Despite much debate, there remains no clear consensus on how the kidney becomes vascularized. In general, blood vessels form by one of two processes: vasculogenesis (assembly of vessels de novo from mesodermal precursors) and angiogenesis (branching of new vessels from existing ones). In principle, renal endothelia may assemble through vasculogenesis-only mechanisms (improbable and not supported), angiogenesis-only mechanisms (conceivable but overlooked), or a combination of both processes (the option favored by most accounts; a summary of pertinent reviews since 1995 is at http://dx.doi.org/10.7488/ds/2307).
Although the combination option is favored, opinions are inconsistent regarding the relative contribution of each mechanism. We challenge this prevailing viewpoint and argue that the renal vasculature (specifically, the endothelial component) instead develops through angiogenesis-only mechanisms.
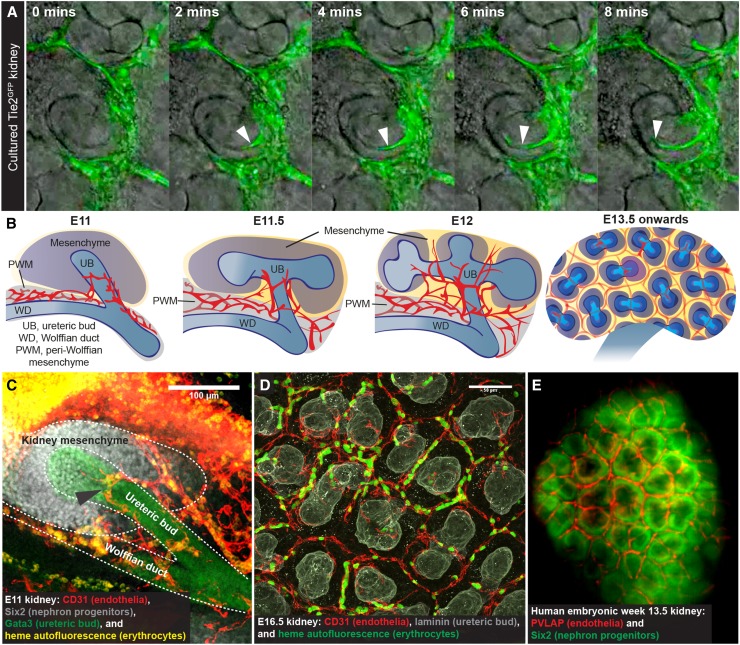
An early proponent for kidney vasculogenesis was Herring,1 who speculated that glomerular capillaries are generated from in situ precursors within the cleft of the S-shaped nephron. This concept persists, despite electron microscopy and immunohistochemistry images suggesting that these early glomerular capillaries instead stem from local, preexisting vessels.2,3 Of course, still images only show cellular arrangement in the kidney at a single moment (at the point of fixation) and cannot prove or disprove dynamic processes, such as angiogenesis or vasculogenesis. Here, time-lapse imaging of cultured embryonic kidneys has provided insights, showing fluorescence-tagged endothelia forming via angiogenesis4 and migrating from preexisting vessels into the S-shaped cleft5 (Figure 1A).
Figure 1.
Evidence for the “angiogenesis-only” hypothesis in kidney vascularization. (A) Earliest glomerular endothelia forming into the cleft of an S-shaped nephron from preexisting vessels in time-lapse culture (white arrowheads). Modified from ref. 5, with permission. (B) Model of early kidney vascularization in vivo. Some argue that the first vessels form via vasculogenesis, but our results suggest otherwise.6 Blood vessels are shown in red. PWM, peri-Wolffian mesenchyme; UB, ureteric bud; WD, Wolffian duct. (C) The embryonic day 11 (E11) kidney is vascularized by systemically connected vessels surrounding the ureteric bud (black arrowhead; these vessels carry erythrocytes and connect to major arteries), which calls into question whether avascular kidneys can be dissected at any age. Scale bar, 100 μm. Modified from ref. 6, with permission. (D) Representative image of the vascular network at the periphery of the E16.5 kidney. Some argue that these peripheral vessels form via vasculogenesis; however, they carry blood, are always enclosed by basement membrane, and connect with preexisting vessels that can be traced to renal arteries.6 Scale bar, 50 μm. (E) As with mouse embryonic kidneys, peripheral vessels in human embryonic kidneys arrange as polygonal networks around nephron progenitors (prepared from data accessed at https://transparent-human-embryo.com7).
More recent support for vasculogenesis comes from crosstransplantation experiments. In transplanted embryonic kidneys, both host- and graft-derived vessels usually developed, and observations of graft-derived vessels were taken as support for vasculogenesis. However, these studies assumed that the transplanted kidney rudiments were avascular, even when using embryonic day 12 (E12)–E12.5 mouse kidneys. We find these to be highly vascularized (Figure 1B). In fact, even the earliest kidney contains blood vessels (E11) (Figure 1C). More significantly, even if truly avascular kidneys were transplanted and vasculogenesis occurred, this does not necessarily mean that it is the normal mechanism of kidney vascularization. Transplanted kidneys are put into artificial environments that may encourage vasculogenesis—it is unclear how this relates to “natural” development, and we suggest that investigating kidney vascularization during normal development in vivo might be more revealing.
In vivo, mouse kidney vascularization starts by E11, when systemically connected vessels wrap around the ureteric bud6 (the precursor of collecting ducts and ureter). Over the next day, blood vessels continue to disperse throughout the kidney mesenchyme in connection with extrinsic vessels6 (Figure 1B). Later, vessels at the border of the kidney form polygonal networks around populations of nephron progenitor cells6 (they organize this way in human embryos as well7; https://transparent-human-embryo.com/?p=1001) (Figure 1, D and E), whereas cortical and medullary vessels are simultaneously developing.
On the basis of our data, normal kidney vascularization relies on growth and remodeling of preexisting vessels (angiogenesis) and does not depend on vasculogenesis at any point. When assessing the entire three-dimensional vascular tree, isolated endothelia are never observed.6 Even when immunostaining for stem cell leukemia, a marker of the most primitive endothelial progenitors,8 staining is observed only within mature vessels that are traceable to the renal artery.6 Other studies, however, suggest that a small population of renal endothelia is stroma derived,9 and the mechanisms by which these cells contribute to vascular development should be explored.
Uncovering the mechanisms of kidney vascularization may allow us to better recapitulate developmental processes to vascularize kidney organoids. Although organoids are generally improving, they lack anatomically realistic vascular systems. On the basis of the “angiogenesis-only” hypothesis, we suggest that, rather than trying to induce vasculogenesis, methodologic advances should promote invasion of renal organoids by exogenous vessels in both culture and transplantation settings.
Illustrating the need for kidney organoid invasion by exogenous vessels, van den Berg et al.10 generated pluripotent stem cell–derived kidney organoids and showed that long periods of culture (where exogenous vessels are lacking) led to gradual loss of endothelia, whereas transplantation into prevascularized host tissue (beneath the murine renal capsule) led to the development of a functional perfused vasculature.
van den Berg et al.10 proposed that maturation of large kidney organoids in culture is limited due to hypoxia and metabolic deficiencies. To facilitate continued maturation in culture, it may be possible to generate perfused engineered vessels that can invade the organoid. Perfused vessels could supply oxygen and nutrients to the organoid, while also generating shear forces to encourage vessel maturation. This could be achieved by connecting engineered vessels to a pumping system, where the flow input could be stringently controlled.
Improved in vitro vascularization and maturation before transplantation would allow for the engraftment of a functionally enhanced organoid. On transplantation, host-derived blood vessels can invade and connect with kidney organoid–derived vessels.10 If a vascularized organoid with functioning glomeruli and nephrons can be transplanted and its flow can quickly be re-established via anastomoses with the host’s vasculature, the regenerative potential of kidney organoids may begin to be realized.
Disclosures
None.
Acknowledgments
We thank Karen Chapman and Peter Hohenstein for their useful comments. This work was supported by the Medical Research Council (MR/K501293/1).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Herring PT: The development of the Malpighian bodies of the kidney, and its relation to pathological changes which occur in them. J Pathol 6: 459–496, 1900 [Google Scholar]
- 2.Kazimierczak J: A study of scanning (SEM) and transmission (TEM) electron microscopy of the glomerular capillaries in developing rat kidney. Cell Tissue Res 212: 241–255, 1980 [DOI] [PubMed] [Google Scholar]
- 3.Naruse K, Fujieda M, Miyazaki E, Hayashi Y, Toi M, Fukui T, et al.: An immunohistochemical study of developing glomeruli in human fetal kidneys. Kidney Int 57: 1836–1846, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Halt KJ, Pärssinen HE, Junttila SM, Saarela U, Sims-Lucas S, Koivunen P, et al.: CD146(+) cells are essential for kidney vasculature development. Kidney Int 90: 311–324, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Saarela U, Akram SU, Desgrange A, Rak-Raszewska A, Shan J, Cereghini S, et al.: Novel fixedz-direction (FiZD) kidney primordia and an organoid culture system for time-lapse confocal imaging. Development 144: 1113–1117, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munro DAD, Hohenstein P, Davies JA: Cycles of vascular plexus formation within the nephrogenic zone of the developing mouse kidney. Sci Rep 7: 3273, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belle M, Godefroy D, Couly G, Malone SA, Collier F, Giacobini P, et al.: Tridimensional visualization and analysis of early human development. Cell 169: 161–173.e12, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Drake CJ, Brandt SJ, Trusk TC, Little CD: TAL1/SCL is expressed in endothelial progenitor cells/angioblasts and defines a dorsal-to-ventral gradient of vasculogenesis. Dev Biol 192: 17–30, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Sims-Lucas S, Schaefer C, Bushnell D, Ho J, Logar A, Prochownik E, et al.: Endothelial progenitors exist within the kidney and lung mesenchyme. PLoS One 8: e65993, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, et al.: Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports 10: 751–765, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]