Abstract
Background Reabsorption of amino acids (AAs) across the renal proximal tubule is crucial for intracellular and whole organism AA homeostasis. Although the luminal transport step is well understood, with several diseases caused by dysregulation of this process, the basolateral transport step is not understood. In humans, only cationic aminoaciduria due to malfunction of the basolateral transporter y+LAT1/CD98hc (SLC7A7/SLC3A2), which mediates the export of cationic AAs, has been described. Thus, the physiologic roles of basolateral transporters of neutral AAs, such as the antiporter LAT2/CD98hc (SLC7A8/SLC3A2), a heterodimer that exports most neutral AAs, and the uniporter TAT1 (SLC16A10), which exports only aromatic AAs, remain unclear. Functional cooperation between TAT1 and LAT2/CD98hc has been suggested by in vitro studies but has not been evaluated in vivo.
Methods To study the functional relationship of TAT1 and LAT2/CD98hc in vivo, we generated a double-knockout mouse model lacking TAT1 and LAT2, the catalytic subunit of LAT2/CD98hc (dKO LAT2-TAT1 mice).
Results Compared with mice lacking only TAT1 or LAT2, dKO LAT2-TAT1 mice lost larger amounts of aromatic and other neutral AAs in their urine due to a tubular reabsorption defect. Notably, dKO mice also displayed decreased tubular reabsorption of cationic AAs and increased expression of y+LAT1/CD98hc.
Conclusions The LAT2/CD98hc and TAT1 transporters functionally cooperate in vivo, and y+LAT1/CD98hc may compensate for the loss of LAT2/CD98hc and TAT1, functioning as a neutral AA exporter at the expense of some urinary loss of cationic AAs. Cooperative and compensatory mechanisms of AA transporters may explain the lack of basolateral neutral aminoacidurias in humans.
Keywords: AGAT, mitochondriopathy, tubulopathy, protein deposits, fibrosis
Renal reabsorption accounts for >98% of recovery of most circulating amino acids (AAs) filtered in the glomerulus. To this end, AAs are actively transported across epithelial cells of the renal proximal tubule by AA transporters located in their apical and basolateral membranes1 (Figure 1A). Primary inherited aminoacidurias caused by loss-of-function mutations demonstrate the role of some transporters in renal reabsorption.2 Thus, mutations in the apical transporters rBAT/b0,+AT (SLC3A1/SLC7A9),3,4 B0AT1 (SLC6A19),5,6 EAAC1 (SLC1A1),7 and PAT2 (SLC36A2) alone or in combination with mutations in IMINO (SLC6A20)8 cause cystinuria, Hartnup disorder, dicarboxylic aminoaciduria, and iminoglycinuria, respectively. Knockout mouse models of these transporters mimic these human aminoacidurias.9–12
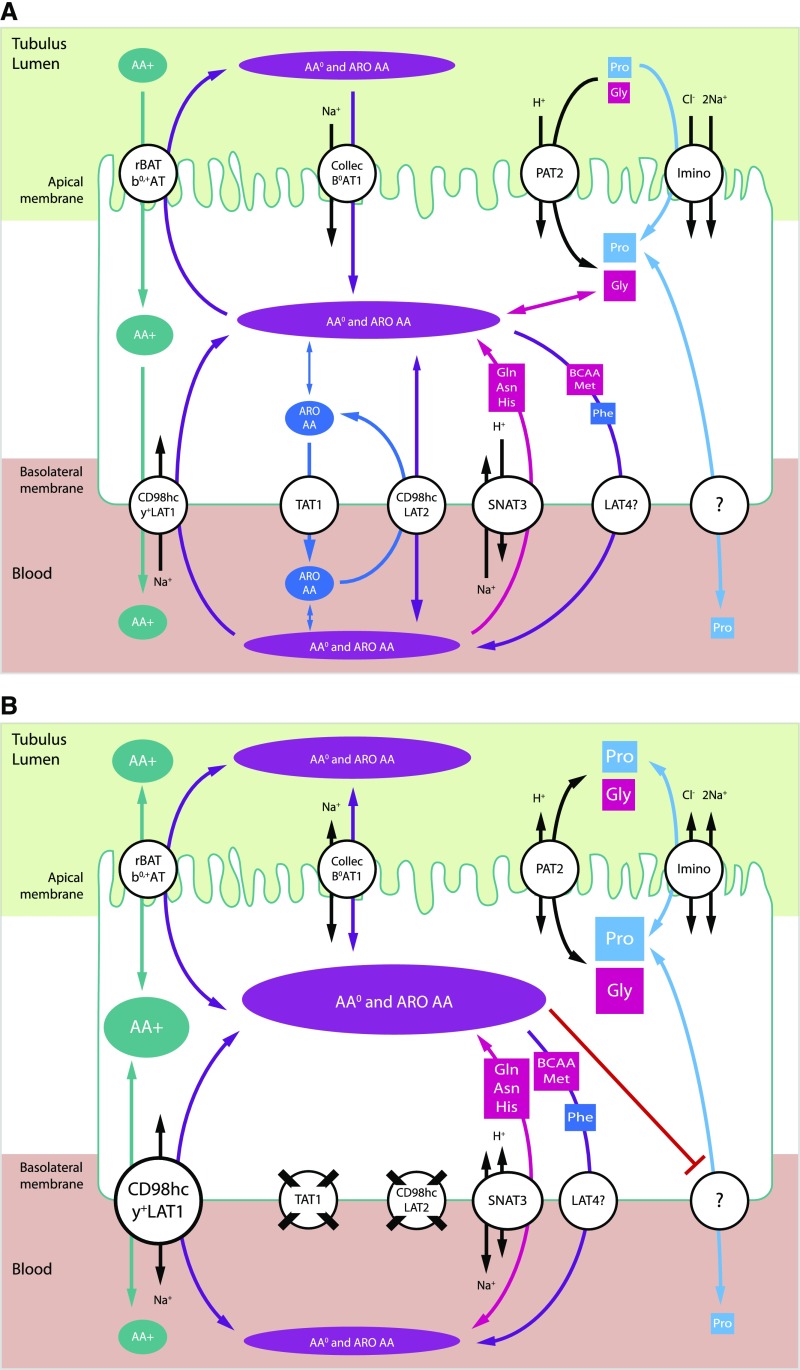
Figure 1.
Proposed model for renal reabsorption of neutral and cationic AAs in mice, showing cooperation of LAT2/CD98hc and TAT1 transporters. Schematic representation of an epithelial cell from the proximal tubule in (A) wild-type and (B) dKO LAT2-TAT1 mice. Arrow head size indicates favored transport direction, and different shape sizes for AAs are used to indicate corresponding concentrations in tubule lumen, cell, and blood. Data presented in this work support a role of TAT1 and LAT2/CD98hc in the basolateral efflux of aromatic and nonaromatic neutral AAs. Ablation of LAT2 and TAT1 resulted in hyperexcretion of neutral and cationic AAs and upregulation of y+LAT1/CD98hc heterodimers. The increased intracellular content of neutral and aromatic AAs is hypothesized to compete with cationic AAs for transport via y+LAT1/4Fh2 that therefore may contribute to the basolateral efflux of aromatic and nonaromatic neutral AAs. It is also proposed that the increased content of neutral and aromatic AAs might block apical B0AT1 and PAT2 transporters. It is speculated that the missing transporter(s) for Pro basolateral efflux might share transport with neutral AAs. Basolateral LAT4 and reversion of basolateral SNAT3 are candidates for efflux of the indicated AA substrates during renal reabsorption. ?, unknown transporter; AA+, cationic amino acids; AA0, neutral amino acids; ARO, aromatic amino acids; Collec, collectrin.
The molecular bases of renal reabsorption of AAs at the basolateral membrane are less well understood. The study of the only known human aminoaciduria involving a basolateral transporter demonstrated the role of y+LAT1/CD98hc (SLC7A7/SLC3A2) in lysinuric protein intolerance and in renal reabsorption of cationic AA.13,14 Ablation of y+LAT1 also resulted in cationic aminoaciduria and large neonatal lethality in mice.15
For neutral AAs, three knockout mouse models of basolateral transporters have been reported, two of which showed a mild phenotype in AA renal reabsorption, whereas the third (LAT4 [Slc43a2] knockout) was postnatally lethal.16 Ablation of the aromatic AA uniporter TAT1 (Slc16a10) caused, next to a substantial aromatic aminoaciduria, also a moderate aminoaciduria of other neutral AAs that became exacerbated under a protein-rich diet.17 It is interesting to note that the plasma concentration of aromatic AAs was strongly increased despite their urinary loss, presumably due to the absence of TAT1 from hepatocytes that normally function as a metabolic sink for aromatic AAs.17 The knockout of LAT2, the catalytic subunit of LAT2/CD98hc (Slc7a8/Slc3a2) heterodimer, showed a very mild aminoaciduria.18 Redundancy and compensatory mechanisms most probably underlie these mild phenotypes of hyperexcretion of AAs in urine.
TAT1 and LAT2/CD98hc have been shown to functionally cooperate in a cellular model.19 TAT1 is a uniporter that mediates downhill transport of L-aromatic AAs (Tyr, Phe, and Trp)20,21 and LAT2/CD98hc is an obligatory exchanger of any L-neutral AA beside proline.22,23 The coexpression of TAT1 was shown to enable glutamine to efflux via LAT2/CD98hc in exchange with aromatic AAs effluxed via TAT1 in Xenopus oocytes.19 TAT1 and LAT2 are expressed in the basolateral membrane of the same epithelial cells in the kidney proximal tubule.19 Thus, these two transporters might cooperate in renal reabsorption using common substrates (Figure 1A). To study their functional relationship in vivo, a double-knockout mouse model (dKO LAT2-TAT1) has been generated. Here, we show that dKO LAT2-TAT1 mice presented a synergistic defect of the tubular reabsorption (TR) of neutral AAs (aromatic and nonaromatic), indicating their cooperation. However, the substantial residual TR of neutral AAs suggested compensation by other transporters. Cationic aminoaciduria and upregulation of the cationic and neutral AA antiporter y+LAT1/CD98hc suggest its involvement in compensatory reabsorption of neutral AAs (Figure 1B).
Methods
A full, detailed description of all materials and methods used is provided in the Supplemental Material.
Mouse Model Generation, Genotyping, and Experimental Diets
Single loss-of-function mouse models for LAT2 (null knockout)24–26 and TAT1 (premature STOP codon at position Y88)17 were crossed to obtain double heterozygous mice and backcrossed to get the F2 generation, including dKO LAT2-TAT1 (dKO) mice. Genotype was confirmed by PCR and/or Sanger sequencing. For exacerbation of renal phenotype, a protein-rich diet was used (40% casein). As control, mice were fed with a 20% casein diet. C57BL/6J mice were maintained in a 12-hour light/dark cycle, with free access to food and water. Experimental diets were maintained for 8 days, with free access to water. Males and females were used for initial characterization of the dKO mouse model; only male mice were included in renal function studies.
Mouse Sample Collection and Analyses
Mice were individually housed in metabolic cages for 4 days during which experimental diet was maintained. Twenty-four-hour urine samples, blood plasma, and organs of interest were harvested. AAs and creatinine were determined in urine and plasma. Kidney total RNA was analyzed by RT-qPCR by using UPL probes (Roche LifeScience, Switzerland) in a microfluidic chip (Fluidigm, CA). LAT2, TAT1, and y+LAT1 proteins were detected from total membranes extracted from kidneys as detailed in the Supplemental Material.
Results
General Features of the Double-Knockout LAT2-TAT1 Mice
Single ablation mouse models for LAT2 (null knockout) (KO LAT2)26,25 and TAT1 (premature STOP codon at position Y88; TAT1 Y88*; KO TAT1)17,24 in pure genetic background C57BL/6J were crossed to obtain the full range of possible genotypes including the double TAT1 and LAT2 knockout mouse model (dKO LAT2-TAT1). The observed frequencies did not follow the expected Mendelian distribution of genotypes, with less than half of the expected frequency for dKO LAT2-TAT1 (Supplemental Table 1).
During initial characterization, mice were kept under standard diet (14% protein content of vegetal origin). In these conditions, dKO LAT2-TAT1 homozygote mice showed lower body weight compared with wild-type mice, this difference being more robust in males than in females (Supplemental Figure 1). Body weights of LAT2 and TAT1 single KO male mice were in between wild-type and dKO LAT2-TAT1 weight curves (Supplemental Figure 1A). Lower body weight has been previously reported for an LAT2 knockout mouse model18 but not for KO TAT1 mice,17 possibly due to the higher protein content of the diet (20% casein). The lower body weight of dKO LAT2-TAT1 mice is the consequence of a general lower weight of all organs, with the exception of the brain, which showed a small but statistically significant higher relative weight (Supplemental Figure 2). Reduced food intake was not the basis of the lower weight of dKO LAT2-TAT1 mice (Supplemental Figure 3).
Ablation of TAT1 and LAT2 Transporters in the Kidneys of the Studied Mouse Models
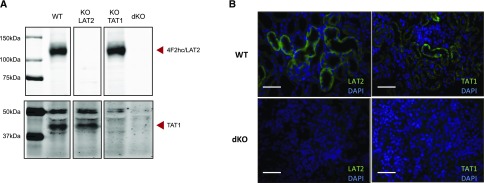
Protein bands corresponding to both transporters were not detectable in renal total membranes of the corresponding single KO mice (Figure 2A), as it has been reported for KO TAT1 mice.17 As expected, the dKO LAT2-TAT1 mice presented no TAT1 and LAT2 protein bands in renal total membranes. Immunofluorescence showed basolateral expression of TAT1 and LAT2 compatible with expression in proximal tubule epithelial cells that is lacking in dKO LAT2-TAT1 mice (Figure 2B).
Figure 2.
Confirmation of ablation of LAT2 and TAT1 protein expression in kidney of single and double LAT2 and TAT1 knockout mice. (A) Total membranes from kidney were analyzed by western blot for LAT2 and TAT1 in mice of all genotypes studied (WT and dKO denote wild-type mice and dKO LAT2-TAT1 homozygotes, respectively). Images correspond to a representative sample for each genotype. Arrow heads point at bands of interest (LAT2/CD98hc and TAT1), as indicated. Because gels were run in nonreducing conditions, LAT2 antibody detected LAT2/CD98hc heterodimer. Samples (50 μg of protein) were loaded into all lanes and run in an 8% acrylamide gel. (B) Immunofluorescence signals of LAT2 and TAT1 (green) and nuclei (DAPI in blue) of kidney cortex sections of mice with the same genotypes as in (A). Antibodies against mouse LAT236 and TAT119 were used as indicated in the Supplemental Material. Bar=50 µm.
AA Hyperexcretion in Urine of Single and Double LAT2 and TAT1 Knockout Mice
Urinary AA excretion was measured in 3-month-old male mice under normal and protein-rich diet (20% or 40% protein of animal origin). During this short diet (11 days), the weight of the KO TAT1 and dKO LAT2-TAT1 remained lower than that of the other genotypes (Supplemental Figure 4) and, as previously reported for KO TAT1 mice, water intake and urine flow were increased in all genotypes under protein-rich diet (Supplemental Figure 5), presumably due to increased urea excretion.17
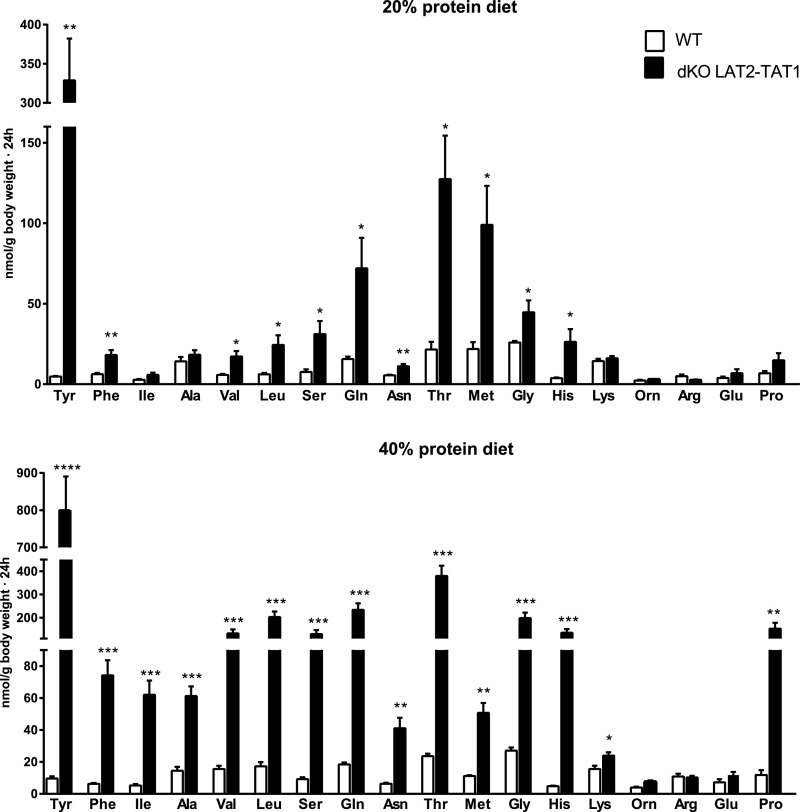
dKO LAT2-TAT1 mice presented urinary hyperexcretion of almost all neutral AAs that was more dramatic under protein-rich diet and was highest for tyrosine (common substrate for TAT1 and LAT2). Surprisingly, the cationic AA lysine and proline were also hyperexcreted, although they are not substrates of these transporters (Figure 3). AA hyperexcretion was much lower in the single ablation mouse models (Supplemental Table 2). None of the tested models showed hyperexcretion of the anionic AA glutamate, which is not a substrate of TAT1 and LAT2 (Figure 3, Supplemental Table 2).
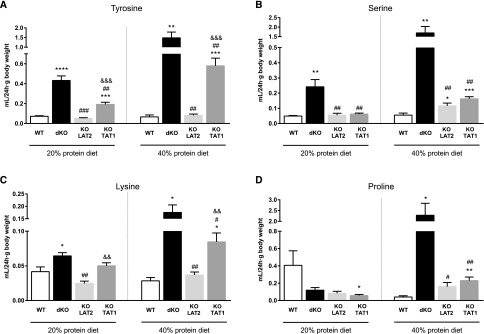
Figure 3.
Double LAT2 and TAT1 knockout mice show urinary hyperexcretion of aromatic, neutral and cationic amino acids. Excretion expressed as nmols of the indicated AA per gram of body weight in 24-hour urine samples of wild-type (WT) and dKO LAT2-TAT1 homozygote mice. Data (mean±SEM) from male mice at 3–4 months of age and after 11 days with the indicated experimental diet. Number of animals analyzed: for 20% protein diet, five WT and five dKO LAT2-TAT1 mice; for 40% protein diet, five WT and 11 dKO LAT2-TAT1 mice. Significant statistical differences (t test) between dKO LAT2-TAT1 and WT values are indicated: *P≤0.05; **P≤0.01; ***P≤0.001. AAs are indicated with the three-letter code.
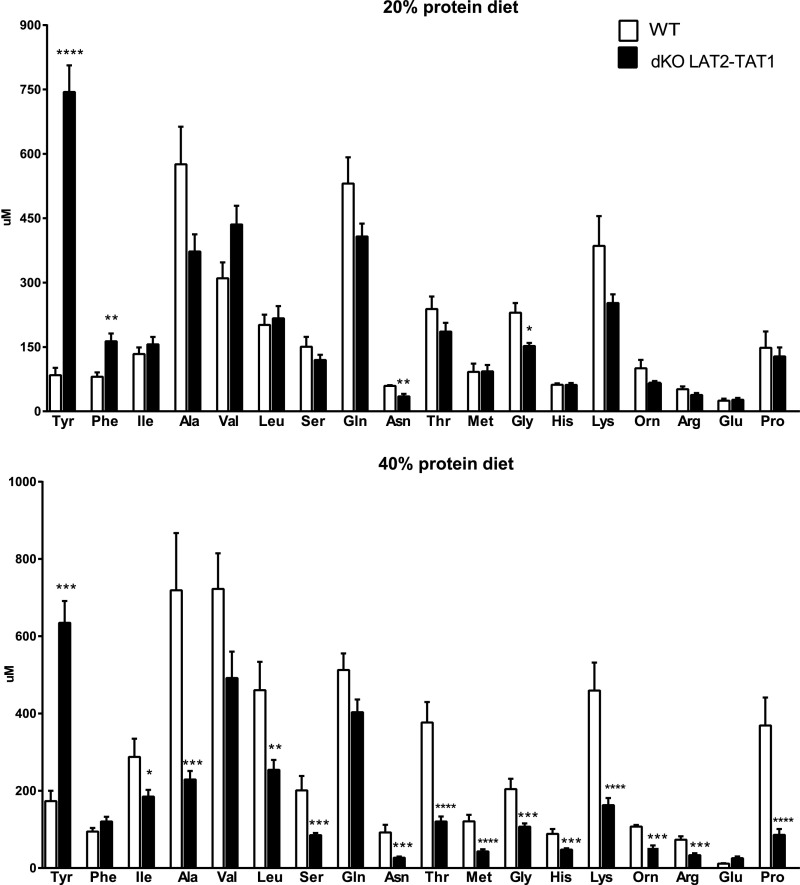
Plasma AA concentration measurements revealed a strong increase only for tyrosine in dKO LAT2-TAT1, as previously reported in KO TAT1 mice, that may be attributed to decreased hepatic uptake and thus catabolism (Figure 4 for wild-type and dKO LAT2-TAT1 mice; all genotypes in Supplemental Table 3).17 In contrast, most of the other neutral AAs and cationic AAs presented a decreased plasma concentration in dKO LAT2-TAT1 mice that was more marked under protein-rich diet (Figure 4). To estimate the amount of a given AA excreted in urine relative to the concentration of this AA in the plasma we calculated the renal clearance of the different AAs (RC; the hypothetic volume of plasma from which a substance is completely removed per minute by the kidney; Supplemental Material and Supplemental Table 4). The higher RC values revealed an increased urinary loss, irrespective of the plasma concentration, of the aromatic AA Tyr (substrates of TAT1 and LAT2) upon TAT1, but not LAT2, ablation, whereas upon ablation of both transporters, excretion increased further (Figure 5A). The high absolute amount of Tyr excretion in TAT1 KO and dKO mice is also related to its high plasma level due to defective hepatic uptake and thus metabolism in the absence of TAT1 (see the introduction and Mariotta et al.17). In the case of Phe, the other tested aromatic AA, a similar effect was observed under protein-rich diet only (Supplemental Table 4). For neutral nonaromatic AAs that are substrates of LAT2, but not of TAT1 (as serine), RC was slightly, but significantly, increased under protein-rich diet in both single KO mouse models compared with wild-type mice (Figure 5B). In contrast, in dKO LAT2-TAT1 mice, RC of neutral nonaromatic AAs was further strongly increased, specifically 2–7-fold under 20% protein diet and 10–40-fold under 40% diet compared with wild-type mice (Supplemental Table 4). This exacerbated excretion in double KO mice compared with single KO mice was observed for all neutral AA substrates of LAT2 and not of TAT1 (Supplemental Table 4; see also Figure 5B for serine values). These results thus indicate a synergistic interaction of TAT1 and LAT2/CD98 for the renal reabsorption of nonaromatic neutral AAs. Surprisingly, a similar effect was also observed under protein-rich diet for the cationic AA lysine and the imino acid proline (Figure 5, C and D).
Figure 4.
Double LAT2 and TAT1 knockout mice show increased tyrosine but decreased concentration of neutral and cationic amino acids. Plasma concentration (µM) of the indicated AAs (three-letter code) in wild-type (WT) and dKO LAT2-TAT1 homozygote mice. Data (mean±SEM) from male mice at 3–4 months of age and after 11 days with the indicated experimental diet. Number of animals analyzed as in Figure 2. Significant statistical differences (t test) between dKO LAT2-TAT1 and WT values are indicated: *P≤0.05; **P≤0.01; ***P≤0.001; ****P<0.001.
Figure 5.
Higher increase of RC of tyrosine, serine, lysine and proline in double knockout mice compared to single knockouts. RC (ml/24 hr·g body weight) of mouse homozygotes of all of the indicated genotypes (WT denotes wild type and dKO denotes dKO LAT2-TAT1). Data (mean±SEM) are from male mice at 3–4 months of age after 11 days with the indicated experimental diet. Number of animals analyzed: for 20% protein diet, five animals in each group; for 40% protein diet, five WT, 11 dKO, six KO LAT2, and eight KO TAT1. Statistical significance (t test) is indicated as follows: * is used to compare versus WT, # to compare versus dKO, and & to compare versus KO LAT2. Number of symbols indicates P values as follows: one symbol for P≤0.05; two for P≤0.01; three for P≤0.001; and four for P<0.001.
AA TR in Single and Double LAT2 and TAT1 Knockout Mice
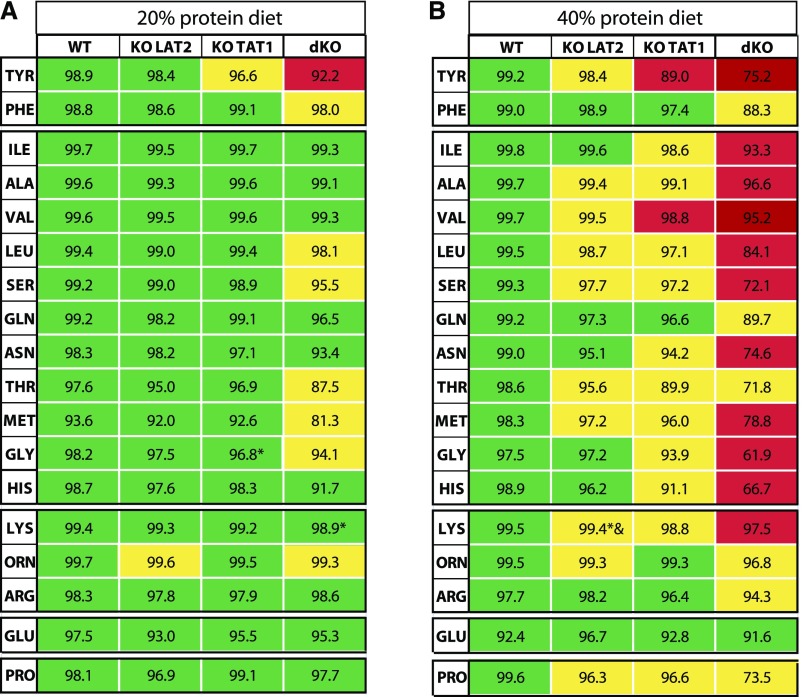
AA tubular reabsorption (TR) (Figure 6, Supplemental Table 5) was estimated (Supplemental Material) after determination of the GFR (Supplemental Material), on the basis of the 24-hour urinary flow (Supplemental Figure 5B) and creatinine plasma and urinary concentrations (Supplemental Figure 6, B–D). The eGFR was similar in all mouse models regardless of the diet (Supplemental Figure 6A). Therefore, the genotype-dependent differences in urinary AA excretion (clearance) discussed above can be fully attributed to differences in TR and thus fractional excretion of AAs (Figures 5 and 6). This confirms that the combined lack of both LAT2 and TAT1 leads to a clear defect of neutral and cationic AA reabsorption. In view of the substantial remaining neutral AA TR, the intriguing urinary loss of cationic AAs suggests the hypothesis that (a) transporter(s) able to accommodate both neutral and cationic AAs might compensate to some extent for the ablation of LAT2 and TAT1 on the expense of a urinary loss of cationic AAs.
Figure 6.
TR of amino acids is more affected in double LAT2 and TAT1 knockout than in single knockout mice. The percentage (mean) of TR, shown inside each cell of the table, was estimated for the indicated AAs (three-letter code) in homozygous mice for the indicated genotypes for (A) 20% protein content in the diet and (B) 40% protein content in the diet. WT denotes wild type and dKO denotes dKO LAT2-TAT1 homozygotes. Male mice at 3–4 months of age after 11 days with the indicated experimental diet were studied. The number of mice is five for all groups under 20% protein diet, and under 40% protein diet as follows: WT, four; KO LAT2, five; KO TAT1, five; dKO, six. For each particular AA, a three-color scale is used on the basis of both statistically significant differences from the group in the preceding column (after t test analysis of data) and % of TR, with green as higher % and red as lower TR. * and & indicate statistical differences compared with WT and KO LAT2, respectively. A complete version of these data, with mean±SEM of TR and statistical significance, is shown in Supplemental Table 5.
Expression of Neutral AA Transporters in the Kidneys of Single and Double LAT2 and TAT1 Knockout Mice
To detect transporters potentially compensating the defective reabsorption activities in our mouse models, the mRNA expression of neutral AA transporters was determined in kidney. The expression of 31 isoforms of 22 AA transporter subunits was determined with nanofluidic chips. Results are summarized in Figure 7 as a heatmap-like table showing in green and red, respectively, the up- and downregulations of all gene products for each genotype and condition, using samples of wild-type mice under 20% protein-diet as reference. As expected, LAT2 mRNA was absent in the mouse models with LAT2 ablation and, interestingly, mice with the Y88* TAT1 mutation showed a strong decrease in TAT1 mRNA suggesting non–sense-mediated decay. In contrast, mRNA expression of most other transporters was not affected in single KO mouse models.
Figure 7.
Upregulation of Slc7a7 mRNA upon ablation of LAT2 in kidney. AA transport system and protein and gene names are indicated. The expression of particular mRNA variants (1, 2, or 3) or the complete set of variants detected together (C) was analyzed in homozygous male mice for the indicated genotypes. When not listed, variants have not been described for that transporter gene, with the exception of CD98hc mRNA variants that have not been analyzed. Green/red table cells indicate gene up-/downregulation, respectively, in comparison with the control condition (wild-type mice under 20% protein diet). Gray table cells indicate no significant changes in gene expression. Expression results of the ablated transporters, TAT1 and LAT2, are shown separately at the bottom of the table, confirming the loss-of-function of each model. ACTB was used as a reference gene (see the Methods section). Data are from 5–8 male mice per experimental group at 3–4 months of age and after 11 days with the indicated experimental diet. Statistical significance of expression ratios is indicated according to Boostratio,37 as follows: ~ for P≤0.1 (trend); *P≤0.05; **P≤0.01; ***P≤0.001; and ****P<0.001. No expression was detected for: Slc38a1 isoform 3, Slc43a1 isoform 3, Slc6a15 common design for all variants, and Slc7a9 isoform 2. WT denotes wild type and dKO denotes dKO LAT2-TAT1 homozygotes.
Intriguingly, however, y+LAT1 mRNA variant 1 was consistently overexpressed when LAT2 was ablated, irrespective of the dietary protein content and unlike variants 2 and 3 (Figure 7, Supplemental Figure 7A). In addition, SNAT3 mRNA variants 1 and 3 were upregulated (3–4 fold over wild-type levels) in the kidneys of KO TAT1 mice but only under protein-rich diet (Figure 7, Supplemental Figure 7B). On the contrary, SNAT2 mRNA and SNAT3 mRNA (variant 3) were downregulated in KO LAT2 and dKO LAT2-TAT1 mice. Similarly, SNAT2, ASCT2, and b0,+AT mRNAs were downregulated in wild-type mice under protein-rich diet.
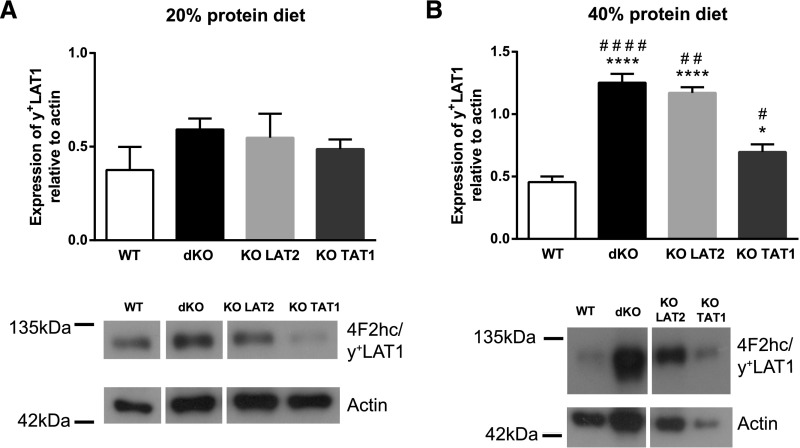
The robust upregulation of y+LAT1 mRNA variant 1 in LAT2-deficient mouse models was further investigated and no effect on total y+LAT1 mRNA expression (i.e., considering all variants together) was observed (Figure 7, Supplemental Figure 7A). This appears to be due to the very low expression of the variant 1 in comparison with the variants 2 and 3 (data not shown). At the protein level, a trend in increased expression of y+LAT1 was observed under normal protein diet (Figure 8A). In contrast, under protein-rich diet y+LAT1 was clearly overexpressed in kidneys of dKO and KO LAT2 animals, to a lesser extent in KO TAT1 mice, and not in wild-type mice (Figure 8B). These results indicate a complex regulation of y+LAT1 and suggest that upregulation of y+LAT1 in these models could work as a compensatory mechanism to the loss-of-function of LAT2 and TAT1 when mice are fed a protein-rich diet.
Figure 8.
y+LAT1 protein expression in kidney is upregulated upon ablation of LAT2 and TAT1 in mice under high protein diet. Expression was quantified in kidneys from homozygous male mice for the indicated genotypes and under diet with (A) 20% or (B) 40% protein content. y+LAT1 protein expression relative to actin as reference protein (upper panels) and a representative image of the immunoblots (lower panels) are shown for all genotypes in (A) and (B). Kidney total membranes (50 μg protein) were run in 10% acrylamide gels in nonreducing conditions. In these conditions, y+LAT1 band corresponds to y+LAT1/CD98hc heterodimer. Data (mean±SEM) are from 5–6 mice per group at 3–4 months of age and after 11 days with the indicated experimental diet. Statistical significance (t tests) is shown with * to indicate differences compared with wild-type and # to indicate differences versus same genotype in 20% protein diet (one symbol P≤0.05; two symbols P≤0.01; three symbols P≤0.001; and four symbols P<0.001). WT, wild-type.
In an attempt to corroborate our hypothesis, we generated two new double-knockout mouse models by crossing a newly generated tamoxifen-inducible (CRE recombinase under ubiquitin C promoter) y+LAT1 knockout mouse (S. Bodoy and M. Palacín, unpublished observations) with the KO LAT2 and the KO TAT1 mice (data not shown). Unfortunately, the high mortality rate (approximately 60% within 2 weeks after induction with tamoxifen) and the deficient health state of surviving animals (dramatic decrease in body weight and GFR) precluded an accurate assessment of AA reabsorption in these new double-knockout mice (y+LAT1-LAT2 and y+LAT1-TAT1).
Discussion
AA TR in the single and the newly generated double LAT2 and TAT1 knockout mice studied here support that: (1) TAT1 and LAT2 participate in renal reabsorption of aromatic AAs, (2) TAT1 and LAT2 have a synergistic functional role in the reabsorption of nonaromatic neutral AAs, and (3) other basolateral transporters compensate for the lack of TAT1 and LAT2 in the renal reabsorption of aromatic and nonaromatic neutral AAs (Figure 1B). Defective TR of some neutral AAs in the single LAT2 KO mice stressed with protein-rich diet (Figure 6) is the first demonstration of the role of LAT2/CD98hc in AA renal reabsorption. Urine hyperexcretion of neutral AAs in LAT2 ablated mice under normal diet, previously reported18 and confirmed here (Figure 3), is only caused by the increased concentration of these AAs in plasma with no effect on TR. The stronger defect in TR of Tyr (substrate for TAT1 and LAT2) in TAT1 KO mice compared with LAT2 KO mice supports a higher contribution of TAT1 in the renal reabsorption of this AA. The synergistic effect of simultaneous TAT1 and LAT2 ablation on TR of aromatic AAs (Tyr and Phe) strongly suggests that LAT2/CD98hc compensates the ablation of TAT1.
Functional coupling of uniporter TAT1 and antiporter LAT2/CD98hc for the basolateral efflux of a non-TAT1 substrate was previously reported in Xenopus oocytes expressing these two human transporters.19 Both transporters are expressed in the basolateral membrane of the murine renal proximal tubule (Figure 2A), in agreement with a previous report,19 and their functional coupling could thus affect TR of neutral AAs. Supporting this hypothesis, namely that the efflux of nonaromatic neutral AAs via LAT2 may, to some extent, be driven by aromatic AAs recycling via TAT1, their TR was synergistically decreased in the double compared with the single LAT2 and TAT1 knockout mice (Figure 6). However, the fact that the lack of TAT1 alone had much less effect on nonaromatic neutral AA reabsorption than the double KO shows that the functional coupling of the LAT2 exchanger is not restricted to TAT1, but probably extends to (an)other basolateral transporter(s), for instance to LAT4 (Slc43a2) which is also a uniporter transporting some essential AAs.16,27 Additionally, the fact that the efflux of these nonaromatic neutral AAs that are not substrates of TAT120 (and mostly also not of LAT4) was much less reduced in LAT2 single KO than in double KO mice indicates that (an)other transporter(s) partially compensate(s) for the lack of efflux via LAT2/CD98hc (Figure 1B). Indeed, >60% of the TR of neutral and aromatic AAs remains in dKO LAT2-TAT1 mice even in the more stressing condition of protein-rich diet (Figure 6). At first sight, it is intriguing that under these high-protein diet conditions no increase in plasma AA concentration was observed, that would explain the strongly increased aminoaciduria. This discrepancy is, however, due to the fact that urine was collected over 24 hours, whereas blood was collected only once during the postabsorptive phase, namely a few hours after the switch to the inactive light phase. Thus, because plasma AA levels are transiently higher during the nocturnal absorptive phase (data not shown), it is proposed that it is the stronger transient blood AA increase (excursion) taking place under high-protein diet that causes a massively increased AA spillover and thus a high aminoaciduria.
Our data suggest that the antiporter y+LAT1/CD98hc compensates, to some extent, the absence of LAT2 and TAT1 for TR of neutral AAs (Figure 1B). This antiporter mediates the electroneutral exchange of cationic AAs with neutral AAs plus sodium with 1:1:1 stoichiometry.28–30 Thus, the inward chemical gradient of sodium favors the efflux of cationic AAs and the influx of neutral AAs. Indeed, loss-of-function mutations in y+LAT1 (SLC7A7) cause lysinuric protein intolerance characterized by hyperexcretion of cationic AAs.31 Interestingly, under the stress of protein-rich diet, a small but significant defect in the TR of cationic AAs was observed in the single LAT2 and TAT1 KO mice that was further increased in the dKO LAT2-TAT1 mice (Figure 6), suggesting that intracellular accumulated neutral AAs compete for efflux with cationic AAs. The only renal basolateral transporter known to share neutral and cationic AA substrates is y+LAT1/CD98hc.8 Moreover, y+LAT1 protein was upregulated in the single and double LAT2 and TAT1 KO mice under protein-rich diet (Figures 7 and 8). Reversion of y+LAT1/CD98hc transport activity (i.e., efflux of neutral AAs plus sodium instead of cationic AA efflux) could be accomplished if the ratio of intracellular neutral/cationic AAs increases to compensate the chemical gradient of sodium (Figure 1B). Indeed, y+LAT1/CD98hc has been proposed as the main Arg influx mediator in human alveolar macrophages, which show little expression of cationic AA uniporters, e.g., CAT1 (SLC7A1) and CAT2b (SLC7A2).32 Unfortunately, double ablation of y+LAT1 (induced after weaning) and LAT2 or TAT1 led to high mortality and surviving animals showed defective GFR (data not shown), hindering their use to assess the expected compensation in renal reabsorption of neutral AAs.
A noticeable surprise in this study is the defect of the TR of proline (Pro) in the single and double LAT2 and TAT1 mice under protein-rich diet (Figure 6). Pro is not a substrate for LAT2 or TAT1,20,22,23 which suggests that the intracellular accumulation of neutral AAs blocks reabsorption of Pro at the apical membrane or efflux at the basolateral membrane (Figure 1B). The study of iminoglycinuria has defined the participation of two main transporters on the apical reabsorption of Pro, the Na+ and Cl− cotransporter of Pro Imino (SLC6A20), and the H+ cotransporter of Pro and glycine (Gly) PAT2 (SLC36A2).8,33 The intracellular accumulation of Gly in TAT1 KO and dKO LAT2-TAT1 mice might block or reverse the uptake of Pro via PAT2 (Figure 1B). To our knowledge, it is not known which basolateral transporters mediate the efflux of Pro in renal reabsorption (Figure 1).2,8 The fact that TR of Pro is affected in LAT2 KO, where Gly TR is not affected, opens the possibility that the missing basolateral Pro transporter might share transport with neutral AAs.
This work supports a functional cooperation between TAT1 and LAT2/CD98hc for the renal reabsorption of neutral AAs and additionally suggests a compensation by y+LAT1/CD98 in case of their defect. The large percentage of TR remaining after knocking out LAT2 and TAT1 is at odds with the larger effect of loss-of-function of the apical neutral AA transporters B0AT1, PAT2, and Imino.8,34 Compensations by other transporters for the basolateral efflux of neutral AAs most probably explain why no neutral aminoaciduria due to the defect of a basolateral transporter has been uncovered. SNAT3, the Na+ cotransporter and H+ antiporter of the neutral AAs Gln, Asn, and His, and LAT4, the uniporter for neutral branched-chain AAs, Met and Phe, expressed in the proximal tubule,16,27,35 might also be at the basis of this compensation (Figure 1B). Kidney proximal tubule–specific ablation of these basolateral AA transporters will be necessary to fully understand the molecular basis of neutral AA reabsorption.
Disclosures
None.
Supplementary Material
Acknowledgments
We want to thank Laura Gónzalez for assistance with handling mice. The Department of Clinical Biochemistry of Sant Joan de Déu Hospital is part of the Centro de Investigación Biomédica en Red de Enfermedades Raras-Instituto de Salud Carlos III (CIBERER-ISCIII) and ‘Centre Daniel Bravo de Diagnòstic i Recerca en Malalties Minoritàries’.
Financial support of the research: Spanish Ministry of Science and Innovation SAF2015-64869-R-FEDER (to M.P.); Spanish Health Institute Carlos III Grant Fondo de Investigación en Salud (FIS) PI13/00121-R-FEDER and PI16/00267-R-FEDER (to V.N.); Generalitat de Catalunya Grants SGR2009-1490 (to V.N.), SGR2009-1355 (to M.P.); Swiss National Science Foundation grant 310030_166430 (to F.V.).
Double knock-out (dKO) generation: C.V., E.B.-K., E.P., and S.B. Mouse management: E.P. and C.V. Quantitative Reverse transcription -polymerase chain reaction (RT-qPCR) design and analysis: C.V. and M.L.d.H. Western blot (WB): E.B.-K., M.L.d.H., S.B., and C.V. Immunofluorescence: E.B.-K. Amino acid and creatinine analysis: A.O. and R.A. Renal function data analysis: C.V. and S.B. Study design: M.P., F.V., V.N., and A.Z. Manuscript draft writing: M.P., C.V., V.N., and F.V. Manuscript review: all authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017111205/-/DCSupplemental.
References
- 1.Makrides V, Camargo SM, Verrey F: Transport of amino acids in the kidney. Compr Physiol 4: 367–403, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Bröer S, Palacín M: The role of amino acid transporters in inherited and acquired diseases. Biochem J 436: 193–211, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Calonge MJ, Gasparini P, Chillarón J, Chillón M, Gallucci M, Rousaud F, et al.: Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nat Genet 6: 420–425, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Feliubadaló L, Font M, Purroy J, Rousaud F, Estivill X, Nunes V, et al.; International Cystinuria Consortium : Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo,+AT) of rBAT. Nat Genet 23: 52–57, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Kleta R, Romeo E, Ristic Z, Ohura T, Stuart C, Arcos-Burgos M, et al.: Mutations in SLC6A19, encoding B0AT1, cause Hartnup disorder. Nat Genet 36: 999–1002, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Seow HF, Bröer S, Bröer A, Bailey CG, Potter SJ, Cavanaugh JA, et al.: Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat Genet 36: 1003–1007, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bailey CG, Ryan RM, Thoeng AD, Ng C, King K, Vanslambrouck JM, et al.: Loss-of-function mutations in the glutamate transporter SLC1A1 cause human dicarboxylic aminoaciduria. J Clin Invest 121: 446–453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bröer S, Bailey CG, Kowalczuk S, Ng C, Vanslambrouck JM, Rodgers H, et al.: Iminoglycinuria and hyperglycinuria are discrete human phenotypes resulting from complex mutations in proline and glycine transporters. J Clin Invest 118: 3881–3892, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peghini P, Janzen J, Stoffel W: Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J 16: 3822–3832, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feliubadaló L, Arbonés ML, Mañas S, Chillarón J, Visa J, Rodés M, et al.: Slc7a9-deficient mice develop cystinuria non-I and cystine urolithiasis. Hum Mol Genet 12: 2097–2108, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Peters T, Thaete C, Wolf S, Popp A, Sedlmeier R, Grosse J, et al.: A mouse model for cystinuria type I. Hum Mol Genet 12: 2109–2120, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Rose AJ, Sijmonsma TP, Bröer A, Pfenninger A, Herzig S, et al.: Mice lacking neutral amino acid transporter B(0)AT1 (Slc6a19) have elevated levels of FGF21 and GLP-1 and improved glycaemic control. Mol Metab 4: 406–417, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borsani G, Bassi MT, Sperandeo MP, De Grandi A, Buoninconti A, Riboni M, et al.: SLC7A7, encoding a putative permease-related protein, is mutated in patients with lysinuric protein intolerance. Nat Genet 21: 297–301, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Torrents D, Mykkänen J, Pineda M, Feliubadaló L, Estévez R, de Cid R, et al.: Identification of SLC7A7, encoding y+LAT-1, as the lysinuric protein intolerance gene. Nat Genet 21: 293–296, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Sperandeo MP, Annunziata P, Bozzato A, Piccolo P, Maiuri L, D’Armiento M, et al.: Slc7a7 disruption causes fetal growth retardation by downregulating Igf1 in the mouse model of lysinuric protein intolerance. Am J Physiol Cell Physiol 293: C191–C198, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Guetg A, Mariotta L, Bock L, Herzog B, Fingerhut R, Camargo SMR, et al.: Essential amino acid transporter Lat4 (Slc43a2) is required for mouse development. J Physiol 593: 1273–1289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariotta L, Ramadan T, Singer D, Guetg A, Herzog B, Stoeger C, et al.: T-type amino acid transporter TAT1 (Slc16a10) is essential for extracellular aromatic amino acid homeostasis control. J Physiol 590: 6413–6424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun D, Wirth EK, Wohlgemuth F, Reix N, Klein MO, Grüters A, et al.: Aminoaciduria, but normal thyroid hormone levels and signalling, in mice lacking the amino acid and thyroid hormone transporter Slc7a8. Biochem J 439: 249–255, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Ramadan T, Camargo SMR, Herzog B, Bordin M, Pos KM, Verrey F: Recycling of aromatic amino acids via TAT1 allows efflux of neutral amino acids via LAT2-4F2hc exchanger. Pflugers Arch 454: 507–516, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kim DK, Kanai Y, Chairoungdua A, Matsuo H, Cha SH, Endou H: Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. J Biol Chem 276: 17221–17228, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Ramadan T, Camargo SMR, Summa V, Hunziker P, Chesnov S, Pos KM, et al.: Basolateral aromatic amino acid transporter TAT1 (Slc16a10) functions as an efflux pathway. J Cell Physiol 206: 771–779, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Pineda M, Fernández E, Torrents D, Estévez R, López C, Camps M, et al.: Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem 274: 19738–19744, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, et al.: LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem 274: 34948–34954, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Espino Guarch M, Font-Llitjós M, Murillo-Cuesta S, Errasti-Murugarren E, Celaya AM, Girotto G, et al.: Mutations in L-type amino acid transporter-2 supportSLC7A8as a novel gene involved in age-related hearing loss. eLife 7: e31511, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Núñez B, Martínez de Mena R, Obregon MJ, Font-Llitjós M, Nunes V, Palacín M, et al.: Cerebral cortex hyperthyroidism of newborn mct8-deficient mice transiently suppressed by lat2 inactivation. PLoS One 9: e96915, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Font-Llitjós M: Renal reabsoprtion of amino acids: SLC7A9 mutations analysis, the type B cystinuria gene and characterization of a knockout murin model Slc7a8, Barcelona, Spain, University of Barcelona, 2005. Available at http://hdl.handle.net/10803/977. Accessed April 25, 2013 [Google Scholar]
- 27.Bodoy S, Martín L, Zorzano A, Palacín M, Estévez R, Bertran J: Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem 280: 12002–12011, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Torrents D, Estévez R, Pineda M, Fernández E, Lloberas J, Shi YB, et al.: Identification and characterization of a membrane protein (y+L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y+L. A candidate gene for lysinuric protein intolerance. J Biol Chem 273: 32437–32445, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer R, Rossier G, Spindler B, Meier C, Kühn L, Verrey F: Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family. EMBO J 18: 49–57, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanai Y, Fukasawa Y, Cha SH, Segawa H, Chairoungdua A, Do Kyung K, et al.: Transport properties of a system y L neutral and basic amino acid transporter. J Biol Chem 275: 20787–20793, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Ogier de Baulny H, Schiff M, Dionisi-Vici C: Lysinuric protein intolerance (LPI): A multi organ disease by far more complex than a classic urea cycle disorder. Mol Genet Metab 106: 12–17, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Rotoli BM, Dall’asta V, Barilli A, D’Ippolito R, Tipa A, Olivieri D, et al.: Alveolar macrophages from normal subjects lack the NOS-related system y+ for arginine transport. Am J Respir Cell Mol Biol 37: 105–112, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Vanslambrouck JM, Bröer A, Thavyogarajah T, Holst J, Bailey CG, Bröer S, et al.: Renal imino acid and glycine transport system ontogeny and involvement in developmental iminoglycinuria. Biochem J 428: 397–407, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Böhmer C, Bröer A, Munzinger M, Kowalczuk S, Rasko JEJ, Lang F, et al.: Characterization of mouse amino acid transporter B0AT1 (slc6a19). Biochem J 389: 745–751, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bröer S: Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88: 249–286, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Fernández E, Jiménez-Vidal M, Calvo M, Zorzano A, Tebar F, Palacín M, et al.: The structural and functional units of heteromeric amino acid transporters. The heavy subunit rBAT dictates oligomerization of the heteromeric amino acid transporters. J Biol Chem 281: 26552–26561, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Clèries R, Galvez J, Espino M, Ribes J, Nunes V, de Heredia ML: BootstRatio: A web-based statistical analysis of fold-change in qPCR and RT-qPCR data using resampling methods. Comput Biol Med 42: 438–445, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.