Abstract
Purpose of Review
Bone marrow adipocytes (BMAs) have distinct molecular properties and physiologic responses depending on their location within the skeleton.
Recent Findings
This concept was introduced in the 1970s and validated more recently in the contexts of cold exposure, sympathetic tone, hematopoiesis, diabetes, lactation, fasting and caloric restriction.
Summary
In this brief review, we discuss the concept of regulated vs constitutive BMAs, explore their evolutionary and microenvironmental origins, define the site-specific molecular features of BMAs, and discuss the translational implications of the dual bone marrow adipose tissue hypothesis.
Keywords: bone marrow adipocyte, adipose, marrow fat, yellow marrow, hematopoiesis
Introduction
Bone marrow adipocytes (BMAs) decrease in size and/or number in response to interventions such as cold exposure, fasting, lactation and anemia (1–6). The magnitude of these responses depends on the location of the BMAs within the skeleton. This is the core observation that led to the hypothesis that there is more than one functional category, or type, of BMAs. However, despite the first publications on this topic more than 40 years ago (4,5), the field is still working to understand its implications for both skeletal and systemic pathophysiology. In this review we will discuss the concept of regulated vs constitutive BMAs, explore their evolutionary and microenvironmental origins, define the site-specific molecular features of BMAs, and discuss the translational implications of the dual bone marrow adipose tissue hypothesis.
Defining regulated vs constitutive bone marrow adipocytes
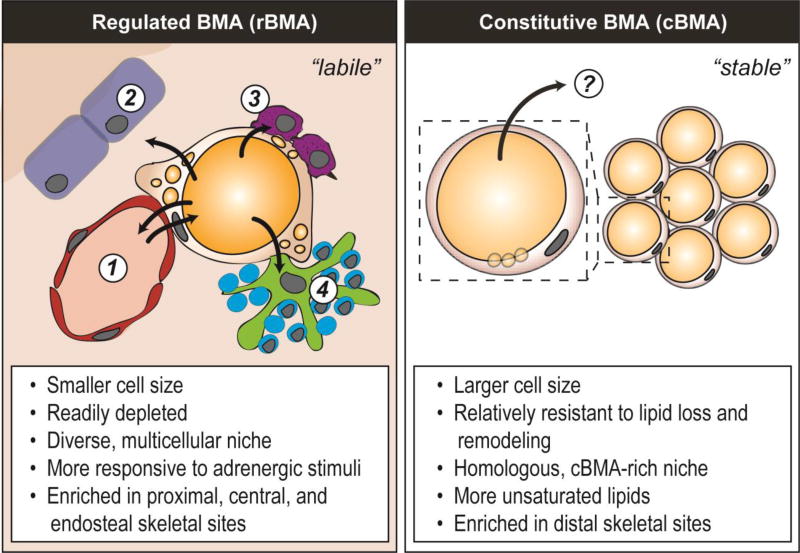
The initial report of BMA subtypes is credited to Dr. Mehdi Tavassoli and was based on site-specific responses of BMAs to phenylhydrazine-induced hemolysis in rabbits (4). He wrote that, “Unlike those of yellow marrow, fat cells of red marrow are in a state of flux. When hemopoietic tissue expands (…), lipid contained in the fat cells undergoes resorption, and the fat cell mass is reduced (…). On the other hand, fat cells of the yellow marrow (…) are not easily displaced by the expansion of hemopoietic tissue” (5). Dr. Tavassoli called these cells ‘labile’ and ‘stable’. This observation of site-specific BMA responses has since been recapitulated in rodents after 21-day cold exposure or a 48-hour fast (1,3). In addition, transgenic mice with PTRF deficiency, a model of congenital generalized lipodystrophy, have differential loss of BMAT (1). In each of these cases, cells within the red, hematopoietic marrow were decreased in size or depleted while cells in the yellow, fatty marrow remained relatively unchanged. These cells have since been termed regulated and constitutive bone marrow adipocytes (rBMA and cBMA) (7,8). Regulated BMAs are equivalent to Dr. Tavassoli’s ‘labile’ BMAs and defined histologically as single cells interspersed within the hematopoietic bone marrow (Figure 1). They form gradually throughout life and accumulate with aging. Constitutive BMAs (also known as ‘stabile’ BMAs), by contrast, form early in development, are larger in size, and appear histologically as densely packed groups of adipocytes with little intervening hematopoiesis (Figure 1).
Figure 1. Regulated vs constitutive bone marrow adipocytes.
Regulated bone marrow adipocytes (rBMAs) exist in a highly cellular, generally peri-vascular niche which contains a diversity of populations including: (1) endothelial cells and perivascular progenitors, (2) osteoblasts and bone lining cells, (3) granulocyte/macrophage lineage cells, and (4) erythroblast islands. In addition to releasing fatty acids into the bloodstream, these diverse surrounding populations represent possible utilizers of rBMA energy reserves (arrows outward). By contrast, constitutive BMAs are located in a relatively homogeneous niche that is packed with adipocytes and relatively inaccessible to hematopoietic and osteogenic cell populations. It remains unclear when and where the lipid from the cBMAs is utilized, however, evidence to date suggests that these cells may serve as a last-resort energy reserve in times of tertiary starvation.
Regulated BMAs are generally enriched in the proximal and central parts of the skeleton owing to the hematopoietic nature of these regions. This includes, for example, BMAs in the area starting above the tibia/fibula junction (in rodents) and extending into the femur, pelvis, sternum, ribs, and thoracic/lumbar vertebrae (1,9). Constitutive BMAs are enriched in the distal skeletal regions including the hands, feet, distal tibia, and tail vertebrae (when present). It is worth noting here that the term ‘enriched’ is key; in higher vertebrates with larger marrow spaces, it is easy to find areas of the skeleton where regions of rBMAs and cBMAs exist side-by-side. In rabbits, for example, cores of cBMA-like yellow marrow run through the center of the femur and tibia (10,11). This core is surrounded by a ring of red marrow with interspersed rBMAs which is then encased by the cortical bone. In humans, it is well established that during development, yellow marrow fills into mid-diaphysis of the femur of the mature adult (9,12). However, it is unknown if the endocortical ring of rBMA-enriched red marrow is present. This point requires further clarification and could have implications for both hematopoiesis and cortical bone turnover. In Tavassoli’s experiments with rabbits, for example, the outer rBMAs of the femur and tibia were preferentially depleted with phenylhydrazine while the core cBMAs extending through the mid-tibia were preserved (5). It remains unclear whether one BMA subtype represents a more immature version of the other, whether they are derived from distinct lineages, or whether their unique responses are simply a product of their divergent microenvironments.
Adipocyte size is a commonly reported biological variable, which reflects relative rates of lipid storage, release and utilization. In rodents and rabbits, the size of the BMAT adipocyte is notably constrained relative to that of peripheral white adipose tissues (1,3,9). In rats and mice, the average size of rBMAs is consistently smaller than cBMAs (1,3). In addition, relative to cBMAs in the tail vertebrae, rBMAs in the distal femur of mice have an increased number of small, accessory lipid droplets in the peripheral cytoplasm around their core unilocular lipid globule (3,13) (Figure 1). This increase in lipid surface area may be important for enhancing the responsiveness of rBMAs to lipolytic stimuli. In rabbits, similar size BMAs were found throughout the femur, tibia, humerus, radius, and ulna (11). However, unlike the work in rodents, the analysis regions were not oriented to red marrow and may have included the core cBMAs in addition to the rBMAs near the endocortical surface (11). Thus, in addition to clarifying species-specific effects of mechanical loading, nutrition, hormonal regulation, etc - it will be important to distinguish between these areas to map region-specific BMA-responses.
Which came first – cell autonomy or microenvironment?
Our findings to date raise the question: in the early stages of differentiation, were all BMAs the same? Conversely, if taken out of their microenvironmental context, does one BMA become equivalent to another? In vertebrates, bone marrow adipose tissue (BMAT) first evolved in the bony fishes, presenting simultaneously in skeletal regions with adjacent hematopoiesis (rBMA-like) and without (cBMA-like) (reviewed in (14)). Beyond fish, BMAs are present in all higher vertebrates studied to date including amphibians, reptiles, birds, and mammals (14). This suggests that, from an evolutionary perspective, all BMAs appeared around the same time – though some were introduced into hematopoietic-rich contexts while others were relegated to purely osseous portions of the skeleton. In mammals such as rabbits, regions of cBMAs and rBMAs are defined at birth, even before lipid accumulation in the adipocytes, and are distinguished by their general architecture and differences in cellularity (10). This suggests that the BMA progenitors, particularly in areas of yellow marrow, are patterned embryonically. Experiments which transplant whole red or yellow bone marrow to ectopic sites further support this concept. Transplantation initiates a regenerative process which mimics de novo bone marrow development, including re-derivation of mature cells (15). In this setting, transplantation of rBMA-rich red marrow results in formation of a hematopoietic bone marrow nodule (15,16). Conversely, transplanted cBMA-rich yellow marrow develops, once again, into a fat-filled bone nodule in which BMAT adipocytes displace the hematopoiesis (15,16). This suggests that there exists inherent developmental patterning to the nature of the BMAT niche that is preserved even after transplantation (10,15,16).
The BMA progenitor was originally defined microscopically as a perivascular cell (17). BMA progenitors have since been characterized as derived from non-hematopoietic (CD45−), non-endothelial (CD31−) lineages expressing factors including osterix, Sca1 and leptin receptor (18–20). In the adult mouse, BMA progenitors are distributed throughout the limb skeleton (18). However, despite their generalized presence, rBMAs have been shown to accumulate preferentially in the metaphyses and along the endocortical surface of the diaphysis during aging and high-fat diet-induced expansion (1,21). This suggests that local regulatory signals, in addition to the presence of appropriate progenitors, are necessary to drive differentiation of mature BMAs (18). Lastly, work by Mizoguchi et al implies that cBMAs, which begin to fill with lipid days after birth (9,22), may be derived initially from a transient, primitive wave of progenitor stem cells, while rBMAs may differentiate throughout life from a definitive adult progenitor population (19). That said, there must be a persistent source of cBMA progenitors that can be recruited during aging and turnover and more work is needed to clarify this point and the turnover rates of both rBMAs and cBMAs. Taken together, published evidence to date suggests that rBMAs and cBMAs are evolutionarily similar in that they both appeared in vertebrates around the time that hematopoiesis was relegated to the skeleton. However, they may derived from unique embryonic and adult progenitor pools. Moreover, the development of new BMAs and their phenotypic specification undoubtedly depends on factors within the local microenvironment.
Do functional demands dictate divergent responses within bone?
In addition to possible differences in progenitors, the distinct features of the rBMA and cBMA microenvironments likely dictate the unique biochemical and physiologic properties of these cell types. Three-dimensional electron microscopy of the BMA niche reveals intimate associations of rBMAs with endothelial-lined vasculature, erythroblast islands, and myeloid/granulocyte-lineage cells in addition to extension of endoplasmic reticulum and lipid droplet-rich cytoplasm toward osteoblast-lined bone surfaces (13) (Figure 1). These interactions connect the BMA to peripheral tissues through the systemic circulation and also to a diverse set of cells within the local microenvironment. It has been estimated that one rBMA is capable of interacting with over 100 hematopoietic cells both through direct cell-cell contact and indirect interactions with core macrophages of the erythroid islands (13). Indeed, maintenance of the rBMA niche is critical for physiologic myelo-erythroid maturation (23). This helps to explain why increasing the bioenergetic needs of one cell type, for example during acute anemia, results in lipid hydrolysis by the rBMA and an overall decrease in cell size (2). By contrast, lack of an appropriate stimulus contributes to increased lipid esterification and storage, leading to BMAT expansion. Indeed, it is easy to envision Tavassoli’s concept of lipid ‘flux’, with rBMAs serving as an energy-rich hub within the stromal-reticular network of the bone marrow.
By contrast, cBMAs are densely packed, closely associated with one another with little intervening hematopoiesis (1,5). Evolutionary analysis and lineage tracing reveal that BMAT adipocytes are more similar to WAT adipocytes than to brown adipose tissue (BAT) (14,18). Specifically, both BMAs and WAT adipocytes are present in diverse vertebrate species including fish, amphibians, reptiles, birds and mammals (14,24). This is unlike brown adipose tissue which is only present in mammals (24). In birds, for example, thermogenesis is generally relegated to the muscle (25). However, despite its similarities to WAT, traditional lipolysis-stimulating signals including acute fasting and caloric restriction fail to mobilize the energy reserves of cBMAs, contributing to the concept that BMAs make up a distinct adipose tissue depot (3,11,26–29). Spatially, when compared to rBMAs, the opportunities for energy utilization by cBMAs are further limited by lack of ‘needy’ surrounding cell populations. The mechanisms underlying their robust resistance to peripheral stimuli remain unknown, however, this may be an important evolutionary survival mechanism (30). Indeed, it has been proposed that cBMAs protect the body from death, providing a last resort energy reserve during the final stages of starvation (30).
Constitutive BMAs generally exist in environments that have decreased amounts of hematopoiesis, however, recent evidence suggests that, like rBMAs (31), they are capable of supporting blood cell production and hematopoietic progenitor maintenance (32). Transplantation experiments further demonstrate that cBMAs can take on an rBMA-like role of hematopoietic support in ectopic bone marrow explants or developing tail vertebrae when the appropriate stimuli are applied (33). This includes simultaneously increasing the temperature of the cBMA region in addition to sustained induction of hematopoiesis with phenylhydrazine (4,33,34). These interventions are much more effective in developing cBMAs, for example in young vertebrae or regenerating transplants, suggesting that the phenotypic fate of the cell is more plastic early in differentiation. To date, there is no direct evidence of rBMAs obtaining a cBMA-like phenotype. However, during BMAT expansion in the proximal tibia and femur the number of cells often increases and displaces hematopoiesis, histologically resembling constitutive BMAT. Additional work is needed to clarify the molecular properties of the BMAs in these pathophysiologic states.
The composition of the BMAT extracellular matrix (ECM) may also dictate its response to peripheral cues (reviewed in (14)). The ECM interacts with cellular receptors such as integrins to activate intracellular signaling pathways and establishes local signaling gradients in both time and space by sequestering (or presenting) molecules like growth factors and cytokines to cell surface receptors. The mechanical rigidity of the matrix also mediates cell spreading and motility. Whole human BMAT from tibia or the 1st metatarsal (also known as cBMA-enriched ‘yellow marrow’) has a liquified, jelly-like consistency that does not hold its shape after removal from the bone marrow cavity (personal observation). By contrast, subcutaneous WAT is springy and rubbery with defined lobules, which maintain their morphology after isolation. This may be due to the fact that WAT is rich in collagenous matrix while whole BMAT is not (13,35,36). The impact of these unique matrices on BMAT function and resistance of the cBMAs to dissolution remains unknown.
Molecular differences between types of bone marrow adipose tissue
This subsection will review what we know about site-specific differences in the molecular cell biology of the BMA, including secretory profile, receptor expression, intracellular signaling pathways, lipid composition, and gene expression.
Fatty Acid and Protein Secretion
BMAs are secretory cells, capable of modifying the actions of both surrounding cells and distant tissues (reviewed in (8,37,38)). This point is exemplified in electron micrographs which reveal that BMAs extend endoplasmic reticulum-rich processes toward regions of cellular activity in the bone marrow (13). Vascular catheterization experiments also show that bone marrow contributes free fatty acids to the peripheral circulation at baseline and after stimulation with isoproterenol, presumably through induction of lipolysis by BMAs (39). In addition to fatty acids, BMAs have been shown to secrete factors such as adiponectin, leptin, stem cell factor (SCF) (32), and RANKL (40) (reviewed in (38)). However, it remains relatively unknown whether the secretory profile of rBMAs and cBMAs is distinct. Three observations of site-specificity, or lack thereof, exist to date. First, it was reported that secretion of SCF by BMAT adipocytes provides crucial support for hematopoietic progenitor cells (32). Though absolute amounts of secreted SCF per cell were not quantified, this was true in both limbs and tail vertebrae, implying similar mechanisms of SCF-mediated hematopoietic support by rBMAs and cBMAs (32). Second, cursory observations of whole human BMAT explants showed that adiponectin expression was higher from tibial BMAT relative to peripheral WAT (26). By contrast, adiponectin expression in femoral BMAT was similar to WAT (41). This may indicate that cBMAs have higher adiponectin production than rBMAs. Third, work with isolated rat adipocytes reveals that acute adrenergic-stimulated glycerol release, a marker of triacylglycerol breakdown via lipolysis, was greater in rBMA-like cells from femur and proximal tibia than in cBMAs from the tail vertebrae (3). This may be due to the increased numbers of small lipid droplets in the peripheral cytoplasm of rBMAs which increases the surface area available for intracellular lipases (13,42). Together, these results show situations in which secretion is of similar effect (SCF), increased in cBMAs (adiponectin), or increased in rBMAs (fatty acids). This reinforces the notion that each BMA is capable of releasing a diverse, but likely well-defined, set of secreted factors that is dependent on local and systemic needs.
Surface receptors and intracellular signaling
Lipid storage and breakdown in adipocytes is controlled by pathways including insulin and β-adrenergic signaling, respectively.
Intraperitoneal injection of insulin for 15-minutes causes phosphorylation of Akt at serine 473, but not threonine 308, in distal tibia BMAT (rat) or whole tail vertebrae (mouse) (E.L.S., personal observation). Conversely, deficiency of the insulin receptor in BMAT adipocytes with adiponectin-cre causes a ~50% reduction in BMA volume within the distal tibia due to a decrease in adipocyte size (43). These results demonstrate that BMAs in the assessed regions are capable of responding to insulin and require insulin signals for maximal lipid storage. Regulated BMAs in the proximal tibia and/or femur were not examined in these reports. Thus, potential differences in insulin responsivity between regulated and constitutive BMAs remain unknown. While insulin promotes lipid storage, β-adrenergic signals induce lipolysis, lipid droplet remodeling, and fatty acid release. This has been shown to occur more readily in rBMAs when compared to cBMAs as discussed above (3). It is worth noting, however, that the BMA response to adrenergic stimuli in vivo and ex vivo is significantly less than the response by iWAT regardless of skeletal site (3). This implies that, as a population, BMAT is less susceptible to conventional lipolytic stimuli through β-adrenergic receptors and is consistent with the documented resistance of BMAs, particularly in constitutive regions, to fasting (3,11,26–29). Future work is needed to define the receptors and/or signals that can stimulate maximal BMA lipid hydrolysis.
Lipids
Performic acid Schiff (PFAS) lipid staining maps a spatial profile for rBMAs (PFAS positive) and cBMAs (PFAS negative) in rabbits that is consistent with developmental patterns (5,10). Specifically, in rabbits, PFAS negative cBMAs are located in the distal tibia and the central core of adipocytes which extends through the center of the proximal tibia and the femur; PFAS negative cBMAs are also found in the tarsal bones of the foot (5). By contrast, also in rabbits, PFAS positive rBMAs are located throughout the red marrow-filled bones including the ribs, sternum, lumbar/thoracic vertebrae, proximal end of the femur and along the length of the femur near the endosteum (5). During phenylhydrazine-induced hemolysis, PFAS positive rBMAs are depleted while PFAS negative cBMAs are retained (5). The PFAS stain was originally thought to be based on the proportion of unsaturated lipids. However, in rats, lipidomic analysis of isolated, purified BMAs shows that rBMAs have an increased proportion of saturated fatty acids while cBMAs have increases in unsaturated lipid species (1). This is opposing Tavassoli’s initial interpretation of the PFAS stain but may be explained by inaccurate assumptions about the PFAS stain mechanism at the time (5). Indirect analyses of lipid composition in humans with 1H-MRS further reinforce the notion that lipid saturation in bone marrow is highest in rBMA-enriched skeletal regions of the femur relative to the cBMA-enriched distal tibia (1). Increases in bone marrow lipid saturation (measured indirectly with 1H-MRS) have since been observed in patients with osteoporosis, fragility fractures, and type 2 diabetes mellitus (44–46), indicating that this may serve as a biomarker of disease and/or play a direct role in disease pathogenesis. However, a limitation of this measurement is that it encompasses both intracellular BMA lipid and lipid in the marrow serum, which may have unique patterns of saturation with divergent implications (47).
Gene expression
To date, gene expression has been compared between purified, primary BMAs and WAT adipocytes in mice (48,49), rats (1) and humans (31,50). These studies show that BMAs express key adipogenic transcription factors including PPARγ, C/EBPα, and C/EBPβ and target genes including GAPDH, lipoprotein lipase, adiponectin, FABP4, and leptin (50). A small-scale, but direct comparison of purified rBMA and cBMA gene expression in rats revealed that expression of PPARγ was similar between the BMAs regardless of skeletal site, but expression of C/EBPα and C/EBPβ was elevated in cBMAs from tail vertebrae (1). BMAs have also been consistently found to express genes Dio2 and CIDEA which are more characteristic of brown or beige adipocytes (50–52). Though their functional similarity to beige adipocytes remains controversial, it seems clear that BMAs have a unique genetic signature relative to WAT and BAT. For example, higher levels of desaturases are reportedly expressed by cBMAs relative to iWAT adipocytes – potentially contributing to increases in lipid unsaturation (1). Changes in gene expression of the pool of BMAs from tibia and femur with aging or obesity may also provide clues about region-specific signatures. Though it is a point that requires additional clarification, we hypothesize that age- and diet-associated cells are rBMA-like. In aged mice (12- and 18-months old), for example, BMAs within the limbs collectively have increased expression of genes involved in fatty acid synthesis and lipolysis, indicative of increased capability for lipid turnover (48). Similarly, in obesity, upregulation of genes including hormone sensitive lipase and the β2-adrenergic receptor were noted in the pool of limb BMAs after 6-months of high-fat diet relative to control chow, indicating potential for enhanced adrenergic responsivity (49).
Translational implications of the dual BMAT hypothesis
Spatial patterning of rBMAs and cBMAs is a defined developmental event which is conserved in vertebrate species ranging from fish and amphibians to birds and mammals (10,14). The skeleton is not the primary site of hematopoiesis in fish, implying that BMAT may have developed to balance bone formation in addition to hematopoiesis. During development, BMA expansion (presumably cBMA-like cells) is positively associated with bone accrual. By contrast, regions of rBMAs form gradually throughout life and accumulation of BMAT with age and disease has been associated with bone loss and fracture risk. In at least some circumstances, osteoblasts and BMAs are derived from the same progenitor population (reviewed in (53)), and PPARγ phosphorylation acts as a key fate switch in this process (54). In addition to exhaustion of the progenitor pool, correlations between pathophysiologic BMAT acquisition could also reflect direct actions of rBMAs on the skeleton. For example, through increases in pro-osteoclastogenic cytokine secretion (55) or inhibition of osteoblast formation (56). Tumors also tend to metastasize to regions of red marrow, and thus regions of rBMAs (12). These adipocytes may represent a more readily accessible fuel source for tumor growth than cBMAs. This is evidenced by recent work showing that a higher frequency of small BMAT adipocytes correlates with myeloma occurrence and drug resistance (57), implying that myeloma tumor cells can utilize BMAT adipocytes to fuel their growth. Thus, pharmacologic intervention to inhibit BMAT expansion or the pathologic features of BMAs, making them more inert and/or cBMA-like while still preserving their hematopoietic-supporting capabilities, may limit disease progression and promote healing.
Conclusions
The main advantage of the dual BMAT hypothesis is the recognition that BMAs exist in multiple states, some of which provide positive and potentially critical support to the body. Incorporating this concept into our work and cataloging any divergent responses will help to determine when and where to deplete or regulate BMAs to promote overall skeletal and systemic health. Though it seems indisputable that BMAs behave differently depending on their surroundings, there are key limitations. Assignment of a regulated or constitutive designation to regions of BMAs is convenient, but is likely an over-simplification of overall BMAT physiology. Future work is needed to define the spectrum of cellular morphology and biochemistry that defines BMA physiology in states of health and disease. In addition, a key step moving forward will be to determine if, when and where BMAs evolve from necessary to detrimental, and whether BMAs can be harnessed to promote local or systemic health.
Acknowledgments
This work was funded by the National Institutes of Health including a pilot grant from the Washington University Musculoskeletal Research Center P30-AR057235 (C.S.C.), R24-DK092759 (O.A.M.), and R00-DE024178 (E.L.S.).
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Clarissa S. Craft, Ziru Li, Ormond A. MacDougald, and Erica L. Scheller each declare no conflicts of interest.
Bibliography
- 1**.Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015 Aug 6;6:7808. doi: 10.1038/ncomms8808. Defined regulated and constitutive bone marrow adipocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bathija A, Davis S, Trubowitz S. Marrow adipose tissue: response to erythropoiesis. Am J Hematol. 1978;5(4):315–321. doi: 10.1002/ajh.2830050406. [DOI] [PubMed] [Google Scholar]
- 3*.Scheller EL, Khandaker S, Learman BS, Cawthorn WP, Anderson LM, Pham HA, et al. Bone marrow adipocytes resist lipolysis and remodeling in response to β-adrenergic stimulation. Bone. 2018 doi: 10.1016/j.bone.2018.01.016. Details molecular mechanisms underlying reduced responses of rBMAs and cBMAs to fasting and adrenergic cues. [DOI] [PMC free article] [PubMed]
- 4.Tavassoli M, Maniatis A, Crosby WH. Induction of sustained hemopoiesis in fatty marrow. Blood. 1974 Jan;43(1):33–38. [PubMed] [Google Scholar]
- 5**.Tavassoli M. Marrow adipose cells. Histochemical identification of labile and stable components. Arch Pathol Lab Med. 1976 Jan;100(1):16–18. Original reference for the dual bone marrow adipose tissue hypothesis. [PubMed] [Google Scholar]
- 6.Bornstein S, Brown SA, Le PT, Wang X, DeMambro V, Horowitz MC, et al. FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology. 2014 Sep;155(9):3516–3526. doi: 10.1210/en.2014-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cawthorn WP, Scheller EL. Editorial: bone marrow adipose tissue: formation, function, and impact on health and disease. Front Endocrinol (Lausanne) 2017 May 29;8:112. doi: 10.3389/fendo.2017.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardouin P, Rharass T, Lucas S. Bone marrow adipose tissue: to be or not to be a typical adipose tissue? Front Endocrinol (Lausanne) 2016 Jun 30;7:85. doi: 10.3389/fendo.2016.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014 Apr;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bigelow CL, Tavassoli M. Fatty involution of bone marrow in rabbits. Acta Anat (Basel) 1984;118(1):60–64. doi: 10.1159/000145823. [DOI] [PubMed] [Google Scholar]
- 11.Cawthorn WP, Scheller EL, Parlee SD, Pham HA, Learman BS, Redshaw CM, et al. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology. 2016 Feb;157(2):508–521. doi: 10.1210/en.2015-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14(1):10–19. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 13*.Robles H, Park S, Joens M, Fitzpatrick J, Craft CS, Scheller EL. Characterization of the bone marrow adipocyte niche with three-dimensional electron microscopy. Bone. 2018 doi: 10.1016/j.bone.2018.01.020. High-resolution visualization of the rBMA and cBMA niche. [DOI] [PMC free article] [PubMed]
- 14.Craft CS, Scheller EL. Evolution of the Marrow Adipose Tissue Microenvironment. Calcif Tissue Int. 2016 Jul 1;100(5):1–15. doi: 10.1007/s00223-016-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tavassoli M, Crosby WH. Bone marrow histogenesis: a comparison of fatty and red marrow. Science. 1970 Jul 17;169(3942):291–293. doi: 10.1126/science.169.3942.291. [DOI] [PubMed] [Google Scholar]
- 16.Tavassoli M. Hemopoiesis in ectopically implanted bone marrow. Kroc Found Ser. 1984;18:31–54. [PubMed] [Google Scholar]
- 17.Biermann A, Graf von Keyserlingk D. Ultrastructure of reticulum cells in the bone marrow. Acta Anat (Basel) 1978;100(1):34–43. doi: 10.1159/000144879. [DOI] [PubMed] [Google Scholar]
- 18*.Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell. 2017 Jun 1;20(6):771–784.e6. doi: 10.1016/j.stem.2017.02.009. Definition of the BMA progenitor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014 May 12;29(3):340–349. doi: 10.1016/j.devcel.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014 Aug 7;15(2):154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheller EL, Khoury B, Moller KL, Wee NK, Khandaker S, Kozloff KM, et al. Changes in Skeletal Integrity and Marrow Adiposity during High-Fat Diet and after Weight Loss. Front Endocrinol (Lausanne) 2016 Jul 27;7:102. doi: 10.3389/fendo.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emery JL, Follett GF. Regression of bone-marrow haemopoiesis from the terminal digits in the foetus and infant. Br J Haematol. 1964 Oct;10:485–489. doi: 10.1111/j.1365-2141.1964.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 23*.Boyd AL, Reid JC, Salci KR, Aslostovar L, Benoit YD, Shapovalova Z, et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat Cell Biol. 2017 Nov;19(11):1336–1347. doi: 10.1038/ncb3625. The BMA supports myelo-erythropoiesis and hematopoietic equilibrium. [DOI] [PubMed] [Google Scholar]
- 24.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007 Oct 19;131(2):242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Pond CM. The Evolution of Mammalian Adipose Tissue. In: Symonds ME, editor. Adipose Tissue Biology. New York, NY: Springer New York; 2012. pp. 227–269. [Google Scholar]
- 26.Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014 Aug 5;20(2):368–375. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010 Sep;25(9):2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavassoli M. Differential response of bone marrow and extramedullary adipose cells to starvation. Experientia. 1974 Apr 15;30(4):424–425. doi: 10.1007/BF01921701. [DOI] [PubMed] [Google Scholar]
- 29.Bathija A, Davis S, Trubowitz S. Bone marrow adipose tissue: response to acute starvation. Am J Hematol. 1979;6(3):191–198. doi: 10.1002/ajh.2830060303. [DOI] [PubMed] [Google Scholar]
- 30.Devlin MJ. Why does starvation make bones fat? Am J Hum Biol. 2011 Oct;23(5):577–585. doi: 10.1002/ajhb.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattiucci D, Maurizi G, Izzi V, Cenci L, Ciarlantini M, Mancini S, et al. Bone marrow adipocytes support hematopoietic stem cell survival. J Cell Physiol. 2018 Feb;233(2):1500–1511. doi: 10.1002/jcp.26037. [DOI] [PubMed] [Google Scholar]
- 32*.Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017 Aug;19(8):891–903. doi: 10.1038/ncb3570. BMAs secrete factors which support hematopoiesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bigelow CL, Tavassoli M. Studies on conversion of yellow marrow to red marrow by using ectopic bone marrow implants. Exp Hematol. 1984 Aug;12(7):581–585. [PubMed] [Google Scholar]
- 34.Maniatis A, Tavassoli M, Crosby WH. Factors affecting the conversion of yellow to red marrow. Blood. 1971 May;37(5):581–586. [PubMed] [Google Scholar]
- 35.Tavassoli M. Marrow adipose cells. Ultrastructural and histochemical characterization. Arch Pathol. 1974 Sep;98(3):189–192. [PubMed] [Google Scholar]
- 36.Tavassoli M. Ultrastructural development of bone marrow adipose cell. Acta Anat (Basel) 1976;94(1):65–77. doi: 10.1159/000144545. [DOI] [PubMed] [Google Scholar]
- 37.Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab. 2016 Jun;27(6):392–403. doi: 10.1016/j.tem.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulston RJ, Cawthorn WP. Bone marrow adipose tissue as an endocrine organ: close to the bone? Hormone molecular biology and clinical investigation. 2016 Oct 1;28(1):21–38. doi: 10.1515/hmbci-2016-0012. [DOI] [PubMed] [Google Scholar]
- 39.Tran MA, Lac DT, Berlan M, Lafontan M. Interplay of alpha-2 and beta adrenoceptors in the control of free fatty acid release from bone marrow adipose tissue. J Pharmacol Exp Ther. 1984 Jul;230(1):228–231. [PubMed] [Google Scholar]
- 40.Takeshita S, Fumoto T, Naoe Y, Ikeda K. Age-related marrow adipogenesis is linked to increased expression of RANKL. J Biol Chem. 2014 Jun 13;289(24):16699–16710. doi: 10.1074/jbc.M114.547919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheller EL, Burr AA, MacDougald OA, Cawthorn WP. Inside out: Bone marrow adipose tissue as a source of circulating adiponectin. Adipocyte. 2016 Sep;5(3):251–269. doi: 10.1080/21623945.2016.1149269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanchette-Mackie EJ, Scow RO. Lipolysis and lamellar structures in white adipose tissue of young rats: lipid movement in membranes. J Ultrastruct Res. 1981 Dec;77(3):295–318. doi: 10.1016/s0022-5320(81)80026-3. [DOI] [PubMed] [Google Scholar]
- 43.Qiang G, Whang Kong H, Xu S, Pham HA, Parlee SD, Burr AA, et al. Lipodystrophy and severe metabolic dysfunction in mice with adipose tissue-specific insulin receptor ablation. Molecular metabolism. 2016 Jul;5(7):480–490. doi: 10.1016/j.molmet.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013 Aug;28(8):1721–1728. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005 Aug;22(2):279–285. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 46.Yu EW, Greenblatt L, Eajazi A, Torriani M, Bredella MA. Marrow adipose tissue composition in adults with morbid obesity. Bone. 2017 Apr;97:38–42. doi: 10.1016/j.bone.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pino AM, Miranda M, Figueroa C, Rodríguez JP, Rosen CJ. Qualitative aspects of bone marrow adiposity in osteoporosis. Front Endocrinol (Lausanne) 2016 Oct 25;7:139. doi: 10.3389/fendo.2016.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011 May 5;12:212. doi: 10.1186/1471-2164-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu LF, Shen WJ, Ueno M, Patel S, Azhar S, Kraemer FB. Age-related modulation of the effects of obesity on gene expression profiles of mouse bone marrow and epididymal adipocytes. PLoS ONE. 2013 Aug 14;8(8):e72367. doi: 10.1371/journal.pone.0072367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poloni A, Maurizi G, Serrani F, Mancini S, Zingaretti MC, Frontini A, et al. Molecular and functional characterization of human bone marrow adipocytes. Exp Hematol. 2013 Jun;41(6):558–566.e2. doi: 10.1016/j.exphem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012 Feb;50(2):546–552. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lecka-Czernik B, Stechschulte LA, Czernik PJ, Sherman SB, Huang S, Krings A. Marrow adipose tissue: skeletal location, sexual dimorphism, and response to sex steroid deficiency. Front Endocrinol (Lausanne) 2017 Aug 4;8:188. doi: 10.3389/fendo.2017.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tencerova M, Kassem M. The Bone Marrow-Derived Stromal Cells: Commitment and Regulation of Adipogenesis. Front Endocrinol (Lausanne) 2016 Sep 21;7:127. doi: 10.3389/fendo.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ge C, Zhao G, Li B, Li Y, Cawthorn WP, MacDougald OA, et al. Genetic inhibition of PPARγ S112 phosphorylation reduces bone formation and stimulates marrow adipogenesis. Bone. 2018 Feb;107:1–9. doi: 10.1016/j.bone.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Fan Y, Hanai JI, Le PT, Bi R, Maridas D, DeMambro V, et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 2017 Mar 7;25(3):661–672. doi: 10.1016/j.cmet.2017.01.001. BMAs can secrete bone-modifying factors including RANKL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu LF, Shen WJ, Zhang ZH, Wang LJ, Kraemer FB. Adipocytes decrease Runx2 expression in osteoblastic cells: roles of PPARγ and adiponectin. J Cell Physiol. 2010 Nov;225(3):837–845. doi: 10.1002/jcp.22291. [DOI] [PubMed] [Google Scholar]
- 57**.Lu W, Weng W, Zhu Q, Zhai Y, Wan Y, Liu H, et al. Small bone marrow adipocytes predict poor prognosis in acute myeloid leukemia. Haematologica. 2018 Jan;103(1):e21–e24. doi: 10.3324/haematol.2017.173492. Utilization of lipid from BMAs may predict poor clinical prognosis of malignancy. [DOI] [PMC free article] [PubMed] [Google Scholar]