI] [PubMed] [Google Scholar]
- 63. Labarthe F, Khairallah M, Bouchard B, Stanley WC, Des Rosiers C.. Fatty acid oxidation and its impact on response of spontaneously hypertensive rat hearts on adrenergic stress: benefits of a medium-chain fatty acid. Am J Physiol 2005;288:H1425–H1436. [DOI] [PubMed] [Google Scholar]
- 64. Stapel B, Kohlhaas M, Ricke-Hoch M, Haghikia A, Erschow S, Knuuti J, Silvola JM, Roivainen A, Saraste A, Nickel AG, Saar JA, Sieve I, Pietzsch S, Müller M, Bogeski I, Kappl R, Jauhiainen M, Thackeray JT, Scherr M, Bengel FM, Hagl C, Tudorache I, Bauersachs J, Maack C, Hilfiker-Kleiner D.. Low STAT3 expression sensitizes to toxic effects of β-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur Heart J 2017;38:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Byrne NJ, Levasseur J, Sung MM, Masson G, Boisvenue J, Young ME, Dyck JR.. Normalization of cardiac substrate utilization and left ventricular hypertrophy precede functional recovery in heart failure regression. Cardiovasc Res 2016;110:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer h.. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation 2002;106:407–411. [DOI] [PubMed] [Google Scholar]
- 67. Güçlü A, Knaapen P, Harms HJ, Vonk AB, Stooker W, Groepenhoff H, Lammertsma AA, van Rossum AC, Germans T, van der Velden J.. Myocardial efficiency is an important determinant of functional improvement after aortic valve replacement in aortic valve stenosis patients: a combined PET and CMR study. Eur Heart J Cardiovasc Imaging 2015;16:882–889. [DOI] [PubMed] [Google Scholar]
- 68. Gehmlich K, Dodd MS, Allwood JW, Kelly M, Bellahcene M, Lad HV, Stockenhuber A, Hooper C, Ashrafian H, Redwood CS, Carrier L, Dunn WB.. Changes in the cardiac metabolome caused by perhexiline treatment in a mouse model of hypertrophic cardiomyopathy. Mol Biosyst 2015;11:564–573. [DOI] [PubMed] [Google Scholar]
- 69. Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G, Ahmed I, Maher AR, Kaur K, Taylor J, Henning A, Ashrafian H, Watkins H, Frenneaux M.. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation 2010;122:1562–1569. [DOI] [PubMed] [Google Scholar]
- 70. George CH, Mitchell AN, Preece R, Bannister ML, Yousef Z.. Pleiotropic mechanisms of action of perhexiline in heart failure. Expert Opin Ther Pat 2016;26:1049–1059. [DOI] [PubMed] [Google Scholar]
- 71. Yin X, Dwyer J, Langley SR, Mayr U, Xing Q, Drozdov I, Nabeebaccus A, Shah AM, Madhu B, Griffiths J, Edwards LM, Mayr M.. Effects of perhexiline-induced fuel switch on the cardiac proteome and metabolome. J Mol Cell Cardiol 2013;55:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. de Jong KA, Lopaschuk GD.. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol 2017;33:860–871. [DOI] [PubMed] [Google Scholar]
- 73. Zervou S, Yin X, Nabeebaccus AA, O’Brien BA, Cross RL, McAndrew DJ, Atkinson RA, Eykyn TR, Mayr M, Neubauer S, Lygate CA.. Proteomic and metabolomic changes driven by elevating myocardial creatine suggest novel metabolic feedback mechanisms. Amino Acids 2016;48:1969–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL.. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 2001;103:1649–1655. [DOI] [PubMed] [Google Scholar]
- 75. Dessy C, Moniotte S, Ghisdal P, Havaux X, Noirhomme P, Balligand JL.. Endothelial beta3-adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium-dependent hyperpolarization. Circulation 2004;110:948–954. [DOI] [PubMed] [Google Scholar]
- 76. Liggett SB, Freedman NJ, Schwinn DA, Lefkowitz RJ.. Structural basis for receptor subtype-specific regulation revealed by a chimeric beta 3/beta 2-adrenergic receptor. Proc Natl Acad Sci USA 1993;90:3665–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gauthier C, Leblais V, Kobzik L, Trochu JN, Khandoudi N, Bril A, Balligand JL, Le Marec H.. The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 1998;102:1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Belge C, Hammond J, Dubois-Deruy E, Manoury B, Hamelet J, Beauloye C, Markl A, Pouleur AC, Bertrand L, Esfahani H, Jnaoui K, Götz KR, Nikolaev VO, Vanderper A, Herijgers P, Lobysheva I, Iaccarino G, Hilfiker-Kleiner D, Tavernier G, Langin D, Dessy C, Balligand JL.. Enhanced expression of β3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation 2014;129:451–462. [DOI] [PubMed] [Google Scholar]
- 79. Musialek P, Rigg L, Terrar DA, Paterson DJ, Casadei B.. Role of cGMP-inhibited phosphodiesterase and sarcoplasmic calcium in mediating the increase in basal heart rate with nitric oxide donors. J Mol Cell Cardiol 2000;32:1831–1840. [DOI] [PubMed] [Google Scholar]
- 80. Lee DI, Vahebi S, Tocchetti CG, Barouch LA, Solaro RJ, Takimoto E, Kass DA.. PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol 2010;105:337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Krüger M, Kötter S, Grützner A, Lang P, Andresen C, Redfield MM, Butt E, Dos Remedios CG, Linke WA.. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res 2009;104:87–94. [DOI] [PubMed] [Google Scholar]
- 82. Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B.. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res 2008;102:242–249. [DOI] [PubMed] [Google Scholar]
- 83. Hermida N, Michel L, Esfahani H, Dubois-Deruy E, Hammond J, Bouzin C, Markl A, Colin H, Steenbergen AV, De Meester C, Beauloye C, Horman S, Yin X, Mayr M, Balligand JL.. Cardiac myocyte β3-adrenergic receptors prevent myocardial fibrosis by modulating oxidant stress-dependent paracrine signaling. Eur Heart J 2018;39:888–898. [DOI] [PubMed] [Google Scholar]
- 84. Bundgaard H, Liu CC, Garcia A, Hamilton EJ, Huang Y, Chia KK, Hunyor SN, Figtree GA, Rasmussen HH.. β(3) adrenergic stimulation of the cardiac Na+-K+ pump by reversal of an inhibitory oxidative modification. Circulation 2010;122:2699–2708. [DOI] [PubMed] [Google Scholar]
- 85. Vanhoutte L, Guilbaud C, Martherus R, Bouzin C, Gallez B, Dessy C, Balligand JL, Moniotte S, Feron O.. MRI assessment of cardiomyopathy induced by β1-adrenoreceptor autoantibodies and protection through β3-adrenoreceptor overexpression. Sci Rep 2017;7:43951.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dessy C, Saliez J, Ghisdal P, Daneau G, Lobysheva II, Frérart F, Belge C, Jnaoui K, Noirhomme P, Feron O, Balligand JL.. Endothelial beta3-adrenoreceptors mediate nitric oxide-dependent vasorelaxation of coronary microvessels in response to the third-generation beta-blocker nebivolol. Circulation 2005;112:1198–1205. [DOI] [PubMed] [Google Scholar]
- 87. Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM.. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 2015;21:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu PP, Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, Kass DA, Shen YT, Vatner SF, Morgans DJ.. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science 2011;331:1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bakkehaug JP, Kildal AB, Engstad ET, Boardman N, Næsheim T, Rønning L, Aasum E, Larsen TS, Myrmel T, How OJ.. Myosin activator omecamtiv mecarbil increases myocardial oxygen consumption and impairs cardiac efficiency mediated by resting myosin ATPase activity. Circ Heart Fail 2015;8:766–775. [DOI] [PubMed] [Google Scholar]
- 90. Kampourakis T, Zhang X, Sun YB, Irving M.. Omecamtiv mercabil and blebbistatin modulate cardiac contractility by perturbing the regulatory state of the myosin filament. J Physiol 2018;596:31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kawas RF, Anderson RL, Ingle SRB, Song Y, Sran AS, Rodriguez HM.. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem 2017;292:16571–16577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, Henze M, Kawas R, Oslob JD, Rodriguez HM, Song Y, Wan W, Leinwand LA, Spudich JA, McDowell RS, Seidman JG, Seidman CE.. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 2016;351:617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I.. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 2013;123:2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kumarswamy R, Bauters C, Volkmann I, Maury F, Fetisch J, Holzmann A, Lemesle G, de Groote P, Pinet F, Thum T.. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res 2014;114:1569–1575. [DOI] [PubMed] [Google Scholar]
- 95. Vakrou S, Fukunaga R, Foster DB, Sorensen L, Liu Y, Guan Y, Woldemichael K, Pineda-Reyes R, Liu T, Tardiff JC, Leinwand LA, Tocchetti CG, Abraham TP, O’Rourke B, Aon MA, Abraham MR.. Allele-specific differences in transcriptome, miRNome, and mitochondrial function in two hypertrophic cardiomyopathy mouse models. JCI Insight 2018;3:94493. [DOI] [PMC free article] [PubMed] [Google Scholar]