Abstract
Objectives
To evaluate the effectiveness and safety of adjunctive olanzapine treatment for low weight adolescents with anorexia nervosa (AN).
Methods
A non-randomized open-label trial was conducted between 2010 and 2014. Participants received standard treatment and were invited to take olanzapine at study enrollment. Participants could accept, continue, or discontinue olanzapine as treatment progressed. Weight and psychological outcomes were monitored.
Results
Of 239 adolescents assessed, 65 met inclusion criteria, 38 enrolled in the study, and 32 were retained for analysis. Twenty-two participants took olanzapine (medication group) and ten participants did not (comparison group). Participants in the medication group demonstrated a higher rate of weight gain compared to those who did not receive olanzapine (p = .012). No serious adverse events were noted, although seven participants (31.8%) discontinued olanzapine due to a side effect.
Conclusion
Preliminary results suggest that olanzapine may help facilitate weight gain in adolescents with AN. The importance of medical monitoring over the course of treatment is discussed. Evaluation of the Efficacy and Safety of Olanzapine for Anorexia Nervosa in Children and Adolescents; http://clinicaltrials.gov; NCT01184443.
Keywords: anorexia nervosa, Olanzapine, adolescent
Résumé
Objectifs
Évaluer l’efficacité et l’innocuité d’un traitement d’appoint par olanzapine pour les adolescents de faible poids souffrant d’anorexie mentale (AM).
Méthodes
Un essai ouvert non randomisé a été mené entre 2010 et 2014. Les participants ont reçu un traitement standard et ont été invités à prendre de l’olanzapine lors de l’inscription à l’étude. Les participants pouvaient accepter, continuer ou cesser l’olanzapine à mesure que le traitement progressait. Le poids et les résultats psychologiques ont été surveillés.
Résultats
Sur les 239 adolescents évalués, 65 satisfaisaient aux critères d’inclusion, 38 se sont inscrits à l’étude, et 32 ont été retenus pour analyse. Vingt-deux participants ont pris de l’olanzapine (groupe du médicament) et 10 participants n’en ont pas pris (groupe de comparaison). Les participants du groupe du médicament ont démontré un taux plus élevé de prise de poids comparativement à ceux qui n’ont pas reçu d’olanzapine (p = 0,012). Aucun effet indésirable sérieux n’a été noté, bien que 7 participants (31,8 %) aient cessé l’olanzapine en raison d’un effet secondaire.
Conclusion
Les résultats préliminaires suggèrent que l’olanzapine peut aider à faciliter la prise de poids chez les adolescents souffrant d’AM. L’importance de la surveillance médicale en cours de traitement est discutée. Evaluation of the Efficacy and Safety of Olanzapine for Anorexia Nervosa in Children and Adolescents; http://clinicaltrials.gov; NCT01184443.
Mots clés: anorexie mentale, olanzapine, adolescent
Anorexia nervosa (AN) is a complex, potentially chronic illness that often begins in adolescence and which is characterized by the interplay of debilitating cognitive, emotional, and physical processes associated with serious weight loss. Treatment of AN is multidisciplinary and includes medical, nutritional and psychological rehabilitation (Watson & Bulik, 2013).
The past decade has seen an increased interest in the use of second-generation antipsychotic (SGA) medications for the treatment of AN (Aigner, Treasure, Kaye, & Kasper, 2011; McElroy, Guerdjikova, Mori, & Keck, 2015). Of the SGAs, olanzapine has the most published evidence of effectiveness in AN (McElroy et al., 2015; van den Heuvel & Jordaan, 2014), and is the only antipsychotic with Class B evidence (i.e. limited positive evidence from controlled studies), as determined by the World Federation of Societies of Biological Psychiatry (Aigner et al., 2011). To date, there is no Class A evidence (i.e. full evidence from controlled studies) for the psychopharmacological treatment of AN (Aigner et al., 2011).
Stip and Lungu (2015) review literature that suggests evidence of dysfunction in the salience network (SN) (especially in the anterior cingulate cortex) in those with eating disorders (EDs). They present evidence from neuroimaging in patients with schizophrenia to show that olanzapine acts on the SN, and thus may act by enhancing the reactivity of the anterior cingulate cortex and SN in the response to reward value of food in those with AN. In addition, Lord et al (2017) found evidence in mice to suggest that olanzapine causes hyperphagia and weight gain through antagonism of the serotonin 2C receptor (5-HT2c). As well as causing increased appetite and weight gain in AN, olanzapine is also hypothesized to reduce anxiety and agitation, and to mitigate obsessions or delusions regarding weight and body shape (Bissada, Tasca, Barber, & Bradwejn, 2008; Lock & Gowers, 2005). Olanzapine may offer a particular advantage over other SGAs due to its more favorable side effect profile for this population (i.e. more likely to cause sedation and weight gain, while less likely to cause extrapyramidal symptoms, QTc interval prolongation on electrocardiogram, or elevated prolactin levels) (Zhu & Walsh, 2002).
Several case reports describing olanzapine use in adults, adolescents, and children diagnosed with AN have observed the association of olanzapine with weight gain, clinically notable decreases in levels of agitation, decreased obsessionality, improvements in sleep and general functioning, and, overall improved compliance with treatment (Boachie, Goldfield, & Spettigue, 2003; Dennis, Le Grange, & Bremer, 2006; Ercan, Copkunol, Cýkoethlu, & Varan, 2003; Jensen & Mejlhede, 2000; La Via, Gray, & Kaye, 2000; Mehler et al., 2001). In trials measuring outcomes specific to EDs, results are mixed; some studies but not all reported that olanzapine facilitates weight gain and/or improves psychological symptoms (Attia et al., 2011; Bissada et al., 2008; Brambilla et al., 2007; Kafantaris et al., 2011; Malina et al., 2003; Mondraty et al., 2005). We are aware of only three published open-label trials (Barbarich et al., 2004; Leggero et al., 2010; Powers, Santana, & Bannon, 2002), one RCT of olanzapine versus chlorpromazine (Mondraty et al., 2005), one placebo-controlled pilot study (Kafantaris et al., 2011) and two placebo-controlled RCTs (Bissada et al., 2008; Brambilla et al., 2007) which have evaluated the efficacy of olanzapine in the treatment of AN. None of the open-label trials included a control group, the Kafantaris study (2011) found no difference between the olanzapine and placebo groups, and only Leggero et al. (2010) examined a strictly pediatric age group in an open trial of 13 adolescents.
In an attempt to clarify the role of olanzapine in the treatment of AN, a recent meta-analysis of all randomized controlled trials (RCTs) examining the use of SGAs in AN was completed. In this review, the authors found no significant differences at endpoint in mean body mass index (BMI) change or anorectic symptoms between a pooled group of individuals treated with olanzapine compared to a control group (Dold, Aigner, Klabunde, Treasure, & Kasper, 2015). This conclusion was consistent with findings published in two earlier reviews (Kishi, Kafantaris, Sunday, Sheridan, & Correll, 2012; Lebow, Sim, Erwin, & Murad, 2013).
Very few studies have examined the benefits and risks of olanzapine for AN in children and adolescents (Kafantaris et al., 2011; Leggero et al., 2010). The primary objective of this study therefore, was to evaluate the effectiveness of olanzapine when administered in combination with standard care to low weight adolescents diagnosed with AN. It was hypothesized that those adolescents treated with olanzapine would achieve a higher rate of increase in weight and percentage of treatment goal weight (TGW) compared to those who did not receive olanzapine.
A secondary objective was to investigate whether olanzapine is effective for treating psychological symptoms. We hypothesized that participants receiving olanzapine would demonstrate improved mood and anxiety over time, as well as reduced ED cognitions and behaviors, compared to the control sample. Finally, this study evaluated the safety of olanzapine use in adolescents with AN. A minor frequency of adverse events was hypothesized given the relatively low dosing and the short duration of use.
Methods
Trial Design and Program Characteristics
This open-label study was conducted between 2010 and 2014 by a specialized ED team located in a children’s hospital in Canada. Our program treats children and adolescents eight to 18 years of age with moderate to severe EDs. The multidisciplinary team offers medical care, nutritional rehabilitation, and psychotherapy. The ED program is guided by the principles of Maudsley family-based treatment (FBT) (FBT; Lock & Fitzpatrick, 2007), such that each patient is assigned a therapist who offers a combination of FBT plus individual therapy. This study was approved by our hospital’s Research Ethics Board, the Clinical Trials arm of Health Canada, and was listed at ClinicalTrials.gov (NCT01184443). The research institute’s data safety board oversaw safety monitoring for the trial.
Participants
Following a comprehensive assessment by our ED team, adolescents ages 11 to 17 years of age who met inclusion but not exclusion criteria, were invited to participate. (For inclusion and exclusion criteria see Table 1.) Patients deemed incapable or rendered involuntary under the Mental Health Act were excluded from the trial. All participants and at least one parent/legal guardian provided written informed consent after receiving a complete description of the study.
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion Criteria | |
|---|---|
| 1 | Fulfilling the diagnostic criteria for AN (restrictive or binge-eating/purging subtype) or Eating disorder not otherwise specified (EDNOS), according to the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, text revision), and weighing 85% or less of his or her TGW. |
| 2 | Aged 11 to 17 years, inclusive. |
| 3 | Receiving medical and psychological treatment from the Eating Disorder Program at our center. |
| Exclusion Criteria | |
| 4 | Currently receiving treatment with any other antipsychotic medication, mood stabilizer, or stimulant. |
| 5 | Known diagnosis of diabetes, impaired glucose tolerance, hyperlipidemia, hepatic dysfunction, substance dependence, narrow angle glaucoma, paralytic ileus, pancreatitis, or any other medical illness that would be considered to significantly impact treatment or recovery from the eating disorder. |
| 6 | Inability to comply with trial requirements including lack of comprehension of English. |
| 7 | Pregnant or breast-feeding. |
| 8 | Known allergy or known sensitivity to products in olanzapine. |
| 9 | Other unspecified reasons that, in the opinion of the investigator, make the patient unsuitable for enrolment. |
| 10 | Liver function tests (AST/ALT) > 2× normal. |
| 11 | Electrocardiogram: QTc > 450 msec or arrhythmia other than sinus bradycardia; conduction abnormalities prolonged QTc or other. |
| 12 | LDL-C > 4.9 mmol/L; (10) Total cholesterol/HDL ratio > 6. |
| 13 | Random glucose > 11 mmol/L (or Fasting glucose ≥ 6.1 mmol/L). |
| 14 | Neutrophil count < 0.5 × 109/L. |
Note that all of these patients would now meet DSM-5 criteria for anorexia nervosa.
ALT = Alanine aminotransferase. AST = Aspartate aminotransferase. HDL = High density lipoprotein cholesterol. LDL-C = Low density lipoprotein cholesterol. QTc = Corrected QT interval. TGW = Treatment goal weight.
Procedure
To approximate real-world treatment conditions, a non-randomized open-label effectiveness trial was conducted. All participants received standard care from our ED team, including psychological, nutritional, and medical treatment, and all were offered adjunctive treatment with the medication olanzapine. The generic product, Teva-olanzapine (Teva Canada Limited) was used, and was provided free of charge to participants enrolled in the study. Participants who chose at any point to take olanzapine belonged to the medication group, and those who opted not to take olanzapine were assigned to the comparison group.
Medication group
For participants in the medication group, olanzapine doses replicated the ED program’s standard clinical practice with respect to dose administered and dosage adjustments being based on individual need and tolerability as determined by the treating clinician, rather than on an ‘a priori’ dosing schedule.
Comparison group
Participants in the comparison group were eligible to switch into the medication group up until the end of week 8 in the study. This accommodation allowed participants who were not ready or willing to start olanzapine at the point treatment was initiated, to change their minds and request treatment with olanzapine. Comparison group participants who started olanzapine after treatment initiation were not included in the comparison cohort and instead were assigned (for the purposes of analysis) to a third group: the switch group.
Outcomes
Our primary outcomes were rate of change in weight (kg) across the 12 weeks of the trial (i.e. weight gain) as well as rate of change of percentage of TGW. TGW (previously known as ideal body weight, or ‘healthy weight,’) was determined by the adolescent health physicians on the team, using a combination of factors including the patient’s pediatric growth curve history, premorbid weight, and menstrual history. (Although we did collect data on BMI and rate of change of BMI, TGW is considered the more useful value in the assessment and treatment of pediatric EDs, especially for those patients whose TGW is at a higher BMI.) Weight was assessed weekly for the first 4 weeks of the study and biweekly thereafter. Secondary outcomes were psychological measures: self-reported depression, anxiety and ED symptoms were assessed at baseline and weeks 4, 8, and 12 using the reliable and valid Children’s Depression Inventory (CDI; Kovacs 1992) the Multidimensional Anxiety Scale for Children (MASC; March 1997), the Eating Disorder Inventory-3 (EDI-3; Garner 2004), which examines ED cognitions, and the Eating Disorder Examination Questionnaire-Adolescent (EDEQ-A; Carter, Stewart, & Fairburn, 2001), which examines ED symptoms and behaviors.
Medical and safety monitoring included: electrocardiogram (ECG), hematology and biochemistry results, patient-reported side effects, and clinician-assessed extrapyramidal symptoms. The Division of AIDS (DAIDS) grading system was used to classify abnormal ALT results (“Division of AIDS table for grading the severity of adult and pediatric adverse events,” 2014). The DAIDS system is widely used in clinical trials. Grades range from 1 (mild event) to 5 (death).
Patient-reported side effects were assessed using an adverse event checklist (AEC) developed by the investigators. The AEC asked participants to rate the following symptoms as absent or present: dizziness, somnolence, agitation, restlessness, headache, dry mouth, constipation, and stiff muscles. Participants were encouraged to specify any other symptoms in a blank space provided on the AEC. Study physicians followed up on any endorsed symptoms. The purpose of the AEC was to increase the dialogue around possible adverse effects. Extrapyramidal symptoms were assessed by a physician using the Extrapyramidal Symptom Rating Scale (ESRS; Chouinard & Margolese, 2005). The ESRS assesses four medication-induced movement disorders: Parkinsonism, akathisia, dystonia, and tardive dyskinesia (Chouinard & Margolese, 2005). The first portion of the ESRS is a physician-rated questionnaire based on patient self-report. Each of the following symptoms is rated on a scale from 0 (absent) to 3 (severe): slowness/weakness; difficulty walking/balancing; stiffness; restlessness; tremors; oculogyric crisis/abnormal sustained posture; and abnormal involuntary movement. The second portion of the ESRS is comprised of a physical exam assessing symptoms of the four medication-induced movement disorders.
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS Inc.) version 22.0 with a two-tailed alpha level of p < .05 was used for all analyses. Mixed-effect models with random intercepts and/or slopes were used to test the weight-related outcomes of change in weight and TGW across time, and the psychological outcomes of depression, anxiety, and ED cognitions, behaviors, and severity. Analyses were conducted excluding participants who started olanzapine later in their treatment (i.e. excluding those eight participants who switched from the comparison to medication group).
Results
Recruitment
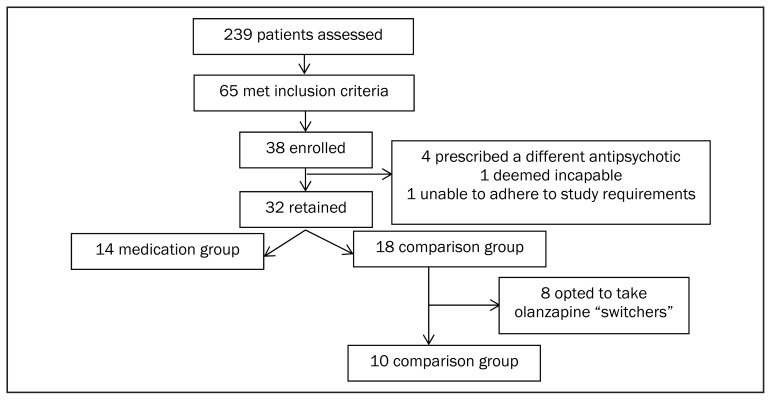
Participant flow is presented in Figure 1. Of 239 patients assessed between 2010 and 2014, 65 (27%) met full study inclusion/exclusion criteria. (We initially aimed to recruit 68 participants, but terminated the study due to poor recruitment.) Thirty-eight participants enrolled in the trial and 32 were retained for analyses. Six participants were excluded for the following reasons: one patient was deemed incapable, one patient was unable to adhere to study requirements, and four patients were prescribed a different SGA. Fourteen participants accepted olanzapine at the time of study entry and were assigned to the medication group. Eighteen chose not to take olanzapine at study entry and were assigned to the comparison group. Of the 18 comparison group participants, eight then changed their minds and requested olanzapine as part of their treatment (switch group), leaving ten participants in the comparison group.
Figure 1.
Participant flow through study arms.
Participant Characteristics
Participant baseline characteristics are presented in Table 2. The final sample of 32 participants had a mean age of 15.48 years (SD = 1.45), and the majority of participants were female (n = 29; 90.6%). Twenty-eight patients (87.5%) met criteria for DSM-IV-TR AN restrictive subtype or eating disorder not otherwise specified (EDNOS) restrictive subtype, and four (12.5%) had a diagnosis of AN binge-eating/purging subtype. The majority of participants (n = 27; 84.4 %) were inpatients at the time of recruitment, and 15.6% (n = 5) were outpatients. Inpatients and outpatients did not differ at baseline on key variables such as age, percent of TGW, or treatment group (i.e. medication versus comparison). All participants received individual and family therapy from an experienced ED therapist.
Table 2.
Descriptive statistics for the final sample. Means (M) and standard deviations (SD) of baseline measurements
| Comparison | Medication | Switch | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Measure | n | M | SD | n | M | SD | n | M | SD |
| Age (years) | 10 | 15.31 | 1.53 | 14 | 15.88 | 1.53 | 8 | 15.06 | 1.82 |
| BMI (kg/m2) | 10 | 16.76 | 0.93 | 14 | 16.25 | 1.67 | 8 | 17.12 | 1.84 |
| BMI z-score | 10 | −1.52 | 0.73 | 14 | −2.13 | 1.03 | 8 | −1.35 | 0.91 |
| TGW (kg) | 10 | 57.56 | 4.70 | 14 | 55.99 | 8.12 | 8 | 55.63 | 9.74 |
| % of TGW | 10 | 77.15 | 6.17 | 14 | 77.27 | 5.19 | 8 | 81.57 | 1.69 |
| CDI total t-score | 9 | 74.00 | 15.10 | 14 | 65.21 | 18.65 | 8 | 69.88 | 11.84 |
| MASC total t-score | 9 | 62.89 | 10.19 | 14 | 59.36 | 16.22 | 8 | 66.75 | 7.40 |
| EDEQ-A total score | 9 | 3.35 | 1.51 | 12 | 3.55 | 1.84 | 7 | 3.05 | 1.22 |
| EDI-3 drive for thinness | 8 | 20.38 | 6.21 | 13 | 17.15 | 11.20 | 7 | 19.14 | 7.76 |
| EDI-3 body dissatisfaction | 8 | 21.25 | 11.62 | 13 | 21.23 | 11.72 | 7 | 25.43 | 7.81 |
| EDS3 total score | 6 | 37.83 | 10.06 | 10 | 32.95 | 8.87 | 6 | 29.13 | 10.48 |
BMI = Body mass index; CDI = Children’s Depression Inventory; EDEQ-A = Eating Disorder Examination Questionnaire for Adolescents; EDI-3 = Eating Disorder Inventory-3; EDS3 = Eating Disorder Symptom Severity Scale; TGW = Treatment goal weight; MASC = Multidimensional Anxiety Scale for Children; Switch group = participants who began in the comparison group then elected to take olanzapine. Baseline for this group is defined as the time at which olanzapine was initiated.
Change in Weight across Time
Mean weekly weight gain for the comparison group was 0.66 kg/week over 12 weeks, and the mean weekly weight gain for the medication group was 1.09kg/week over 12 weeks. Mean differences in weight change scores are presented in Table 3. A mixed-effects regression analysis indicated that both the medication and comparison groups experienced significant gains in weight across time (β = 0.91; t = 4.70, df = 22.27, p < .001), with medication group participants gaining more weight per week (12.22 kg over 10 weeks) than those in the comparison group (who gained 7.55 kg over ten weeks) (β = 0.60; t = 2.35, df = 22.84, p = .028).
Table 3.
Mean weekly increase in weight (kg) and percent of treatment goal weight (TGW) for comparison and medication groups
| Comparison | Medication | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Week range | n | Weight M (SD) | % of TGW M (SD) | n | Weight M (SD) | % of TGW M (SD) |
| Baseline to week 1 | 10 | 1.07 (0.67) | 1.84 (1.11) | 13 | 1.24 (1.16) | 2.24 (1.99) |
| Week 1 to week 2 | 10 | 0.77 (0.63) | 1.39 (1.15) | 13 | 1.21 (0.93) | 2.12 (1.43) |
| Week 2 to week 3 | 10 | 1.08 (0.65) | 1.90 (1.17) | 12 | 1.40 (1.25) | 2.66 (2.44) |
| Week 3 to week 4 | 10 | 0.81 (0.78) | 1.45 (1.37) | 11 | 1.55 (0.93) | 2.70 (1.19) |
| Week 4 to week 6* | 7 | 1.51 (1.27) | 2.67 (2.23) | 9 | 2.72 (0.86) | 5.07 (2.12) |
| Week 6 to week 8* | 6 | 1.27 (0.93) | 2.26 (1.75) | 9 | 1.98 (1.05) | 3.50 (1.61) |
| Week 8 to week 10* | 5 | 1.04 (1.41) | 1.71 (2.43) | 5 | 2.12 (0.68) | 3.64 (1.36) |
| Week 10 to week 12* | 6 | .33 (0.23) | 0.60 (0.40) | 3 | 0.87 (1.61) | 1.59 (2.91) |
2-week interval
TGW = Treatment Goal Weight
Note: Values for medication group participants are only those values while they were taking olanzapine (hence decrease in sample size as study progresses). Total sample size is 24, but because some values are missing, weekly n does not add up to 24.
Change in Percent of Treatment Goal Weight across Time
Mean weekly change in percent of TGW was 1.15%/week over 12 weeks for the comparison group, and 1.96%/week over 12 weeks for the medication group. Mean differences in percent of TGW scores are presented in Table 3. A mixed-effects regression (random intercepts and slopes) indicated that both the medication and comparison groups experienced significant gains in percentage of TGW across time (β = 1.60; t = 4.65, df = 22.08, p < .001), with medication group participants approaching their TGW at a faster rate than those in the comparison group (β = 1.12; t = 2.45, df = 22.64, p = .022).
Participants who Switched from the Comparison to the Medication Group
The eight participants who switched from the comparison to the medication group were examined more closely. Of the eight who switched, five participants switched within two weeks of enrolling in the study, two switched within five weeks, and one participant switched at eight weeks. We wanted to examine rate of weight gain before and after starting olanzapine. To do so, we looked at participants who switched within five weeks and the one participant who switched at week eight. (Participants who switched within two weeks were not examined as we did not have enough time points to calculate a reliable mean rate of weight gain before versus after olanzapine). For the three participants examined, mean rate of weight gain before starting olanzapine was 1.01 kg/week, and mean rate of weight gain after starting olanzapine was 1.42 kg/week.
Psychological Symptoms
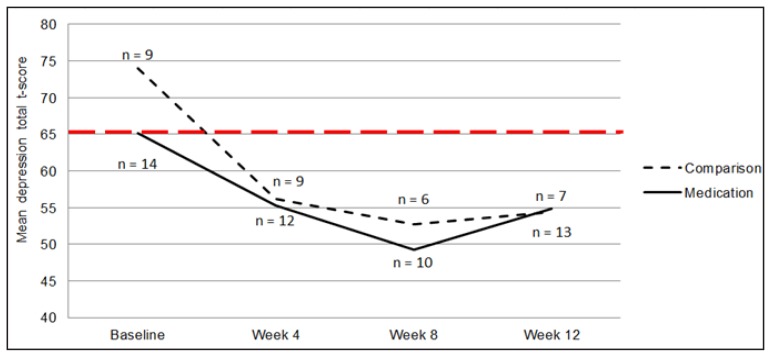
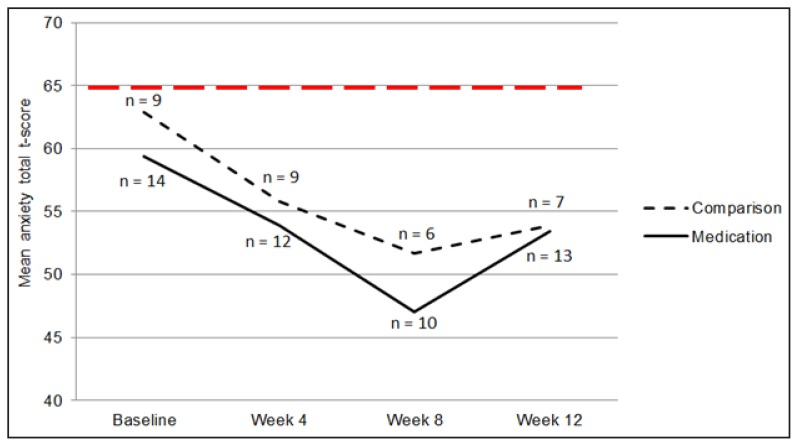
Psychological outcomes, including mood, anxiety, and ED cognitions and behaviors, were assessed at baseline and at weeks 4, 8, and 12. Mixed-effects model analyses indicated that all participants experienced a significant decrease in depression (β = −6.04; t = −3.29, df = 59.42, p = .002; random intercepts model; Figure 2) and anxiety (β = −3.22; t = −2.19, df = 23.50, p = .039; random intercepts and slopes model; Figure 3) throughout the 12-week study period. However, the two groups did not differ in rate of change of depression scores (β = −8.02; t = −0.97, df = 54.19, p = .355) or anxiety scores (β = −4.60; t = −0.71, df = 21.55, p = .487). Similarly, participants experienced a significant decrease in self-reported ED behaviors (β = −0.56; t = −3.03, df = 53.89, p = .004; random intercepts model), but no differences between groups were seen (β = −0.10; t = −0.12, df = 46.93, p = .904). Drive for thinness decreased from baseline to week 12 (β = −11.73; t = −3.03, df = 14.35, p = .009; random intercepts model), but no differences between groups were found (β = −9.77; t = −1.35, df = 24.13, p = .190). Body dissatisfaction did not change throughout the study (β = −8.76; t = −1.57, df = 14.32, p = .137; random intercepts model), and did not differ between groups (β = −9.86; t = −0.97, df = 22.10, p = .343).
Figure 2.
Mean depression t-scores across time, as measured by the Children’s Depression Inventory. The clinical cut-off is a t-score of 65, and is shown by a bold, dotted, horizontal line
Figure 3.
Mean anxiety t-score across time, as measured by the Multidimensional Anxiety Scale for Children. The clinical cut-off is a t-score of 65, and is shown by a bold, dotted, horizontal line.
Figure 2: Mean depression t-scores across time, as measured by the Children’s Depression Inventory. The clinical cut-off is a t-score of 65, and is shown by a bold, dotted, horizontal line.
Figure 3. Mean anxiety t-score across time, as measured by the Multidimensional Anxiety Scale for Children. The clinical cut-off is a t-score of 65, and is shown by a bold, dotted, horizontal line.
Adverse Effects (this discussion includes switch group participants)
There were no serious adverse effects reported throughout the study timeframe. Serum fasting glucose, HbA1C, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), triglycerides, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase, amylase, prolactin, and complete blood counts with differentials were checked at baseline, and weeks 2, 4, 8, and 12. The majority of participants, including those in the comparison group, (n = 19/32; 59.4% of participants from both groups) experienced a biochemistry result outside the reference range at some point throughout the study. Participants in the medication/switch group experienced a higher frequency of abnormal values deemed clinically significant by a study physician when compared to the comparison group. Although none of the differences reached statistical significance, they are clinically meaningful as all of the adverse effects occurred in the medication/switch group. Eight medication/switch group participants (8/22 = 27.3%) experienced elevated ALT levels while taking olanzapine. Two participants had a DAIDS Grade 2 or moderate ALT elevation (2.6–5× upper limit of normal (ULN)), and six had a DAIDS Grade 1 or mild ALT elevation (1.25–2.5 × ULN). Three of the same participants (3/22 = 13.6%) experienced a concurrent elevated AST value.
A total of seven participants, all in the medication group (7/22 = 31.8%), experienced an asymptomatic elevated serum prolactin result while taking olanzapine. The abnormal prolactin values were experienced earlier in the trial: six participants had an elevated prolactin level at week 2, and one participant at week 4.
Five participants in the medication/switch group experienced an elevated total cholesterol value (5/22 = 22.7%), three of whom also experienced an elevated LDL value (3/22 = 13.6%), and four of whom also experienced elevated HDL values (4/22 = 18.2%). One participant in the medication/switch group (1/22 = 4.6%) had an elevated triglyceride value at week 4. One participant (1/22 = 4.6%) experienced a DAIDS Grade 1 elevated CK value while taking olanzapine at week 12. One participant (1/22 = 4.6%) experienced a DAIDS Grade 2 elevated amylase value at week 6. No participants experienced abnormal fasting glucose or HbA1C values.
All participants were also asked to complete a self-report checklist of possible side-effects using the AEC. Side effects that were examined included: dizziness, somnolence, agitation, restlessness, headache, dry mouth, constipation, and stiff muscles. Subjectively, patients on olanzapine tended to experience less agitation (13.0% versus 33.3%), headache (21.7% versus 33.3%), and constipation (30.4% versus 33.3%) than the comparison group. However, they reported more dizziness (30.4% versus 22.2%), muscle stiffness (39.1% versus 22.2%), somnolence (34.8% versus 33.3%), and dry mouth (26.1% versus 22.2%). Subjective restlessness was approximately equal between the groups (21.7% in medication group and 22.2% in comparison group).
Extrapyramidal Symptoms
Physicians monitored the presence of extrapyramidal symptoms for participants taking olanzapine (medication + switch group participants) using the ESRS. The following number of participants taking olanzapine received a mild rating by a physician on each of the following self-reported symptoms: slowness/weakness = 1; difficulty walking/balancing = 0; stiffness = 0; restlessness = 9; tremors = 1; oculogyric crisis/abnormal sustained posture = 0; and abnormal involuntary movement = 0. One participant received a moderate rating of restlessness. In terms of the physical exam for Parkinsonism and akathisia, 3 patients had a very mild tremor in an upper limb (rating = 1), one patient had bradykinesia (rating = 1), two patients experienced rigidity (rating = 1), six patients had akathisia (rating = 1) and one patient had akathisia rated as a 2. Symptoms of dystonia were very rare, with one patient experiencing very mild dystonia in the upper limbs. No patients experienced dyskinetic movements. Taken together, the above-rated symptoms resulted in the following clinical global impression of severity ratings: Parkinsonism = 0 for all patients; akathisia = 1 for two patients; dystonia = 1 for one patient; and dyskinesia = 0 for all patients.
Electrocardiograms
Participants in both groups received regular ECGs to monitor for conduction abnormalities, especially corrected QT (QTc) prolongation. QTc values were machine-calculated, then manually verified and corrected if necessary. One participant in each group experienced prolonged QTc, defined as QTc > 460 ms: 470 ms for the comparison group participant, and 462 ms for the medication group participant.
Olanzapine Treatment Course
Participants in the medication group were on olanzapine for a mean of 55.18 days (SD = 21.09, range 16 – 86 days). The mean number of days between study enrollment and olanzapine treatment initiation was 1.07 days (SD = 1.33) for those who elected to take olanzapine immediately (n = 14), and 18.88 days (SD = 15.30) for those who changed their minds and started olanzapine treatment later (i.e. the switch group; n = 8). Starting doses ranged from 1.25 mg/day to 5 mg/day, and dose increase increments ranged from 1.25 mg/day to 2.5 mg/day. Treatment doses ranged from 2.5 mg to 15 mg daily (physicians were told to follow their regular standard of care in prescribing doses). Of the 22 participants who took olanzapine, one participant received a maximum dose of 15 mg/day, two received 10 mg/day, two received 7.5 mg/day, nine received a maximum dose of 5 mg per day, one received 3.75 mg/day, and seven received 2.5 mg/day (average max dose = 5.28 mg/day). Nine (40.9%) discontinued olanzapine treatment at their physician’s recommendation because they had reached their TGW; seven (31.8%) because the treating physician recommended stopping the medication because of an adverse effect, most commonly elevated liver function tests and elevated cholesterol values (Table 4); and one (4.5%) participant discontinued olanzapine on her own as she felt it was not helping her. Five participants (22.7%) remained on olanzapine for the length of the study.
Table 4.
Details of the seven participants who discontinued olanzapine due to an adverse effect
| Participant | Abnormal lab | Olanzapine details | Weight restored as per physician | % of TGW | |
|---|---|---|---|---|---|
| Days of treatment* | Max dose (mg/day) | ||||
| 1 | ↑ ALT ↑ AST |
60 | 5 | No | 91.58 |
| 2 | ↑ ALT ↑ AST |
33 | 2.5 | No | 95.41 |
| 3a | ↑ ALT ↑ AST |
26 | 2.5 | No | 82.67 |
| 4b | ↑ cholesterol ↑ triglycerides |
28 | 5 | Yes | 94.34 |
| 5 | ↑ cholesterol ↑ HDL ↑ LDL ↑ prolactin |
34 | 5 | No | 81.40 |
| 6 | ↑ cholesterol ↑ HDL ↑ prolactin |
16 | 2.5 | No | 84.81 |
| 7c | ↑ amylase | 37 | 2.5 | No | 95.79 |
Number of days the participant was taking olanzapine when a side effect resulted in the discontinuation of olanzapine treatment
Participant also had a viral infection at the time of the blood work
Participant was deemed weight restored by a study physician at the time, but elevated cholesterol was the primary reason for olanzapine discontinuation
Participant’s baseline level of amylase was elevated
ALT = Alanine aminotransferase. AST = Aspartate aminotransferase. HDL = High density lipoprotein cholesterol. LDL = Low density lipoprotein. TGW = Treatment goal weight.
Discussion
There is currently no approved psychopharmacological treatment for AN (Aigner et al., 2011; Flament, Bissada, & Spettigue, 2012). Despite the lack of evidence, many ED experts in the field use olanzapine ‘off label’ for adults and adolescents suffering from AN, especially in the most severely ill patients (Couturier & Spettigue, 2011).
Our study, albeit not randomized, is the largest trial to date of olanzapine to treat AN in adolescents. Our goal was to examine the effectiveness of adjunctive olanzapine for the treatment of adolescent AN under real-world treatment conditions, where patients and families are included in treatment decisions, and where medication regimens are flexible. As demonstrated in this trial, a proportion of participants (n = 8) who elected not to take olanzapine at the beginning of treatment later changed their minds; some after only a few days, and some after a few weeks. Our non-conventional study design enabled the collection of valuable information about this sub-set of participants. One possible limitation of such a study is that those participants who chose to take medication may be more motivated for recovery and thus less “ill” in some respects. In fact, although not statistically different, the medication group tended to have more symptoms of depression at baseline, whereas weight, anxiety, drive for thinness, body dissatisfaction, and ED urges and symptoms appeared comparable. This is only the second trial, after Leggero et al. (2010), and the largest, to demonstrate an increased rate of weight gain in youth with AN treated with olanzapine in addition to standard treatment (including medical management, nutritional rehabilitation, and/or individual and family therapy). This knowledge has implications in terms of olanzapine helping to shorten the time to weight restoration, and possibly helping to shorten the length of hospital admissions. Future research should include a longer follow-up to examine maintenance of weight gain over time.
One weakness of this study is the amount of missing data. Many patients had reached their TGW by week 8, such that the clinicians opted to stop olanzapine by this time. As a result, there were 21 patients on olanzapine in week 1, 13 patients on olanzapine in weeks 6–8, and only four patients taking olanzapine by weeks 10–12. While the clinicians often reported that they had stopped using the olanzapine as a result of abnormal laboratory values (e.g. high ALT, high cholesterol, high prolactin), it should be noted that most of these patients (57.1%) were over 90% of their TGW when the clinician stopped the medication. As a result, we were unable to report results for weights, laboratory values, or side effects at week 12, due to the small numbers still left taking olanzapine at that time. We thus chose to compare the two groups at week 4, and to a lesser extent at week 8.
Another limitation of this study is the lack of a structured medication titration schedule. Although our goal was to approximate real-world treatment conditions where medication regimens are flexible, the absence of a structured medication regimen prohibits us from drawing conclusions on the association between olanzapine dosage and weight gain. Given that researchers have demonstrated in adults that olanzapine has different pharmacodynamic properties at different doses, with higher doses being associated with higher D2 receptor occupancy than 5-HT2A receptor occupancy (Schmitt et al., 2002), it is possible that the pattern of results would have been different had we implemented a structured medication titration schedule.
Although some case studies in children and adolescents (Boachie et al., 2003) and the RCT in adults by Bissada et al. (2008) have reported decreased AN preoccupations or obsessions, we were unable to demonstrate a difference in ED thoughts or urges between the two groups, possibly due to low power. This has implications for longer-term recovery: weight restoration may not be sustainable if the core ED psychopathology does not improve. Both groups improved significantly on measures of depression and anxiety, suggesting that weight gain and non-pharmacological treatment play important roles.
Although olanzapine was generally well tolerated and although adverse effects were not serious, they seemed to be more common in the medication/switch group (although differences were not statistically significant). Our findings are consistent with other published pediatric studies that have found an increased likelihood of high triglycerides, total cholesterol, prolactin, and ALT/AST with olanzapine (Pringsheim, Panagiotopoulos, Davidson, & Ho, 2011) especially second generation antipsychotics. In general laboratory abnormalities were mild, but the high number of abnormal laboratory values would suggest that adolescents being treated with olanzapine should be closely monitored, especially for liver function tests and prolactin levels.
Conclusion
This open-label trial is one of the first studies, and the largest, to show an association between weight gain and adjunctive olanzapine treatment in adolescents with AN, compared to adolescents with AN who chose not to take olanzapine. The mean maximum dose of olanzapine utilized was 5.28 mg/day and the medication was well tolerated in this population. Future multi-center double-blind placebo-controlled trials are needed to further advance our knowledge of the safety and effectiveness of olanzapine for adolescents with AN.
Acknowledgments / Conflicts of Interest
This work was supported by the W. Garfield Weston Foundation. The sponsors had no role in the study design; the collection, analysis, and interpretation of the data; writing of the report; or the decision to submit the article for publication. The authors do not have any conflicts of interest to disclose. The authors would like to thank the W. Garfield Weston Foundation for their support. Partial results from this open-label trial were presented at the 62nd Annual Meeting of the American Academy of Child and Adolescent Psychiatry in San Antonio, Texas on October 29, 2015, and at the International Conference on Eating Disorders in Boston, Massachusetts on April 25, 2015.
References
- Aigner M, Treasure J, Kaye W, Kasper S. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Pharmacological Treatment of Eating Disorders. World Journal of Biological Psychiatry. 2011;12(6):400–443. doi: 10.3109/15622975.2011.602720. [DOI] [PubMed] [Google Scholar]
- Attia E, Kaplan AS, Walsh BT, Gershkovich M, Yilmaz Z, Musante D, Wang Y. Olanzapine versus placebo for out-patients with anorexia nervosa. Psychological Medicine. 2011;41(10):2177–2182. doi: 10.1017/S0033291711000390. [DOI] [PubMed] [Google Scholar]
- Barbarich NC, McConaha CW, Gaskill J, La Via M, Frank GK, Achenbach S, … Kaye WH. An open trial of olanzapine in anorexia nervosa. Journal of Clinical Psychiatry. 2004;65(11):1480–1482. doi: 10.4088/JCP.v65n1106. [DOI] [PubMed] [Google Scholar]
- Bissada H, Tasca GA, Barber AM, Bradwejn J. Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: A randomized, double-blind, placebo-controlled trial. American Journal of Psychiatry. 2008;165(10):1281–1288. doi: 10.1176/appi.ajp.2008.07121900. [DOI] [PubMed] [Google Scholar]
- Boachie A, Goldfield GS, Spettigue W. Olanzapine use as an adjunctive treatment for hospitalized children with anorexia nervosa: Case reports. International Journal of Eating Disorders. 2003;33(1):98–103. doi: 10.1002/eat.10115. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Garcia CS, Fassino S, Daga GA, Favaro A, Santonastaso P, … Monteleone P. Olanzapine therapy in anorexia nervosa: Psychobiological effects. International Clinical Psychopharmacology. 2007;22(4):197–204. doi: 10.1097/YIC.0b013e328080ca31. [DOI] [PubMed] [Google Scholar]
- Carter JC, Stewart DA, Fairburn CG. Eating disorder examination questionnaire: Norms for young adolescent girls. Behaviour Research & Therapy. 2001;39(5):625–632. doi: 10.1016/s0005-7967(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Chouinard G, Margolese HC. Manual for the Extrapyramidal Symptom Rating Scale (ESRS) Schizophrenia Research. 2005;76(2–3):247–265. doi: 10.1016/j.schres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Couturier J, Spettigue W. Pharmacotherapy for eating disorders in children and adolescents. In: Le Grange D, Lock J, editors. Eating disorders in children and adolescents: A clinical handbook. The Guilford Press; 2011. pp. 402–418. [Google Scholar]
- Dennis K, Le Grange D, Bremer J. Olanzapine use in adolescent anorexia nervosa. Eating and Weight Disorders. 2006;11(2):e53–e56. doi: 10.1007/BF03327760. [DOI] [PubMed] [Google Scholar]
- Division of AIDS table for grading the severity of adult and pediatric adverse events. Division of AIDS. 2014. Retrieved from https://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf.
- Dold M, Aigner M, Klabunde M, Treasure J, Kasper S. Second-generation antipsychotic drugs in anorexia nervosa: A meta-analysis of randomized controlled trials. Psychotherapy and Psychosomatics. 2015;(84):110–116. doi: 10.1159/000369978. [DOI] [PubMed] [Google Scholar]
- Ercan ES, Copkunol H, Cýkoethlu S, Varan A. Olanzapine treatment of an adolescent girl with anorexia nervosa. Human Psychopharmacology. 2003;18(5):401–403. doi: 10.1002/hup.492. [DOI] [PubMed] [Google Scholar]
- Flament MF, Bissada H, Spettigue W. Evidence-based pharmacotherapy of eating disorders. The International Journal of Neuropsychopharmacology. 2012;15(2):189–207. doi: 10.1017/S1461145711000381. [DOI] [PubMed] [Google Scholar]
- Garner DM. The Eating Disorder Inventory-3 Professional Manual. Lutz, Florida: Psychological Assessment Resources Inc; 2004. [Google Scholar]
- Jensen VS, Mejlhede A. Anorexia nervosa: Treatment with olanzapine. The British Journal of Psychiatry. 2000;177(87):573–576. doi: 10.1192/bjp.177.1.87. [DOI] [PubMed] [Google Scholar]
- Kafantaris V, Leigh E, Hertz S, Berest A, Schebendach J, Meyer Sterling W, … Malhorta AK. A placebo-controlled pilot study of adjunctive olanzapine for adolescents with anorexia nervosa. Journal of Child and Adolescent Psychopharmacology. 2011;21(3):207–212. doi: 10.1089/cap.2010.0139. [DOI] [PubMed] [Google Scholar]
- Kishi T, Kafantaris V, Sunday S, Sheridan EM, Correll CU. Are antipsychotics effective for the treatment of anorexia nervosa? The Journal of Clinical Psychiatry. 2012;73(6):e757–e766. doi: 10.4088/JCP.12r07691. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children’s Depression Inventory (CDI) Manual. New York, NY: Multi-Health Systems Inc; 1992. [Google Scholar]
- La Via MC, Gray N, Kaye WH. Case reports of olanzapine treatment of anorexia nervosa. International Journal of Eating Disorders. 2000;27(3):363–366. doi: 10.1002/(sici)1098-108x(200004)27:3<363::aid-eat16>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lebow J, Sim La, Erwin PJ, Murad MH. The effect of atypical antipsychotic medications in individuals with anorexia nervosa: A systematic review and meta-analysis. International Journal of Eating Disorders. 2013;46(4):332–339. doi: 10.1002/eat.22059. [DOI] [PubMed] [Google Scholar]
- Leggero C, Masi G, Brunori E, Calderoni S, Carissimo R, Maestro S, Muratori F. Low-dose olanzapine monotherapy in girls with anorexia nervosa, restricting subtyp: Focus on hyperactivity. Journal of Child and Adolescent Psychopharmacology. 2010;20(2):127–133. doi: 10.1089/cap.2009.0072. [DOI] [PubMed] [Google Scholar]
- Lock J, Fitzpatrick KK. Evidenced-based treatments for children and adolescents with eating disorders: Family therapy and family-facilitated cognitive-behavioral therapy. Journal of Contemporary Psychotherapy. 2007;37(3):145–155. doi: 10.1007/s10879-007-9049-x. [DOI] [Google Scholar]
- Lock J, Gowers S. Effective interventions for adolescents with anorexia nervosa. Journal of Mental Health. 2005;14(6):599–610. doi: 10.1080/09638230500400324. [DOI] [Google Scholar]
- Lord CC, Wyler SC, Wan R, Castorena CM, Ahmed N, Mathew D, … Elmquist JK. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. Journal of Clinical Investigation. 2017;127(9):3402–3406. doi: 10.1172/JCI93362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malina A, Gaskill J, McConaha C, Frank GK, LaVia M, Scholar L, Kaye WH. Olanzapine treatment of anorexia nervosa: A retrospective study. The International Journal of Eating Disorders. 2003;33(2):234–237. doi: 10.1002/eat.10122. [DOI] [PubMed] [Google Scholar]
- March JS. Multidimensional Anxiety Scale for Children Technical Manual. Toronto, Ontario: Multi-Health Systems Inc; 1997. [Google Scholar]
- McElroy SL, Guerdjikova AI, Mori N, Keck PE. Psychopharmacologic treatment of eating disorders: Emerging findings. Current Psychiatry Reports. 2015;17(5):1–7. doi: 10.1007/s11920-015-0573-1. [DOI] [PubMed] [Google Scholar]
- Mehler C, Wewetzer C, Schulze U, Warnke A, Theisen F, Dittmann RW. Olanzapine in children and adolescents with chronic anorexia nervosa: A study of five cases. European Child & Adolescent Psychiatry. 2001;10(2):151–157. doi: 10.1007/s007870170039. [DOI] [PubMed] [Google Scholar]
- Mondraty N, Birmingham CL, Touyz S, Sundakov V, Chapman L, Beumont P. Randomized controlled trial of olanzapine in the treatment of cognitions in anorexia nervosa. Australasian Psychiatry : Bulletin of Royal Australian and New Zealand College of Psychiatrists. 2005;13(1):72–75. doi: 10.1111/j.1440-1665.2004.02154.x. [DOI] [PubMed] [Google Scholar]
- Powers PS, Santana CA, Bannon YS. Olanzapine in the treatment of anorexia nervosa: An open label trial. International Journal of Eating Disorders. 2002;32(2):146–154. doi: 10.1002/eat.10084. [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Panagiotopoulos C, Davidson J, Ho J. Evidence-based recommendations for monitoring safety of second generation antipsychotics in children and youth. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2011;20(3):218–233. 1Retrieved from http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L362335340%5Cnhttp://www.cacap-acpea.org/%5Cnhttp://resolver.ebscohost.com/openurl?sid=EMBASE&issn=17198429&id=doi:&atitle=Evidence-based+recommendations+for+monitoring+safety+of+second+ge. [PMC free article] [PubMed] [Google Scholar]
- Schmitt GJE, Meisenzahl EM, Dresel S, Tatsch K, Rossmuller B, Frodl T, … Moller HJ. Striatal dopamine D2 receptor binding of risperidone in schizophrenic patients as assessed by 123I-iodobenzamide SPECT: A comparative study with olanzapine. Journal of Psychopharmacology. 2002;16(3):200–206. doi: 10.1177/026988110201600302. [DOI] [PubMed] [Google Scholar]
- Stip E, Lungu OV. Salience network and olanzapine in schizophrenia: Implications for treatment in anorexia nervosa. Canadian Journal of Psychiatry. 2015;60(3):S35–S39. [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel LL, Jordaan GP. The psychopharmacological management of eating disorders in children and adolescents. Journal of Child & Adolescent Mental Health. 2014;26(2):125–137. doi: 10.2989/17280583.2014.909816. [DOI] [PubMed] [Google Scholar]
- Watson HJ, Bulik CM. Update on the treatment of anorexia nervosa: Review of clinical trials, practice guidelines and emerging interventions. Psychological Medicine. 2013;43(12):2477–500. doi: 10.1017/S0033291712002620. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Walsh BT. In review: Pharmacologic treatment of eating disorders. Canadian Journal of Psychiatry. 2002;47(3):227–234. doi: 10.1177/070674370204700302. [DOI] [PubMed] [Google Scholar]