Abstract
In metazoans, thousands of intracellular proteins are modified with O-linked β-N-acetylglucosamine (O-GlcNAc) in response to a wide range of stimuli and stresses. In particular, a complex and evolutionarily conserved interplay between O-GlcNAcylation and oxidative stress has emerged in recent years. Here, we review the current literature on the connections between O-GlcNAc and oxidative stress, with a particular emphasis on major signaling pathways, such as KEAP1/NRF2, FOXO, NFκB, p53 and cell metabolism. Taken together, this work sheds important light on the signaling functions of protein glycosylation and the mechanisms of stress responses alike and illuminates how the two are integrated in animal cell physiology.
Keywords: KEAP1, NRF2, O-GlcNAc, oxidative stress
Introduction
O-GlcNAcylation is a reversible form of glycosylation on serine and threonine side-chains of intracellular proteins in a wide variety of animal and plant species. In mammals, O-GlcNAc is added to substrates from a uridine diphosphate (UDP)-GlcNAc donor by a single O-GlcNAc transferase (OGT) and is removed by a single glycoside hydrolase, O-GlcNAcase (OGA). In many organisms, including mammals, O-GlcNAcylation is essential for cell viability and embryonic development. Consistent with this critical role, O-GlcNAc is thought to participate in a long list of cellular functions. For example, global O-GlcNAc levels fluctuate in response to many noxious stimuli, including heat shock, nutrient depletion, endoplasmic reticulum dysfunction and redox imbalance (Zachara et al. 2004; Zachara and Hart 2004; Groves et al. 2013; Reeves et al. 2014). Moreover, (de)glycosylation of particular substrates governs stress responses themselves, pointing to a complex, reciprocal interplay between stress pathways and O-GlcNAc signaling. However, in most cases, the regulatory mechanisms and most important O-GlcNAc substrates remain enigmatic. Here, we review the connections between O-GlcNAc and oxidative stress in metazoans as a prototypical example of stress signaling. Understanding this crosstalk is essential for building an integrated model of the oxidative stress response and also serves as a useful case study for elucidating how O-GlcNAcylation participates in a critical aspect of cellular homeostasis.
Oxidative stresses affect O-GlcNAcylation
In aerobic environments, cells frequently encounter stress caused by excessive oxidants, most prominently reactive oxygen species (ROS), including the superoxide anion, hydroxyl radicals and hydrogen peroxide (Schieber and Chandel 2014). Indeed, various species of ROS are generated by mitochondrial respiration, central metabolism and environmental stimuli (Holmstrom and Finkel 2014). ROS can act not only as signal transduction molecules, but can also damage proteins, nucleic acids and lipids, resulting in stress (Circu and Aw 2010; Panieri and Santoro 2016). The accumulation of oxidative damage and the resulting dysregulation of cellular processes increase the danger of genome instability, cell death and tumorigenesis (Cui 2012; Costa et al. 2014; Sies et al. 2017).
Animal cells have evolved several well-known mechanisms for defending against oxidative damage, including redox buffering by glutathione and NADP/NADPH, the regeneration of thioredoxin, the action of ROS-detoxifying enzymes, and the sequestration of iron, an essential redox-active metal (Gorrini et al. 2013; Panieri and Santoro 2016). Interestingly, the level of O-GlcNAcylation also responds to oxidative stress. For example, Hart and colleagues reported that treatment of COS7 cells or mouse embryonic fibroblasts (MEFs) with hydrogen peroxide or arsenite enhanced global O-GlcNAcylation (Zachara et al. 2004; Zachara and Hart 2004). Extending these observations to intact tissues, Peternelj et al. (2015) subsequently found that global O-GlcNAcylation is induced in rat white gastrocnemius muscle after exercise and recovery in the presence of diethyl maleate, which raises ROS levels by depleting glutathione. Moreover, the authors observed that exercise and recovery triggered fluctuations in the mRNA levels of OGT, OGA and isoforms of glutamine:fructose-6-phosphate amidotransferase (GFAT), the rate-limiting enzyme in the hexosamine biosynthetic pathway (HBP), though the mechanistic underpinnings of this relationship remain unclear (Peternelj et al. 2015). Conversely, manipulating O-GlcNAcylation can also alter intracellular ROS levels. For instance, Goldberg et al. (2011) showed that knockdown of OGT lowers hyperglycemia-triggered ROS in murine glomerular mesangial cells, perhaps in part through the modulation of the stress-induced MAP kinase p38. As these examples illustrate, the crosstalk between O-GlcNAcylation and oxidative stress signaling is complex and reciprocal. In the following sections, we discuss how O-GlcNAc responds to different oxidative stress stimuli and how O-GlcNAcylation impacts on phenotypic outcomes under oxidative stress conditions.
Hydrogen peroxide
In 2016, the Zachara group performed quantitative, proteome-wide profiling of hydrogen peroxide-induced changes in O-GlcNAc in MEFs (Lee et al. 2016). Importantly, O-GlcNAcylated proteins were affinity-enriched by a ‘G5 Lectibody’ approach, using a combination of O-GlcNAc-binding lectins and monoclonal antibodies to purify proteins of interest. In this work, hydrogen peroxide treatment increased the enrichment of ~26% of identified proteins, whereas 11% or 17% of identified proteins were depleted at 1- and 2-h time-points, respectively. In parallel, the authors showed that 2-h hydrogen peroxide increased the O-GlcNAcylation of high molecular weight proteins (>72 kDa). The differentially O-GlcNAcylated proteins identified by the authors participate in a range of cellular processes, including chromatin remodeling (SWItch/sucrose non fermenting complex), transcriptional control (mediator complex), RNA and stress granule biogenesis and post-translational signaling (14-3-3 family).
In recent complementary work, the same group used quantitative proteomics and a proximity biotinylation strategy to analyze the oxidative stress-dependent OGA interactome (Groves et al. 2017). Consistent with their prior study, the authors found that hydrogen peroxide treatment increased global O-GlcNAcylation in human U2OS osteosarcoma cells. Surprisingly, however, total OGA protein levels and activity increased under the same conditions. A possible explanation for this apparent paradox was provided by the discovery that hydrogen peroxide treatment promoted the interaction between OGA and the metabolic enzyme fatty acid synthase (FAS), reducing OGA activity by ~85%. These results suggest that hydrogen peroxide may downregulate global OGA activity through its sequestration by FAS. Indeed, the authors found that FAS overexpression further augments global hydrogen peroxide-induced O-GlcNAc levels. However, the biochemical details of OGA/FAS interaction, as well as the functional significance of most other hydrogen peroxide-induced changes to the OGA interactome, remain to be characterized.
Other groups have made analogous observations in complementary experimental systems. For example, Nagy and colleagues reported that a 2-h treatment of hydrogen peroxide modestly induced global O-GlcNAc levels and GFAT mRNA in SH-SY5Y neuroblastoma cells, suggesting that this response is conserved across multiple cell types (Katai et al. 2016). On the other hand, in retinal ganglion cells, a 1-h hydrogen peroxide treatment reduced global O-GlcNAcylation, but supplementation with glucosamine (which promotes UDP-GlcNAc biosynthesis and O-GlcNAcylation) reduced hydrogen peroxide-induced cell death, suggesting that the protective effects of O-GlcNAc may be more universal than the ability to regulate O-GlcNAc in response to this particular stimulus (Chen, Huang et al. 2015).
Given the pleiotropic effects of hydrogen peroxide, more work will be required to dissect the kinetics, dose-response relationship, cell type-specific events and key glycoprotein substrates that trigger protective, stress-induced increases in O-GlcNAc. However, one recent study provided a potentially important clue to this mode of signaling. Han et al. (2017) demonstrated that hydrogen peroxide treatment (as well as genotoxic or nutrient stress) induced the O-GlcNAcylation of the SIRT1 sirtuin deacetylase on Ser549. Interestingly, SIRT1 glycosylation potentiated its deacetylase activity and enhanced cellular stress resistance, at least in part by promoting the deacetylation of the tumor suppressor protein p53. While SIRT1 Ser549 glycosylation was essential for these effects, the authors found evidence of additional, as-yet unidentified O-GlcNAcylation sites on SIRT1 as well, suggesting that other stimuli or stresses may influence SIRT1 activity through glycosylation at distinct sites. Clearly, we have more to learn about the role of O-GlcNAcylation in hydrogen peroxide stress, with respect to both SIRT1 in particular and other, uncharacterized glycosylation changes in additional signaling pathways.
Hypoxia and ischemia
In any pathological condition of vascular insufficiency, including most solid tumors (Dewhirst and Chi 2013), hypoxia and ischemia are prominent features. After a hypoxic or ischemic event, tissue reperfusion and re-oxygenation increase oxidative stress, a disease-relevant example of redox imbalance (Giordano 2005). Changes in O-GlcNAc signaling have been implicated in this context as well. For example, the Jones group has addressed the pathophysiological connection between ischemia-induced ROS and O-GlcNAcylation in neonatal rat cardiac myocytes (NRCMs). In one study, the authors demonstrated that adenovirus-mediated overexpression of OGA (AdOGA) in NRCMs increased cytotoxicity after hypoxia/re-oxygenation, whereas inhibition of OGA by siRNA or the small molecule PUGNAc produced the opposite effect. These effects correlated with lower mitochondrial membrane potential and higher calcium overload in AdOGA-infected NRCMs, suggesting a possible mechanism for the cytoprotective effects of O-GlcNAcylation in this tissue (Ngoh et al. 2009). The same lab subsequently showed that global O-GlcNAcylation was reduced after 40 min of myocardial ischemia, but temporarily increased in the ischemic zone at 1 h after reperfusion. Similarly, O-GlcNAcylation in NRCMs was decreased soon after hypoxia but increased at 6 h after re-oxygenation. Interestingly, adenovirus-mediated OGT overexpression or PUGNAc treatment decreased ROS production after hypoxia/re-oxygenation or hydrogen peroxide treatment, whereas OGA overexpression produced the opposite result (Ngoh et al. 2011). This work confirms the protective effects of increased O-GlcNAcylation in a well-established model of cardiovascular disease.
The mechanisms linking ischemic insults to O-GlcNAc changes remain incompletely understood. However, one potentially important player is the hypoxia-inducible factor 1α (HIF-1 α), a transcription factor stabilized under low oxygen conditions. A report by the Reginato group (Ferrer et al. 2014) demonstrated that OGT facilitates HIF-1α stabilization and the expression of its target gene, GLUT1, during hypoxia in both in vitro and in vivo breast cancer models. Conversely, the authors found that OGT depletion raised the levels of several metabolites, including α-ketoglutarate (α-KG), which in turn destabilizes HIF-1α protein by proline hydroxylation, recruitment of the von Hippel–Lindau protein (VHL), and subsequent VHL-mediated degradation. Because of the importance of hypoxia and HIF-1α in a wide variety of human diseases, these results suggest that an OGT/HIF-1α regulatory connection may be important in diverse pathological contexts. Testing this possibility will be a priority for future studies.
Oxidative stress signaling pathways regulated by O-GlcNAc
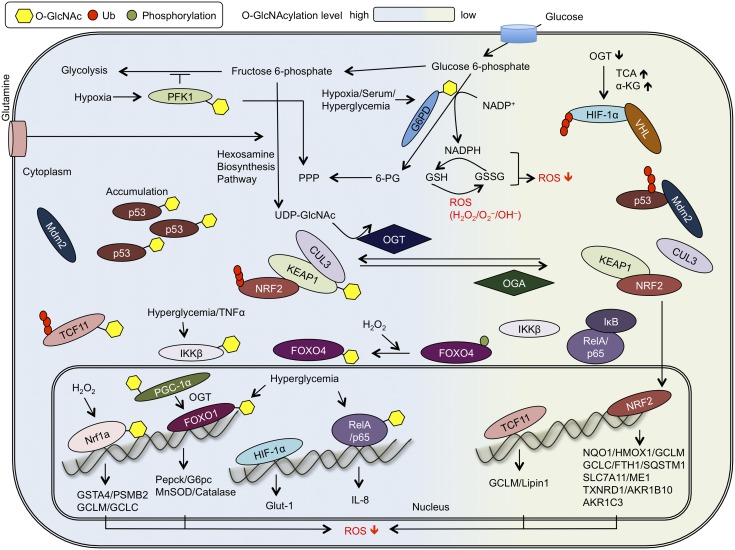
Here, we focus on individual signaling pathways directly regulated by O-GlcNAcylation during redox stress. To provide a broad perspective on how O-GlcNAc cycling affects redox homeostasis, we review studies across different aspects of the antioxidant response in diverse model systems (Figure 1).
Fig. 1.
O-GlcNAcylation regulates oxidative stress signaling pathways. Summary of selected connections among O-GlcNAcylation and major redox stress signaling pathways described in the text. OGT glycosylates PFK1 and G6PD, leading to the inhibition of glycolysis while promoting flux through the pentose phosphate pathway (PPP). O-GlcNAcylation of IKKβ and RelA/p65 enhances RelA/p65 transactivation activity. O-GlcNAcylation of p53 reduces its polyubiquitination via decreased interaction with MDM2. KEAP1 is likely constitutively O-GlcNAcylated to facilitate its interaction with CUL3 and subsequent NRF2 polyubiquitination. Upon KEAP1 deglycosylation, an NRF2-mediated antioxidant response is activated. OGT inhibition increases TCF11 protein level and target gene expression, and Nrf1a O-GlcNAcylation, stimulated by hydrogen peroxide exposure, also promotes its transcriptional activation. OGT inhibition increases HIF-1α polyubiquitination by VHL, suppressing HIF-1α signaling, whereas OGT overexpression increases the expression of the HIF-1α target gene GLUT1. FoxO4 O-GlcNAcylation is enhanced upon hydrogen peroxide treatment and FoxO1 O-GlcNAcylation promotes expression of stress response genes. Please see text for details and references.
Central carbon metabolism
Because O-GlcNAc is a nutrient-sensitive modification, it is perhaps no surprise that O-GlcNAcylation and redox signaling are intimately connected through core cellular metabolism, itself a major source of endogenous ROS. In one key example, Hsieh-Wilson and colleagues reported that the glycolytic enzyme phosphofructokinase 1 (PFK1) is an OGT substrate (Yi et al. 2012). PFK1 phosphorylates fructose-6-phosphate to generate fructose 1,6-biphosphate, which is then cleaved by aldolase to produce glyceraldehyde 3-phosphate for glycolysis. Increased PFK1 activity is associated with higher glycolytic flux (Yalcin et al. 2009; Mor et al. 2011) and several studies have shown that PFK1 activity is governed in part by various post-translational modifications (PTMs; Smerc et al. 2011, Yi et al. 2012, Li et al. 2016). Extending these observations, the Hsieh-Wilson Lab found that OGT overexpression or PUGNAc treatment decreased glycolytic rates, lactate production and PFK1 activity alike (Yi et al. 2012). In dissecting the mechanism of these observations, the authors found that O-GlcNAcylation of PFK1 at Ser529 affects its oligomerization and represses PFK1 activity under hypoxic conditions. Interestingly, O-GlcNAcylation of PFK1 on Ser529 redirects glucose flux from glycolysis to the pentose phosphate pathway (PPP), leading to higher levels of NADPH and reduced glutathione (GSH). Moreover, the authors also showed that OGT-overexpressing H1299 cells exhibited both lower ROS upon treatment with the oxidizing agent diamide and resistance to hydrogen peroxide-induced cell death. This mode of metabolic regulation may contribute to cancer cell growth and proliferation because the authors showed that ablation of PFK1 Ser529 glycosylation inhibited cancer cell division in vitro and tumor formation in vivo. Taken together, these results demonstrate that redox stress resistance can be regulated by the glycosylation of a key glycolytic enzyme, with important implications for understanding both normal cell physiology and cancer metabolism.
In another example of crosstalk, Rao et al. (2015) reported that glucose-6-phosphate dehydrogenase (G6PD) activity and oligomerization are also regulated by O-GlcNAc cycling. G6PD is the rate-limiting enzyme of the PPP, catalyzing the conversion of glucose-6-phosphate to 6-phosphogluconate. Therefore, G6PD contributes to the regeneration of NADPH, a major ROS scavenger. The authors found that OGT glycosylates G6PD at Ser84 and showed that A549 cells expressing a Ser84Val G6PD mutant (compared with WT control) exhibited lower PPP metabolite levels (e.g., 6-phosphogluconate), without affecting TCA or glycolytic metabolites. Overexpression of OGT in cells expressing WT G6PD increased the relative amounts of NADPH and GSH but failed to do so in S84V G6PD-expressing cells. Consistently, A549 cells expressing a Ser84Val G6PD mutant were sensitized to hydrogen peroxide, hypoxia and diamide, relative to WT-expressing controls. Importantly, the authors provided in vivo evidence that O-GlcNAcylation of G6PD is increased in human lung malignancies, suggesting that these observations, like those with PFK1 glycosylation, may have direct relevance to oncogenesis or cancer treatment.
The KEAP1/NRF2 axis
NRF2 is a transcription factor and a master regulator of the cellular response to oxidative stress and xenobiotics (Jaramillo and Zhang 2013; Menegon et al. 2016). In unstressed cells, NRF2 associates with a complex comprising the cytoplasmic KEAP1 adaptor protein and CUL3 E3 ubiquitin ligase, which ubiquitinates NRF2, targeting it for proteasome-mediated destruction (Jaramillo and Zhang 2013). During stress, various toxicants (e.g., electrophiles or oxidizing agents) covalently modify redox-active cysteines in KEAP1, impairing the ability of KEAP1/CUL3 to ubiquitinate NRF2 (Jaramillo and Zhang 2013; Menegon et al. 2016). Free NRF2 then migrates to the nucleus, where it binds to promoters containing antioxidant response elements (AREs) and upregulates antioxidant defense genes, such as the glutathione biosynthetic pathway, xenobiotic efflux pumps and drug-metabolizing enzymes (Hayes et al. 2010; Jaramillo and Zhang 2013; Menegon et al. 2016).
Several biochemical and functional connections between O-GlcNAcylation and NRF2 have been reported. For example, genetic knockdown of OGT induced NRF2 target gene expression in cultured human cell lines (Chu et al. 2014), human embryonic stem cells (Andres et al. 2017) and the murine forebrain (Wang et al. 2016). Consistent with these results, two other studies observed correlations between global O-GlcNAcylation, NRF2 levels and the cellular antioxidant response. In particular, α-lipoic acid (LA) treatment decreased global O-GlcNAcylation in the streptozotocin (STZ)-induced diabetic rat kidney (Arambasic et al. 2013) and rat liver (Dinic et al. 2013). Moreover, these authors observed an increase in NRF2 nuclear translocation and elevated mRNA/protein levels of antioxidant enzymes, such as manganese superoxide dismutase (SOD), copper/zinc SOD and catalase. Together, these reports hint at an inverse correlation between global O-GlcNAcylation and NRF2 signaling.
Consistent with this notion, a recent report by the Slawson group found that very long (3-week) treatment of SH-SY5Y neuroblastoma cells with the OGA inhibitor Thiamet-G and the UDP-GlcNAc precursor glucosamine (GlcN) reduced NRF2 protein and NRF2 target gene expression (Tan et al. 2017). Conversely, genetic ablation of OGT in the murine liver caused the opposite effect, with increased NRF2 levels and activity. In this case, the authors reported that increased global O-GlcNAcylation suppressed ROS levels while slightly increasing the NAD+/NADH ratio. These observations pointed to a likely functional connection between O-GlcNAc signaling and the NRF2 pathway but the relevant OGT substrates and biochemical mechanisms remained unclear (Tan et al. 2014, 2017).
In recent work, we unexpectedly discovered that the pharmacological inhibition or genetic knockdown of OGT in numerous human cell types elicits an antioxidant response by activating the NRF2 pathway (Chen, Chi et al. 2017; Chen, Smith et al. 2017). Interestingly, we did not observe increased ROS production or a reduced:oxidized glutathione imbalance upon OGT inhibition, suggesting that NRF2 activation is due to a specific O-GlcNAc-mediated signal, and not to nonspecific stress. Indeed, we found that KEAP1 physically interacts with OGT and is thereby O-GlcNAcylated under unstressed conditions at eleven candidate sites that we mapped by mass spectrometry. A panel of site-specific glycosylation-null mutants identified KEAP1 Ser104 as the most functionally relevant site. Abrogation of KEAP1 glycosylation, by inhibiting OGT or by mutating KEAP1 Ser104, reduced the interaction of KEAP1 with CUL3, resulting in a loss of NRF2 ubiquitination. Thus, our data indicate that, under homeostatic conditions, site-specific O-GlcNAcylation of KEAP1 at Ser104 is required for its optimal activity, mediating NRF2 ubiquitination and restraining the NRF2 pathway. Consistent with this idea, we also found that KEAP1 O-GlcNAcylation correlates with glucose availability, as hypoglycemic conditions triggered KEAP1 deglycosylation and subsequent NRF2 signaling. Inhibition of OGA blocked hypoglycemia-induced KEAP1 deglycosylation and prevented NRF2 induction, indicating that glucose availability is sensed in part through site-specific KEAP1 O-GlcNAcylation. Overall, these results revealed a new connection between nutrient sensing and redox metabolism through the conduit of KEAP1 glycosylation.
NRF2 belongs to the cap’n’collar (Cnc) protein family, which includes the related mammalian transcription factors NFE2, NRF1 and NRF3, as well as orthologs in other animals (Sykiotis and Bohmann 2010). Interestingly, the functional connections among O-GlcNAc and Cnc family transcription factors extend beyond NRF2 itself, and even beyond mammalian systems. For example, Hanover and colleagues first reported that SKN-1, the sole Cnc family member in Caenorhabditis elegans, translocated to nucleus in ogt-1(ok430)-null worms with kinetics similar to that of sodium azide-induced SKN-1 nuclear translocation in a WT strain (Hanover et al. 2005). Like mammalian NRF2, nematode SKN-1 responds to oxidative stress to upregulate the expression of detoxifying enzymes (Walker et al. 2000, An and Blackwell 2003, An et al. 2005; Inoue et al. 2005). However, no KEAP1 ortholog has been identified in C. elegans, suggesting that the regulation of SKN-1 and NRF2 by O-GlcNAc are likely distinct. It was reported previously that DAF-2 (insulin/insulin-like growth factor receptor) activation facilitates AKT-mediated phosphorylation of SKN-1 and inhibits SKN-1 from translocating to the nucleus (Cohen and Dillin 2008; Tullet et al. 2008), keeping the pathway off. Interestingly, Li et al. (2017) subsequently found that SKN-1 is O-GlcNAcylated. In this work, the authors discovered that O-GlcNAc cycling also regulates SKN-1 nuclear localization, demonstrating that SKN-1 mislocalizes in ogt-1(ok1474) or oga-1(ok1207) null worms upon treatment with the ROS inducer tert-butyl hydroperoxide. The translocation of SKN-1 and expression of its target genes (e.g., gcs-1, gst-4 and gst-7) are induced in oga-null worms. Importantly, SKN-1 interacts with OGT and is O-GlcNAcylated at Ser470 and Thr493, and reconstitution of skn-1-null worms with an S470A/T493A SKN-1 mutant reduced lifespan under oxidative stress conditions. These effects may be due to a lost regulatory interplay between glycosylation and phosphorylation because the authors demonstrated that phosphorylation of SKN-1 at Ser483 by GSK-3 is repressed by its oxidative stress-induced O-GlcNAcylation.
Two recent reports showed that mammalian NRF1 is glycosylated as well (Chen, Liu et al. 2015; Han et al. 2017). NRF1 (NFE2L1) is expressed as several isoforms, including NRF1a, NRF1b, LCRF1 and TCF11. Like NRF2, NRF1 interacts with ARE-containing promoters, but the PTMs of NRF1 and NRF2 are distinct. Chen, Liu et al. (2015) reported that OGT interacts with TCF11 and promotes its ubiquitination in 293T cells. Genetic depletion of OGT increased both TCF11 protein level and the mRNA and protein of its downstream targets GCLM and lipin1, whereas OGT overexpression decreased TCF11 protein. These results suggest that the NRF1 isoform TCF11 is O-GlcNAcylated, but specific glycosylation sites have not yet been mapped. In separate work, Han et al. (2017) found that NRF1a interacts with OGT and the heavily glycosylated transcriptional coactivator host cell factor 1 (HCF1). NRF1a is O-GlcNAcylated, especially under oxidative stress conditions (i.e., arsenic or tert-butylhydroquinone treatment), although, once again, the glycosylated residues have not been pinpointed. Nevertheless, these observations likely have functional significance, because NRF1a polyubiquitination was reduced and its half-life increased by transient overexpression of OGT or PUGNAc treatment, both of which boost global O-GlcNAc levels. Interestingly, several recent reports have also suggested a role for ER lumenal glycosylation of NRF1 in regulating its function (Radhakrishnan et al. 2014; Tomlin et al. 2017; Widenmaier et al. 2017). It remains unknown whether and how the distinct O-GlcNAcylation and secretory pathway glycosylation of NRF1 are coordinated.
Taken together, these studies indicate an evolutionarily conserved connection between O-GlcNAc cycling and redox stress signaling through the Cnc transcription factor family. It is tempting to speculate that this mode of regulation has been preserved from worms to mammals in the case of SKN-1/NRF1 glycosylation, whereas the analogous regulation of the NRF2 pathway has shifted to the O-GlcNAcylation of KEAP1, an upstream regulator in mammals that is absent from C. elegans. Testing this model would be an interesting object of future study.
The FOXO family
The mammalian forkhead box (FOX) class O transcription factors, including FoxO1, FoxO3, FoxO4 and FoxO6, are important regulators of oxidative stress signaling and glucose metabolism (Nakae et al. 2008). As with NRF2, crosstalk among FOXO family members and O-GlcNAcylation is an evolutionarily conserved feature of redox stress signaling. In C. elegans, DAF-16 (an ortholog of FoxO) was found to be inhibited by the insulin/IGF-1-like signaling pathway. In the presence of insulin-like peptide, DAF-2 is activated and triggers a phosphorylation cascade involving AGE-1, PDK1, AKT1/2, and DAF-16. Phosphorylation of DAF-16 prevents its nuclear accumulation and thereby blocks the upregulation of its target genes. Interestingly, several studies reported genetic interactions between OGT and the insulin/IGF-1-like signaling pathway in C. elegans (Hanover et al. 2005; Forsythe et al. 2006; Lee et al. 2010; Love et al. 2010; Rahman et al. 2010). For instance, Hanover and colleagues found that dauer formation in daf-2 worms is affected by O-GlcNAc cycling in a temperature-sensitive manner (Hanover et al. 2005; Forsythe et al. 2006). In addition, multiple groups reported a connection between O-GlcNAc cycling and lifespan, a phenotype heavily influenced by DAF-2/DAF-16 signaling. Rahman et al. (2010) reported that ogt-1 mutant animals have a shorter lifespan and Love et al. (2010) discovered that oga-1 mutation extended lifespan. Importantly, both effects were suppressed in a daf-16 mutant background. Moreover, daf-16;oga-1 double mutant animals exhibited reduced survival after treatment with the ROS inducer paraquat, as compared with single oga-1 mutants. Similarly, ogt-1;daf-2 double mutant animals lost the survival advantage of daf-2 single mutants under oxidative stress conditions. These results indicate that genetic interactions between OGT/OGA and the DAF-2/DAF-16 pathway influence lifespan and oxidative stress resistance. However, DAF-16 nuclear localization is slightly increased in both ogt-1 and oga-1 mutants, and whether DAF-16 is directly O-GlcNAcylated in worms remains unclear. Complementary biochemical and genetic experiments will be required to elucidate the precise mechanism by which O-GlcNAcylation regulates DAF-16 activity in C. elegans.
Hyperglycemia is a prominent feature of uncontrolled diabetes and glucose control is one of the best strategies to prevent long-term diabetic complications. One prevailing hypothesis is that dysregulated O-GlcNAcylation may contribute directly to hyperglycemia-induced diabetic symptoms. Indeed, hyperglycemia is known to induce mitochondrial superoxide and activate the hexosamine biosynthesis pathway (Du et al. 2000; Nishikawa et al. 2000). Interestingly, insulin resistance can be recapitulated by elevating O-GlcNAcylation level as well (Marshall et al. 1991; McClain et al. 2002; Vosseller et al. 2002). Furthermore, the O-GlcNAc/FoxO axis may underlie these observations. For example, Housley et al. (2008) found that the DAF-16 ortholog FoxO1 is O-GlcNAcylated at on several residues in Fao rat hepatoma cells. In this context, OGT enhances FoxO1 transactivation via O-GlcNAcylation at Thr317. FoxO1 target genes involved in gluconeogenesis (e.g., phosphoenolpyruvate carboxykinase (Pepck) and the Glucose-6-Phosphatase catalytic subunit (G6pc)) and the oxidative stress response (e.g., MnSOD and catalase) are also induced upon exposure to high glucose (25 mM). Notably, while high glucose enhances O-GlcNAcylation of FoxO1, insulin treatment reduces its O-GlcNAcylation. The authors also discovered that the transcriptional coactivator PGC1α, which binds directly to FoxO1, is O-GlcNAcylated at Ser333. The PGC1α/OGT complex promotes O-GlcNAcylation of FoxO1 and FoxO3 (Housley et al. 2009), but the functional relevance of these observations is not yet certain. In parallel, another study suggested that FoxO4 is O-GlcNAcylated in 293 cells, though the specific O-GlcNAc sites were not identified (Ho et al. 2010). In this system, the authors found that hydrogen peroxide treatment induces an OGT/FoxO4 interaction, resulting in increased FoxO4 glycosylation and decreased FoxO4 phosphorylation at Ser193, a known AKT target site (Brownawell et al. 2001). In addition, OGT expression promotes FoxO4 transactivation activity while OGA expression decreases it, suggesting a direct functional connection between FoxO4 and O-GlcNAc cycling. Additional biochemical and cellular studies will be necessary to understand the molecular details and complex interplay between O-GlcNAcylation and phosphorylation in regulating FoxO activity during oxidative stress.
NFκB
The canonical NFκB transcriptional pathway is induced by pro-inflammatory molecules, such as tumor necrosis factor α (TNFα) and interleukin-1 (IL-1). Activation of the TNF or IL-1 receptor induces phosphorylation of the IKK (IκB kinase, an inhibitor of κB kinase) complex, which leads to the subsequent phosphorylation and degradation of IκB. The RelA (also called p65)/p50 complex (or RelB/p52, in an alternative pathway) then dissociates from IκB and translocates into nucleus to induce the expression of cytokines, chemokines, adhesion molecules and antioxidant enzymes (Lawrence 2009).
In addition to pro-inflammatory signals, the NFκB pathway is also regulated by ROS through PTMs on NFκB subunits. For example, oxidation or glutathionylation of p50 at Cys62 reduces its DNA binding activity (Pineda-Molina et al. 2001; Morgan and Liu 2011). More recently, O-GlcNAc has been shown to regulate the NFκB pathway as well. For instance, Yang et al. (2008) reported that RelA/p65 is O-GlcNAcylated in rat vascular smooth muscle cells and MEFs. OGA overexpression reduced NFκB p65 transactivation activity, whereas OGT overexpression increased it. The authors further found that RelA/p65 O-GlcNAcylation at Thr352 regulates its interaction with IκBα and transcriptional activity. In subsequent work, Allison et al. (2012) also reported that RelA/p65 is O-GlcNAcylated in 293T cells, with specific O-GlcNAc sites regulating its transactivation activity by affecting the acetylation of RelA by p300. The authors showed that glycosylation of RelA/p65 Thr305 affects its acetylation on Lys310. NFκB transactivation activity was repressed by silencing OGT in the presence of TNFα stimulation, and cells expressing Thr205Ala or Lys310Arg mutant RelA/p65 were sensitized to TNFα or etoposide, as compared with cells expressing WT RelA/p65. Consistent with these results, another study found that hyper-O-GlcNAcylation of RelA/p65 confers resistance to apoptosis in pancreatic cells (Ma et al. 2013). Together, these studies demonstrate a clear functional connection between NFκB protein O-GlcNAcylation and downstream stress resistance.
Other components of the NFκB pathway are regulated by O-GlcNAc signaling as well. For example, Kawauchi et al. (2009) reported that IKKβ is O-GlcNAcylated at Ser733. Similarly, Ramakrishnan et al. (2013) showed that c-Rel is O-GlcNAcylated at Ser350 in lymphocytes, and this modification influences the binding of c-Rel to DNA containing the CD28 response element. We have also observed the concerted transcriptional downregulation of several NFκB target genes (e.g., IL-8 and COX-2) in response to OGT inhibition (Chen, Smith et al. 2017), though the relevant OGT substrate(s) in this case remain unidentified.
In sum, these studies demonstrate that multiple components of the NFκB pathway are directly regulated by O-GlcNAcylation, with functional relevance for gene expression and phenotypic responses. Although NFκB signaling is well-known to influence cell survival in response to redox stress and other noxious stimuli, more work will be required to determine whether the influence of O-GlcNAc cycling is critical for NFκB-mediated redox stress resistance in particular.
p53
ROS are a major cause of DNA damage, leading to the activation of the tumor suppressor protein p53 (Sablina et al. 2005; Liu and Xu 2011). p53 induces a variety of transcription-dependent and -independent cellular responses, including DNA repair, cell cycle arrest, apoptosis, and ferroptosis (Kastenhuber and Lowe 2017). p53 activity is tightly regulated, and PTMs provide one important layer of control (Dai and Gu 2010). Yang et al. (2006) first reported that p53 is O-GlcNAcylated at Ser149 in human breast cancer cells. The nonspecific OGA inhibitor streptozotocin (STZ) enhanced p53 O-GlcNAcylation and reduced its polyubiquitination by interrupting its binding to MDM2, an E3 ubiquitin ligase. Cells expressing a Ser149Ala mutant of p53 exhibited moderate resistance to STZ treatment combined with the genotoxic drug doxorubicin, as compared with doxorubicin alone. In more recent, complementary work, Shtraizent et al. (2017) discovered a novel form of crosstalk between p53 O-GlcNAcylation and the metabolic enzyme mannose phosphate isomerase (MPI) in zebrafish. MPI interconverts mannose-6-phosphate and fructose-6-phosphate, regulating the levels of a key upstream substrate for the HBP. The authors found that p53 accumulated when MPI was depleted in zebrafish, MEFs, and human liver cancer cells, and this effect was caused by increased flux through the HBP and subsequent p53 O-GlcNAcylation.
Although these studies point to a functional connection between O-GlcNAcylation and p53, the role of this mode of regulation in the redox stress response remains to be investigated. Interestingly, however, O-GlcNAcylation is reported to regulate several DNA damage-sensing and repair pathways beyond p53, suggesting that O-GlcNAc may be relevant in the p53 response to diverse stimuli (Yang et al. 2006; Miura et al. 2012; Chen and Yu 2016). This possibility will be an important focus for future studies.
Concluding remarks
Many studies have demonstrated that critical regulators of redox homeostasis are governed by dynamic O-GlcNAcylation in a wide range of tissues, organisms and pathophysiological contexts. Moreover, changes in O-GlcNAcylation can decisively affect phenotypic outcomes under redox stress conditions. Together, these observations point to a complex and reciprocal interplay between a nutrient-sensitive form of glycosylation and a stress caused largely by byproducts of aerobic metabolism.
However, it remains incompletely understood how O-GlcNAc signaling integrates metabolic and oxidative stress cues to respond to noxious stimuli. Though some key O-GlcNAcylated substrates have been discovered, more work is needed to determine the complete ‘parts list’ of glycoproteins that transduce these signals. In addition, a better understanding is needed of the kinetics and stoichiometry of dynamic O-GlcNAc cycling during oxidative stress, both on specific substrates of interest, and globally within cells and tissues. We expect that recent advances in chemical and mass spectrometry-based technologies will greatly accelerate these efforts (Tarrant et al. 2012; Myers et al. 2013; Raj et al. 2016). Indeed, future studies may provide a more comprehensive understanding of the role of O-GlcNAc in oxidative stress signaling and suggest new opportunities to manipulate these responses for therapeutic benefit in diseases as diverse as cancer, diabetes and neurodegeneration.
Abbreviations
- O-GlcNAc
O-linked β-N-acetylglucosamine
- OGT
O-GlcNAc transferase
- OGA
O-GlcNAcase
- ROS
reactive oxygen species
- MEF
mouse embryonic fibroblast
- GFAT
glutamine:fructose-6-phosphate amidotransferase
- HBP
hexosamine biosynthetic pathway
- FAS
fatty acid synthase
- NRCMs
neonatal rat cardiac myocytes
- AdOGA
adenovirus-mediated overexpression of OGA
- HIF-1 α
hypoxia-inducible factor 1α
- α-KG
α-ketoglutarate
- VHL
von Hippel–Lindau protein
- PPP
pentose phosphate pathway
- PFK1
phosphofructokinase 1
- G6PD
glucose-6-phosphate dehydrogenase
- AREs
antioxidant response elements
- SOD
superoxide dismutase
- HCF1
host cell factor 1
- FOX
forkhead box
- Pepck
phosphoenolpyruvate carboxykinase
- TNFα
tumor necrosis factor α
- STZ
streptozotocin
- MPI
mannose phosphate isomerase.
Funding
Research in the Boyce Lab has been supported by the Rita Allen Foundation, the Sidney Kimmel Foundation, the Concern Foundation, the Mizutani Foundation, the Duke Cancer Institute and National Institute of General Medical Sciences (1R01GM118847 and 1R01GM117473). Research in the Chi Lab has been supported by the Duke Cancer Institute, the Department of Defense (W81XWH-12-1-0148, W81XWH-14-1-0309 and W81XWH-15-1-0486) and National Cancer Institute (1R01CA125618). Po-Han Chen is a Hung-Taiwan Duke fellow.
Conflict of interest statement
None declared.
References
- Allison DF, Wamsley JJ, Kumar M, Li D, Gray LG, Hart GW, Jones DR, Mayo MW. 2012. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-kappaB acetylation and transcription. Proc Natl Acad Sci U S A. 109:16888–16893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JH, Blackwell TK. 2003. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 17:1882–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, Blackwell TK. 2005. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci U S A. 102:16275–16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres LM, Blong IW, Evans AC, Rumachik NG, Yamaguchi T, Pham ND, Thompson P, Kohler JJ, Bertozzi CR. 2017. Chemical modulation of protein O-GlcNAcylation via OGT inhibition promotes human neural cell differentiation. ACS Chem Biol. 12:2030–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arambasic J, Mihailovic M, Uskokovic A, Dinic S, Grdovic N, Markovic J, Poznanovic G, Bajec D, Vidakovic M. 2013. Alpha-lipoic acid upregulates antioxidant enzyme gene expression and enzymatic activity in diabetic rat kidneys through an O-GlcNAc-dependent mechanism. Eur J Nutr. 52:1461–1473. [DOI] [PubMed] [Google Scholar]
- Brownawell AM, Kops GJ, Macara IG, Burgering BM. 2001. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol Cell Biol. 21:3534–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-H, Chi J-T, Boyce M. 2017. KEAP1 has a sweet spot: a new connection between intracellular glycosylation and redox stress signaling in cancer cells. Mol Cell Oncol. 4:e1361501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Huang YS, Chen JT, Chen YH, Tai MC, Chen CL, Liang CM. 2015. Protective effects of glucosamine on oxidative-stress and ischemia/reperfusion-induced retinal injury. Invest Ophthalmol Vis Sci. 56:1506–1516. [DOI] [PubMed] [Google Scholar]
- Chen J, Liu X, Lu F, Liu X, Ru Y, Ren Y, Yao L, Zhang Y. 2015. Transcription factor Nrf1 is negatively regulated by its O-GlcNAcylation status. FEBS Lett. 589:2347–2358. [DOI] [PubMed] [Google Scholar]
- Chen PH, Smith TJ, Wu J, Siesser PF, Bisnett BJ, Khan F, Hogue M, Soderblom E, Tang F, Marks JR et al. 2017. Glycosylation of KEAP1 links nutrient sensing to redox stress signaling. EMBO J. 36:2233–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yu X. 2016. OGT restrains the expansion of DNA damage signaling. Nucleic Acids Res. 44:9266–9278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CS, Lo PW, Yeh YH, Hsu PH, Peng SH, Teng YC, Kang ML, Wong CH, Juan LJ. 2014. O-GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci U S A. 111:1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu ML, Aw TY. 2010. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 48:749–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Dillin A. 2008. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 9:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. 2014. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 25:23–32. [DOI] [PubMed] [Google Scholar]
- Cui X. 2012. Reactive oxygen species: the achilles’ heel of cancer cells? Antioxid Redox Signal. 16:1212–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Gu W. 2010. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol Med. 16:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst MW, Chi JT. 2013. Understanding the tumor microenvironment and radioresistance by combining functional imaging with global gene expression. Semin Radiat Oncol. 23:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinic S, Arambasic J, Mihailovic M, Uskokovic A, Grdovic N, Markovic J, Karadzic B, Poznanovic G, Vidakovic M. 2013. Decreased O-GlcNAcylation of the key proteins in kinase and redox signalling pathways is a novel mechanism of the beneficial effect of alpha-lipoic acid in diabetic liver. Br J Nutr. 110:401–412. [DOI] [PubMed] [Google Scholar]
- Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. 2000. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 97:12222–12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN, Reginato MJ. 2014. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell. 54:820–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ME, Love DC, Lazarus BD, Kim EJ, Prinz WA, Ashwell G, Krause MW, Hanover JA. 2006. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc Natl Acad Sci U S A. 103:11952–11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano FJ. 2005. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 115:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg H, Whiteside C, Fantus IG. 2011. O-linked beta-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am J Physiol Endocrinol Metab. 301:E713–E726. [DOI] [PubMed] [Google Scholar]
- Gorrini C, Harris IS, Mak TW. 2013. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 12:931–947. [DOI] [PubMed] [Google Scholar]
- Groves JA, Lee A, Yildirir G, Zachara NE. 2013. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones. 18:535–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JA, Maduka AO, O’Meally RN, Cole RN, Zachara NE. 2017. Fatty acid synthase inhibits the O-GlcNAcase during oxidative stress. J Biol Chem. 292:6493–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Gu Y, Shan H, Mi W, Sun J, Shi M, Zhang X, Lu X, Han F, Gong Q et al. 2017. a. O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat Commun. 8:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JW, Valdez JL, Ho DV, Lee CS, Kim HM, Wang X, Huang L, Chan JY. 2017. b. Nuclear factor-erythroid-2 related transcription factor-1 (Nrf1) is regulated by O-GlcNAc transferase. Free Radic Biol Med. 110:196–205. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Forsythe ME, Hennessey PT, Brodigan TM, Love DC, Ashwell G, Krause M. 2005. A Caenorhabditis elegans model of insulin resistance: altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci U S A. 102:11266–11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. 2010. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 13:1713–1748. [DOI] [PubMed] [Google Scholar]
- Ho SR, Wang K, Whisenhunt TR, Huang P, Zhu X, Kudlow JE, Paterson AJ. 2010. O-GlcNAcylation enhances FOXO4 transcriptional regulation in response to stress. FEBS Lett. 584:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom KM, Finkel T. 2014. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 15:411–421. [DOI] [PubMed] [Google Scholar]
- Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. 2008. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 283:16283–16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, Hart GW. 2009. A PGC-1alpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem. 284:5148–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. 2005. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 19:2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo MC, Zhang DD. 2013. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 27:2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber ER, Lowe SW. 2017. Putting p53 in Context. Cell. 170:1062–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katai E, Pal J, Poor VS, Purewal R, Miseta A, Nagy T. 2016. Oxidative stress induces transient O-GlcNAc elevation and tau dephosphorylation in SH-SY5Y cells. J Cell Mol Med. 20:2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi K, Araki K, Tobiume K, Tanaka N. 2009. Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci U S A. 106:3431–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T. 2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kim KY, Lee J, Paik YK. 2010. Regulation of Dauer formation by O-GlcNAcylation in Caenorhabditis elegans. J Biol Chem. 285:2930–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Miller D, Henry R, Paruchuri VD, O’Meally RN, Boronina T, Cole RN, Zachara NE. 2016. Combined antibody/lectin-enrichment identifies extensive changes in the O-GlcNAc sub-proteome upon oxidative stress. J Proteome Res. 15:4318–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu X, Wang D, Su L, Zhao T, Li Z, Lin C, Zhang Y, Huang B, Lu J et al. 2017. O-GlcNAcylation of SKN-1 modulates the lifespan and oxidative stress resistance in Caenorhabditis elegans. Sci Rep. 7:43601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TY, Sun Y, Liang Y, Liu Q, Shi Y, Zhang CS, Zhang C, Song L, Zhang P, Zhang X et al. 2016. ULK1/2 constitute a bifurcate node controlling glucose metabolic fluxes in addition to autophagy. Mol Cell. 62:359–370. [DOI] [PubMed] [Google Scholar]
- Liu D, Xu Y. 2011. p53, oxidative stress, and aging. Antioxid Redox Signal. 15:1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love DC, Ghosh S, Mondoux MA, Fukushige T, Wang P, Wilson MA, Iser WB, Wolkow CA, Krause MW, Hanover JA. 2010. Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proc Natl Acad Sci U S A. 107:7413–7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Vocadlo DJ, Vosseller K. 2013. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem. 288:15121–15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S, Bacote V, Traxinger RR. 1991. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 266:4706–4712. [PubMed] [Google Scholar]
- McClain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. 2002. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci U S A. 99:10695–10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon S, Columbano A, Giordano S. 2016. The dual roles of NRF2 in cancer. Trends Mol Med. 22:578–593. [DOI] [PubMed] [Google Scholar]
- Miura Y, Sakurai Y, Endo T. 2012. O-GlcNAc modification affects the ATM-mediated DNA damage response. Biochim Biophys Acta. 1820:1678–1685. [DOI] [PubMed] [Google Scholar]
- Mor I, Cheung EC, Vousden KH. 2011. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol. 76:211–216. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Liu ZG. 2011. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 21:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SA, Daou S, Affar el B, Burlingame A. 2013. Electron transfer dissociation (ETD): the mass spectrometric breakthrough essential for O-GlcNAc protein site assignments-a study of the O-GlcNAcylated protein host cell factor C1. Proteomics. 13:982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae J, Oki M, Cao Y. 2008. The FoxO transcription factors and metabolic regulation. FEBS Lett. 582:54–67. [DOI] [PubMed] [Google Scholar]
- Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. 2009. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res. 104:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoh GA, Watson LJ, Facundo HT, Jones SP. 2011. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 40:895–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP et al. 2000. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 404:787–790. [DOI] [PubMed] [Google Scholar]
- Panieri E, Santoro MM. 2016. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 7:e2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peternelj TT, Marsh SA, Strobel NA, Matsumoto A, Briskey D, Dalbo VJ, Tucker PS, Coombes JS. 2015. Glutathione depletion and acute exercise increase O-GlcNAc protein modification in rat skeletal muscle. Mol Cell Biochem. 400:265–275. [DOI] [PubMed] [Google Scholar]
- Pineda-Molina E, Klatt P, Vazquez J, Marina A, Garcia de Lacoba M, Perez-Sala D, Lamas S. 2001. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 40:14134–14142. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, den Besten W, Deshaies RJ. 2014. p97-dependent retrotranslocation and proteolytic processing govern formation of active Nrf1 upon proteasome inhibition. eLife. 3:e01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Stuchlick O, El-Karim EG, Stuart R, Kipreos ET, Wells L. 2010. Intracellular protein glycosylation modulates insulin mediated lifespan in C.elegans. Aging (Albany NY). 2:678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj R, Lercher L, Mohammed S, Davis BG. 2016. Synthetic nucleosomes reveal that GlcNAcylation modulates direct interaction with the FACT complex. Angew Chem, Int Ed Engl. 55:8918–8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan P, Clark PM, Mason DE, Peters EC, Hsieh-Wilson LC, Baltimore D. 2013. Activation of the transcriptional function of the NF-kappaB protein c-Rel by O-GlcNAc glycosylation. Sci Signal. 6:ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X, Duan X, Mao W, Li X, Li Z, Li Q, Zheng Z, Xu H, Chen M, Wang PG et al. 2015. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat Commun. 6:8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RA, Lee A, Henry R, Zachara NE. 2014. Characterization of the specificity of O-GlcNAc reactive antibodies under conditions of starvation and stress. Anal Biochem. 457:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. 2005. The antioxidant function of the p53 tumor suppressor. Nat Med. 11:1306–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. 2014. ROS function in redox signaling and oxidative stress. Curr Biol. 24:R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtraizent N, DeRossi C, Nayar S, Sachidanandam R, Katz LS, Prince A, Koh AP, Vincek A, Hadas Y, Hoshida Y et al. 2017. MPI depletion enhances O-GlcNAcylation of p53 and suppresses the Warburg effect. eLife. 6:e22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H, Berndt C, Jones DP. 2017. Oxidative stress. Annu Rev Biochem. 86:715–748. [DOI] [PubMed] [Google Scholar]
- Smerc A, Sodja E, Legisa M. 2011. Posttranslational modification of 6-phosphofructo-1-kinase as an important feature of cancer metabolism. PLoS One. 6:e19645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis GP, Bohmann D. 2010. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci Signal. 3:re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EP, McGreal SR, Graw S, Tessman R, Koppel SJ, Dhakal P, Zhang Z, Machacek M, Zachara NE, Koestler DC et al. 2017. Sustained O-GlcNAcylation reprograms mitochondrial function to regulate energy metabolism. J Biol Chem. 292:14940–14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EP, Villar MT, Lu EL, Selfridge J, Artigues JE, Swerdlow A, Slawson C. RH. 2014. Altering O-linked beta-N-acetylglucosamine cycling disrupts mitochondrial function. J Biol Chem. 289:14719–14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant MK, Rho HS, Xie Z, Jiang YL, Gross C, Culhane JC, Yan G, Qian J, Ichikawa Y, Matsuoka T et al. 2012. Regulation of CK2 by phosphorylation and O-GlcNAcylation revealed by semisynthesis. Nat Chem Biol. 8:262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin FM, Gerling-Driessen UIM, Liu Y, Flynn RA, Vangala JR, Lentz CS, Clauder-Muenster S, Jakob P, Mueller WF, Ordoñez-Rueda D et al. 2017. Inhibition of NGLY1 inactivates the transcription factor Nrf1 and potentiates proteasome inhibitor cytotoxicity. ACS Cent Sci. 3:1143–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. 2008. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 132:1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosseller K, Wells L, Lane MD, Hart GW. 2002. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 99:5313–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, See R, Batchelder C, Kophengnavong T, Gronniger JT, Shi Y, Blackwell TK. 2000. A conserved transcription motif suggesting functional parallels between Caenorhabditis elegans SKN-1 and Cap’n’Collar-related basic leucine zipper proteins. J Biol Chem. 275:22166–22171. [DOI] [PubMed] [Google Scholar]
- Wang AC, Jensen EH, Rexach JE, Vinters HV, Hsieh-Wilson LC. 2016. Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration. Proc Natl Acad Sci U S A. 113:15120–15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenmaier SB, Snyder NA, Nguyen TB, Arduini A, Lee GY, Arruda AP, Saksi J, Bartelt A, Hotamisligil GS. 2017. NRF1 Is an ER Membrane Sensor that Is Central to Cholesterol Homeostasis. Cell. 171:1094–1109 e1015. [DOI] [PubMed] [Google Scholar]
- Yalcin A, Telang S, Clem B, Chesney J. 2009. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 86:174–179. [DOI] [PubMed] [Google Scholar]
- Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW. 2006. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 8:1074–1083. [DOI] [PubMed] [Google Scholar]
- Yang WH, Park SY, Nam HW, Kim DH, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW. 2008. NFkappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci U S A. 105:17345–17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA 3rd, Peters EC, Driggers EM, Hsieh-Wilson LC. 2012. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 337:975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachara NE, Hart GW. 2004. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 1673:13–28. [DOI] [PubMed] [Google Scholar]
- Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. 2004. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 279:30133–30142. [DOI] [PubMed] [Google Scholar]