Abstract
Background Frail kidney transplant (KT) recipients may be particularly vulnerable to surgical stressors, resulting in delirium and subsequent adverse outcomes. We sought to identify the incidence, risk factors, and sequelae of post-KT delirium.
Methods We studied 125,304 adult KT recipients (1999–2014) to estimate delirium incidence in national registry claims. Additionally, we used a validated chart abstraction algorithm to identify post-KT delirium in 893 adult recipients (2009–2017) from a cohort study of frailty. Delirium sequelae were identified using adjusted logistic regression (length of stay ≥2 weeks and institutional discharge [skilled nursing or rehabilitation facility]) and adjusted Cox regression (death-censored graft loss and mortality).
Results Only 0.8% of KT recipients had a delirium claim. In the cohort study, delirium incidence increased with age (18–49 years old: 2.0%; 50–64 years old: 4.6%; 65–75 years old: 9.2%; and ≥75 years old: 13.8%) and frailty (9.0% versus 3.9%); 20.0% of frail recipients aged ≥75 years old experienced delirium. Frailty was independently associated with delirium (odds ratio [OR], 2.05; 95% confidence interval [95% CI], 1.02 to 4.13; P=0.04), but premorbid global cognitive function was not. Recipients with delirium had increased risks of ≥2-week length of stay (OR, 5.42; 95% CI, 2.76 to 10.66; P<0.001), institutional discharge (OR, 22.41; 95% CI, 7.85 to 63.98; P<0.001), graft loss (hazard ratio [HR], 2.73; 95% CI, 1.14 to 6.53; P=0.03), and mortality (HR, 3.12; 95% CI, 1.76 to 5.54; P<0.001).
Conclusions Post-KT delirium is a strong risk factor for subsequent adverse outcomes, yet it is a clinical entity that is often missed.
Keywords: geriatric nephrology, kidney transplantation, transplant outcomes, clinical, epidemiology

Delirium, an acute decline and fluctuation in cognitive function and attention after a stressor,1–5 is a common surgical complication characterized by an acute onset and fluctuating course of inattention, impaired consciousness, and disturbance of cognition.6 This possibly preventable complication7 may manifest as disorientation, memory impairment, disturbance in the sleep-wake cycle, hallucinations or illusions, delusions, psychomotor disturbance, inappropriate behavior, and/or emotional lability. Delirium is dependent on a complex inter-relationship between vulnerable patients, like older and frail patients,3,8–14 and exposure to stressors, like anesthesia and surgery. Delirium occurs across all ages and levels of cognition.3 Although delirium is common in many surgical populations, the incidence differs vastly by type of surgery.3,15 The incidence, risk factors, and sequelae of delirium among kidney transplant (KT) recipients are unclear.
Delirium is likely common among KT recipients given that older,16 frail,17 and cognitively impaired patients with ESRD routinely undergo KT. Frailty occurs in approximately 20% of KT recipients.17 Furthermore, we have estimated that, at the time of admission, 11.7% of deceased donor KT recipients and 8.1% of live donor recipients have cognitive impairment,18 a delirium risk factor in other surgical populations.13,19 These recipients are likely vulnerable to complications after surgery, like delirium,20–23 which may then lead to adverse long-term outcomes, like mortality and graft loss, in this population.20,24,25
Given the burden of cognitive impairment and frailty at the time of transplantation and an aging population of KT recipients, post-KT delirium likely occurs frequently. First, we ascertained the incidence of delirium using national claims data. Second, using a longitudinal cohort of KT recipients, we abstracted data on post-KT delirium to estimate the incidence of delirium and characterize delirium risk factors with a particular focus on age, frailty status, and cognitive function. Finally, we tested whether post-KT delirium was associated with subsequent mortality and graft loss.
Methods
National Cohort of KT Recipients
We studied 125,304 adult KT recipients with Medicare as the primary payer between January 1, 1999 and December 31, 2014 as reported to the Organ Procurement and Transplantation Network and linked to Medicare claims by the US Renal Data System. International Classification of Diseases 9 (ICD-9) codes for delirium were identified from in-patient claims throughout the entire set of KT hospitalization on the basis of a published algorithm26: 290.11, 290.3, 290.41, 291.0, 292.81, 293.0, 293.1, and 780.09. Post-KT dementia diagnosis27 was identified through the following ICD-9 codes: 331.0, 331.1, 331.2, 331.7, 290.0, 290.1, 290.10, 290.12, 290.13, 290.20, 290.21, 290.40, 290.42, 290.43, 294.0, 294.1, 294.8, 797, and 331.0. We tested whether a diagnosis of delirium was associated with an increased risk of a subsequent diagnosis of dementia using a Kaplan–Meier approach.
Longitudinal Cohort Study of KT Recipients
This study also used data from a longitudinal cohort study at the Johns Hopkins Hospital (n=893; Baltimore, MD) between August 2009 and September 2017, which has been described elsewhere.17,20,23,24,28–31 Briefly, participants were enrolled at admission for KT, and they (1) underwent assessments for frailty and cognitive function as described below, (2) reported depressive symptoms (Centers for Epidemiology Study Depression Scale) and quality of life, and (3) consented to medical record abstraction. The clinical and research activities being reported are consistent with the Declaration of Helsinki and the Declaration of Istanbul. The Institutional Review Board of Johns Hopkins Hospital approved this study, and all participants provided written informed consent.
Recipient and Transplant Factors
In the longitudinal cohort study, recipient factors (age, sex, race/ethnicity, comorbidities, education, and years on dialysis) and transplant factors (donor type [deceased or live donor], panel reactive antibody, cold ischemia time, delayed graft function, induction agent [none, IL-2 receptor blockers, or antithymocyte globulin], and maintenance immunosuppression [tacrolimus, cyclosporin, sirolimus, everolimus, azathioprine, mycophenolate mofetil, and prednisone) were abstracted from the medical record. The number of comorbidities from the Charlson Comorbidity Index (adapted for ESRD32) was both self-reported and abstracted from the medical record.
Chart-Based Delirium
In the longitudinal cohort study, post-KT delirium was identified retrospectively using a validated instrument for chart review, which was designed to maximize the sensitivity for delirium identification26; among hospitalized patients, this method has 74% sensitivity, 83% specificity, and 82% agreement with interview-based methods for identifying delirium.26 Briefly, trained and blinded abstractors overseen by a physician researcher searched all sections of the medical chart (including physician progress notes, nursing notes, and consultation notes) throughout the entire set of KT hospitalization records to identify evidence of acute onset of mental status changes.
Global Cognitive Function
The Modified Mini-Mental State (3MS) examination, a validated assessment of global cognitive function,33,34 was administered to study participants at admission for KT. Scores for the 3MS examination range between zero and 100 (lower scores indicate worse cognition) on the basis of responses to 15 components, including temporal and spatial orientation, multistage commands, and recall. We defined cognitive impairment as a 3MS score <80 (−1 SD).
Frailty
We studied the physical frailty phenotype as defined by Fried et al.35 in older adults and as defined by our group in ESRD and KT populations.17,20,23,24,28,30,31,36–39 This phenotype is on the basis of five components: shrinking (self-report of unintentional weight loss of >10 pounds in the past year on the basis of dry weight), weakness (grip strength below an established cutoff on the basis of sex and body mass index), exhaustion (self-report), low physical activity (kilocalories per week below an established cutoff), and slowed walking speed (walking time of 15 feet below an established cutoff by sex and height).35 Participants with three or more of the components were considered frail.
Incidence of Post-KT Delirium
First, we estimated the claims-based cumulative incidence (percentage) of delirium among the 125,304 KT recipients in the national cohort. Second, we explored the cumulative incidence of post-KT delirium in our longitudinal cohort study on the basis of the chart abstraction during the KT hospitalization. These cumulative incidences were stratified by age and frailty status.
Risk Factors for Post-KT Delirium
Using the longitudinal cohort study data, the final model to identify risk factors for chart-based delirium was selected for optimal parsimony by minimizing the Akaike Information Criterion of the logistic regression model. The potential risk factors that were considered were age, sex, race/ethnicity, education, donor type, number of comorbidities from the Charlson Comorbidity Index, years on dialysis, preemptive KT, cause of ESRD, body mass index, panel reactive antibody, cold ischemia time, self-reported quality of life, global cognitive function, depressive symptoms, and frailty. We also tested whether either induction (none, IL-2 receptor blockers, or antithymocyte globulin) and/or standard triple regimen (prednisone, tacrolimus, and mycophenolate mofetil) was independently associated with post-KT delirium in the final model. We also calculated the area under the receiver operating characteristic curve (AUC) as a measure of the model’s predictive ability.
Delirium and Adverse Outcomes
In the longitudinal cohort study, we estimated the independent association of delirium and length of stay (LOS) using negative binomial regression (ratio of time) when LOS was the outcome and logistic regression (odds ratio) when LOS of 2 weeks or longer was the outcome. A 2-week LOS was chosen as a threshold for prolonged LOS to be consistent with previous publications.20 Additionally, we used logistic regression to estimate the odds ratio of institutional discharge (discharge to skilled nursing facility or rehabilitation center) by post-KT delirium. Perioperative mortality was defined as mortality during admission for KT. Cox proportional hazards regression was used to assess the independent association between delirium and mortality/death-censored graft loss. All models were adjusted for the risk factors for delirium as identified using the methods described above.
Statistical Analyses
Differences in recipient and transplant characteristics by chart-based delirium were assessed using the chi-squared test for categorical factors and ANOVA for continuous factors. Differences in the survivor function were assessed using the log rank test. Proportional hazards were confirmed visually by graphing the log-log plot of survival and statistically using Schoenfeld residuals. We used a two-sided α of 0.05 to indicate a statistically significant difference. All analyses were performed using Stata 14.2/MP for Linux (College Station, TX).
Results
National Registry Estimates of Delirium
Among 125,304 KT recipients, the mean age was 50.3 years old (SD=13.7), 38.6% were women, 31.5% were black, and 21.6% received live donor transplants. In this national cohort of KT recipients, 973 had a claim for delirium, such that the cumulative incidence of delirium on the basis of ICD-9 codes was 0.8%. The 5-year cumulative incidence of diagnosed with dementia was 9.8% among KT recipients with delirium compared with 2.1% among recipients without a diagnosis of delirium (log rank P value <0.001).
Longitudinal Cohort Study Population
Among 893 KT recipients, the mean age was 52.5 years old (SD=14.2; range, 18–86), 39.0% were women, and 41.2% were black. The median LOS was 9 days (interquartile range, 7–13 days), and 37.9% received live donor transplants. At admission for KT, 16.4% were frail, and 9.5% had cognitive impairment; older recipients (age ≥65 years old) were more likely to be frail (prevalence of frailty in older recipients versus younger recipients: 21.9% versus 14.8%; P=0.03) and have cognitive impairment (prevalence of cognitive impairment in older recipients versus younger recipients: 17.2% versus 7.4%; P<0.001) at the time of KT.
Cumulative Incidence of Post-KT Delirium
There were 42 patients with delirium (4.7%) post-KT; eight (19.0%) were the hypoactive subtype, seven (16.7%) were the hyperactive subtype, and 27 (64.3%) were the mixed subtype. The cause of delirium was not listed in the chart for 34 (81.0%) of the KT recipients with delirium. For the remaining eight patients with delirium, the chart noted that the possible causes included two cases of ischemic stroke, one case of embolic stroke, one case of stroke and sepsis, one case of sepsis, one case of hyponatremia, and two cases of adverse drug reactions (alprazolam and steroids/narcotics).
Delirium was more common in recipients who were frail (9.0% versus 3.9%; P=0.01), and incidence increased with age: 2.0% for recipients age 18–49 years old; 4.6% for those age 50–64 years old; 9.2% for those age 65–75 years old; and 13.8% for those age 75 years old and older (P<0.001). Furthermore, frail recipients age 50–64 years old (9.8% versus 3.5%), 65–74 years old (16.2% versus 7.1%), and 75 years old or older (20.0% versus 12.5%) had a greater cumulative incidence of delirium than their nonfrail counterparts (Figure 1).
Figure 1.
The incidence of post-kidney transplant (KT) delirium increased by recipient age and frailty status.
Risk Factors for Post-KT Delirium
KT recipients with delirium were older on average (60.4 versus 52.2 years old; P<0.001) and had a greater number of comorbidities (2.6 versus 1.5; P<0.001) (Table 1). They were also more likely to be frail (31.0% versus 15.6%; P=0.02), have cognitive impairment (19.1% versus 9.1%; P=0.05), have an activities of daily living disability (23.8% versus 4.9%; P<0.001), and have instrumental activities of daily living disability (38.1% versus 13.8%; P<0.001). Finally, deceased donor KT recipients were more likely to experience delirium (81.0% versus 61.2%; P=0.01).
Table 1.
Characteristics of 893 kidney transplant recipients by post-kidney transplant delirium
| Characteristic | No Delirium | Delirium |
|---|---|---|
| N (%) | 851 (95.3) | 42 (4.7) |
| Recipient characteristics | ||
| Women | 38.9 | 40.5 |
| Black | 40.4 | 57.1 |
| Age, yr | 52.2 (14.1) | 60.4 (14.4) |
| Years on dialysis | 2.9 (3.6) | 3.6 (6.1) |
| BMI | 28.4 (6.9) | 28.3 (4.9) |
| High school education or less | 40.4 | 42.9 |
| Time on dialysis, yr | 2.9 (3.6) | 3.6 (6.1) |
| Cause of ESRD | ||
| Diabetes | 16.2 | 33.3 |
| Hypertension | 31.5 | 45.2 |
| Glomerular disease | 26.6 | 9.5 |
| Cystic disease | 10.6 | 0 |
| Other | 15.1 | 11.9 |
| Frail | 15.6 | 31.0 |
| Cognitive impairment | 9.1 | 19.1 |
| Global cognitive function | 92.1 (10.3) | 88.2 (10.2) |
| Poor HRQOL | 8.8 | 11.9 |
| Depressive symptoms | 11.3 | 16.7 |
| ADL disability | 4.9 | 23.8 |
| IADL disability | 13.8 | 38.1 |
| Comorbidities | ||
| Charlson Comorbidity Index | 1.5 (1.7) | 2.6 (2.2) |
| Myocardial infarction | 5.2 | 14.3 |
| Peripheral vascular disease | 5.9 | 11.9 |
| Cerebral vascular disease | 2.1 | 0 |
| Dementia | 0 | 0 |
| Chronic lung conditions | 7.3 | 11.9 |
| Rheumatologic disorders | 20.6 | 28.6 |
| Peptic ulcers | 3.6 | 7.1 |
| Diabetes | 30.2 | 50.0 |
| Moderate/severe liver disease | 2.0 | 4.8 |
| Metastatic cancer | 0 | 0 |
| Leukemia | 0.2 | 0 |
| Lymphoma | 0.1 | 0 |
| HIV | 2.0 | 0 |
| Transplant characteristics | ||
| Deceased donor | 61.2 | 81.0 |
| Not on standard triple therapy | 8.7 | 17.1 |
| DGF | 22.9 | 40.5 |
| PRA>80% | 14.7 | 4.8 |
| Cold ischemia time, ha | 26 (19–33) | 24 (18–34) |
Characteristics are presented as percentages for binary variables and means (SD) for continuous variables except cold ischemia time, in which the medians (interquartile ranges) are presented. BMI, body mass index; HRQOL, health-related quality of life; ADL, activities of daily living; IADL, instrumental activities of daily living; DGF, delayed graft function; PRA, panel reactive antibody.
For deceased donor recipients.
Age, frailty status, donor type, a score of two or more on the Charlson Comorbidity Index, and years on dialysis were included in the final model of delirium risk factors (Table 2); AUC for the model was 0.73. Age 65 years old or older (adjusted odds ratio [aOR], 2.65; 95% confidence interval [95% CI], 1.36 to 5.18; P=0.004), frailty (aOR, 2.05; 95% CI, 1.02 to 4.13; P=0.04), and the presence of two or more comorbidities (aOR, 1.93; 95% CI, 1.01 to 3.71; P=0.05) were independently associated with post-KT delirium. Cognitive impairment was not selected by the Akaike Information Criterion approach for inclusion in the model; however, when included in a sensitivity analysis, it was not associated with delirium (aOR, 1.57; 95% CI, 0.67 to 3.66; P=0.30).
Table 2.
Risk prediction models for post-kidney transplant delirium among kidney transplant recipients
| Risk Factor | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Age 65 yr or older | 2.65 (1.36 to 5.18) | 0.004 |
| Frail | 2.05 (1.02 to 4.13) | 0.04 |
| Deceased donor KT | 2.03 (0.91 to 4.52) | 0.10 |
| Two or more on the Charlson Comorbidity Index | 1.93 (1.01 to 3.71) | 0.05 |
| Years on dialysis | 1.06 (0.99 to 1.15) | 0.10 |
All hazard ratios are from a single adjusted Cox proportional hazards model for delirium. The area under the receiver operating characteristic curve for the model is 0.73. 95% CI, 95% confidence interval; KT, kidney transplant.
Neither induction agent was associated with post-KT delirium (all P>0.05). KT recipients not receiving a standard triple therapy regimen were at increased odds of post-KT delirium (aOR, 2.47; 95% CI, 1.07 to 5.68; P=0.03); however, including immunosuppression regimen did not improve delirium prediction (AUC=0.73).
LOS
The median time to discharge differed between KT recipients with and without delirium (16 versus 8 days; P<0.001) (Figure 2). LOS was 2.49-fold (95% CI, 2.13 to 2.91; P<0.001) longer for KT recipients with delirium after adjustment. Furthermore, the odds of a 2-week LOS were 5.42-fold (95% CI, 2.76 to 10.66; P<0.001) higher among KT recipients with delirium after adjustment (Table 3). There was no difference in the effect of delirium on the odds of a 2-week LOS between recipients who were and were not frail (P value for interaction =0.52).
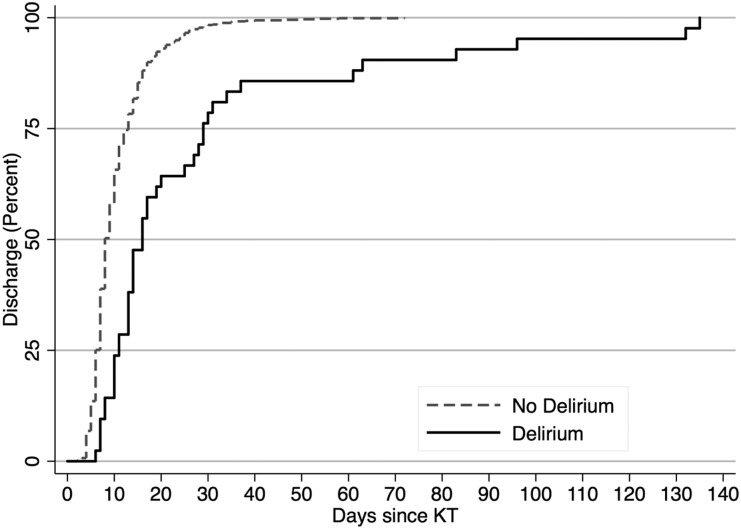
Figure 2.
The time to discharge after kidney transplantation was longer for recipients with post-KT delirium. The median time to discharge differed between KT recipients with and without delirium (16 versus 8 days; P<0.001).
Table 3.
The association between post-kidney transplant (KT) delirium and length of stay, discharge location, death-censored graft loss, and mortality
| Sequelae | Unadjusted (95% CI) | Adjusted (95% CI) |
|---|---|---|
| Length of stay | ||
| Days, RR | 2.67 (2.27 to 3.14) | 2.49 (2.13 to 2.91) |
| 2 wk or Longer length of stay, OR | 5.89 (3.09 to 11.21) | 5.42 (2.76 to 10.66) |
| Institutional discharge, OR | 30.11 (11.42 to 79.36) | 22.41 (7.85 to 63.98) |
| Death-censored graft loss, HR | 2.81 (1.21 to 6.54) | 2.73 (1.14 to 6.53) |
| Mortality, HR | 4.72 (2.71 to 8.20) | 3.12 (1.76 to 5.54) |
All models are adjusted for the recipient, transplant, and donor factors listed in Table 2. 95% CI, 95% confidence interval; RR, ratio of time; OR, odds ratio; HR, hazard ratio.
Discharge Location
Three KT recipients died during admission for KT, one of whom had delirium. The risk of perioperative mortality was 2.4% among those with delirium and 0.2% among those without delirium (P<0.001). Among the 890 participants without perioperative mortality, 19 (2.1%) were discharged to a skilled nursing facility or rehabilitation facility (institutional discharge). Institutional discharge was more common among KT recipients who had experienced delirium (ten [24.4%] versus nine [1.1%]; P<0.001). KT recipients with delirium were at a 22-fold (aOR, 22.41; 95% CI, 7.85 to 63.98; P<0.001) increased odds of institutional discharge after adjustment. There was no difference in the effect of delirium on institutional discharge between recipients who were and were not frail (P value for interaction =0.39).
Graft Loss and Mortality
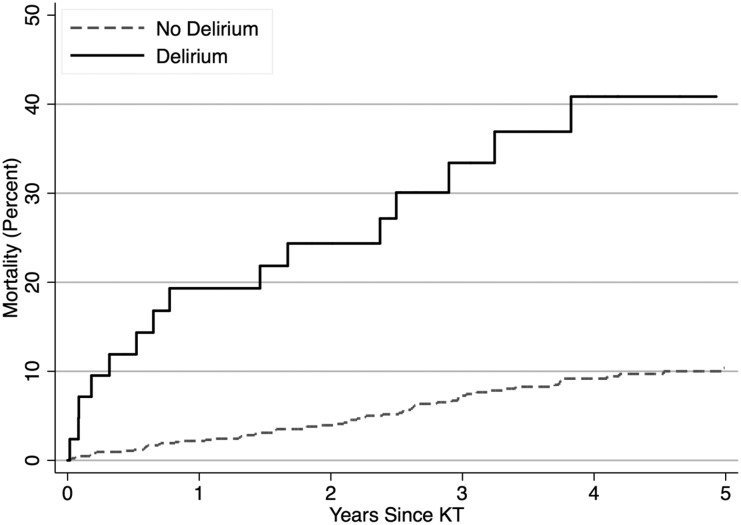
Sixty-two recipients experienced graft loss over a mean of 3.52 years of follow-up, and 94 died over a mean of 3.69 years of follow-up. The unadjusted risks of graft loss (P=0.01) and mortality (P<0.001) were increased among KT recipients with delirium (Figure 3, Table 3). The 1-, 3-, and 5-year risks of death-censored graft loss were greater among KT recipients with delirium (Table 4): 1 year (13.0% versus 2.3%), 3 year (16.1% versus 5.5%), and 5 year (16.1% versus 9.2%). Similarly, the 1-, 3-, and 5-year mortality risks were greater among KT recipients with delirium: 1 year (19.3% versus 2.2%), 3 year (33.4% versus 7.3%), and 5 year (40.9% versus 10.4%). KT recipients with delirium were at a 2.73-fold (95% CI, 1.14 to 6.53; P=0.03) increased risk of death-censored graft loss and a 3.12-fold (95% CI, 1.76 to 5.54; P<0.001) increased risk of post-KT mortality after adjustment.
Figure 3.
The cumulative incidence of mortality was greater among recipients with post-kidney transplant (KT) delirium. The log rank P value was <0.001.
Table 4.
Risk of death-censored graft loss and mortality for kidney transplant recipients with and without post-kidney transplant delirium
| Sequelae | Risk, % | ||
|---|---|---|---|
| 1 yr | 3 yr | 5 yr | |
| Death-censored graft loss | |||
| No delirium | 2.3 | 5.5 | 9.2 |
| Delirium | 13.0 | 16.1 | 16.1 |
| Mortality | |||
| No delirium | 2.2 | 7.3 | 10.4 |
| Delirium | 19.3 | 33.4 | 40.9 |
The risks (cumulative incidences) are expressed as percentages, and they are estimated using a Kaplan–Meier approach.
There were no differences in the strength of the associations between delirium and death-censored graft loss by age (P value for interaction =0.27), frailty (P value for interaction =0.45), or cognitive impairment (P value for interaction =0.60). Similarly, there were no differences in the strength of the association between delirium and mortality by age (P value for interaction =0.20), frailty (P value for interaction =0.96), or cognitive impairment (P value for interaction =0.85).
Discussion
The cumulative incidence of delirium ranged from 2.0% for recipients age 18–49 years old to 13.8% for those age ≥75 years old in a cohort study of 893 KT recipients. Delirium was rarely captured in the medical claims; however, when captured by claims, it was associated with subsequent diagnosis of dementia. We found that delirium occurred in KT recipients of all ages but was most common in those who were older and frail: 20.0% of frail recipients age ≥75 years old experienced delirium. Recipients with delirium were at a 5.42-fold higher odds of ≥2-week LOS, 22.41-fold increased risk of institutional discharge, 2.73-fold increased risk of death-censored graft loss, and 3.12-fold increased risk of mortality after adjustment.
Among older adults, delirium is considered to be the most common surgical complication,40 occurring in up to 50% of older surgical patients,3,15 but only 0.8% of older patients had a claim for delirium. We have extended these findings to a surgical population of KT recipients, many of whom were younger, and found a similar cumulative incidence of claims-based delirium. Additionally, we identified KT-specific risk factors for post-KT delirium, including receipt of a deceased donor KT, and lack of standard triple therapy was an additional risk factor for post-KT delirium. Furthermore, we have found that the risk factors for delirium differ between older surgical patients and KT recipients.41 Most notably, we found that frailty was a risk factor for delirium, whereas cognitive impairment was not, suggesting that there is less concern for delirium superimposed on cognitive impairment in this population; frailty was also more common in this population than cognitive impairment. Cognitive impairment was univariately associated with delirium, but this finding was not significant after adjusting for other factors, like age and frailty. One possible reason that we did not find an association between cognitive impairment and delirium is that KT recipients are a highly selective group of patients with ESRD who are screened to be free of dementia, such as is indicated in Table 1. However, in the national registry data, a claim for delirium during the KT hospitalization was associated with subsequent diagnosis of dementia; this association should be further explored in a prospective cohort that includes an in-depth neuropsychologic assessments to identify dementia.
The majority of studies of postoperative delirium have focused on older adults and found that, in this population, delirium is associated with poor outcomes in other surgical settings. For example, in one study of older (age >70 years old) elective surgery patients, delirium was associated with an increased risk of prolonged LOS and institutional discharge.5 Previous studies have found that older surgical patients with delirium experience an increased risk of mortality, functional decline, prolonged LOS, and institutional discharge as well as increased use of hospital resources and higher health care costs.1–5,14,15,42–48 We have extended these findings to a novel population of KT recipients of older age and provided initial evidence that delirium is associated with adverse transplant-specific outcomes, like graft loss. Our study suggests that delirium may be a serious complication for KT recipients of all ages, because it is associated with markers of poor outcomes, like prolonged LOS, institutional discharge, graft loss, and mortality.
Delirium may be an important post-transplant complication that may warrant further investigation into whether prevention of delirium reduces the risk of poor outcomes, like dementia, longer LOS, institutional discharge, graft loss, and mortality. Previous research in other surgical settings notes that delirium is preventable in up to 40% of patients.49,50 Preventing delirium could be one pathway for transplant centers to improve their long-term outcomes if the association is determined to be causal. In fact, the American Geriatrics Society has put forth a Best Practices Statement41 on postoperative delirium in older adults that includes evidence-based recommendations for delirium screening and diagnosis, measures to prevent delirium, and treatment of delirium; however, these best practices should be tailored to an ESRD population undergoing KT if the associations of delirium and longer LOS, institutional discharge, graft loss, and mortality are determined to be causal. Interventions that were identified in this Best Practices Statement41 and may prevent post-KT delirium may include using the Bispectral Index to monitor and control the depth of anesthesia as well as avoidance of postoperative medications that induce delirium; however, these interventions must be tested during KT.
A limitation of this study was that the chart abstraction of delirium was retrospective. However, the abstractors were blinded to the outcome status of the participants, and they only abstracted data on delirium that were entered into the charts during the KT hospitalizations (i.e., before the outcomes of this study). As a sensitivity analyses, we excluded the one patient with an unclear start of delirium in the chart, and all results were consistent. Additionally another limitation of our study was the small sample size of our single-center, longitudinal cohort population relative to the national data on transplant recipients. However, studies of post-KT delirium are reliant on chart abstractions26 or in-person assessments,6 because delirium is rarely captured in medical claims. Furthermore, the reference standard diagnosis for delirium includes cognitive testing supplemented by collateral historical information about acute changes.51 Future studies should explore the prevalence and sequelae of an in-person assessment of delirium as well as a prospective measurement of cognitive impairment and dementia. We defined delirium using a chart-based method, resulting in missed patients with hypoactive delirium. Although this method of identifying patients with delirium has good sensitivity and specificity compared with an in-person assessment,26 it is known that this method often misses hypoactive symptoms (decreased arousal, delayed awakening from anesthesia, slowed thinking and speech, decreased movements, new difficulties maintaining posture, sleeping during the day, decreased appetite, or new incontinence),41 because these are more commonly missed in the medical chart. Similarly, the cause of delirium is often multifactorial,3 and it is not often identified in clinical practice or recorded in the chart. Furthermore, our cohort study did not include a comprehensive assessment of inpatient medications, limiting our ability to identify medications that were associated with delirium. Strengths of this study include the validated chart abstraction of delirium, measurement of frailty, and global cognitive impairment and up to 8 years of follow-up for the long-term outcomes. We were also able to adjust for a wider range of comorbidities than is available in the national registry.
In summary, post-KT delirium occurs in KT recipients of all ages, but the cumulative incidence increases with age. The evident dearth of medical claims for delirium suggests that it is a clinical entity that is often missed. Older KT recipients who were frail were the most likely to experience delirium, and this incidence rises to 20%. Furthermore, delirium was independently associated with a longer LOS, institutional discharge, graft loss, and mortality. These findings underscore the vulnerability of frail KT recipients and may suggest that this population is at risk of postoperative complications, which then increase their risk of subsequent long-term outcomes, possibly even including dementia. Transplant centers should be aware of the risks associated with post-KT delirium and implement interventions to reduce the risk of delirium.
Disclosures
None.
Supplementary Material
Acknowledgments
Funding for this study was provided, in part, by National Institute of Diabetes and Digestive and Kidney Disease and National Institute on Aging grants F32AG053025 (principal investigator [PI]: C.E.H.), K76AG057020 (PI: C.H.B.), R01DK102981 (PI: D.C.B.), R21AG050850 (PI: K.J.N.), K24DK101828 (PI: D.L.S.), R01DK096008 (PI: D.L.S.), K01AG043501 (PI: M.M.-D.), R01AG055781 (PI: M.M.-D.), and R01DK114074 (PI: M.M.-D.).
The data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Marcantonio ER, Goldman L, Mangione CM, Ludwig LE, Muraca B, Haslauer CM, et al.: A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 271: 134–139, 1994 [PubMed] [Google Scholar]
- 2.Saczynski JS, Inouye SK, Kosar C, Tommet D, Marcantonio ER, Fong T, et al. : Cognitive and brain reserve and the risk of postoperative delirium in older patients. Lancet Psychiatry 1: 437–443, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RG, Saczynski JS: Delirium in elderly people. Lancet 383: 911–922, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults : American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 63: 142–150, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, et al.: Effect of delirium and other major complications on outcomes after elective surgery in older adults. JAMA Surg 150: 1134–1140, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI: Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med 113: 941–948, 1990 [DOI] [PubMed] [Google Scholar]
- 7.Bettelli G, Neuner B: Postoperative delirium: A preventable complication in the elderly surgical patient. Monaldi Arch Chest Dis 87: 842, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Assmann P, Kievit P, van der Wulp K, Verkroost M, Noyez L, Bor H, et al.: Frailty is associated with delirium and mortality after transcatheter aortic valve implantation. Open Heart 3: e000478, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culley DJ, Flaherty D, Fahey MC, Rudolph JL, Javedan H, Huang CC, et al.: Poor performance on a preoperative cognitive screening test predicts postoperative complications in older orthopedic surgical patients. Anesthesiology 127: 765–774, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oldham MA, Hawkins KA, Yuh DD, Dewar ML, Darr UM, Lysyy T, et al.: Cognitive and functional status predictors of delirium and delirium severity after coronary artery bypass graft surgery: An interim analysis of the Neuropsychiatric Outcomes After Heart Surgery study. Int Psychogeriatr 27: 1929–1938, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown CH 4th, Morrissey C, Ono M, Yenokyan G, Selnes OA, Walston J, et al.: Impaired olfaction and risk of delirium or cognitive decline after cardiac surgery. J Am Geriatr Soc 63: 16–23, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang CK, Chu CL, Chou MY, Lin YT, Lu T, Hsu CJ, et al.: Interrelationship of postoperative delirium and cognitive impairment and their impact on the functional status in older patients undergoing orthopaedic surgery: A prospective cohort study. PLoS One 9: e110339, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong TG, Hshieh TT, Wong B, Tommet D, Jones RN, Schmitt EM, et al.: Neuropsychological profiles of an elderly cohort undergoing elective surgery and the relationship between cognitive performance and delirium. J Am Geriatr Soc 63: 977–982, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown CH 4th, Max L, LaFlam A, Kirk L, Gross A, Arora R, et al. : The association between preoperative frailty and postoperative delirium after cardiac surgery. Anesth Analg 123: 430–435, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasgupta M, Dumbrell AC: Preoperative risk assessment for delirium after noncardiac surgery: A systematic review. J Am Geriatr Soc 54: 1578–1589, 2006 [DOI] [PubMed] [Google Scholar]
- 16.McAdams-DeMarco MA, James N, Salter ML, Walston J, Segev DL: Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc 62: 2235–2242, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Ying H, Olorundare I, King EA, Haugen C, Buta B, et al.: Individual frailty components and mortality in kidney transplant recipients. Transplantation 101: 2126–2132, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas AG, Ruck J, Shaffer A, Haugen C, Ying H, Warsame F, et al. : Cognitive impairment and graft loss in kidney transplant recipients. Am J Transplant 18: 39–40, 2018 [Google Scholar]
- 19.Gosselt AN, Slooter AJ, Boere PR, Zaal IJ: Risk factors for delirium after on-pump cardiac surgery: A systematic review. Crit Care 19: 346, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdams-DeMarco MA, King EA, Luo X, Haugen C, DiBrito S, Shaffer A, et al.: Frailty, length of stay, and mortality in kidney transplant recipients: A national registry and prospective cohort study. Ann Surg 266: 1084–1090, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL: Early hospital readmission after kidney transplantation: Patient and center-level associations. Am J Transplant 12: 3283–3288, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Grams ME, McAdams Demarco MA, Kucirka LM, Segev DL: Recipient age and time spent hospitalized in the year before and after kidney transplantation. Transplantation 94: 750–756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAdams-DeMarco MA, Law A, Salter ML, Chow E, Grams M, Walston J, et al.: Frailty and early hospital readmission after kidney transplantation. Am J Transplant 13: 2091–2095, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, et al.: Frailty and mortality in kidney transplant recipients. Am J Transplant 15: 149–154, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAdams-Demarco MA, Grams ME, King E, Desai NM, Segev DL: Sequelae of early hospital readmission after kidney transplantation. Am J Transplant 14: 397–403, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr, Leslie DL, Agostini JV: A chart-based method for identification of delirium: Validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc 53: 312–318, 2005 [DOI] [PubMed] [Google Scholar]
- 27.McAdams-DeMarco MA, Bae S, Chu N, Gross AL, Brown CH 4th, Oh E, et al. : Dementia and Alzheimer’s disease among older kidney transplant recipients. J Am Soc Nephrol 28: 1575–1583, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAdams-DeMarco MA, Law A, Tan J, Delp C, King EA, Orandi B, et al.: Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation 99: 805–810, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nastasi AJ, McAdams-DeMarco MA, Schrack J, Ying H, Olorundare I, Warsame F, et al.: Pre-kidney transplant lower extremity impairment and post-kidney transplant mortality. Am J Transplant 18: 189–196, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAdams-DeMarco MA, Isaacs K, Darko L, Salter ML, Gupta N, King EA, et al.: Changes in frailty after kidney transplantation. J Am Geriatr Soc 63: 2152–2157, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAdams-DeMarco MA, Ying H, Olorundare I, King EA, Desai N, Dagher N, et al.: Frailty and health-related quality of life in end stage renal disease patients of all ages. J Frailty Aging 5: 174–179, 2016 [PMC free article] [PubMed] [Google Scholar]
- 32.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Teng EL, Chui HC: The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48: 314–318, 1987 [PubMed] [Google Scholar]
- 34.McDowell I, Kristjansson B, Hill GB, Hébert R: Community screening for dementia: The Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol 50: 377–383, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al.; Cardiovascular Health Study Collaborative Research Group : Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: M146–M156, 2001 [DOI] [PubMed] [Google Scholar]
- 36.McAdams-DeMarco MA, Law A, Salter ML, Boyarsky B, Gimenez L, Jaar BG, et al.: Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc 61: 896–901, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, et al.: Frailty and delayed graft function in kidney transplant recipients. Arch Surg 147: 190–193, 2012 [DOI] [PubMed] [Google Scholar]
- 38.McAdams-DeMarco MA, Suresh S, Law A, Salter ML, Gimenez LF, Jaar BG, et al.: Frailty and falls among adult patients undergoing chronic hemodialysis: A prospective cohort study. BMC Nephrol 14: 224, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAdams-DeMarco MA, Tan J, Salter ML, Gross A, Meoni LA, Jaar BG, et al.: Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol 10: 2181–2189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcantonio ER, Juarez G, Goldman L, Mangione CM, Ludwig LE, Lind L, et al.: The relationship of postoperative delirium with psychoactive medications. JAMA 272: 1518–1522, 1994 [PubMed] [Google Scholar]
- 41.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults : Postoperative delirium in older adults: Best practice statement from the American Geriatrics Society. J Am Coll Surg 220: 136–148.e1, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M: Postoperative delirium in the elderly: Risk factors and outcomes. Ann Surg 249: 173–178, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Raats JW, van Eijsden WA, Crolla RM, Steyerberg EW, van der Laan L: Risk factors and outcomes for postoperative delirium after major surgery in elderly patients. PLoS One 10: e0136071, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown CH 4th, Laflam A, Max L, Lymar D, Neufeld KJ, Tian J, et al.: The impact of delirium after cardiac surgical procedures on postoperative resource use. Ann Thorac Surg 101: 1663–1669, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee HB, Oldham MA, Sieber FE, Oh ES: Impact of delirium after hip fracture surgery on one-year mortality in patients with or without dementia: A case of effect modification. Am J Geriatr Psychiatry 25: 308–315, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sher Y, Mooney J, Dhillon G, Lee R, Maldonado JR: Delirium after lung transplantation: Association with recipient characteristics, hospital resource utilization, and mortality [published online ahead of print April 11, 2017]. Clin Transplant doi:10.1111/ctr.12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elsamadicy AA, Wang TY, Back AG, Lydon E, Reddy GB, Karikari IO, et al.: Post-operative delirium is an independent predictor of 30-day hospital readmission after spine surgery in the elderly (≥65years old): A study of 453 consecutive elderly spine surgery patients. J Clin Neurosci 41: 128–131, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Moskowitz EE, Overbey DM, Jones TS, Jones EL, Arcomano TR, Moore JT, et al. : Post-operative delirium is associated with increased 5-year mortality. Am J Surg 214: 1036–1038, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Inouye SK, Bogardus ST Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al.: A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 340: 669–676, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM: Reducing delirium after hip fracture: A randomized trial. J Am Geriatr Soc 49: 516–522, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Neufeld KJ, Nelliot A, Inouye SK, Ely EW, Bienvenu OJ, Lee HB, et al.: Delirium diagnosis methodology used in research: A survey-based study. Am J Geriatr Psychiatry 22: 1513–1521, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.