Abstract
Genetic variants related to dopamine functioning (e.g., the ANKK1/TaqIa polymorphism within the DRD2 gene and the Val158Met polymorphism within the COMT gene) have previously been shown to predict cognitive flexibility and learning (e.g., Colzato et al., 2010; Stelzel et al., 2010). Additionally, researchers have found that these genetic variants may also predict second language learning (Mamiya et al., 2016), although this relationship may change across the lifespan (Sugiura et al., 2011). The current study examined the role of the ANKK1/TaqIa and Val158Met polymorphisms along with age of second language acquisition (AoA) in order to predict levels of bilingual proficiency in Spanish-English bilinguals. Results indicated a three-way interaction such that the relationship between the genetic variants and bilingual proficiency depended on AoA. At earlier AoAs, having the genetic variant associated with higher levels of subcortical dopamine (A1+) predicted the highest levels of bilingual proficiency. At later AoAs, individuals with the genetic variant associated with cortical dopamine levels that are balanced between stability and flexibility (Val/Met) predicted the highest levels of bilingual proficiency. These results fit with theories about the development of language as a subcortical process early in life and as a cortical process later in life (Hernandez & Li, 2007), as well as the importance of both stability and flexibility in bilingual language development (Green & Abutalebi, 2013). Finally, this study raises questions about the direction of causality between bilingualism and cognitive control, which is central to the debate over the "bilingual advantage."
Keywords: Bilingualism, Proficiency, DRD2, COMT
1. Introduction
Acquiring skills or knowledge is based on the appropriate environmental stimuli and on neural systems, such as the dopamine system, which allow the brain to adapt to these environmental stimuli. Wong, Morgan-Short, Ettlinger, and Zheng (2012) were the first to suggest that acquiring a second language is no different, and that individual differences in the functioning of the dopamine system, as indicated by genetic variants in dopamine-related genes such as the D2 dopamine receptor gene (DRD2) and Catechol-O-methyltransferase gene (COMT), may predict second language learning. The current study tested this hypothesis using genotypes of single nucleotide polymorphisms (SNPs) located within the DRD2 and COMT genes, as well as age of second language learning, to predict proficiency and balance in the first and second languages of adult bilinguals.
1.1 Neurobiological Theories of Language Learning
One of the current neurobiological theories of language learning is the Declarative/Procedural model (Ullman, 2016), which suggests that language is built using the same memory systems as other types of knowledge. Specifically, knowledge of words and idiosyncratic information about language is stored as declarative memory, which is associated with the medial temporal lobes. Procedural memory, on the other hand, is related to learning the phonological and syntactic rules of language, and involves connections between the basal ganglia and frontal cortex. This model makes predictions about the neural bases of different language functions (e.g., phonology, semantics, syntax), but does not consider the role of neural development, which likely influences language learning when it occurs at different ages, as is often the case with second language learning.
A theory of language learning that does consider age of acquisition was proposed by Hernandez and Li (2007) in their Sensorimotor Hypothesis. This theory takes into account the neural development at the onset of language learning to account for the neural and cognitive systems involved in learning that language. For example, when a second language is introduced in childhood, subcortical regions of the brain are still developing, and children tend to approach new information in a sensorimotor fashion (e.g., exploring objects through touch and physical interaction). Later in life, the cortex maintains some plasticity, and learning occurs most often through reading or listening, which are cognitive, rather than sensorimotor, strategies. This theory suggests that learning a second language early in life may lead to adaptations in subcortical structures such as the basal ganglia, whereas learning a second language later in life may lead to adaptations in cortical structures.
What the Procedural/Declarative model and the Sensorimotor hypothesis have in common is an important role of the basal ganglia and frontal cortex in language learning. Theories about language impairments have also begun to suggest that that these regions may underlie developmental language disorders such as specific language impairment and dyslexia (Krishnan, Watkins, & Bishop, 2016). This focus on basal ganglia and frontal regions opens up questions about the role of dopamine, which is involved in connections between these two regions, in learning a second language.
In addition to these more general theories regarding the neurobiology of language, Stocco, Yamasaki, Natalenko, and Prat (2014) propose a theory about "bilingual brain training" that specifically connects bilingual language experiences to basal ganglia and frontal cortex functioning based on the conditional routing model. According to this theory, the basal ganglia acts to override automatic cortico-cortical responses in situations where a non-automatic response is preferred, such as in situations of language or task switching. In other words, connections between the basal ganglia and frontal regions are responsible for flexibility in adapting to new tasks. The researchers who developed this theory relate it to bilingualism in situations where one needs to flexibly switch between languages. The language currently in use may produce cortico-cortical responses that are automatic, but the basal ganglia can override these responses in favor of the language not currently in use in order for the speaker to switch languages.
In sum, these three theories about the neurobiology of languages (the Declarative/Procedural Model, the Sensorimotor Hypothesis, and the Bilingual Brain Training Framework) suggest that connections between the basal ganglia and frontal cortex are important for rule-based or procedural knowledge of language, for learning a language at different ages, and for flexibly using the two languages based on context (i.e., switching between the two languages when appropriate). Dopamine may play a role in this relationship through a variety of cognitive functions such as learning (Bäckman & Nyberg, 2013; Knecht et al., 2004), cognitive flexibility (Dang, Donde, Madison, O’Neil, & Jagust, 2012; Steenbergen, Sellaro, Hommel, & Colzato, 2015), and motivation (Frank, Doll, Oas-Terpstra, & Moreno, 2009; Kasanova et al., 2017). The current study will examine the role of dopamine in the basal ganglia and frontal cortex in order to understand bilingual proficiency as a function of subcortical and cortical dopamine levels at different ages based on these theories.
1.2 Genetic Variants in the DRD2 and COMT Genes
Variation in both the DRD2 gene and the COMT gene has been suggested to be associated with individual differences in language learning because of the role of both genes in the dopamine system turnover, which allows the brain to flexibly adapt to environmental cues. This cognitive flexibility is associated with single nucleotide polymorphisms (SNPs) located within each of these genes. The first of these, ANKK1/Taq1A (rs1800497) is located within the DRD2 gene. The DRD2 gene codes for D2 dopamine receptors that are found subcortically, specifically in the striatum. Typically, two genotypes are identified for this polymorphism: A1+ (i.e. carrying at least one A1 allele) and A1− (carrying no A1 alleles). Individuals with the A1+ genotype show a reduction in D2 receptors, which leads to increased subcortical dopamine (Laakso et al., 2005). Research by Stelzel, Basten, Montag, Reuter, and Fiebach (2010) indicates that individuals with the A1+ genotype, also called "A1 carriers," showed greater flexibility during cognitive tasks. A1 carriers in their study responded more quickly and made fewer errors on a cognitive flexibility task compared with non-carriers. Of note, other studies have found advantages for non-carriers in other tasks, including long-term memory (Persson et al., 2015), associative memory (Papenberg et al., 2017), and the trail-making test (Fagundo et al., 2014).
Vaughn and colleagues (2016) extended this work by examining the relationship between neural activity and DRD2 genotype in bilingual participants who performed a cognitive flexibility task, a language production task, and an inhibition task. fMRI data from the bilingual sample was analyzed using multiple regression where DRD2 genotype, language proficiency, and age of second language acquisition were entered as predictors of neural activity during each of the tasks. DRD2 genotype predicted neural activity during both the cognitive flexibility task and the language production task, but not the inhibition task. These findings suggest that subcortical dopamine may be involved not only in cognitive flexibility, but also in some aspects of language use for bilinguals.
Additional support for the association between bilingualism and this DRD2 polymorphism comes from Hernandez, Greene, Vaughn, Francis, and Grigorenko (2015). This study found that bilingual and monolingual college students differed in the prevalence of A1 carrier status: bilingual students showed twice the proportion of the A1+ genotype relative to monolingual peers. Hernandez and colleagues suggested that carrying the A1 allele may confer some advantage to those individuals that leads them to more successfully learn English, resulting in the pursuit of a college education at higher rates than non-carrier bilinguals.
In addition to the abovementioned polymorphism in the DRD2 gene, a second single nucleotide polymorphism Val158Met (rs4680) located within the COMT gene also plays a role in the levels of dopamine in the brain. Specifically, one of the functions of the COMT enzyme is to break down prefrontal dopamine. The polymorphism involves one or two of the valine (Val) alleles being substituted with methionine (Met) alleles. The Met substitution results in poorer COMT enzyme functioning, which then leads to increased prefrontal dopamine (Chen et al., 2004). Variation in this COMT SNP has been associated with individual differences in cognitive flexibility, with individuals who have the Val/Val genotype showing the most flexible task performance, individuals with the Met/Met genotype showing the least flexible/most stable task performance, and Val/Met individuals presenting with an intermediate level of flexibility (Colzato, Waszak, Nieuwenhuis, Posthuma, & Hommel, 2010; Ettinger et al., 2008; Markant, Cicchetti, Hetzel, & Thomas, 2014; Rosa, Dickinson, Apud, Weinberger, & Elvevåg, 2010; Schulz et al., 2012).
One interesting question is the extent to which the levels of cortical and subcortical dopamine collectively influence cognitive flexibility. Based on the field’s knowledge of the role of the DRD2 and COMT genes in these two specific polymorphisms in dopamine turnover, individuals whose genotypes are Met/Met and A1+ are likely to have the highest levels of dopamine availability both subcortically and cortically, whereas individuals whose genotypes are Val/Val and A1− would have the lowest levels of dopamine availability both cortically and subcortically (see Table 1). Research by Garcia-Garcia, Barcelo, Clemente, and Escera (2011) finds that optimal cognitive performance does not occur when both cortical and subcortical dopamine levels are high or both are low, but when there is a balance in the levels of cortical and subcortical dopamine. These researchers compared working memory in terms of behavioral and neural responses across different genotypes of the DRD2 and COMT SNPs. The researchers observed the poorest working memory performance in the groups whose genotypes suggest very high or very low levels of dopamine both cortically and subcortically (i.e., Met/Met A1+ and Val/Val A1−). The two groups with the best working memory performance were those who had more balanced levels of dopamine (Met/Met A1− and Val/Val A1+). A similar study by Berryhill and colleagues (2013) found similar patterns for accuracy on a working memory task, but an advantage only for the Met/Met A1− group in terms of response times for the same task. These studies lead to the conclusion that neither very high levels of dopamine nor very low levels of dopamine are ideal for cognitive performance; however, they are some of the only studies that has examined both the DRD2 and COMT genotypes in relation to cognitive task performance. The current study, like that by Garcia-Garcia and colleagues (2011) and Berryhill and colleagues (2013) will include both genotypes as well as their interaction to predict bilingual proficiency.
Table 1.
Assumed levels of cortical and subcortical dopamine for each genotype combination
| Cortical Dopamine | Subcortical Dopamine | |
|---|---|---|
| Val/Val A1− | Low | Low |
| Val/Val A1+ | Low | High |
| Val/Met A1− | Balanced | Low |
| Val/Met A1+ | Balanced | High |
| Met/Met A1− | High | Low |
| Met/Met A1+ | High | High |
1.3 Dopamine Availability and Learning
Research on learning suggests that the genotypes associated with flexibility (Val/Val and A1+) are also associated with learning-related improvements in performance and learning-related changes in the brain. Bellander and colleagues (2015) found that individuals with the Val/Val genotype showed larger gains in working memory with training than individuals with the Met/Met genotype, though their baseline working memory performance before training was worse. Similarly, Söderqvist, Matsson, Peyrard-Janvid, Kere, and Klingberg (2014) found that A1 carriers showed similar improvements with working memory training compared with non-carriers.
In terms of second language learning, Mamiya, Richards, Coe, Eichler, and Kuhl (2016) found changes in white matter tracts of individuals learning a second language when those individuals carried the Val/Val genotype or the Val/Met genotype, but not the Met/Met genotype. Together, these studies suggest that the “flexibility” associated with each the Val/Val and A1+ genotypes can also be interpreted as an ability to adapt to training or learning.
Finally, it is important to note that the relationship between these genotypes and learning may change across the lifespan. Sugiura and colleagues (2011) found that in children between six and eight years old, individuals who carried a Met allele (Val/Met or Met/Met) presented with better language ability than individuals with the Val/Val genotype. However, in children who were ten years old, the two genotype groups performed equally. Therefore, at younger ages or earlier stages of learning, individuals with the Val/Val genotype may show poorer performance on some language or cognitive tasks than individuals with a Met allele, but the Val/Val group may also show the greatest gains in performance with appropriate training. One potential implication for the current study is that Val/Val individuals may have greater flexibility in learning a second language relative to carriers of the Met allele.
1.4 Current Study
The current study sought to extend the understanding of how these SNPs on the DRD2 and COMT genes (Taq1A and Val158Met) interact with age of second language acquisition to predict adult bilingual proficiency. In this study, bilingual proficiency will be defined in a way that accounts for both L1 and L2 proficiency and the balance between the two proficiencies. As seen in previous studies, it was expected that the genotypes associated with cognitive flexibility (Val/Val and A1+) would predict the highest and most balanced levels of proficiency, though this relationship may change when the second language is learned earlier in life. Additionally, following Garcia-Garcia and colleagues (2011), high and balanced levels of proficiency were expected from the following groups: Val/Met (regardless of DRD2 genotype), Val/Val A1+, Met/Met A1−, as these groups have “balanced” levels of cortical and subcortical dopamine. Conversely, the Val/Val A1− group, with low levels of both cortical and subcortical dopamine, and the Met/Met A1+ group, with high levels of both cortical and subcortical dopamine, were expected to have lower and less balanced levels of proficiency.
2. Method
2.1 Participants
One hundred seventeen Spanish-English bilinguals between the ages of 18 and 34 (mean = 22.41, SD = 3.94) provided data for the current study. Self-reported age of English acquisition (AoA) ranged from 0 to 17 (mean = 6.54, SD = 3.12). SES was measured using parental education and ranged from 1, i.e., parents have less than an elementary school education, to 6, i.e., parents have graduate degrees (mean = 3.31, SD = 1.43).
2.2 Materials
2.2.1 Language History Questionnaire
The language history questionnaire was an in-house questionnaire that asked participants to report basic demographic information, health information, language history, educational history, and socioeconomic status information. This form was used for exclusion of participants who are left-handed, have uncorrected vision or hearing problems, psychological problems, or those who are taking psychoactive medications. Additionally, the questionnaire was used to exclude individuals who did not learn Spanish as a first language or those who have extensive experience with languages other than English or Spanish. For participants who qualified for the study, this form was used to gather their age of English acquisition (AoA) and socioeconomic status (SES). For AoA, participants reported age of first exposure to English. For SES, participants reported their parents’ education levels on a scale of 1 – 6, where 1 = less than an elementary school education and 6 = graduate degree. SES was defined as the education level of a single parent when only one parent’s education level was reported (N = 4) or the average education level of two parents when both parents’ education levels were reported (N = 113).
2.2.2 Woodcock-Muñoz Language Survey – Revised
Participants completed the picture vocabulary and passage comprehension subtests of the Spanish and English versions of the Woodcock-Muñoz Language Survey - Revised (Woodcock, Muñoz-Sandoval, Ruef, & Alvarado, 2005). In the picture vocabulary subtest, participants named pictures in the appropriate language. In the passage comprehension subtest participants read incomplete sentences and filled in the missing word. Both subtests started with easier items and increased in difficulty. If participants missed a whole page of items (4–6 items), the researcher ended the testing. All testing began at the basic adult level. The English and Spanish version of this test were designed to be administered to bilingual participants, and thus did not contain overlapping items (e.g., naming the same picture in the English version and the Spanish version). Picture naming and passage comprehension scores in each language were summed for each participant to create a composite measure of English proficiency and a composite measure of Spanish proficiency. In total, the English proficiency measure included 92 items (59 items on the picture vocabulary subtest and 33 items on the passage comprehension subtest), and the Spanish proficiency measure included 89 items (58 items on the picture vocabulary subtest and 31 items on the passage comprehension subtest). The composite measures of proficiency in each language were calculated as a percentage out of 100 so that the two languages could be compared on an equivalent scale.
2.2.3 DNA Samples
Participants provided 2mL saliva samples into Oragene (OG-500) kits from DNA Genotek. This kit consisted of a tube that participants filled with saliva. This is a simple, non-invasive way to collect DNA samples.
2.3 Procedure
After consenting to participate in the research study, participants completed the language history questionnaire. The researchers used this form to ensure that participants did not meet any of the exclusion criteria mentioned previously, and then administered the Woodcock-Munoz Language Survey – Revised in English and Spanish. Participants then completed some measures of cognitive control, which will not be discussed here. Finally, participants provided a DNA sample in the tubes provided. They were then compensated for their participation.
2.4 Analyses
2.4.1 Calculating Proficiency
Proficiency in each language was calculated by summing the scores on the picture vocabulary and passage comprehension subtests. The researchers then created a score of “bilingual proficiency” using the following calculation: . This method of calculating bilingual proficiency gives equal weight to both languages, but also leads to higher scores for individuals who are more balanced. In other words, someone with an L1 proficiency of 55 and an L2 proficiency of 70 would receive a lower “bilingual proficiency” score than someone with an L1 proficiency of 62 and an L2 proficiency of 63, even though the sum of the two proficiencies for these individuals would be equal. This means that higher scores on the scale of bilingual proficiency represent individuals who have high levels of proficiency in each language, and do not appear to have one “dominant” language. Lower scores would be obtained by individuals with lower levels of proficiency in each language, or those who have a dominant language and a weaker language. The key to understanding the bilingual proficiency score is to recognize that the multiplier on the right is a fraction and equals 1.0 when L1 = L2. Thus, the bilingual proficiency score equals L1 + L2 when L1 = L2, but is otherwise less than L1 + L2.
2.4.2 Genetic Analyses
DNA kits from participants of this study were analyzed with a larger sample of participants that included both monolinguals and bilinguals. Genomic DNA for the larger sample was isolated from 190 Oragene Saliva Collection Kits and assessed for quality. In this larger sample, genotyping was performed on a total of 188 DNA samples, 7 of which were randomly chosen as technical replicates to verify protocol efficacy. Of the remaining 181 participants, 58 were monolinguals, and therefore excluded from the current study. Five bilingual participants were dropped for missing data (e.g., did not report parental education, AoA, etc.)
A custom GoldenGate® Genotyping Assay Panel (Illumina Inc.) was created to extract the genotypes of 96 SNPs. Raw data were generated at the Yale Center for Genomic Analysis using Illumina’s standard GoldenGate genotyping protocols and scanned on an Illumina iScan. Raw data were then clustered using Illumina’s GenomeStudio software generating allelic differentiation for each marker.
The two SNPs analyzed as part of the current study were selected based on the previous research described in the introduction. These two SNPS were the ANKK1/Taq1a polymorphism (rs1800497) on the DRD2 gene and the Val158Met polymorphism (rs4680) on the COMT gene. Although many more SNPs were genotyped, the current study lacks the power to conduct genome-wide analyses. The analyses presented here are hypothesis-driven, based on two SNPs that have been commonly studied in relation to dopamine and cognitive functioning in healthy, young adult samples.
2.4.3 Statistical Analyses
A multiple regression model was conducted to analyze the genetic interaction, as well as the role of AoA on the bilingual proficiency measure, while controlling for SES differences across participants. The regression model included 12 predictors, which are listed in Table 2.
Table 2.
Regression Coefficients Predicting Bilingual Proficiency
| B | SE | β | |
|---|---|---|---|
| Intercept (Met/Met A1+) | 160.10 | 7.69 | 0.00*** |
| A1− | −20.75 | 10.31 | −0.95* |
| Val/Met | −19.74 | 8.06 | −0.97* |
| Val/Val | 3.09 | 10.17 | 0.14 |
| AoA | −2.22 | 1.09 | −0.68* |
| SES | 0.40 | 0.61 | 0.06 |
| Val/Met A1− | 32.88 | 11.66 | 1.19* |
| Val/Val A1− | −6.14 | 14.56 | −0.18 |
| A1− AoA | 3.80 | 1.33 | 1.50* |
| Val/Met AoA | 3.95 | 1.17 | 1.57** |
| Val/Val AoA | 0.16 | 1.54 | 0.05 |
| Val/Met A1− AoA | −6.41 | 1.51 | −1.91*** |
| Val/Val A1− AoA | −0.73 | 1.96 | −0.18 |
Notes.
p < 0.05.
p < 0.02.
p < 0.001. R2 = 0.28.
3. Results
3.1 Descriptive Statistics
Participants were fairly balanced in terms of their proficiency; the multiplier in the bilingual proficiency measure ranged from 0.94 to 1.0 (mean = 0.996, SD = 0.01). The range of English proficiency was between 49% and 96% (mean = 74.06%, SD = 7.41), and the range in Spanish proficiency was from 59% to 88% (mean = 76.62%, SD = 6.08). Bilingual proficiency ranged from 118.66 to 176.54 (mean = 150.10, SD = 10.23). Table 3 shows the number of participants and descriptive statistics for each genotype group. ANOVAs conducted on the measures of bilingual proficiency (F(5,111) = 0.15, p = 0.98), AoA (F(5,111) = 0.94, p = 0.46), and SES (F(5,111) = 0.15, p = 0.98) showed no significant differences based on DRD2 or COMT genotype or their interaction.
Table 3.
Characteristics of the samples of each of the genotype combinations.
| Genotype Combination | n | Bilingual Proficiency | AoA | SES |
|---|---|---|---|---|
| Val/Val A1− | 12 | 145.38 (6.12) | 7.67 (2.96) | 3.46 (1.41) |
| Val/Val A1+ | 26 | 151.81 (7.50) | 6.12 (1.68) | 3.19 (1.40) |
| Val/Met A1− | 19 | 147.46 (8.85) | 7.11 (3.89) | 3.16 (1.38) |
| Val/Met A1+ | 41 | 152.14 (10.90) | 6.05 (3.29) | 3.37 (1.45) |
| Met/Met A1− | 6 | 153.14 (14.91) | 7.83 (5.31) | 3.58 (1.36) |
| Met/Met A1+ | 13 | 147.04 (13.64) | 6.46 (2.44) | 3.31 (1.76) |
3.2 Multiple Regression
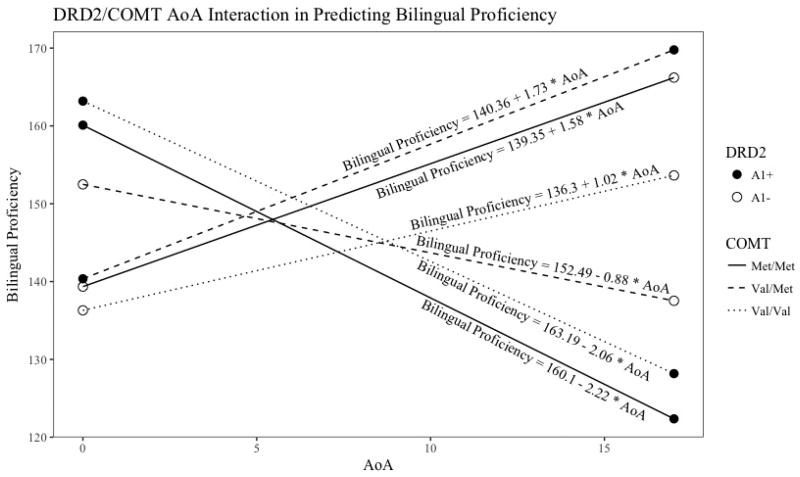
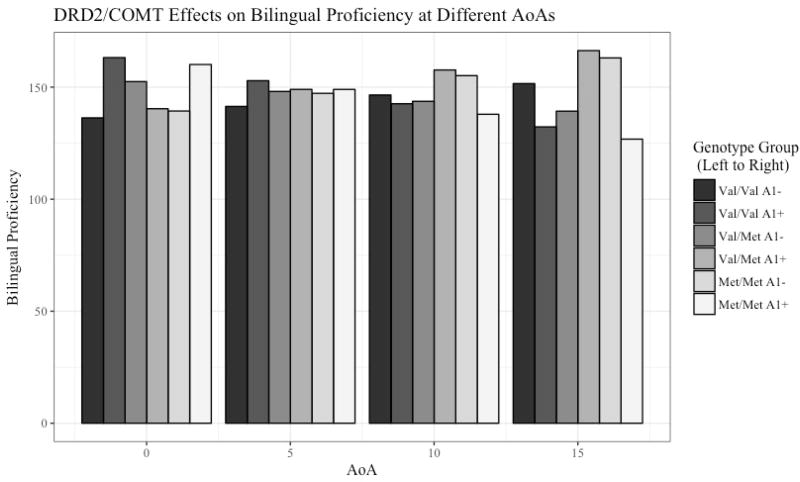
The multiple regression revealed a significant three-way interaction between age of English acquisition, DRD2 genotype, and COMT genotype in predicting bilingual proficiency (see Figure 1 and Figure 2). The relationship between the SES covariate and bilingual proficiency was not significant (standardized beta = 0.05, p = 0.52). The overall multiple regression model explained 28% of the variance in bilingual proficiency (F (12,104) = 3.39, p < 0.001).
Figure 1.
Representing of the significant three-way interaction in the multiple regression model as a line graph.
Figure 2.
Representing the significant three-way interaction in the multiple regression model as a bar graph.
4. Discussion
The current study found a significant three-way interaction between DRD2 genotype, COMT genotype, and AoA (see Figure 1 and Figure 2). The results did not match the hypotheses based on the study by Garcia-Garcia and colleagues (2011), where the Val/Val A1+ group and Met/Met A1− groups were expected to have the highest bilingual proficiency because of their balanced levels of cortical and subcortical dopamine. This could be a result of a variety of differences between the current study and the previous study. The current study used a bilingual proficiency measure, whereas the previous study measured working memory. The current study also included the Val/Met genotype group, while the previous study included only the two homozygous groups of the COMT genotype. Finally, the current study examined the interaction of these genotypes with AoA, which was not a factor in the previous study. To date, the study by Garcia-Garcia and colleagues is one of the only studies that has considered the interaction between these two SNPs in predicting cognitive functioning in healthy adults, so there is a need for similar studies to be conducted that address some of the differences between the current study and the previous study. Although the hypotheses based on the previous study were not supported, the significant three-way interaction still has important implications for second language learning and adult bilingual proficiency.
One way to attempt to interpret this complex interaction is to look at which genotype groups show improved bilingual proficiency with later ages of acquisition, and which genotype groups show improved bilingual proficiency with earlier ages of acquisition. Earlier age of acquisition appears to be better for individuals who present with the following genotypes: Val/Val A1+, Met/Met A1+, and Val/Met A1−. Later age of acquisition appears to be better for individuals who present with the other genotypes: Val/Val A1−, Met/Met A1−, and Val/Met A1+. Notably, the relationship among DRD2 genotype, AoA, and bilingual proficiency is different for the Val/Met group; the Val/Val and Met/Met groups show similar interactions among these variables.
Looking first at the consistencies between the Val/Val and Met/Met groups, having higher levels of subcortical dopamine (A1+) seems to be beneficial for acquiring two languages early in life, but not later in life. This fits with theories about the development of language as a sensorimotor or procedural-learning-based process that relies on the basal ganglia early in life, whereas later language learning may rely on executive attention and working memory processes that rely on areas such as the prefrontal cortex (e.g., Chandrasekaran, Koslov, & Maddox, 2014; Hernandez & Li, 2007). Support for this view comes from evidence that simultaneous bilinguals show increases in the size of the bilateral putamen, right caudate, and left pallidum compared to monolinguals (Burgaleta, Sanjuán, Ventura-Campos, Sebastian-Galles, & Ávila, 2016). Therefore, high levels of subcortical dopamine seems most beneficial to second language learning at early AoAs, but prefrontal dopamine may be more important for second language learning at later AoAs.
For participants with later AoAs, the Val/Met A1+ group has the highest levels of bilingual proficiency. This may reflect the more balanced levels of cortical dopamine observed in this group. Carriers of the Met/Met genotype have previously been considered “stable,” and carriers of the Val/Val genotype have previously been considered “flexible” (Colzato, Waszak, Nieuwenhuis, Posthuma, & Hommel, 2010; Ettinger et al., 2008; Markant, Cicchetti, Hetzel, & Thomas, 2014; Rosa, Dickinson, Apud, Weinberger, & Elvevåg, 2010; Schulz et al., 2012). Depending on the situation, being too flexible or too stable may not be ideal. The current findings are consistent with the adaptive control hypothesis of Green and Abutalebi (2013), which suggests that different control processes are needed for different language environments. For example, in a dense code-switching environment, being very flexible can be advantageous, but in a single language environment, being very stable would be ideal. In a dual language environment, in which a bilingual may need to switch languages when speaking with different individuals, it is ideal to be neither too stable nor too flexible so that the language selected is always appropriate to the speaker. Because stability and flexibility both have a place in bilingual conversations depending on the language context, having a balance between these two skills is likely to be most adaptive for developing proficiency. Our results are consistent with the view that balance in flexibility and stability seems to become more important at later ages of acquisition, when there is more cortical involvement in language learning, as opposed to the importance of the subcortical dopamine at earlier ages of acquisition (Chandrasekaran, Koslov, & Maddox, 2014; Hernandez & Li, 2007).
4.1 Limitations and Future Directions
The current study is one of the first attempts to understand the role of genetic background in developing bilingual proficiency, but it is not without limitations. One limitation was the use of a cross-sectional approach to examine individual differences in language development across the lifespan. Future research may wish to examine the relationship between genetic background and bilingual proficiency longitudinally in order to control for additional individual differences. A second limitation is that this study focused exclusively on Spanish-English bilinguals. Future research should determine whether these findings generalize to other bilingual populations, as well as how they compare to monolingual language development. A third limitation was the measurement of English and Spanish proficiency using the Woodcock-Muñoz Language Survey - Revised. This test is thought to assess cognitive academic language proficiency (CALP) more than basic interpersonal communicative skills (BICS; Shrank, Fletcher, & Alvarado, 1996). CALP may be linked to higher-level cognition more than BICS; therefore, future studies should use a variety of measures of bilingual proficiency in order to better understand these relationships. Finally, the current study is based on many other studies investigating the TaqIa/ANKK1 and Val158Met SNPs. Since DRD2 and COMT are not the only genes involved in the dopamine system, future studies should investigate other dopamine-related genes in order to create a more complete picture of this dynamic, lifelong relationship between genetic background and environment in predicting the development of bilingual proficiency.
4.2 Implications for theories of bilingual language development
The results of this study have important implications for two recurring questions regarding second language learning. The first asks what the ideal age is to teach a child a second language, and the second asks why some children pick up languages more easily than others. The current study suggests that there is not a straightforward answer to either of these questions; at different ages, there may be different strategies for learning a second language, which may be a match or mismatch with what works best for each child. The purpose of this study is not to diagnose children as good or poor language learners, but to point out that age of learning is one of many factors that may predict bilingual proficiency, and that genetic background may be another factor that should receive more attention from researchers in the future.
Furthermore, the association between the COMT and DRD2 polymorphisms and bilingual proficiency observed in the current study has implications for understanding the cognitive functions involved in acquiring a second language. Both of these SNPs have previously been associated with cognitive flexibility, which suggests that, at least when learning a second language later in life, cognitive flexibility may contribute to achieving bilingual proficiency. One interpretation of this relationship is that in order to develop a high degree of proficiency in two languages, one must be willing and able to switch between the two languages as needed. These SNPs, and dopamine functioning more generally, have also been associated with other cognitive functions, such as learning (Bäckman & Nyberg, 2013; Knecht et al., 2004) and motivation (Frank, Doll, Oas-Terpstra, & Moreno, 2009; Kasanova et al., 2017). Therefore, these results could also be interpreted as better learning of each language or higher motivation surrounding maintaining bilingual proficiency in adulthood.
Some researchers have suggested that bilingualism serves as a form of "brain training" for more general cognitive control abilities, such as flexibility (Antoniou, Gunasekera, & Wong, 2013; Stocco, Yamasaki, Natalenko, & Prat, 2012). This view assumes that bilingualism influences cognitive control outcomes. The results of the current study provide an alternative to this perspective, suggesting instead that genetic variants associated with flexibility may influence language proficiency outcomes. Thus, the direction of causality may not be just from bilingualism to cognitive control, but also from cognitive control to bilingualism, as has been suggested previously by Festman, Rodriguez-Fornells, and Munte (2010), Li and Grant (2015), and Hernandez and colleagues (2015). Further studies are needed to further flesh out this intricate relationship.
Supplementary Material
Highlights.
-
▪
Spanish-English bilinguals were genotyped for ANKK1/TaqIa and Val158Met SNPs
-
▪
Early bilinguals with the A1+ genotype achieved high proficiency
-
▪
Late bilinguals with the Val/Met genotype achieved high proficiency
-
▪
Becoming a proficient bilingual early in life is related to subcortical dopamine
-
▪
Becoming a proficiency bilingual later in life is related to cortical dopamine
Acknowledgments
We would like to thank Maya R. Greene and Aurora I. Ramos Nuñez for overseeing data collection for this study, and Elena L. Grigorenko and David J. Francis for their feedback about earlier versions of this manuscript.
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R03HD079873 (“Effects of genetic differences and bilingual status on cognitive control”; 2015–2017). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoniou M, Gunasekera GM, Wong PC. Foreign language training as cognitive therapy for age-related cognitive decline: a hypothesis for future research. Neuroscience and Biobehavioral Reviews. 2013;37(10 Pt 2):2689–2698. doi: 10.1016/j.neubiorev.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L. Dopamine and training-related working-memory improvement. Neuroscience & Biobehavioral Reviews. 2013;37:2209–2219. doi: 10.1016/j.neubiorev.2013.01.014. doi: 0.1016/j.neubiorev.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Bellander M, Bäckman L, Liu T, Schjeide B-MM, Bertram L, Schmiedek F, Lövdén M. Lower baseline performance but greater plasticity of working memory for carriers of the val allele of the COMT Val158Met polymorphism. Neuropsychology. 2015;29(2):247–254. doi: 10.1037/neu0000088. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Wiener M, Stephens JA, Lohoff FW, Coslett B. COMT and ANKK1-Taq-Ia genetic polymorphisms influence visual working memory. PLOS One. 2013:e5586. doi: 10.1371/journal.pone.0055862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaleta M, Sanjuán A, Ventura-Campos N, Sebastian-Galles N, Ávila C. Bilingualism at the core of the brain. Structural differences between bilinguals and monolinguals revealed by subcortical shape analysis. NeuroImage. 2016;125:437–445. doi: 10.1016/j.neuroimage.2015.09.073. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran B, Koslov SR, Maddox WT. Toward a dual-learning systems model of speech category learning. Frontiers in Psychology. 2014 doi: 10.3389/fpsyg.2014.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mrna, protein, and enzyme activity in postmortem human brain. American Journal of Human Genetics. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Waszak F, Nieuwenhuis S, Posthuma D, Hommel B. The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158Met polymorphism: evidence for a role of dopamine in the control of task-switching. Neuropsychologia. 2010;48(9):2764–2768. doi: 10.1016/j.neuropsychologia.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Dang LC, Donde A, Madison C, O’Neil JP, Jagust WJ. Striatal dopamine influences the default mode network to affect shifting between object features. Journal of Cognitive Neuroscience. 2012;24:1960–70. doi: 10.1162/jocn_a_00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Collier DA, Powell J, Luzi S, Michel TM, Williams SCR. Catechol-O-methyltransferase (COMT) Val158Met genotype is associated with BOLD response as a function of task characteristic. Neuropsychopharmacology. 2008;33(13):3046–3057. doi: 10.1038/sj.npp.1301658. [DOI] [PubMed] [Google Scholar]
- Fagundo AB, Fernández-Aranda F, de la Torre R, Verdejo-García A, Granero R, Penelo E, Jiménez-Murcia S. Dopamine DRD2/ANKK1 TaqIa and DAT1 VNTR polymorphisms are associated with a cognitive flexibility profile in pathological gamblers. Journal of Psychopharmacology. 2014;18:1170–1177. doi: 10.1177/0269881114551079. [DOI] [PubMed] [Google Scholar]
- Festman J, Rodriguez-Fornells A, Munte TF. Individual differences in control of language interference in late bilinguals are mainly related to general executive abilities. Behavioral and Brain Functions. 2010;6:5. doi: 10.1186/1744-9081-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flege JE, Yeni-Komshian GH, Liu S. Age constraints on second-language acquisition. Journal of Memory and Language. 1999;41(1):78–104. [Google Scholar]
- Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nature Neuroscience. 2009;12:1062–1068. doi: 10.1038/nn.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia M, Barcelo F, Clemente IC, Escera C. COMT and ANKK1 gene-gene interaction modulates contextual updating of mental representations. Neuroimage. 2011;56(3):1641–1647. doi: 10.1016/j.neuroimage.2011.02.053. [DOI] [PubMed] [Google Scholar]
- Green DW, Abutalebi J. Language control in bilinguals: The adaptive control hypothesis. Journal of Cognitive Psychology. 2013;25(5):515–530. doi: 10.1080/20445911.2013.796377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE, Greene MR, Vaughn KA, Francis DJ, Grigorenko EL. Beyond the bilingual advantage: The potential role of genes and environment on the development of cognitive control. Journal of Neurolinguistics. 2015;35:109–119. doi: 10.1016/j.jneuroling.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE, Li P. Age of acquisition: Its neural and computational mechanisms. Psychological Bulletin. 2007;133:638–650. doi: 10.1037/0033-2909.133.4.638. [DOI] [PubMed] [Google Scholar]
- Howes OD, McCutcheon R, Owen MJ, Murrary RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biological Psychiatry. 2017;81:9–20. doi: 10.1016/j.biopsych.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: The influence of maturational state on the acquisition of English as a second language. Cognitive Psychology. 1989;21(1):60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Laakso A, Pohjalainen T, Bergman J, Kajander J, Haaparanta M, Solin O, Hietala J. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharmacogenetics and Genomics. 2005;15(6):387–391. doi: 10.1097/01213011-200506000-00003. [DOI] [PubMed] [Google Scholar]
- Kasanova Z, Ceccarini J, Frank MJ, Amelsvoort TV, Booij J, Heinzel A, Myin-Germeys I. Striatal dopaminergic modulation of reinforcement learning predicts reward-oriented behavior in daily life. Biological Psychology. 2017;127:1–9. doi: 10.1016/j.biopsycho.2017.04.014. [DOI] [PubMed] [Google Scholar]
- Knecht S, Breitenstein C, Bushuven S, Wailke S, Kamping S, Flöel A, Ringelstein EB. Levodopa: Faster and better word learning in normal humans. Annals of Neurology. 2004;56:20–26. doi: 10.1002/ana.20125. [DOI] [PubMed] [Google Scholar]
- Krishnana S, Watkins KE, Bishop DVM. Neurobiological Basis of Language Learning Difficulties. Trends in Cognitive Sciences. 2016;20:701–714. doi: 10.1016/j.tics.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Early language learning and the social brain. Cold Spring Harbor Symposia on Quantitative Biology. 2016;79:211–220. doi: 10.1101/sqb.2014.79.024802. [DOI] [PubMed] [Google Scholar]
- Mamiya PC, Richards TL, Coe BP, Eichler EE, Kuhl PK. Brain white matter structure and COMT gene are linked to second-language learning in adults. Proceedings of the National Academy of Sciences. 2016 doi: 10.1073/pnas.1606602113. 201606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J, Cicchetti D, Hetzel S, Thomas KM. Contributions of COMT Val158Met to cognitive stability and flexibility in infancy. Developmental Science. 2014;17(3):396–411. doi: 10.1111/desc.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M. Dopamine signals and physiological origin of cognitive dysfunction in Parkinson’s disease. Movement Disorders. 2015;30:472–83. doi: 10.1002/mds.26177. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Becker N, Ferencz B, Naveh-Benjamin M, Laukka EJ, Bäckman L, Brehmer Y. Dopamine receptor genes modulate associative memory in old age. Journal of Cognitive Neuroscience. 2017;29:245–253. doi: 10.1162/jocn_a_01048. [DOI] [PubMed] [Google Scholar]
- Persson J, Rieckmann A, Kalpouzos G, Fischer H, Bäckman L. Influences of a DRD2 polymorphism on updating of long-term memory representations and caudate BOLD activity: Magnification in aging. Human Brain Mapping. 2015;36:1325–1334. doi: 10.1002/hbm.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Peters K, Schroeter K, Koebke W, Lenardon D, Bloch B, Hennig J. The influence of the dopaminergic system on cognitive functioning: a molecular genetic approach. Behavioural Brain Research. 2005;164(1):93–99. doi: 10.1016/j.bbr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Rosa EC, Dickinson D, Apud J, Weinberger DR, Elvevåg B. COMT Val158Met polymorphism, cognitive stability and cognitive flexibility: an experimental examination. Behavioral and Brain Functions. 2010;6(1):53. doi: 10.1186/1744-9081-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank FA, Fletcher TV, Alvarado CG. Comparative validity of three English oral language proficiency tests. The Bilingual Research Journal. 1996;20:55–68. [Google Scholar]
- Schulz S, Arning L, Pinnow M, Wascher E, Epplen JT, Beste C. When control fails: influence of the prefrontal but not striatal dopaminergic system on behavioural flexibility in a change detection task. Neuropharmacology. 2012;62(2):1028–1033. doi: 10.1016/j.neuropharm.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. Journal of Neuroscience. 2010;30(42):14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco A, Yamasaki B, Natalenko R, Prat CS. Bilingual brain training: A neurobiological framework of how bilingual experience improves executive function. International Journal of Bilingualism. 2012;18(1):67–92. doi: 10.1177/1367006912456617. [DOI] [Google Scholar]
- Sugiura L, Ojima S, Matsuba-Kurita H, Dan I, Tsuzuki D, Katura T, Hagiwara H. Sound to language: different cortical processing for first and second languages in elementary school children as revealed by a large-scale study using fNIRS. Cerebral Cortex. 2011;21(10):2374–2393. doi: 10.1093/cercor/bhr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderqvist S, Matsson H, Peyrard-Janvid M, Kere J, Klingberg T. Polymorphisms in the dopamine receptor 2 gene region influence improvements during working memory training in children and adolescents. Journal of Cognitive Neuroscience. 2014;26(1):54–62. doi: 10.1162/jocn_a_00478. [DOI] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, Hommel B, Colzato LS. Tyrosine promotes cognitive flexibility: Evidence from proactive vs. reactive control during task switching performance. Neuropsychologia. 2015;69:50–5. doi: 10.1016/j.neuropsychologia.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Stocco A, Yamasaki B, Natalenko R, Prat CS. Bilingual brain training: A neurobiological framework of how bilingual experience improves executive function. International Journal of Bilingualism. 2014;18:67–92. doi: 10.1177/1367006912456617. [DOI] [Google Scholar]
- Tucker-Drob EM, Briley DA, Harden KP. Genetic and Environmental Influences on Cognition Across Development and Context. Current Directions in Psychological Science. 2014;22(5):349–355. doi: 10.1177/0963721413485087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunbridge E, Weickert C, Kleinman J, Herman M, Chen J, Kolachana B, Weinberger D. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cerebral Cortex. 2007;17(5):1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Ullman MT. The declarative/procedural model: A neurobiological model of language learning, knowledge, and use. In: Hickok Gregory, Small Steven L., editors. Neurobiology of Language. Academic Press; 2016. [Google Scholar]
- Vaughn KA, Nuñez AIR, Greene MR, Munson BA, Grigorenko EL, Hernandez AE. Individual differences in the bilingual brain: The role of language background and DRD2 genotype in verbal and non-verbal cognitive control. Journal of Neurolinguistics. 2016;40:112–127. doi: 10.1016/j.jneuroling.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart HA, Roth RM, Saykin AJ, Rhodes CH, Tsongalis GJ, Pattin KA, McAllister TW. COMT Val158Met genotype and individual differences in executive function in healthy adults. Journal of the International Neuropsychological Society. 2011;17(1):174–180. doi: 10.1017/S1355617710001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Morgan-Short K, Ettlinger M, Zheng J. Linking neurogenetics and individual differences in language learning: the dopamine hypothesis. Cortex. 2012;48(9):1091–1102. doi: 10.1016/j.cortex.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, Muñoz-Sandoval AF, Ruef ML, Alvarado CG. Woodcock-Muñoz Language Survey – Revised. Itasca, IL: Riverside Publishing; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.