Abstract
Objectives
Daptomycin non-susceptibility in Staphylococcus aureus can emerge via the accumulation of single or multiple mutations, each resulting in a slight increase in the daptomycin MIC. The daptomycin-non-susceptible phenotype may include other features such as daptomycin tolerance. This study identifies S. aureus genomic regions that frequently develop mutations following prolonged daptomycin exposure but have not been previously associated with daptomycin non-susceptibility.
Methods
Sequence variations in the same eight loci independently observed following 28 day parallel serial passages of S. aureus J01 in daptomycin were introduced in isolation into S. aureus J01. MICs were determined by microbroth dilution. Daptomycin killing and tolerance were determined by kill curve analysis.
Results
Single mutations in snoF, hmp1, sspA, rimP, hepT, rsh, map1 and amaP had only a modest impact on the daptomycin MIC (≤2-fold). In contrast, individual mutation in several of these regions resulted in pronounced changes to daptomycin tolerance.
Conclusions
This study demonstrates that less characterized mutations in S. aureus following daptomycin exposure do not result in significant daptomycin susceptibility changes, but rather allow for enhanced survival characteristics during treatment. This sheds new light on genetic adaptations that may play a role in persistent infection. Further studies are needed to elucidate the prevalence of these mutations in clinical isolates.
Introduction
Daptomycin-non-susceptible (DNS) Staphylococcus aureus strains are observed clinically, particularly during prolonged antibiotic exposure, and are associated with treatment failure. Mutations in six genes, mprF, walKR, rpoBC and cls2, have individually been associated with daptomycin non-susceptibility, with mprF being the most prevalent.1 Although some are sufficient for daptomycin non-susceptibility, none is uniquely necessary for the phenotype. Sequence polymorphisms in other loci are observed in DNS strains, but causality has not been established. These and other sequence polymorphisms may represent alternative pathways to the DNS phenotype and can identify core metabolic functions disturbed in response to daptomycin.
Compensatory responses to daptomycin may modulate growth rates, bypass daptomycin-disrupted pathways and/or promote antibiotic tolerance. Tolerance is distinct from resistance in that growth is restricted but viability is minimally affected.2 Tolerance occurs among S. aureus isolates from persistent and recurrent bacteraemia,3 a phenotype largely absent among isolates from rapidly resolving infection.4 This reflects the discrepancy between a low rate of DNS S. aureus by MIC (∼2%) and the ∼15% rate of persistent bacteraemia with treatment.5,6 This study aimed to determine the effect of polymorphisms in nine additional genomic regions on daptomycin non-susceptibility, tolerance and organism survival upon daptomycin exposure.
Materials and methods
MRSA strains J01 and J03 and in vitro-derived mutants have been described previously7,8 with sequence reads publically available in GenBank (PRJEB8645). J01 was subcultured daily in vitro for 28 days in parallel experiments containing escalating concentrations of daptomycin and static concentrations of either broth or adjunctive antibiotics. Serially passaged strains were renamed ‘SP-’ followed by identifiers for antibiotics included in serial passage and replicate number. Mueller–Hinton broth was supplemented to 50 mg/L calcium and 12.5 mg/L magnesium (MHB50). Brain heart infusion broth was solidified with 1.5% agar. MICs of antibiotics were determined in quadruplicate by microbroth dilution.9 Candidates for allelic replacement were selected based on sequence variation occurring in at least 10% of in vitro-generated DNS genomes and in at least two passages containing different adjunctive antibiotics. Mutations in yycFG/walKR, rpoBC, mprF and cls2 were excluded as they constitute established mediators of the DNS phenotype and have been described elsewhere.8
Targeted allelic replacement was accomplished utilizing the pIMAY-Z system (Table S1, available as Supplementary data at JAC Online).10 If multiple distinct variations were identified in the same locus, amplification from strains with WT growth rates was prioritized to mitigate the influence of growth rate changes on downstream analyses. All allelic replacement strains were confirmed free of second-site mutation by whole-genome sequencing.8
Time–kill curve analyses were performed using static concentrations of 3.8 mg/L daptomycin. Cultures (106 cfu/mL) in MHB50 were shaken (180 rpm, 37°C) in triplicate and viable organisms enumerated by dilution plating. The area under the time–kill curve (AUC) was determined using the trapezoidal method. The lowest measurable quantity was 100 cfu/mL.
Tolerance–kill curve analyses were performed with supratherapeutic concentrations of antibiotic according to the method of Mechler et al.11 Overnight cultures (∼5 × 109 cfu/mL) were brought to 100 mg/L daptomycin (∼100 × MIC) and viable organism counts enumerated via dilution plating at predefined intervals. Minimum durations of exposure required to achieve 2 log and 4 log reductions in viability were calculated (MDK99 and MDK99.99, respectively). Equivalent MDK99 and MDK99.99 values indicates ‘isotolerance’, equivalent MDK99 but different MDK99.99 values indicates ‘heterotolerance’, and different MDK99 values indicates ‘tolerance’.2 MHB50 was supplemented to account for calcium internalization by the high bacterial inoculum (≥15%, data not shown). Samples were washed with normal saline prior to plating. AUC was determined using the trapezoidal method.
Continuous variables were summarized using the mean and SD, and compared using the two-tailed unpaired Student’s t-test. P values of ≤0.05 were considered ‘significant’ following a post hoc Holm–Bonferroni adjustment for multiple comparisons.
Results
Nine loci were identified where mutation emerged independently in at least three DNS isolates (Table S2). Technical challenges precluded further analysis of clpX mutations; therefore, the following data and discussion will exclude clpX and focus on the remaining eight loci. In this subset, 20 sequence polymorphisms were identified. In no region was mutation unique to a specific antibiotic combination exposure, indicating daptomycin selective pressure rather than any influence of other antibiotics. A comprehensive list of mutations is provided in Table S3.
Introduction of mutations in snoF, sspA, hepT or amaP into J01 resulted in a modest increase in daptomycin MIC (from 0.25 to 0.5 mg/L). No changes to daptomycin MIC were observed when targeted mutations were introduced into the DNS J03 genomic background (daptomycin MIC = 2 mg/L) (Table 1).
Table 1.
Daptomycin activity in allelic replacement strains
| Strain background | Allele replaceda | Daptomycin MIC (mg/L) | Doubling time (min) | AUCb |
|---|---|---|---|---|
| J01 | WT | 0.25 | 35 ± 3.0 | 67.1 ± 0.22 |
| J01 | snoF, G28D | 0.5 | 39 ± 2.5 | 75.2 ± 1.08 |
| J01 | hmp1, T275Rfs7 | 0.25 | 36 ± 2.2 | 71.9 ± 1.23 |
| J01 | sspA, D234Y | 0.5 | 31 ± 0.9 | 63.5 ± 0.17 |
| J01 | rimP, L75S | 0.25 | 33 ± 3.4 | 74.7 ± 1.27 |
| J01 | hepT, M309Wfs13 | 0.5 | 49 ± 3.0c | 83.3 ± 0.77c |
| J01 | rsh, L61F | 0.25 | 36 ± 7.2 | 72.2 ± 0.47c |
| J01 | map1, H64Y | 0.25 | 31 ± 5.5 | 69.5 ± 1.26 |
| J01 | amaP, G22R | 0.5 | 27 ± 2.4 | 64.0 ± 0.86 |
| J03 | WT | 2 | 32 ± 2.8 | 76.0 ± 0.90 |
| J03 | snoF, G28D | 2 | 31 ± 9.4 | 108.6 ± 2.75c |
| J03 | hmp1, T275Rfs7 | 2 | 34 ± 8.9 | 84.5 ± 2.71 |
| J03 | sspA, D234Y | 2 | 31 ± 2.9 | 75.9 ± 0.66 |
| J03 | rimP, L75S | 2 | 35 ± 3.0 | 92.7 ± 0.92c |
| J03 | hepT, M309Wfs13 | 2 | 45 ± 3.2c | 96.3 ± 2.14c |
| J03 | rsh, L61F | 2 | 35 ± 3.6 | 106.2 ± 1.27c |
| J03 | map1, H64Y | 2 | 30 ± 1.5 | 99.5 ± 4.13c |
| J03 | amaP, G22R | 2 | 28 ± 2.1 | 65.5 ± 0.60c |
fs, frameshift mutation.
Area under the 24 h growth curve following in vitro exposure of 106 bacteria to 3.8 mg/L daptomycin.
P < 0.05 versus WT progenitor strain.
In kill curve analyses, mutation in either hepT or rsh resulted in significantly less extensive daptomycin killing compared with the J01 parent. In the DNS J03 genomic background, mutations in snoF, rimP, hepT, rsh or map1 resulted in significantly less extensive daptomycin killing (Table 1). Only hepT affected growth, prolonging doubling time by ∼50%.
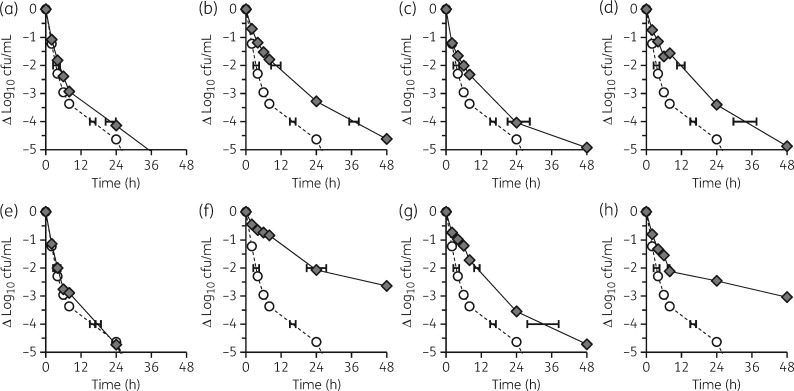
Mutation in any targeted locus, with the exception of hepT, resulted in significantly reduced daptomycin killing compared with J01 in the stationary phase (high inoculum) tolerance assay. Mutation in hmp1, rimP, rsh, map1 or amaP confers tolerance, and mutation in snoF or sspA confers heterotolerance (Figure 1). Similar results were obtained for DNS J03 with minor distinctions of sspA conferring full tolerance and hepT conferring heterotolerance (data not shown).
Figure 1.
Antimicrobial tolerance. Overnight cultures of study strains were exposed to 100 mg/L daptomycin and viable cfu/mL enumerated at predetermined intervals. (a) J01snoF (G28D). (b) J01hmp1 (T275Rfs7). (c) J01sspA (D234Y). (d) J01rimP (L75S). (e) J01hepT (M309Wfs13). (f) J01rsh (L61F). (g) J01map1 (H64Y). (h) J01amaP (G22R). Circles, WT J01; diamonds, allelic replacement strain. Exposures were performed in triplicate from distinct colonies started on different days. Control experiments without added daptomycin demonstrate ≤0.2 log10 cfu/mL change from baseline at all timepoints tested in all strain backgrounds.
Discussion
Daptomycin exposure has multiple physiological effects on staphylococci, resulting in bactericidal antimicrobial activity. Here we demonstrate that mutations in eight loci do not affect daptomycin MICs significantly but do contribute to survival in the presence of daptomycin. Since mutations identified in this work are not generally implicated in either cell envelope homeostasis or regulatory control (Table S4), their role in enhancing cell survival during daptomycin exposure is unclear.
snoF
SnoF is a component of complex I NADH: ubiquinone oxidoreductase. Transposon disruption of snoD in S. aureus has been generated previously, resulting in resistance to host cationic peptide tPMP-1.12 Daptomycin and host cationic peptides both act by disrupting membrane integrity, and cross-resistance has been reported.13
hmp1
SAUSA300_RS04910 encodes an AI-2E-like transmembrane efflux protein. Mutations isolated in hmp1 are predicted to result in loss of function.
sspA
SspA is a well-characterized extracellular protease and virulence factor, V8 protease.14 The D234Y mutation identified in this work localizes to a C-terminal repeat region of unclear function.
rimP
RimP is a ribosome maturation factor involved in production of mature 30S ribosomal subunits. Several DNS strains in this study and others contain mutations in genes encoding additional ribosomal proteins including rplB, rplC, rplP, rplV and rpsU. However, no single subunit or ribosomal maturation factor appears uniquely responsible for mediating the DNS phenotype.
hepT
The SAUSA300_RS07410 gene product resembles GerCC/HepT-like components of heterodimeric polyprenyl synthetases. Its local genomic context suggests involvement in menaquinone biosynthesis.
rsh
SAUSA300_RS08665 encodes Rsh, a bifunctional enzyme combining ppGpp synthesis and hydrolase activities. The modified guanosine nucleotide mediates the stringent response, a cellular program that modifies global transcription to rapidly adapt to cellular stresses.15 Antibiotics can induce ppGpp synthesis and high ppGpp levels can reduce antibiotic activity, including daptomycin.16 Mutations primarily map to the hydrolase domain and consequently are predicted to result in impaired ppGpp degradation. Mutations in rsh occur in small colony variant and persistent infections.
map1
SAUSA300_RS10105 encodes a membrane-associated protein (Map1) of unknown function. Allelic variants include frameshift and nonsense mutations, suggesting loss of function.
amaP
AmaP is as a membrane anchor for alkaline shock protein (Asp23). Loss of either AmaP or Asp23 results in up-regulation of cell envelope stress response genes, including vraSR, a mediator of both daptomycin non-susceptibility and decreased resistance to oxacillin.17
Overall, the contribution of these individual mutations to daptomycin MIC changes was only marginal despite the marked daptomycin MIC elevation in the previously described passaged strains.1 Likewise, we observed a modest protective effect of several targeted mutations in the presence of therapeutically relevant daptomycin concentrations in the growth curve assay.
Antimicrobial tolerance can contribute to clinical treatment failures.2,4 This trait may be shared by the entire population (‘tolerant’) or by only a small subpopulation (‘heterotolerant’).2,4 In the current study, seven out of the eight mutations enhanced survival against concentrations of daptomycin at least 100-fold above the MIC. Strains containing mutations in hmp1, rimP, rsh, map1 or amaP were daptomycin tolerant, whereas two additional loci (snoF and sspA) confer heterotolerance.
rsh mutation and induction of the stringent response has already been implicated in antimicrobial tolerance from both clinical and lab-derived S. aureus.18,19 The relative ease with which rsh mutations were selected in the current study (24% of serial passage strains) and diverse SNP locations suggest a dysregulated stringent response results from direct mutation of RSH.
In summary, our study identifies the effect of eight novel mutations in S. aureus on evolution of daptomycin non-susceptibility. From these data, we posit that these mutations reduce daptomycin activity via adaptations to: (i) redirect around metabolic pathway disruption; (ii) alter growth rate to promote antimicrobial tolerance; (iii) modulate cell envelope characteristics without altering daptomycin susceptibility; or (iv) synergize with classical DNS-conferring mutations. Although this work was performed in only one genetic background, these findings have important implications on the mechanisms of daptomycin tolerance, a continuing challenge in patients with invasive S. aureus infections.
Supplementary Material
Acknowledgments
Funding
This work was supported in part by a grant from the National Institutes of Health (1R01AI132627–01) and by the National Health and Medical Research Council, Australia (fellowship APP1023526).
Transparency declarations
W. E. R. has received research funding support from Merck. All other authors: none to declare.
Supplementary data
Tables S1–S4 are available as Supplementary data at JAC Online.
References
- 1. Tran TT, Munita JM, Arias CA.. Mechanisms of drug resistance: daptomycin resistance. Ann N Y Acad Sci 2015; 1354: 32–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brauner A, Fridman O, Gefen O. et al. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 2016; 14: 320–30. [DOI] [PubMed] [Google Scholar]
- 3. Berti AD, Schroeder JW, Rottier AD. et al. Evolution of antibiotic tolerance during oxacillin, daptomycin and dalbavancin therapy results in breakthrough Staphylococcus aureus bacteremias. Open Forum Infect Dis 2017; 4 Suppl 1: S14. [Google Scholar]
- 4. Conlon BP. Staphylococcus aureus chronic and relapsing infections: evidence of a role for persister cells: an investigation of persister cells, their formation and their role in S. aureus disease. Bioessays 2014; 36: 991–6. [DOI] [PubMed] [Google Scholar]
- 5. Rose WE, Eickhoff JC, Shukla SK. et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 2012; 206: 1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fowler VG, Miro JM, Hoen B. et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293: 3012–21. [DOI] [PubMed] [Google Scholar]
- 7. Berti AD, Wergin JE, Girdaukas GG. et al. Altering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibiotics. Antimicrob Agents Chemother 2012; 56: 5046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berti AD, Baines SL, Howden BP. et al. Heterogeneity of genetic pathways toward daptomycin nonsusceptibility in Staphylococcus aureus determined by adjunctive antibiotics. Antimicrob Agents Chemother 2015; 59: 2799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement M100-S25 CLSI, Wayne, PA, USA, 2015.
- 10. Monk IR, Tree JJ, Howden BP. et al. Complete bypass of restriction systems for major Staphylococcus aureus lineages. MBio 2015; 6: e00308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mechler L, Herbig A, Paprotka K. et al. A novel point mutation promotes growth phase-dependent daptomycin tolerance in Staphylococcus aureus. Antimicrob Agents Chemother 2015; 59: 5366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bayer AS, McNamara P, Yeaman MR. et al. Transposon disruption of the complex I NADH oxidoreductase gene (snoD) in Staphylococcus aureus is associated with reduced susceptibility to the microbicidal activity of thrombin-induced platelet microbicidal protein 1. J Bacteriol 2006; 188: 211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mishra NN, McKinnell J, Yeaman MR. et al. In vitro cross-resistance to daptomycin and host defense cationic antimicrobial peptides in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 2011; 55: 4012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prasad L, Leduc Y, Hayakawa K. et al. The structure of a universally employed enzyme: V8 protease from Staphylococcus aureus. Acta Crystallogr D Biol Crystallogr 2004; 60: 256–9. [DOI] [PubMed] [Google Scholar]
- 15. Wolz C, Geiger T, Goerke C.. The synthesis and function of the alarmone (p)ppGpp in firmicutes. Int J Med Microbiol 2010; 300: 142–7. [DOI] [PubMed] [Google Scholar]
- 16. Hobbs JK, Miller K, Chopra I.. The effect of the stringent response on the bactericidal activity of antibiotics against Staphylococcus aureus In: Abstracts of the Forty-Seventh Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 2007. Abstract C1-1488. Washington, DC: American Society for Microbiology. [Google Scholar]
- 17. Müller M, Reiß S, Schlüter R. et al. Deletion of membrane-associated Asp23 leads to upregulation of cell wall stress genes in Staphylococcus aureus. Mol Microbiol 2014; 93: 1259–68. [DOI] [PubMed] [Google Scholar]
- 18. Geiger T, Goerke C, Fritz M. et al. Role of the (p)ppGpp synthase RSH, a RelA/SpoT homolog, in stringent response and virulence of Staphylococcus aureus. Infect Immun 2010; 78: 1873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao W, Chua K, Davies JK. et al. Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent infection. PLoS Pathog 2010; 6: e1000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.