Abstract
Background
Numerous studies have found increased risk of Clostridium difficile infection (CDI) with increasing age. We hypothesized that increased CDI risk in an elderly population is due to poorer overall health status with older age.
Methods
A total of 174 903 persons aged 66 years and older coded for CDI in 2011 were identified using Medicare claims data. The comparison population consisted of 1 453 867 uninfected persons. Potential risk factors for CDI were identified in the prior 12 months and organized into categories, including infections, acute noninfectious conditions, chronic comorbidities, frailty indicators, and health care utilization. Multivariable logistic regression models with CDI as the dependent variable were used to determine the categories with the biggest impact on model performance.
Results
Increasing age was associated with progressively increasing risk of CDI in univariate analysis, with 5-fold increased risk of CDI in 94–95-year-old persons compared with those aged 66–67 years. Independent risk factors for CDI with the highest effect sizes included septicemia (odds ratio [OR], 4.1), emergency hospitalization(s) (OR, 3.9), short-term skilled nursing facility stay(s) (OR, 2.7), diverticulitis (OR, 2.2), and pneumonia (OR, 2.1). Exclusion of age from the full model had no impact on model performance. Exclusion of acute noninfectious conditions followed by frailty indicators resulted in lower c-statistics and poor model fit. Further exclusion of health care utilization variables resulted in a large drop in the c-statistic.
Conclusions
Age did not improve CDI risk prediction after controlling for a wide variety of infections, other acute conditions, frailty indicators, and prior health care utilization.
Keywords: age, Clostridium difficile, epidemiology, Medicare, risk factor
Clostridium difficile is the most common pathogen causing health care–acquired infections and the leading cause of death associated with gastroenteritis in the United States [1, 2]. The incidence of C. difficile infection (CDI) during an acute care hospital stay increased about 2.7-fold between 2000 and 2012, based on the Healthcare Cost and Utilization Project Nationwide Inpatient Sample [3]. CDI was associated with more than 29 000 deaths in 2011, with an attributable mortality ranging from 5.7% in endemic settings to 16.7% in severe outbreaks since 2000 [4].
Age is considered one of the primary risk factors for CDI in general [5–8] and for severe CDI [9–12]. In the most recent report from the US Emerging Infections Program (EIP), 57% of the estimated CDI cases in 2011 were in the elderly [4]. The incidence of CDI rose dramatically with age, from 47/100 000 in younger adults aged 18–44 years to 148.5 in persons aged 45–64 years, and up to 628/100 000 in persons aged 65 years and older [4]. In the EIP study, there was a more than 13-fold increase in CDI incidence in the elderly compared with younger adults (18–44 years). Despite this, few studies have sought to elucidate the underlying biological reason(s) for the increased incidence of CDI among elderly persons.
One feature that deserves closer analysis is the role of overall health status, including frailty, and risk of CDI. Frailty, the expression of biologic aging, increases susceptibility to a variety of adverse events, including falls, fractures, infections, and ultimately death [13–16]. Frailty also results in increased health care exposure, including emergency department (ED) encounters, hospitalization, and institutionalization [17–19], resulting in increased opportunity for exposure to antibiotics, the most important risk factor for CDI [20].
Although the association between overall health status and increased risk of CDI has not been examined explicitly, a review of the literature reveals hints that the relationship between age and CDI may be more complicated than previously thought. Severity of illness has long been known to be associated with CDI [5, 21, 22]. Rao et al. found that poor functional status was an independent risk factor for severe CDI [23]. More recently, Ticinesi et al. found that multimorbidity was associated with increased risk of CDI [24]. Frailty per se has not been taken into account in prior studies in terms of progressive accumulation of deficits and their impact on CDI risk. Limitations of many prior analyses include relatively small sample sizes, which restrict the ability to control for many underlying conditions, use of summary measures (eg, Charlson index) not designed to determine CDI risk, or relatively geographically confined populations (eg, single hospitals) that may impact the distribution of underlying conditions within the population studied. A better understanding of the impact of overall health status on CDI risk is necessary to understand how best to implement CDI prevention efforts.
We used Medicare claims data to determine whether age remains an important predictor of CDI in an elderly population after taking into account overall health status, including recent acute and chronic illnesses, health care utilization, and indicators of frailty.
METHODS
We used 2010–2012 Medicare claims data from the Centers for Medicare and Medicaid Services Chronic Conditions Data Warehouse (CCW) for all analyses. All patients aged 66 years and older with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD–9–CM) diagnosis code for CDI (008.45) in 2011 in the Inpatient, Outpatient, or Carrier claims files were identified as CDI case patients (100% data). The uninfected comparison group consisted of individuals in the 2011 CCW 5% random sample, excluding those coded for CDI. Individuals were excluded from both groups if they were enrolled at any time during 2010–2011 in a health maintenance organization, lacked complete Part A and Part B coverage, or if they were coded for CDI in the last quarter of 2010 (to identify incident CDI in 2011). Also excluded were 135 329 individuals with no health claims in 2010 and 2011, as there was no evidence for use of health care benefits. The Washington University Human Research Protection Office gave approval to conduct this research with a waiver of informed consent.
Date of Onset and Attribution of CDI
The date of onset of CDI was defined as the first date corresponding to a coded diagnosis of CDI, unless additional information was available to define an earlier date of onset, as previously described [25, 26]. The location of onset and attribution for each CDI episode was determined using an algorithm based on the recommended CDI surveillance definitions [25–27].
For persons without CDI in 2011, an analogous date of onset (termed “index date”) was created to anchor the prior time period to identify comorbidities. After determining the onset date for all persons with CDI in 2011, the distribution function of these dates was determined. This distribution was used to randomly select index dates in the comparison uninfected population to mirror the distribution of onset dates in the CDI population, with the only restriction being that the index date occurred before the death date for uninfected persons who died in 2011.
Conditions Potentially Associated With CDI in the Prior Year
Conditions potentially associated CDI were identified in the year before the index date and grouped into 6 categories to explore their contribution in a model to predict CDI risk. The categories included age in 2-year increments, comorbidities, acute infections, acute noninfectious conditions, health care utilization, and frailty indicators. Comorbidities were defined according to the Elixhauser classification, with modification of the algorithm for complete claims data according to Klabunde et al. [28, 29]. Diagnosis codes on laboratory claims were not used to identify comorbidities or acute infectious or noninfectious conditions, as they may indicate suspected, but not confirmed, conditions. Acute infections were identified using ICD-9-CM diagnosis codes and categorized into infection groups, as described previously [25]. Noninfectious acute conditions were also identified using ICD-9-CM diagnosis codes, including myocardial infarction, gastrointestinal hemorrhage, fractures, and others (Appendix). Only a single outpatient claim coded for an acute infectious or noninfectious condition was required, as acute conditions may not be coded repeatedly over a prolonged period of time. All dates coded for acute infections were used to determine the timing of infection compared with the CDI onset or index date for uninfected persons.
Health care utilization in the year before CDI included surgical procedures, defined by Uniform Billing (UB–04) revenue codes for operating room expenses in inpatient and outpatient files, hospitalization, ED encounters, skilled nursing facility stays, and long-term facility (ie, nursing home) residence. Hospitalizations were categorized as emergency hospitalizations if they originated in the ED (defined by UB-04 revenue codes 0450–0459) or nonemergency hospitalizations. Treat-and-release ED visits were defined by revenue codes 0450–0459 from outpatient facilities. Skilled nursing facility stays were identified using the Skilled Nursing Facility file. Residence in a long-term care facility was identified using method 2 in Goodwin et al., based on the work of Intrator et al. [30, 31].
Indicators suggestive of frailty were identified in the year before the onset date, including dementia, decubitus ulcer, urinary incontinence, senility/frailty, failure to thrive, sleep disturbances, and difficulty walking. In contrast to the criteria for standard comorbidities, only a single inpatient or outpatient claim coded for the frailty indicators was required, as they do not generally require diagnostic testing to establish the diagnosis. The only exception was for Parkinson’s disease, which was identified using the same criteria as the comorbidities.
Analysis
The association of age with risk of CDI in univariate analysis was determined by chi–square and Mann-Whitney U tests. Multivariable logistic regression was used to characterize the independent association of age with risk of CDI, controlling for all comorbidities, acute and chronic noninfectious conditions, health care utilization in the prior year, and acute infections. The variance inflation factor (VIF) was used to identify important collinearity in the full model. No variable had a VIF greater than 2.4 in the full model, suggesting no important collinearity. To determine the impact of inclusion of the 6 categories of potential risk factors on CDI prediction, the individual categories were excluded sequentially, and the impact on model performance was determined by assessing the discrimination of the model using the area under the receiver operating curve (c-statistic), change in the Bayesian Information Criterion (BIC), and the deviance statistic comparing nested models. BIC is a measure used to select the “best” model from a nested set, based on the log likelihood of the models. BIC includes a penalty for increased number of terms in the model and takes into account the sample size in calculating the penalty. Because of this, BIC is more conservative and will select smaller models than the commonly used Akaike Information Criterion [32]. The deviance statistic was used to compare the goodness of fit of nested models rather than the standard Hosmer-Lemeshow test, because with large sample sizes, small deviations can result in rejection of the null hypothesis that the model fits the data [33]. SAS Enterprise Guide, version 7.1 (SAS, Cary, NC), was used for all data management and analysis.
RESULTS
A total of 174 903 persons aged 66 years and older with complete fee-for-service Medicare coverage were identified with at least 1 episode of CDI in 2011 in the Medicare claims files. For all persons, the first episode of CDI in 2011 was selected for further analyses. The first CDI episode was categorized as hospital-onset in 49 755 persons (28.4%), other health care facility–onset in 43 433 (24.8%), community-onset community-associated in 46 738 (26.7%), community-onset health care facility–associated in 21 952 (12.6%), and indeterminate association in 13 025 persons (7.4%). The comparison population consisted of 1 318 538 uninfected persons in the 5% random sample data.
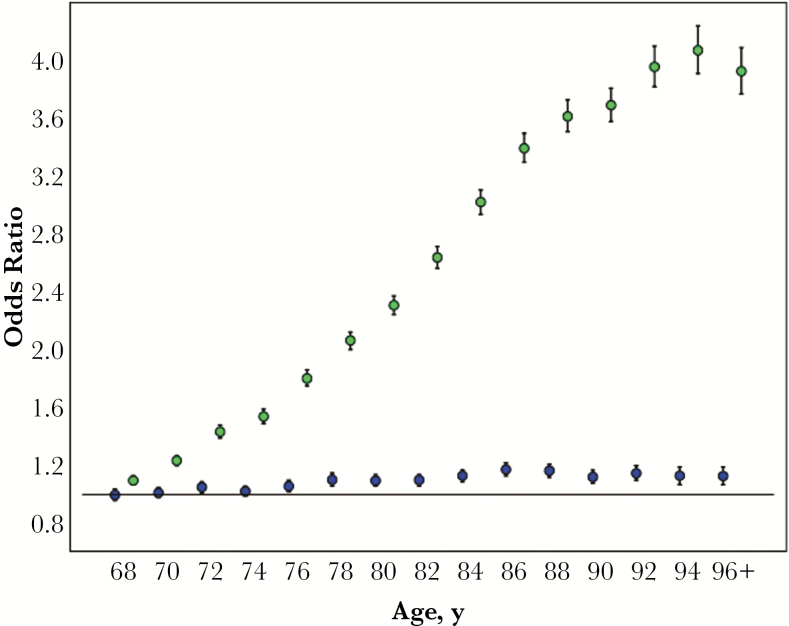
The association of age, sex, and acute and chronic medical and frailty conditions with CDI in univariate and multivariable analysis is shown in Table 1, and the odds ratios for increasing age are displayed in Figure 1. Age was categorized in 2-year increments to show the relationship between risk of CDI and increasing age. In univariate analysis, the risk of CDI increased linearly with increasing age until approximately age 88 years, at which point the risk leveled off. The odds of CDI dropped slightly in the oldest age group (96 years and older), possibly in part due to lower rates of testing for C. difficile in the very old.
Table 1.
Risk Factors for CDI in Univariate and Multivariate Analysis, Including Comorbid Conditions, Acute Infections, Acute Noninfectious Conditions, Health Care Utilization, and Frailty Indicators Present in the Year Before CDI
| Risk Factor | OR | 95% CI | aOR | 95% CI |
|---|---|---|---|---|
| Demographics | ||||
| Age (66–67 ref), y | ||||
| 68–69 | 1.098 | 1.066–1.132 | 0.998 | 0.961–1.036 |
| 70–71 | 1.235 | 1.198–1.273 | 1.013 | 0.976–1.053 |
| 72–73 | 1.434 | 1.391–1.477 | 1.052 | 1.013–1.092 |
| 74–75 | 1.539 | 1.493–1.585 | 1.024 | 0.986–1.063 |
| 76–77 | 1.801 | 1.748–1.855 | 1.059 | 1.020–1.100 |
| 78–79 | 2.063 | 2.003–2.124 | 1.103 | 1.063–1.145 |
| 80–81 | 2.305 | 2.239–2.372 | 1.097 | 1.057–1.138 |
| 82–83 | 2.634 | 2.559–2.710 | 1.102 | 1.062–1.144 |
| 84–85 | 3.016 | 2.931–3.104 | 1.131 | 1.089–1.174 |
| 86–87 | 3.386 | 3.289–3.486 | 1.174 | 1.130–1.220 |
| 88–89 | 3.606 | 3.499–3.716 | 1.166 | 1.121–1.214 |
| 90–91 | 3.683 | 3.567–3.803 | 1.122 | 1.076–1.171 |
| 92–93 | 3.947 | 3.809–4.089 | 1.148 | 1.095–1.203 |
| 94–95 | 4.061 | 3.897–4.232 | 1.131 | 1.071–1.194 |
| ≥96 | 3.917 | 3.759–4.081 | 1.129 | 1.070–1.192 |
| White race | 1.089 | 1.073–1.106 | 1.373 | 1.344–1.403 |
| Female | 1.114 | 1.102–1.125 | 1.053 | 1.038–1.069 |
| Comorbidities | ||||
| Congestive heart failure | 5.921 | 5.853–5.991 | 0.900 | 0.883–0.917 |
| Vascular disease | 3.374 | 3.326–3.423 | 1.000 | 0.979–1.021 |
| Pulmonary circulatory disorder | 6.166 | 6.027–6.309 | 1.121 | 1.086–1.157 |
| Peripheral vascular disease | 3.862 | 3.816–3.908 | 1.095 | 1.076–1.113 |
| Paralysis | 6.412 | 6.259–6.570 | 0.950 | 0.919–0.983 |
| Neurologic disease | 5.523 | 5.437–5.610 | 0.974 | 0.952–0.996 |
| Parkinson’s disease | 3.162 | 3.076–3.251 | 1.023 | 0.985–1.063 |
| Chronic pulmonary disease | 3.493 | 3.453–3.533 | 0.987 | 0.970–1.005 |
| Hypothyroidism | 2.096 | 2.071–2.122 | 1.03 | 1.013–1.047 |
| Chronic renal failure | 5.462 | 5.397–5.529 | 1.271 | 1.248–1.294 |
| Liver disease | 3.737 | 3.602–3.878 | 1.428 | 1.357–1.502 |
| Peptic ulcer disease | 4.216 | 3.665–4.851 | 0.927 | 0.766–1.123 |
| Lymphoma | 3.150 | 3.042–3.262 | 1.636 | 1.559–1.716 |
| Metastatic cancer | 4.195 | 4.074–4.318 | 1.228 | 1.177–1.281 |
| Solid tumor | 1.963 | 1.935–1.992 | 1.115 | 1.091–1.141 |
| Rheumatoid arthritis/collagen vascular disease | 2.298 | 2.250–2.347 | 1.266 | 1.230–1.303 |
| Coagulation disorder | 5.880 | 5.768–5.993 | 1.143 | 1.113–1.174 |
| Obesity | 3.771 | 3.696–3.846 | 1.100 | 1.070–1.132 |
| Blood loss anemia | 6.105 | 5.915–6.302 | 0.763 | 0.732–0.797 |
| Deficiency anemias | 6.732 | 6.660–6.804 | 1.271 | 1.251–1.291 |
| Psychoses | 4.521 | 4.431–4.613 | 0.888 | 0.863–0.914 |
| Depression | 4.918 | 4.848–4.988 | 1.142 | 1.119–1.165 |
| Cardiac | 3.846 | 3.805–3.888 | 1.000 | 0.984–1.016 |
| Prior fluid/electrolyte disorders | 7.504 | 7.411–7.598 | 0.838 | 0.823–0.851 |
| Prior weight loss/malnutrition | 7.799 | 7.658–7.943 | 0.952 | 0.927–0.978 |
| Diabetes | 1.973 | 1.952–1.994 | 0.940 | 0.926–0.955 |
| Hypertension | 3.771 | 3.722–3.821 | 0.987 | 0.970–1.005 |
| Infections | ||||
| Septicemia | 34.006 | 33.245–34.783 | 4.104 | 3.994–4.217 |
| Pneumonia | 13.380 | 13.178–13.585 | 2.054 | 2.012–2.096 |
| Urinary tract infection/prostatitis | 5.029 | 4.969–5.089 | 1.245 | 1.224–1.267 |
| Skin and soft tissue infection | 4.092 | 4.025–4.161 | 1.368 | 1.336–1.402 |
| Surgical site infection | 12.880 | 12.342–13.441 | 1.479 | 1.395–1.567 |
| Bone infection/osteomyelitis | 10.246 | 9.823–10.686 | 1.266 | 1.193–1.344 |
| Organ infection/meningitis | 6.134 | 5.666–6.641 | 1.193 | 1.067–1.334 |
| Sexually transmitted disease/pelvic infection | 1.442 | 1.355–1.533 | 0.982 | 0.902–1.068 |
| Abdominal abscess/peritonitis | 3.879 | 3.812–3.948 | 1.106 | 1.076–1.137 |
| Diverticulitis | 5.095 | 4.987–5.205 | 2.214 | 2.147–2.283 |
| Upper respiratory infection | 1.297 | 1.275–1.319 | 1.010 | 0.987–1.034 |
| Tonsillitis/ocular infection/mastoiditis | 1.554 | 1.471–1.641 | 0.772 | 0.716–0.832 |
| Otitis media | 0.978 | 0.927–1.033 | 0.940 | 0.877–1.007 |
| Oral infection | 2.172 | 1.989–2.371 | 1.430 | 1.269–1.611 |
| Viral infection | 2.778 | 2.689–2.871 | 1.196 | 1.142–1.252 |
| Health care utilization | ||||
| Inpatient surgery | 8.162 | 8.068–8.257 | 1.479 | 1.453–1.506 |
| Outpatient surgery | 1.668 | 1.645–1.692 | 1.099 | 1.078–1.121 |
| Nonelective hospitalization(s) | 20.497 | 20.248–20.748 | 3.907 | 3.836–3.979 |
| Elective hospitalization(s) | 4.873 | 4.814–4.933 | 1.689 | 1.658–1.721 |
| 1 treat-and-release ED encounter | 2.547 | 2.519–2.575 | 1.841 | 1.809–1.872 |
| 2 or more treat-and-release ED encounters | 5.558 | 5.498–5.619 | 1.259 | 1.236–1.283 |
| Nursing home residence | 6.762 | 6.668–6.858 | 1.604 | 1.568–1.640 |
| Short-term skilled nursing facility stay | 15.724 | 15.543–15.907 | 2.679 | 2.634–2.725 |
| Acute noninfectious conditions | ||||
| Acute myocardial infarction | 6.852 | 6.724–6.982 | 1.269 | 1.237–1.302 |
| COPD exacerbation | 5.286 | 5.203–5.369 | 1.198 | 1.169–1.227 |
| Gastrointestinal bleed | 6.276 | 6.194–6.359 | 1.826 | 1.792–1.861 |
| Diverticulosis | 3.022 | 2.984–3.061 | 1.445 | 1.416–1.474 |
| Subdural hematoma | 5.570 | 5.265–5.893 | 1.031 | 0.960–1.107 |
| Cerebrovascular accident | 3.707 | 3.668–3.747 | 1.114 | 1.097–1.132 |
| Closed fracture, lower extremity | 4.608 | 4.529–4.689 | 0.938 | 0.914–0.962 |
| Open fracture, lower extremity | 6.322 | 6.019–6.641 | 0.869 | 0.815–0.926 |
| Closed fracture, other | 3.464 | 3.407–3.522 | 0.986 | 0.963–1.009 |
| Open fracture, other | 4.074 | 3.808–4.358 | 0.871 | 0.797–0.952 |
| Frailty indicators | ||||
| Decubitus ulcer | 13.244 | 13.013–13.478 | 1.727 | 1.686–1.770 |
| Dementia | 4.910 | 4.851–4.970 | 1.206 | 1.183–1.230 |
| Dehydration, past 30 d | 7.332 | 7.235–7.429 | 1.058 | 1.038–1.078 |
| Deep venous thrombosis | 6.147 | 6.050–6.246 | 1.319 | 1.289–1.350 |
| Pulmonary embolism | 5.277 | 5.141–5.416 | 1.069 | 1.031–1.109 |
| Urinary incontinence | 2.727 | 2.686–2.770 | 1.227 | 1.201–1.253 |
| Senility/frailty | 10.281 | 10.121–10.444 | 1.362 | 1.333–1.391 |
| Failure to thrive | 8.144 | 7.936–8.357 | 0.958 | 0.925–0.992 |
| Sleep disturbance | 2.166 | 2.134–2.198 | 1.136 | 1.113–1.160 |
| Difficulty walking | 6.407 | 6.338–6.476 | 1.087 | 1.069–1.105 |
C-statistic of the full model = 0.918.
Abbreviations: aOR, adjusted odds ratio; CDI, Clostridium difficile infection; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; OR, odds ratio.
Figure 1.
Unadjusted and adjusted odds ratios for Clostridium difficile infection by age (years) in the elderly Medicare population. Green circles: unadjusted odds ratios; blue circles: adjusted odds ratios.
In multivariable analysis, the risk factors in the year prior that were associated with >2-fold increased risk of CDI were septicemia (OR, 4.1), emergency hospitalization(s) (OR, 3.9), short-term skilled nursing facility stay(s) (OR, 2.7), diverticulitis (OR, 2.2), and pneumonia (OR, 2.1). Factors associated with moderately increased risk of CDI in the year prior (odds ratios of 1.5–2.0) included 1 ED visit, gastrointestinal hemorrhage, decubitus ulcer, elective hospitalization(s), lymphoma, long-term care facility residence, inpatient surgery, and surgical site infection. Additional factors associated with approximately 40% increased risk of CDI included white race, diverticulosis, liver disease, skin and soft tissue infection, and oral infection.
After adjustment for the large variety of infections at any time in the year before CDI, other chronic and acute conditions, frailty indicators, and health care utilization in the year before CDI, the risk of CDI associated with increasing age decreased dramatically (Table 1, Figure 1). Although the odds of CDI remained significantly elevated compared with the youngest persons (aged 66–67 years), the odds ratios fluctuated slightly from 1.052 to a high of 1.174 in the 86–87-year-old group. The risk of CDI with increasing age remained only slightly elevated when acute infections were restricted to those coded more than 30 days before CDI (Table 1).
To test whether inclusion of age, comorbidities, and other conditions affected model performance, discrimination, fit, and BIC were assessed in the full model (including all variables in Table 1), compared with models with individual variable groups removed. As shown in Table 2, removal of age from the full model had no impact on the c-statistic and resulted in a slight decrease in the BIC, and the deviance statistic remained nonsignificant, indicating that the model performance improved with removal of age. Removal of comorbidities, acute noninfectious conditions, and frailty indicators had little impact on the c-statistic but resulted in small increases in the BIC and significant deviance statistics, indicating that these models did not fit the data as well as the full model. The biggest decrease in the c-statistic occurred after removal of infections (from 0.918 to 0.911) and health care utilization (0.918 to 0.897), along with the largest increases in the BIC, indicating that these models performed more poorly than the full model. The impact on the BIC and c-statistic of removal of only the septicemia variable was about half that of removal of the entire infection category from the full model (Table 2), consistent with the very elevated risk of CDI associated with septicemia.
Table 2.
Comparison of Performance of Full and Reduced Models, Excluding Categories of CDI Predictors
| Model | No. of Variables | BIC | Change in BIC | C-Statistic | Deviance Pa |
|---|---|---|---|---|---|
| Full | 87 | 638 187.4 | 0.918 | 1.0000 | |
| Age | 72 | 638 167.8 | –19.6 | 0.918 | 1.0000 |
| Comorbidities | 60 | 642 135.4 | 3948.0 | 0.917 | .0001 |
| Acute noninfectious conditions | 77 | 645 064.7 | 4968.9 | 0.916 | .0001 |
| Frailty indicators | 77 | 643 156.3 | 6877.3 | 0.917 | .0001 |
| Septicemia | 86 | 651 908.3 | 13 720.9 | 0.915 | .0001 |
| Infectionsb | 79 | 663 514.9 | 25 327.5 | 0.911 | .0001 |
| Health care utilization | 72 | 702 080.9 | 63 893.5 | 0.897 | .0001 |
Abbreviations: BIC, Bayesian Information Criterion; CDI, Clostridium difficile infection.
aAs with other goodness of fit tests, the null hypothesis for the deviance statistic is that the model fits the data. Therefore, P < .05 indicates poor model fit.
bIncludes septicemia.
DISCUSSION
We found that exclusion of age in a multivariable model to predict risk of CDI had no demonstrable impact on model performance after controlling for acute infections, health care utilization, acute noninfectious conditions, and indicators of frailty in the year before CDI. These results suggest that overall health status, including infections, health care utilization, acute conditions in the past year, and frailty indicators are the most important determinants of CDI risk in an elderly population. In the multivariable model, the lack of a dose–response in risk of CDI with increasing age is likely due to the large number of risk factors we were able to control for in the very large Medicare population, including acute conditions common in the elderly, and indicators of frailty. This lack of an age dose–response is consistent with the hypothesis that, after adequately accounting for overall health status, age per se is no longer an important predictor of CDI. This is particularly relevant as individuals “age” at different times in their lives, and thus a younger person with serious medical conditions may have much higher risk of CDI than a healthy older person.
Acute infections were associated with increased risk of CDI, particularly septicemia (4-fold increased odds) and pneumonia (2-fold increased odds). Other serious infections, including surgical site, skin and soft tissue, and oral infections, were associated with moderately increased odds of CDI, consistent with need for antibiotic therapy of these infections. Interestingly, even past viral infections were associated with a small increased risk of CDI, reflecting possible inappropriate use of antibiotics in these patients.
Not surprisingly, health care utilization in the past year was independently associated with increased risk of CDI and improved the fit of the model. Emergency hospitalization was associated with almost 4-fold increased risk of CDI, followed by 2.7-fold increased risk associated with skilled nursing facility stay(s). Our finding of increased risk of CDI with hospitalization is consistent with recent reports by McDonald and colleagues of increased risk of CDI with hospitalization in the past 30 days [34] and with the number of hospitalizations in the past 90 days [35]. Stays in a skilled nursing facility occur following discharge from a hospitalization and require documentation of the need for continuation of nursing care to be reimbursed by Medicare. Thus the increased CDI risk associated with skilled nursing facility stay(s) is consistent with overall health status as the primary driver of CDI risk. In contrast, residence in a long-term care facility was associated with only 1.5-fold increased risk of CDI, after adjustment for other variables in the model. This suggests that while nursing home residence is a risk factor for CDI, it is not as important as acute infectious and noninfectious events and health care utilization associated with those acute events, particularly emergency hospitalization. This finding is consistent with recent surveillance studies in which more than half of the incident cases of CDI with onset in a nursing home occurred within 30 days following hospital discharge [36, 37]. Zarowitz et al. similarly reported that up to 67% of CDI in nursing home residents was attributable to a recent hospitalization, using the Minimum Data Set survey of skilled nursing residents [38]. Using the 2009 Medicare 5% random sample, we previously found the incidence of CDI among nursing home residents to be 10 093/100 000 person-years if they had a prior emergency hospitalization, and only 1505/100 000 person-years if the person did not have any hospitalizations in the previous year [25].
In addition to acute infections and health care encounters, several acute noninfectious conditions were also associated with increased risk of CDI. These conditions, including diverticulosis, gastrointestinal hemorrhage, and myocardial infarction, were likely a primary contributor to the patients’ underlying severity of illness. In contrast, indicators of frailty, including decubitus ulcers and urinary incontinence, were likely markers for advanced underlying illness. Exclusion of acute noninfectious conditions reduced the c-statistic and resulted in an imperfectly fitting model according to the deviance statistic. In the comparison of models with individual categories removed, the biggest impact on model fit was associated with removal of the health care utilization category, followed by infections. The big impact of removal of infections, including septicemia, on model performance was not surprising, as many of the individual infections were associated with high risk of CDI, likely due to antibiotic treatment of the preceding infections with resulting colonic dysbiosis. The bigger impact of removal of the health care utilization category was also not surprising, as serious infections would result in hospitalization, and more than half of hospitalized patients are treated with at least 1 dose of antibiotics, even in the absence of documented infection [39].
Limitations of this study include identification of CDI by ICD-9-CM diagnosis codes, which are not perfectly accurate [40], and the lack of data on antibiotic utilization for all patients, particularly during hospital stays. The use of Medicare claims data restricted analyses to the elderly; whether younger persons with poorer overall health status are at the same risk as much older persons remains to be determined in a future study. In addition, repeating this study using Medicare data from a more recent year with higher utilization of nucleic acid amplification testing would be beneficial, to determine whether the relationship with age remains minimal in the setting of increased sensitivity to identify not just CDI but C. difficile colonization.
Strengths of our study include the very large sample size, generalizability to the fee-for-service US elderly population, and identification of CDI across many institutions and providers. The very large sample size allowed us to control for a very large number of potential risk factors in the multivariable model and separate out the independent effect of increasing age on risk of CDI.
Advancing age is frequently cited as one of the primary risk factors for CDI. This study demonstrates that overall health status, including recent acute infections and even acute noninfectious conditions, is more important than age with respect to CDI risk. Clearly, an 80-year-old with hypertension who has never been hospitalized will be at lower risk for CDI than a 66-year-old person emergently hospitalized for management of congestive heart failure. Markers of poorer overall health status include frailty indicators, recent acute infections, and emergency hospitalizations. These conditions are not difficult to identify and can be used to target CDI prevention activities to patients most likely to benefit.
Acknowledgments
Disclosures. M. A. O.: grants and personal fees from Sanofi Pasteur, during the conduct of the study; personal fees from Pfizer, outside the submitted work. D. S.: none. C. D.: employee of Sanofi. E. R. D.: grants from Sanofi, during the conduct of the study; personal fees from Sanofi, grants and personal fees from Pfizer, personal fees from Synthetic Biologics, personal fees from Valneva, personal fees from Abbott, personal fees from Biofire, grants and personal fees from Rebiotix, grants and personal fees from Merck, outside the submitted work.
Financial support. This work was supported by Sanofi Pasteur. The sponsor participated in study design, interpretation of data, and final review of the manuscript. Access to data and additional services were provided by the Washington University Center for Administrative Data Research, supported in part by grant UL1 TR000448 from the National Center for Advancing Translational Sciences of the National Institutes of Health and grant R24 HS19455 through the Agency for Healthcare Research and Quality.
References
- 1. Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall AJ, Curns AT, McDonald LC, et al. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999-2007. Clin Infect Dis 2012; 55:216–23. [DOI] [PubMed] [Google Scholar]
- 3. Agency for Healthcare Research and Quality. HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Researchand Quality; 2015. http://hcupnet.ahrq.gov. Accessed 15 May 2015. [Google Scholar]
- 4. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis 1990; 162:678–84. [DOI] [PubMed] [Google Scholar]
- 6. Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40:1–15. [DOI] [PubMed] [Google Scholar]
- 7. Beaulieu M, Williamson D, Pichette G, Lachaine J. Risk of Clostridium difficile-associated disease among patients receiving proton-pump inhibitors in a Quebec medical intensive care unit. Infect Control Hosp Epidemiol 2007; 28:1305–7. [DOI] [PubMed] [Google Scholar]
- 8. Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 2011; 365:1693–703. [DOI] [PubMed] [Google Scholar]
- 9. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med 2005; 353:2442–9. [DOI] [PubMed] [Google Scholar]
- 10. Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis 2009; 15:415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khanna S, Aronson SL, Kammer PP, et al. Gastric acid suppression and outcomes in Clostridium difficile infection: a population-based study. Mayo Clin Proc 2012; 87:636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abou Chakra CN, McGeer A, Labbé AC, et al. Factors associated with complications of Clostridium difficile infection in a multicenter prospective cohort. Clin Infect Dis 2015; 61:1781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA 2001; 285:2987–94. [DOI] [PubMed] [Google Scholar]
- 14. Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med 2008; 168:382–9. [DOI] [PubMed] [Google Scholar]
- 15. Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013; 381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kennedy CC, Ioannidis G, Rockwood K, et al. A frailty index predicts 10-year fracture risk in adults age 25 years and older: results from the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 2014; 25:2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian Study of Health and Aging. J Gerontol A Biol Sci Med Sci 2004; 59:1310–7. [DOI] [PubMed] [Google Scholar]
- 18. Rockwood K, Mitnitski A, Song X, et al. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc 2006; 54:975–9. [DOI] [PubMed] [Google Scholar]
- 19. Chamberlain AM, Finney Rutten LJ, Manemann SM, et al. Frailty trajectories in an elderly population-based cohort. J Am Geriatr Soc 2016; 64:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stevens V, Dumyati G, Fine LS, et al. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011; 53:42–8. [DOI] [PubMed] [Google Scholar]
- 21. Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–9. [DOI] [PubMed] [Google Scholar]
- 22. Pham VP, Luce AM, Ruppelt SC, et al. Age-stratified treatment response rates in hospitalized patients with Clostridium difficile infection treated with metronidazole. Antimicrob Agents Chemother 2015; 59:6113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rao K, Micic D, Chenoweth E, et al. Poor functional status as a risk factor for severe Clostridium difficile infection in hospitalized older adults. J Am Geriatr Soc 2013; 61:1738–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ticinesi A, Nouvenne A, Folesani G, et al. Multimorbidity in elderly hospitalised patients and risk of Clostridium difficile infection: a retrospective study with the Cumulative Illness Rating Scale (CIRS). BMJ Open 2015; 5:e009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubberke ER, Olsen MA, Stwalley D, et al. Identification of Medicare recipients at highest risk for Clostridium difficile Infection in the US by population attributable risk analysis. PLoS One 2016; 11:e0146822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olsen MA, Young-Xu Y, Stwalley D, et al. The burden of Clostridium difficile infection: estimates of the incidence of CDI from U.S. administrative databases. BMC Infect Dis 2016; 16:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen SH, Gerding DN, Johnson S, et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 28. Agency for Healthcare Research and Quality. HCUP Comorbidity Software. Healthcare Cost and Utilization Project (HCUP) 2014. http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed 26 September 2014. [DOI] [PubMed]
- 29. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000; 53:1258–67. [DOI] [PubMed] [Google Scholar]
- 30. Goodwin JS, Li S, Zhou J, et al. Comparison of methods to identify long term care nursing home residence with administrative data. BMC Health Serv Res 2017; 17:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Intrator O, Berg K. Benefits of home health care after inpatient rehabilitation for hip fracture: health service use by Medicare beneficiaries, 1987-1992. Arch Phys Med Rehabil 1998; 79:1195–9. [DOI] [PubMed] [Google Scholar]
- 32. Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. New York: Springer-Verlag; 2009. [Google Scholar]
- 33. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med 2013; 32:67–80. [DOI] [PubMed] [Google Scholar]
- 34. Tabak YP, Johannes RS, Sun X, et al. Predicting the risk for hospital-onset Clostridium difficile infection (HO-CDI) at the time of inpatient admission: HO-CDI risk score. Infect Control Hosp Epidemiol 2015; 36:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baggs J, Yousey-Hindes K, Ashley ED, et al. Identification of population at risk for future Clostridium difficile infection following hospital discharge to be targeted for vaccine trials. Vaccine 2015; 33:6241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mylotte JM, Russell S, Sackett B, et al. Surveillance for Clostridium difficile infection in nursing homes. J Am Geriatr Soc 2013; 61:122–5. [DOI] [PubMed] [Google Scholar]
- 37. Hunter JC, Mu Y, Dumyati GK, et al. Burden of nursing home-onset Clostridium difficile infection in the United States: estimates of incidence and patient outcomes. Open Forum Infect Dis 2016; 3:ofv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zarowitz BJ, Allen C, O’Shea T, Strauss ME. Risk factors, clinical characteristics, and treatment differences between residents with and without nursing home- and non-nursing home-acquired Clostridium difficile infection. J Manag Care Spec Pharm 2015; 21:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baggs J, Fridkin SK, Pollack LA, et al. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 2016; 176:1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dubberke ER, Butler AM, Yokoe DS, et al. ; Prevention Epicenters Program of the Centers for Disease Control and Prevention Multicenter study of surveillance for hospital-onset Clostridium difficile infection by the use of ICD-9-CM diagnosis codes. Infect Control Hosp Epidemiol 2010; 31:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]