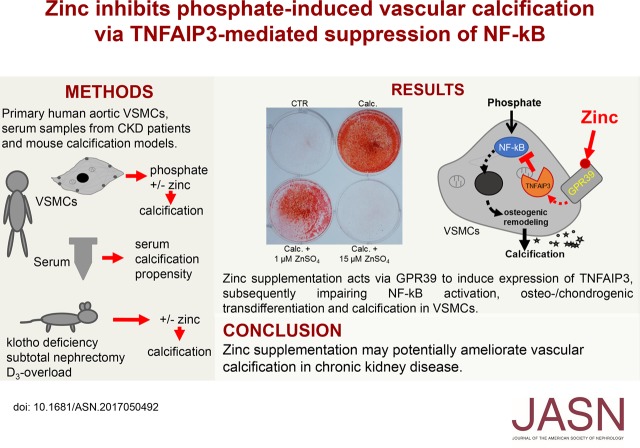
Abstract
Background The high cardiovascular morbidity and mortality of patients with CKD may result in large part from medial vascular calcification, a process promoted by hyperphosphatemia and involving osteo-/chondrogenic transdifferentiation of vascular smooth muscle cells (VSMCs). Reduced serum zinc levels have frequently been observed in patients with CKD, but the functional relevance of this remains unclear.
Methods We performed experiments in primary human aortic VSMCs; klotho-hypomorphic (kl/kl), subtotal nephrectomy, and cholecalciferol-overload mouse calcification models; and serum samples from patients with CKD.
Results In cultured VSMCs, treatment with zinc sulfate (ZnSO4) blunted phosphate-induced calcification, osteo-/chondrogenic signaling, and NF-κB activation. ZnSO4 increased the abundance of zinc-finger protein TNF-α–induced protein 3 (TNFAIP3, also known as A20), a suppressor of the NF-κB pathway, by zinc-sensing receptor ZnR/GPR39-dependent upregulation of TNFAIP3 gene expression. Silencing of TNFAIP3 in VSMCs blunted the anticalcific effects of ZnSO4 under high phosphate conditions. kl/kl mice showed reduced plasma zinc levels, and ZnSO4 supplementation strongly blunted vascular calcification and aortic osteoinduction and upregulated aortic Tnfaip3 expression. ZnSO4 ameliorated vascular calcification in mice with chronic renal failure and mice with cholecalciferol overload. In patients with CKD, serum zinc concentrations inversely correlated with serum calcification propensity. Finally, ZnSO4 ameliorated the osteoinductive effects of uremic serum in VSMCs.
Conclusions Zinc supplementation ameliorates phosphate-induced osteo-/chondrogenic transdifferentiation of VSMCs and vascular calcification through an active cellular mechanism resulting from GPR39-dependent induction of TNFAIP3 and subsequent suppression of the NF-κB pathway. Zinc supplementation may be a simple treatment to reduce the burden of vascular calcification in CKD.
Keywords: TNFAIP3, osteogenic signaling, vascular smooth muscle cells, GPR39, vascular calcification, zinc
Cardiovascular disease is the main cause of death in patients with CKD.1 The high cardiovascular mortality of patients with CKD is associated with medial vascular calcification (VC).2 VC develops in the early stages of CKD and progressively increases with advanced stages of the disease.3 VC develops as part of CKD–mineral bone disorder and is linked with hyperphosphatemia,4 a risk factor for VC and cardiovascular mortality, especially in patients with CKD,5 but also in the general population.6 Phosphate has therefore been considered as a key factor in the development of VC.1
VC has been recognized as an active process, regulated primarily by vascular smooth muscle cells (VSMCs).7 VSMCs are able to alter their phenotype and release matrix vesicles and initiate mineralization of arteries during pathologic conditions, in a process similar to physiologic bone mineralization.1 During hyperphosphatemic stimulation, VSMCs differentiate into cells with an osteo-/chondroblastic phenotype.8 The phenotypical transdifferentiation is characterized by expression of osteogenic transcription factors, such as msh homeobox 2 (MSX2) and core-binding factor α-1 (CBFA1, also termed RUNX2).9 The osteo-/chondroblastic VSMCs express tissue-nonspecific alkaline phosphatase (ALPL), which hydrolyzes the endogenous calcification inhibitor pyrophosphate and thus fosters uncontrolled formation of calcium phosphate crystals.10 The phenotypical transdifferentiation of VSMCs is regulated by complex signaling pathways,11 which are linked to generalized inflammation and are, at least in part, dependent on the transcription factor NF-κB, which has emerged as a key regulator of VC.12
Abnormalities of zinc homeostasis in patients with CKD are well known.13 Plasma zinc levels decline with decreasing renal function,14 and patients on hemodialysis frequently develop zinc deficiency.15 Hypozincemia in patients with CKD may result from both reduced uptake and increased excretion.13
Zinc deficiency has previously been linked to inflammatory processes.16–18 Vascular effects of zinc have been described and zinc deficiency aggravates intimal atherosclerosis in experimental models.19 Accordingly, zinc supplementation reduced the atherosclerotic lesions in rabbits on a high cholesterol diet,20 and the extent of abdominal aneurysm formation in mice.21 Also, in humans, lower zinc bioavailability was correlated with subclinical atherosclerosis.22 Reduced serum zinc levels are also associated with increased carotid intima to media thickness in patients on hemodialysis.23 Although medial VC differs from intimal atherosclerosis, overlapping inflammatory pathways exist.24 A regulatory role of zinc on vascular inflammatory processes may be especially important in patients with CKD and VC. However, the effect of zinc on VC remains unclear. This study was therefore conducted to investigate possible effects of zinc on VC induced by hyperphosphatemia.
Methods
A detailed description of the methods can be found in the Supplemental Material. Primary human aortic VSMCs (HAoSMCs; Thermo Fisher Scientific) were treated with β-glycerophosphate and CaCl2 as calcification medium,25 β-glycerophosphate, hydroxyapatite nanoparticles (<200 nm particle size), zinc sulfate (ZnSO4), zinc chloride (ZnCl2), BAY11–7082, BMS-345541, or parthenolide (Sigma Aldrich), and/or silenced with TNFAIP3, GPR39, or negative control siRNA using the siPORT amine transfection reagent (Thermo Fisher Scientific).26 Lifelong treatment with ZnSO4 (7.18g/l in distilled water) was performed in klotho wild-type and klotho-hypomorphic (kl/kl) mice.27 C57Bl/6 mice were injected subcutaneously with cholecalciferol (Sigma-Aldrich).28 Subtotal nephrectomy was performed in a two-step surgical procedure in DBA mice.28
The mRNA expression was determined by qRT-PCR,29 protein expression and localization by Western blotting and confocal microscopy,30 transcriptional activity by luciferase assay,30 calcification by Alizarin Red staining, von Kossa staining and Calcium assay (BioAssay Systems),28 and ALPL activity by a colorimetric assay (Abcam).9 Serum was obtained from patients with CKD or healthy controls after obtaining informed consent. A second study cohort was used for validation.31 Zinc concentrations were determined by zinc assay (BioAssay Systems). Serum calcification propensity was determined by a nephelometric method, as described by Pasch et al.32
Data are shown as scatterdot plots and arithmetic means±SEM. Statistical analysis was performed by SPSS and JMP software. Normality was tested with the Shapiro–Wilk test. Non-normal datasets were transformed before statistical testing to provide normality according to the Shapiro–Wilk test. Statistical testing was performed by one-way ANOVA followed by the Tukey test (homoscedastic data) or Games–Howell test (heteroscedastic data). Non-normal data were tested by the Steel–Dwass method. Two groups were compared by unpaired two-tailed t-test. For correlations, Spearman correlation test was used. P<0.05 was considered statistically significant.
Results
Effect of Zinc on Phosphate-Induced Osteoinductive Signaling and Calcification of HAoSMCs
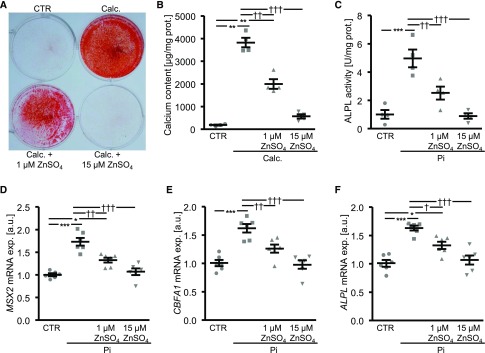
A first series of experiments explored the effects of zinc on osteoinductive signaling and calcification of primary human aortic VSMCs (HAoSMCs) under elevated phosphate conditions. In HAoSMCs, treatment with calcification medium was followed by a significant increase of calcium deposition (Figure 1, A and B), an effect significantly reduced by additional treatment with 1 µM ZnSO4 and completely abrogated by the addition of 15 µM ZnSO4. Furthermore, ZnSO4 significantly inhibited the phosphate-induced alkaline phosphatase activity (Figure 1C) and mRNA expression of osteogenic markers MSX2, CBFA1, and ALPL (Figure 1, D–F) in a concentration-dependent manner. ZnCl2 treatment similarly blunted the expression of phosphate-induced osteogenic markers in HAoSMCs (Supplemental Figure 1). The mRNA expression of ACTA2, a VSMC marker, was significantly downregulated by phosphate, an effect again significantly blunted by ZnSO4 (Supplemental Figure 2).
Figure 1.
ZnSO4 inhibits phosphate-induced osteoinductive signaling and calcification in HAoSMCs. (A) Representative original images showing Alizarin Red staining in HAoSMCs after treatment for 11 days with control or with calcification medium (Calc.) without or with additional treatment with 1 or 15 µM ZnSO4. Images are representative of four independent experiments. The calcified areas are shown as red staining. (B) Scatterdot plots and arithmetic means±SEM (n=4, micrograms per milligram protein) of calcium content in HAoSMCs after treatment for 11 days with control or with Calc. without or with additional treatment with 1 or 15 µM ZnSO4. (C) Scatterdot plots and arithmetic means±SEM (n=4, units per milligram protein) of alkaline phosphatase activity in HAoSMCs after treatment for 7 days with control or with β-glycerophosphate (Pi) without or with additional treatment with 1 or 15 µM ZnSO4. (D–F) Scatterdot plots and arithmetic means±SEM (n=6; arbitrary units, a.u.) of MSX2 (D), CBFA1 (E), and ALPL (F) relative mRNA expression in HAoSMCs after treatment for 24 hours with control or with Pi without or with additional treatment with 1 or 15 µM ZnSO4. *P<0.05; **P<0.01; ***P<0.001 statistically significant versus control treated HAoSMCs; †P<0.05; ††P<0.01; †††P<0.001 statistically significant versus HAoSMCs treated with Calc./Pi alone. CTR, control.
Further experiments explored the effects of ZnSO4 in the presence of small calcium phosphate crystals (hydroxyapatite nanoparticles). As shown in Supplemental Figure 3, hydroxyapatite treatment significantly increased alkaline phosphatase activity and mRNA expression of osteogenic markers and down-regulated ACTA2 mRNA expression in HAoSMCs, effects significantly suppressed in the presence of ZnSO4. In contrast, calcium phosphate precipitation (Supplemental Figure 4A) and hydroxyapatite dissociation (Supplemental Figure 4B) were not modified in the presence of ZnSO4.
Effect of ZnSO4 Supplementation on Phosphate-Induced NF-κB Activation in HAoSMCs
To elucidate the underlying mechanisms of the protective effects of zinc under high phosphate conditions, we investigated the activation of NF-κB. As shown in Figure 2, A–C, phosphate treatment significantly increased IκBα phosphorylation, downregulated IκBα protein abundance, and triggered the nuclear translocation of NF-κB p65 protein together with increased NF-κB–dependent transcriptional activity in HAoSMCs, all effects significantly suppressed in the presence of ZnSO4.
Figure 2.
ZnSO4 inhibits phosphate-induced NF-κB activation and upregulates TNFAIP3 expression in HAoSMCs. (A) Representative original Western blots and scatterdot plots and arithmetic means±SEM (n=6; arbitrary units, a.u.) of normalized phospho-IκBα (Ser32)/IκBα/GAPDH and total IκBα/GAPDH protein ratio in HAoSMCs after treatment for 24 hours with control or with β-glycerophosphate (Pi) without or with additional treatment with 15 µM ZnSO4. (B) Representative confocal microscopy images showing NF-κB p65 protein expression and localization in HAoSMCs after treatment for 24 hours with control or with Pi without or with additional treatment with 15 µM ZnSO4. Images are representative for three independent experiments. NF-κB p65 expression: green labeling, nuclei: purple labeling. Scale bar, 25 μm. (C) Scatterdot plots and arithmetic means±SEM (n=4; a.u.) of NF-κB–dependent transcriptional activity measured by luciferase reporter assay in HAoSMCs after transfection for 48 hours with NF-κB–responsive luciferase/Renilla constructs and treatment for 24 hours with control or with Pi without or with additional treatment with 15 µM ZnSO4. (D) Scatterdot plots and arithmetic means±SEM (n=6; a.u.) of TNFAIP3 relative mRNA expression in HAoSMCs after treatment for 24 hours with control or with Pi without or with additional treatment with 15 µM ZnSO4. (E) Representative original Western blots and scatterdot plots and arithmetic means±SEM (n=5; a.u.) of normalized TNFAIP3/GAPDH protein ratio in HAoSMCs after treatment for 24 hours with control or with Pi without or with additional treatment with 15 µM ZnSO4. *P<0.05; ***P<0.001 statistically significant versus control treated HAoSMCs; †P<0.05; ††P<0.01; †††P<0.001 statistically significant versus HAoSMCs treated with Pi alone. CTR, control.
These effects of ZnSO4 treatment were paralleled by upregulation of the known endogenous inhibitor of NF-κB activity, the zinc-finger protein TNFAIP3 (A20; encoded by the TNFAIP3 gene), at the level of both mRNA and protein expression in HAoSMCs (Figure 2, D and E, Supplemental Figure 3F). TNFAIP3 mRNA expression in HAoSMCs was similarly upregulated by ZnCl2 (Supplemental Figure 1D). Phosphate did not significantly modify TNFAIP3 protein levels, but tended to increase TNFAIP3 mRNA expression in HAoSMCs; however, this effect did not reach statistical significance (Figure 2, D and E).
The inhibitory effects of zinc on phosphate-induced mRNA expression of osteogenic markers in HAoSMCs were mimicked by treatment with NF-κB pathway inhibitors BAY11–7082, an irreversible inhibitor of IKKα and phosphorylation of IκBα, parthenolide, which binds directly and inhibits IKKβ, and BMS-345541 a highly selective inhibitor of IKKα and IKKβ (Supplemental Figure 5).
Role of TNFAIP3 in the Anticalcific Effects of ZnSO4 Supplementation in HAoSMCs
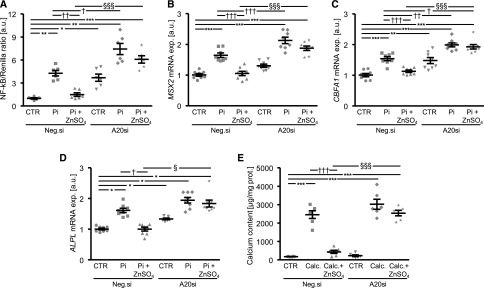
To explore the role of TNFAIP3 in the anticalcific effects of ZnSO4, endogenous TNFAIP3 expression was suppressed by silencing in HAoSMCs (Supplemental Figure 6). The inhibitory effect of ZnSO4 on phosphate-induced NF-κB–dependent transcriptional activity and mRNA expression of osteogenic markers was abolished in HAoSMCs silenced with TNFAIP3 siRNA (Figure 3, A–D). Silencing of TNFAIP3 alone significantly upregulated NF-κB–dependent transcriptional activity and CBFA1 and ALPL mRNA expression in HAoSMCs. Silencing of TNFAIP3 further enhanced the NF-κB–dependent transcriptional activity and MSX2 and CBFA1 mRNA expression in HAoSMCs under high phosphate conditions compared with HAoSMCs exposed to control siRNA. Furthermore, silencing of TNFAIP3 did not significantly modify the calcium deposition in HAoSMCs, but significantly abolished the inhibitory effects of ZnSO4 on phosphate-induced HAoSMCs mineralization (Figure 3E).
Figure 3.
Silencing of TNFAIP3 blunts the protective effects of ZnSO4 on phosphate-induced osteoinductive signaling and calcification in HAoSMCs. (A) Scatterdot plots and arithmetic means±SEM (n=6; arbitrary units, a.u.) of NF-κB–dependent transcriptional activity measured by luciferase reporter assay in HAoSMCs after transfection for 48 hours with NF-κB–responsive luciferase/Renilla constructs, silencing for 48 hours with negative control siRNA (Neg.si) or TNFAIP3 siRNA (A20si) and treatment for 24 hours with control or with β-glycerophosphate (Pi) without or with additional treatment with 15 µM ZnSO4. (B–D) Scatterdot plots and arithmetic means±SEM (n=8; a.u.) of MSX2 (B), CBFA1 (C), and ALPL (D) relative mRNA expression in HAoSMCs after silencing for 48 hours with Neg.si or A20si and treatment for 24 hours with control or with Pi without or with additional treatment with 15 µM ZnSO4. (E) Scatterdot plots and arithmetic means±SEM (n=6, micrograms per milligram protein) of calcium content in HAoSMCs after silencing for 11 days with Neg.si or A20si and treatment with control or with calcification medium (Calc.) without or with additional treatment with 15 µM ZnSO4. *P<0.05; **P<0.01; ***P<0.001 statistically significant versus Neg.si silenced HAoSMCs; †P<0.05; ††P<0.01; †††P<0.001 statistically significant versus Neg.si silenced and Calc./Pi treated HAoSMCs; §P<0.05; §§§P<0.001 statistically significant between Neg.si silenced and A20si silenced Pi+ZnSO4 treated HAoSMCs. CTR, control.
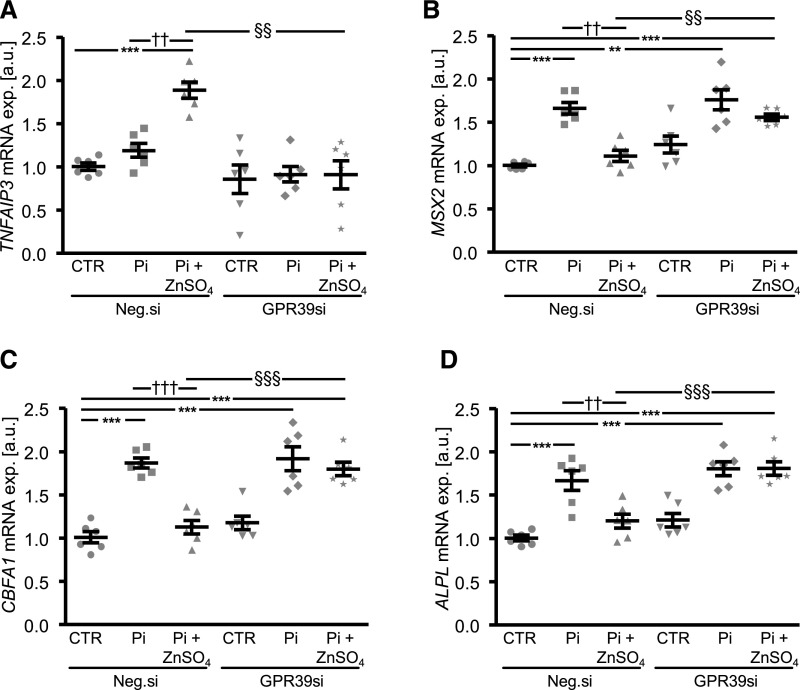
Additional experiments investigated the regulation of TNFAIP3 expression by ZnSO4. To this end, the endogenous expression of zinc-sensing receptor ZnR/GPR39 was inhibited by silencing in HAoSMCs (Supplemental Figure 7). Upregulation of TNFAIP3 mRNA expression under high phosphate conditions in the presence of ZnSO4 was significantly blunted in GPR39-silenced HAoSMCs (Figure 4A). Furthermore, the inhibitory effects of ZnSO4 on phosphate-induced mRNA expression of osteogenic markers were significantly blunted in GPR39-silenced HAoSMCs (Figure 4, B–D).
Figure 4.
Silencing of zinc-sensing receptor ZnR/GPR39 blunts the ZnSO4-induced TNFAIP3 expression and the protective effects of ZnSO4 on phosphate-induced osteoinductive signaling in HAoSMCs. Scatterdot plots and arithmetic means±SEM (n=6; arbitrary units, a.u.) of TNFAIP3 (A), MSX2 (B), CBFA1 (C), and ALPL (D) relative mRNA expression in HAoSMCs after silencing for 48 hours with negative control siRNA (Neg.si) or GPR39 siRNA (GPR39si) and treatment for 24 hours with control or with β-glycerophosphate (Pi) without or with additional treatment with 15 µM ZnSO4. **P<0.01; ***P<0.001 statistically significant versus Neg.si silenced HAoSMCs; ††P<0.01; †††P<0.001 statistically significant versus Neg.si silenced and Pi treated HAoSMCs; §§P<0.01; §§§P<0.001 statistically significant between Neg.si silenced and GPR39si silenced Pi+ZnSO4 treated HAoSMCs. CTR, control.
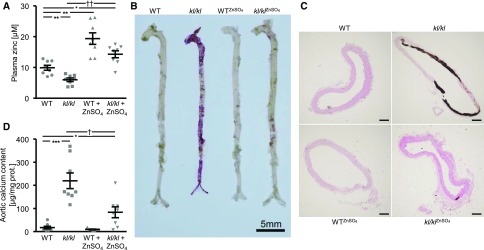
Effects of ZnSO4 Supplementation on VC in kl/kl Mice
To investigate the in vivo effects of ZnSO4 on vascular osteoinduction and calcification, further experiments were performed in hyperphosphatemic kl/kl mice and corresponding wild-type mice after long-term ZnSO4 supplementation. As shown in Figure 5A, kl/kl mice displayed lower plasma zinc levels than wild-type mice. ZnSO4 supplementation increased plasma zinc levels irrespective of the genotype. ZnSO4 did not significantly modify the increase of plasma phosphate and calcium levels, but tended to reduce the increased FGF23 levels in kl/kl mice; however, this effect did not reach statistical significance. The body weight of kl/kl mice was lower compared with wild-type mice, a difference blunted by lifelong treatment with ZnSO4 (Supplemental Table 1).
Figure 5.
ZnSO4 supplementation ameliorates VC in kl/kl mice. (A) Scatterdot plots and arithmetic means±SEM (n=8, micromolar) of plasma zinc levels in kl/kl mice and corresponding wild-type mice (WT) after treatment without or with ZnSO4. (B) Representative original images showing aortic Alizarin Red staining in kl/kl mice and WT mice after treatment without or with ZnSO4. The calcified areas are shown as red staining. Scale bar, 5 mm. (C) Representative original images showing von Kossa staining of thoracic aorta sections from kl/kl mice and WT mice after treatment without or with ZnSO4. The calcified areas are shown as gray/black staining. Scale bar, 100 μm. (D) Scatterdot plots and arithmetic means±SEM (n=7–8, micrograms per milligram protein) of calcium content in the aortic arch of kl/kl mice and WT mice after treatment without or with ZnSO4. *P<0.05; **P<0.01; ***P<0.001 statistically significant versus control treated WT mice; †P<0.05; ††P<0.01 statistically significant versus control treated kl/kl mice. CTR, control.
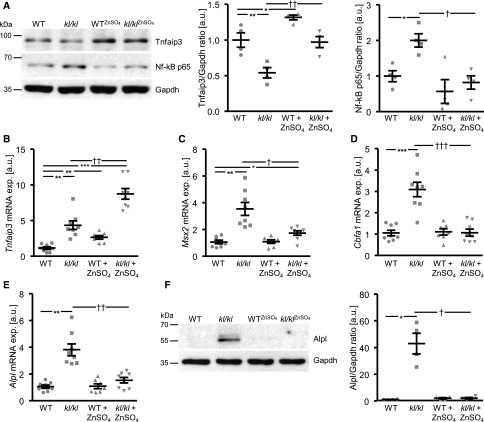
Aortic Alizarin Red staining, von Kossa staining of thoracic aorta sections, and quantification of calcium content in the aortic arch revealed extensive VC in kl/kl mice, but not in wild-type mice (Figure 5, B–D). Treatment with ZnSO4 profoundly reduced VC in kl/kl mice. Tnfaip3 protein expression was significantly lower, but Tnfaip3 mRNA expression significantly higher in aortic tissue of kl/kl mice compared with wild-type mice (Figure 6, A and B). ZnSO4 significantly upregulated aortic Tnfaip3 expression in both kl/kl and wild-type mice. Moreover, NF-κB p65 expression (Figure 6A), the mRNA expression of Msx2, Cbfa1, and Alpl (Figure 6, C–E), and Alpl protein abundance (Figure 6F) were all significantly higher in aortic tissue of kl/kl mice than wild-type mice, effects significantly reduced by ZnSO4.
Figure 6.
ZnSO4 supplementation ameliorates vascular osteoinductive signaling and upregulates aortic Tnfaip3 expression in kl/kl mice. (A) Representative original Western blots and scatterdot plots and arithmetic means±SEM (n=4; arbitrary units, a.u.) of normalized Tnfaip3/Gapdh and NF-κB p65/Gapdh protein ratio in aortic tissue of kl/kl mice and wild-type (WT) mice after treatment without or with ZnSO4. (B–E) Scatterdot plots and arithmetic means±SEM (n=7–8; a.u.) of Tnfaip3 (B), Msx2 (C), Cbfa1 (D), and Alpl (E) relative mRNA expression in aortic tissue of kl/kl mice and WT mice after treatment without or with ZnSO4. (F) Representative original Western blots and scatterdot plots and arithmetic means±SEM (n=4; a.u.) of normalized Alpl/Gapdh protein ratio in aortic tissue of kl/kl mice and WT mice after treatment without or with ZnSO4. *P<0.05; **P<0.01; ***P<0.001 statistically significant versus control treated WT mice; †P<0.05; ††P<0.01; †††P<0.001 statistically significant versus control treated kl/kl mice. CTR, control.
In addition, zinc supplementation blunted the cholecalciferol overload-induced VC and aortic osteo-/chondrogenic transdifferentiation in vivo (Supplemental Figure 8, B–G). Cholecalciferol overload significantly increased plasma zinc concentrations, which strongly increased further after ZnSO4 treatment (Supplemental Figure 8A). However, the cholecalciferol-induced increase of plasma calcium was blunted by ZnSO4 (Supplemental Table 2). Plasma calcium phosphate product showed a high scatter, but was not significantly different between control and ZnSO4-treated mice with cholecalciferol overload (Supplemental Table 2).
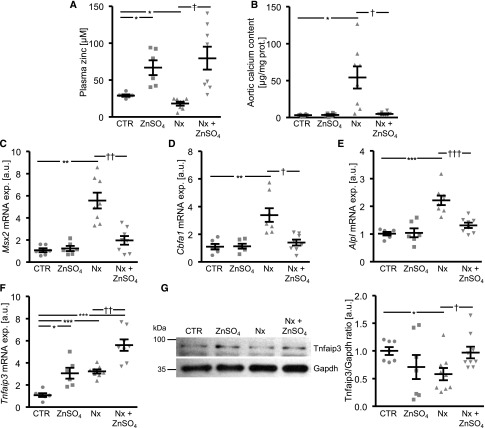
Effects of ZnSO4 Supplementation on VC during Chronic Renal Failure in Mice
Further experiments addressed the anticalcific effects of ZnSO4 after subtotal nephrectomy in phosphate-fed DBA mice. As shown in Figure 7A, plasma zinc levels were significantly lower in mice with subtotal nephrectomy than in control mice. ZnSO4 supplementation increased plasma zinc levels in both groups. The calcification of the aortic arch after subtotal nephrectomy was significantly blunted after ZnSO4 treatment (Figure 7B). Furthermore, ZnSO4 ameliorated the increased aortic mRNA expression of osteogenic markers after subtotal nephrectomy (Figure 7, C–E), effects paralleled by augmentation of aortic Tnfaip3 expression (Figure 7, F and G). The aortic pulse propagation velocity as marker of vascular stiffness was increased after subtotal nephrectomy, an effect blunted by ZnSO4 supplementation (Supplemental Table 3). Subtotal nephrectomy induced an increase of BUN, which further increased during the observational period, an effect abrogated by ZnSO4 treatment (Supplemental Table 3). The DBA mice with subtotal nephrectomy displayed extensive renal calcification, which was blunted by ZnSO4 supplementation (Supplemental Figure 9). Plasma phosphate levels tended to increase with subtotal nephrectomy, but the difference did not reach statistical significance (Supplemental Table 3).
Figure 7.
ZnSO4 supplementation ameliorates VC and osteoinductive signaling in chronic renal failure. (A) Scatterdot plots and arithmetic means±SEM (n=6–8, micromolar) of plasma zinc levels in DBA mice without (CTR) or with subtotal nephrectomy (Nx) and treatment without or with ZnSO4. (B) Scatterdot plots and arithmetic means±SEM (n=6–8, micrograms per milligram protein) of calcium content in the aortic arch of DBA mice without (CTR) or with Nx and treatment without or with ZnSO4. (C–F) Scatterdot plots and arithmetic means±SEM (n=6–8; arbitrary units, a.u.) of Msx2 (C), Cbfa1 (D), Alpl (E), and Tnfaip3 (F), relative mRNA expression in aortic tissue of DBA mice without (CTR) or with Nx and treatment without or with ZnSO4. (G) Representative original Western blots and scatterdot plots and arithmetic means±SEM (n=7–9; a.u.) of normalized Tnfaip3/Gapdh protein ratio in aortic tissue of DBA mice without (CTR) or with Nx and treatment without or with ZnSO4. *P<0.05; **P<0.01; ***P<0.001 statistically significant versus control mice; †P<0.05; ††P<0.01; †††P<0.001 statistically significant versus Nx mice. CTR, control.
Association of Serum Zinc Levels with Serum Calcification Propensity in Patients with CKD
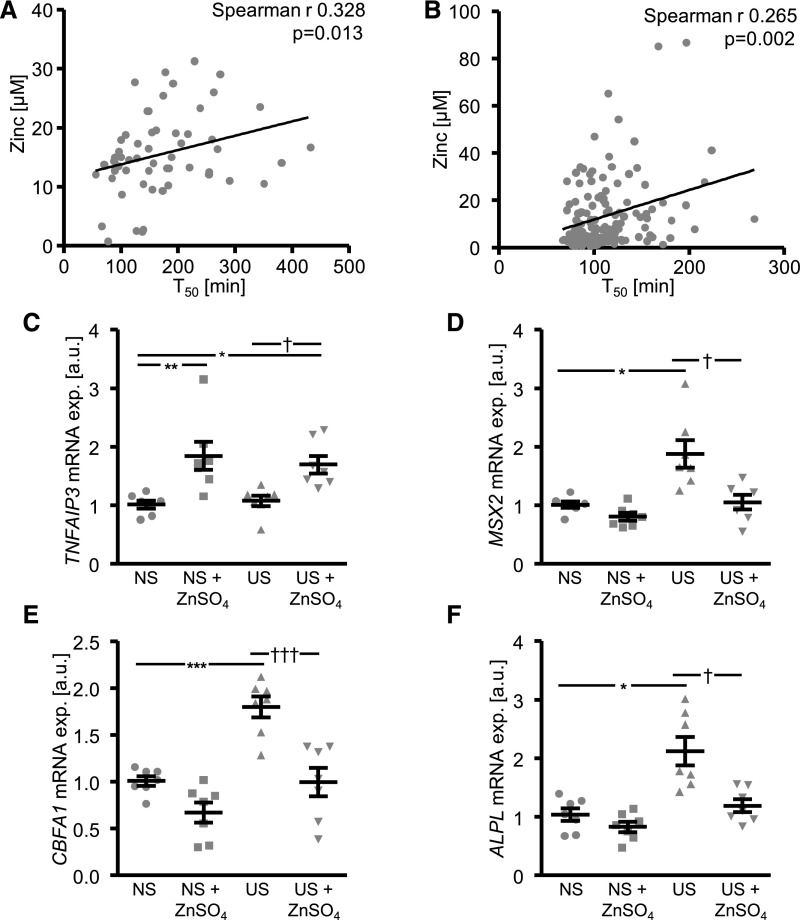
To investigate an association between zinc levels and VC, serum zinc concentrations and serum calcification propensity were determined in patients with known CKD, patients on hemodialysis before dialysis, and healthy volunteers (Supplemental Table 4). Serum zinc levels and half-maximal calciprotein particle maturation time (T50) tended to be lower in patients with CKD compared with controls. However, because of the high scatter in the CKD group, these differences did not reach statistical significance. Serum zinc levels further decreased in patients on dialysis, which also showed further decrease of T50. Addition of exogenous ZnSO4 or ZnCl2 were not able to significantly modify the serum calcification propensity in controls or patients on dialysis (Supplemental Table 5), suggesting that zinc has no ex vivo effects on overall propensity for calcification in serum. Nevertheless, serum zinc levels were significantly correlated with serum calcification propensity in controls and patients with CKD (Figure 8A).
Figure 8.
Serum zinc levels associate with serum calcification propensity in patients with CKD and ZnSO4 supplementation inhibits uremic serum-induced osteoinductive signaling in HAoSMCs. (A) Scatterdot plot of correlation between serum zinc concentrations (micromolar) and serum calcification propensity measured as calciprotein particle maturation time (T50, minute) in human healthy volunteers and patients with CKD (n=57). (B) Scatterdot plot of correlation between serum zinc concentrations (millimolar) and serum calcification propensity measured as calciprotein particle maturation time (T50, minute) in a second cohort of human patients with CKD (n=138). P values are indicated in the figure. (C–F) Scatterdot plots and arithmetic means±SEM (n=7; arbitrary units, a.u.) of TNFAIP3 (C), MSX2 (D), CBFA1 (E), and ALPL (F) relative mRNA expression in HAoSMCs after treatment with normal or uremic serum without or with additional treatment with 15 µM ZnSO4. *P<0.05; **P<0.01; ***P<0.001 statistically significant versus normal serum treated HAoSMCs; †P<0.05; †††P<0.001 statistically significant versus uremic serum treated HAoSMCs. NS, normal serum; US, uremic serum.
To confirm these findings, serum zinc and calcification propensity were determined in another cohort of 138 patients with various stages of renal disease (Supplemental Table 6).31 In this cohort, serum zinc levels were again inversely correlated with serum calcification propensity (Figure 8B).
Effects of ZnSO4 on Osteoinductive Signaling in HAoSMCs during Uremic Conditions
To explore the effects of ZnSO4 during uremic conditions, HAoSMCs were exposed to uremic serum or normal serum collected from patients on hemodialysis before dialysis and from healthy volunteers from the first cohort. As shown in Figure 8C, ZnSO4 significantly upregulated TNFAIP3 mRNA levels in HAoSMCs. Osteogenic markers mRNA expression was significantly higher in HAoSMCs exposed to uremic serum compared with normal serum, and was significantly reduced by ZnSO4 (Figure 8, D–F).
Discussion
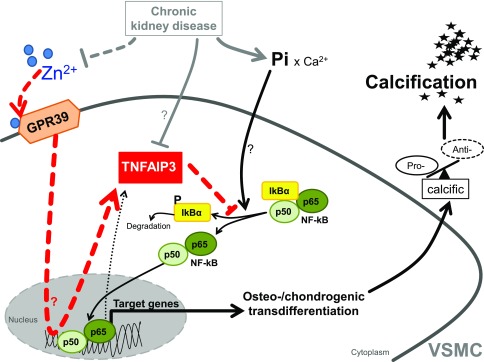
This study shows that zinc supplementation provides powerful protective effects during VC in vitro and in animal models. Low serum zinc levels are correlated with serum calcification propensity in patients with CKD, whereas zinc supplementation ameliorates the osteo-/chondrogenic effects of uremic serum in VSMCs. The inhibitory effect of zinc depends on GPR39 to increase TNFAIP3 expression, which subsequently inhibits NF-κB activation and osteo-/chondrogenic reprogramming in VSMCs during hyperphosphatemia (Figure 9).
Figure 9.
Schematic illustration of phosphate-induced VSMC calcification and its inhibition by zinc supplementation. CKD induces hyperphosphatemia, hypozincemia, and downregulation of vascular Tnfaip3 protein levels by unknown mechanisms. In VSMCs, exposure to elevated phosphate (Pi) levels induces NF-κB (p50/p65) activation. NF-κB is activated via phosphorylation-dependent ubiquitination and degradation of IκBα.50 The active NF-κB transcription factor translocates to the nucleus and induces target gene expression to promote osteo-/chondrogenic transdifferentiation of VSMCs. The osteo-/chondrogenic transdifferentiation of VSMCs induces a procalcific environment causing vascular mineralization. Target genes of NF-κB include also TNFAIP3, presumably as a negative feedback mechanism, an effect that may be insufficient to prevent NF-κB overactivation in CKD. Zinc supplementation increases TNFAIP3 protein levels by upregulation of zinc-sensing receptor ZnR/GPR39-dependent TNFAIP3 gene expression. Increased TNFAIP3 protein levels inhibit NF-κB activation, and therefore suppress the osteo-/chondrogenic transdifferentiation of VSMCs, ameliorating the development of a procalcific environment and subsequent mineralization.
The transcription factor NF-κB has emerged as a key regulator of VSMC osteo-/chondrogenic transdifferentiation.12 NF-κB activation leads to MSX2-dependent ALPL expression,33 upregulation of CBFA1,34 and interference with anticalcific pathways in VSMCs.12,35 Zinc is able to modify NF-κB activity.16 Upregulation of TNFAIP321 expression was essential to mediate the anticalcific effects of zinc. TNFAIP3 is a target gene of NF-κB, which in turn inhibits NF-κB activity through a negative feedback mechanism.36 Accordingly, increased NF-κB activation by calcifying conditions is paralleled by slightly increased TNFAIP3 transcription, which is strongly augmented by zinc supplementation. Interference of TNFAIP3 with NF-κB involves IκBα.37 The increased IκBα phosphorylation and reduced protein levels during calcifying conditions were rescued by zinc supplementation. Zinc-induced TNFAIP3 expression and subsequent inhibition of osteo-/chondrogenic transdifferentiation was dependent on the zinc-sensing receptor ZnR/GPR39.38 Impaired activation of GPR39 during hypozincemia in CKD could therefore lead to unrestrained NF-κB activation and subsequent augmentation of VC.
Plasma zinc levels were reduced in kl/kl27 and subtotal nephrectomy28 mice, but were slightly increased in cholecalciferol-treated mice.26 Zinc levels in respective controls differed between the models, most likely because of the different genetic background of the animals. Nevertheless, plasma zinc levels significantly increased with supplementation, which provided powerful anticalcific effects and upregulated Tnfaip3 expression in all animal models. Contrary to mRNA expression, Tnfaip3 protein expression was reduced in aortic tissue of calcifying mice, whereas TNFAIP3 protein abundance was not modified by phosphate in vitro. Therefore, unknown factors may reduce protein abundance of Tnfaip3 by post-translational regulation during calcifying conditions in vivo. At least in murine brain tissue and myeloid cells, deficiency of TNFAIP3 is sufficient to induce inflammatory processes and NF-κB activation.39,40 The inhibition of NF-κB by upregulation of TNFAIP3 may therefore underlie the protective effects of zinc supplementation in these calcification models. Subtotal nephrectomy mice also displayed a better-maintained renal function after zinc supplementation. Presumably, the zinc-mediated inhibition of calcification in residual renal tissue may underlie the maintained renal function in the subtotal nephrectomy model.
Zinc did not alter the precipitation of calcium phosphate at physiologic concentrations and its anticalcific properties were still intact during in vitro treatment with preformed hydroxyapatite crystals. Addition of zinc to serum of either healthy controls or patients with CKD did not alter serum calcification propensity. These observations suggest that zinc does not directly interfere with calcium phosphate precipitation but acts indirectly via an active cellular mechanism. Nonetheless, an effect of zinc on calcium phosphate precipitation or metabolism in vivo cannot be ruled out. This may be reflected by ameliorated plasma FGF23 levels in the zinc-treated kl/kl mice. In addition, zinc treatment blunted the cholecalciferol-induced increase of plasma calcium without significantly modifying the calcium phosphate product. Clearly, other mechanisms and tissues besides the vasculature may contribute to the protective effects of zinc in vivo. Although ZnCl2 replicated the anticalcific effects of ZnSO4, sulfate may display anticalcific properties by itself.41 Nonetheless, the current observations show a powerful anticalcific effect of zinc supplementation.
Reduced zinc levels are frequently observed in patients with CKD.14 Accordingly, we observed diminished zinc levels in patients with CKD, especially in patients on dialysis, although dialysis may slightly restore zinc levels.42 In two separate cohorts of patients with CKD,32 lower serum zinc levels were significantly associated with an increased serum calcification propensity. Serum calcification propensity is determined by the sum of pro- and anticalcific factors in serum.32 Zinc did not directly modulate serum calcification propensity in a cellfree system, and thus may rather induce cellular mechanisms that subsequently change the balance of pro- and anticalcific factors in serum. Of note, lower values of T50 compared to the reference laboratory were observed.43 We attribute these differences mainly to differences in sample handling procedures and sample processing. Increased serum calcification propensity may be a surrogate for VC and is associated with pulse wave velocity and aortic stiffening.44 The serum calcification propensity is also independently associated with myocardial infarction and mortality in patients on hemodialysis45 and is independent of renal function.46 Therefore, hypozincemia may be associated with an increased risk for calcification in patients with CKD. In fact, addition of zinc blunts the osteogenic effects of uremic serum on VSMC osteo-/chondrogenic transdifferentiation.
These interpretations are however limited, as the findings in patients with CKD are observational and serum calcification propensity may not directly reflect VC. Also, clinical interpretations are limited by differing serum zinc levels in humans and mice. Despite the limitations, our observations suggest a possible beneficial effect of zinc supplementation and prevention of hypozincemia on VC in patients with CKD. Zinc treatment does not seem to carry the risk of major side effects47 and its supplementation may be very cost-effective. Zinc supplementation in patients with CKD has already been safely conducted,48 but correction of hypozincemia may require rather high oral doses of zinc.49 Clearly, further clinical studies are needed to confirm the association of hypozincemia and VC and the potential benefits of zinc supplementation.
In conclusion, the current observations disclose a potentially powerful inhibitory effect of zinc supplementation on VC. The protective effect of zinc is, at least in part, caused by inhibition of NF-κB activity via GPR39-mediated upregulation of TNFAIP3. Correction of hypozincemia may be a simple, yet very effective clinical approach to reduce progression of VC and cardiovascular disease in patients with CKD, and warrants further study.
Disclosures
A.P. is an employee and stockholder of Calciscon AG, which commercializes the calcification propensity test.
Supplementary Material
Acknowledgments
The authors acknowledge the Advanced Medical Bioimaging Core Facility (AMBIO) of the Charité for support in acquisition of the imaging data.
J.V. and I.A. designed the research; J.V., I.A., R.T., T.T.D.L., D.Z., J.M., and M.F. performed the experiments; J.V., N.V., F.B., M.K., A.T., S.P., A.P., K-U.E., J.E.S., F.L., B.P., and I.A. analyzed and interpreted the data; and J.V. and I.A. wrote the manuscript with comments and edits from all authors.
This work was supported by the Berlin Institute of Health (BIH) Translational Postdoc Grant, Deutsche Forschungsgemeinschaft (AL2054/1-1), Else Kröner-Fresenius-Stiftung, DZHK (German Centre for Cardiovascular Research), “Sonnenfeld-foundation,” and the European Union Seventh Framework Programme (FP7/2007-2013–603288-SysVasc).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017050492/-/DCSupplemental.
References
- 1.Paloian NJ, Giachelli CM: A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol 307: F891–F900, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shroff R, Long DA, Shanahan C: Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24: 179–189, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Biyik Z, Selcuk NY, Tonbul HZ, Anil M, Uyar M: Assessment of abdominal aortic calcification at different stages of chronic kidney disease. Int Urol Nephrol 48: 2061–2068, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Moe SM, Chen NX: Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol 19: 213–216, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Collins AJ, Ishani A, Kalra PA: Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 156: 556–563, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Lang F, Ritz E, Voelkl J, Alesutan I: Vascular calcification--is aldosterone a culprit? Nephrol Dial Transplant 28: 1080–1084, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Lang F, Ritz E, Alesutan I, Voelkl J: Impact of aldosterone on osteoinductive signaling and vascular calcification. Nephron, Physiol 128: 40–45, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Alesutan I, Musculus K, Castor T, Alzoubi K, Voelkl J, Lang F: Inhibition of phosphate-induced vascular smooth muscle cell osteo-/chondrogenic signaling and calcification by bafilomycin A1 and methylamine. Kidney Blood Press Res 40: 490–499, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O’Neill WC: Phosphate-induced vascular calcification: Role of pyrophosphate and osteopontin. J Am Soc Nephrol 15: 1392–1401, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Lin ME, Chen TM, Wallingford MC, Nguyen NB, Yamada S, Sawangmake C, et al. : Runx2 deletion in smooth muscle cells inhibits vascular osteochondrogenesis and calcification but not atherosclerotic lesion formation [published online ahead of print Sep 26, 2016]. Cardiovasc Res 10.1093/cvr/cvw205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao G, Xu MJ, Zhao MM, Dai XY, Kong W, Wilson GM, et al. : Activation of nuclear factor-kappa B accelerates vascular calcification by inhibiting ankylosis protein homolog expression. Kidney Int 82: 34–44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan SK: Zinc in kidney disease. J Am Coll Nutr 8: 296–304, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Gilli P, Fagioli F, De Paoli Vitali E, Farinelli A: Is zinc status a problem in the dietary treatment of chronic renal failure? Nephron 40: 382, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Tonelli M, Wiebe N, Hemmelgarn B, Klarenbach S, Field C, Manns B, et al. ; Alberta Kidney Disease Network : Trace elements in hemodialysis patients: A systematic review and meta-analysis. BMC Med 7: 25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster M, Samman S: Zinc and regulation of inflammatory cytokines: Implications for cardiometabolic disease. Nutrients 4: 676–694, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maiguma M, Suzuki Y, Suzuki H, Okazaki K, Aizawa M, Muto M, et al. : Dietary zinc is a key environmental modifier in the progression of IgA nephropathy. PLoS One 9: e90558, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilz S, Dobnig H, Winklhofer-Roob BM, Renner W, Seelhorst U, Wellnitz B, et al. : Low serum zinc concentrations predict mortality in patients referred to coronary angiography. Br J Nutr 101: 1534–1540, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Beattie JH, Gordon MJ, Duthie SJ, McNeil CJ, Horgan GW, Nixon GF, et al. : Suboptimal dietary zinc intake promotes vascular inflammation and atherogenesis in a mouse model of atherosclerosis. Mol Nutr Food Res 56: 1097–1105, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Jenner A, Ren M, Rajendran R, Ning P, Huat BT, Watt F, et al. : Zinc supplementation inhibits lipid peroxidation and the development of atherosclerosis in rabbits fed a high cholesterol diet. Free Radic Biol Med 42: 559–566, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Yan YW, Fan J, Bai SL, Hou WJ, Li X, Tong H: Zinc prevents abdominal aortic aneurysm formation by induction of A20-mediated suppression of NF-κB pathway. PLoS One 11: e0148536, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung SK, Kim MK, Lee YH, Shin DH, Shin MH, Chun BY, et al. : Lower zinc bioavailability may be related to higher risk of subclinical atherosclerosis in Korean adults. PLoS One 8: e80115, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ari E, Kaya Y, Demir H, Asicioglu E, Keskin S: The correlation of serum trace elements and heavy metals with carotid artery atherosclerosis in maintenance hemodialysis patients. Biol Trace Elem Res 144: 351–359, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. : Medial vascular calcification revisited: Review and perspectives. Eur Heart J 35: 1515–1525, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villa-Bellosta R, Millan A, Sorribas V: Role of calcium-phosphate deposition in vascular smooth muscle cell calcification. Am J Physiol Cell Physiol 300: C210–C220, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Alesutan I, Tuffaha R, Auer T, Feger M, Pieske B, Lang F, et al. : Inhibition of osteo/chondrogenic transformation of vascular smooth muscle cells by MgCl2 via calcium-sensing receptor. J Hypertens 35: 523–532: 2017 [DOI] [PubMed] [Google Scholar]
- 27.Voelkl J, Alesutan I, Leibrock CB, Quintanilla-Martinez L, Kuhn V, Feger M, et al. : Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice. J Clin Invest 123: 812–822, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alesutan I, Feger M, Tuffaha R, Castor T, Musculus K, Buehling SS, et al. : Augmentation of phosphate-induced osteo-/chondrogenic transformation of vascular smooth muscle cells by homoarginine. Cardiovasc Res 110: 408–418, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Leibrock CB, Alesutan I, Voelkl J, Pakladok T, Michael D, Schleicher E, et al. : NH4Cl treatment prevents tissue calcification in klotho deficiency. J Am Soc Nephrol 26: 2423–2433, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voelkl J, Alesutan I, Primessnig U, Feger M, Mia S, Jungmann A, et al. : AMP-activated protein kinase α1-sensitive activation of AP-1 in cardiomyocytes. J Mol Cell Cardiol 97: 36–43, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, et al. : Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 95: e3011, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J, et al. : Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 23: 1744–1752, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee HL, Woo KM, Ryoo HM, Baek JH: Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem Biophys Res Commun 391: 1087–1092, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Raaz U, Schellinger IN, Chernogubova E, Warnecke C, Kayama Y, Penov K, et al. : Transcription factor Runx2 promotes aortic fibrosis and stiffness in Type 2 diabetes mellitus. Circ Res 117: 513–524, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheen CR, Kuss P, Narisawa S, Yadav MC, Nigro J, Wang W, et al. : Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res 30: 824–836, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verstrepen L, Verhelst K, van Loo G, Carpentier I, Ley SC, Beyaert R: Expression, biological activities and mechanisms of action of A20 (TNFAIP3). Biochem Pharmacol 80: 2009–2020, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Meng Z, Zhao T, Zhou K, Zhong Q, Wang Y, Xiong X, et al. : A20 ameliorates intracerebral hemorrhage-induced inflammatory injury by regulating TRAF6 polyubiquitination. J Immunol 198: 820–831, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storjohann L, Holst B, Schwartz TW: Molecular mechanism of Zn2+ agonism in the extracellular domain of GPR39. FEBS Lett 582: 2583–2588, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Guedes RP, Csizmadia E, Moll HP, Ma A, Ferran C, da Silva CG: A20 deficiency causes spontaneous neuroinflammation in mice. J Neuroinflammation 11: 122, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, et al. : A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet 43: 908–912, 2011 [DOI] [PubMed] [Google Scholar]
- 41.O’Neill WC, Hardcastle KI: The chemistry of thiosulfate and vascular calcification. Nephrol Dial Transplant 27: 521–526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gómez de Oña C, Martínez-Morillo E, Gago González E, Vidau Argüelles P, Fernández Merayo C, Álvarez Menéndez FV: Variation of trace element concentrations in patients undergoing hemodialysis in the north of Spain. Scand J Clin Lab Invest 76: 492–499, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Keyzer CA, de Borst MH, van den Berg E, Jahnen-Dechent W, Arampatzis S, Farese S, et al. : Calcification propensity and survival among renal transplant recipients. J Am Soc Nephrol 27: 239–248, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith ER, Ford ML, Tomlinson LA, Bodenham E, McMahon LP, Farese S, et al. : Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol 25: 339–348, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasch A, Block GA, Bachtler M, Smith ER, Jahnen-Dechent W, Arampatzis S, et al. : Blood calcification propensity, cardiovascular events, and survival in patients receiving hemodialysis in the EVOLVE trial. Clin J Am Soc Nephrol 12: 315–322, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Seigneux S, Ponte B, Berchtold L, Hadaya K, Martin PY, Pasch A: Living kidney donation does not adversely affect serum calcification propensity and markers of vascular stiffness. Transpl Int 28: 1074–1080, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Pakfetrat M, Shahroodi JR, Zolgadr AA, Larie HA, Nikoo MH, Malekmakan L: Effects of zinc supplement on plasma homocysteine level in end-stage renal disease patients: A double-blind randomized clinical trial. Biol Trace Elem Res 153: 11–15, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Rashidi AA, Salehi M, Piroozmand A, Sagheb MM: Effects of zinc supplementation on serum zinc and C-reactive protein concentrations in hemodialysis patients. J Ren Nutr 19: 475–478, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Tonelli M, Wiebe N, Thompson S, Kinniburgh D, Klarenbach SW, Walsh M, et al. ; Alberta Kidney Disease Network : Trace element supplementation in hemodialysis patients: A randomized controlled trial. BMC Nephrol 16: 52, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang F, Voelkl J: Therapeutic potential of serum and glucocorticoid inducible kinase inhibition. Expert Opin Investig Drugs 22: 701–714, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.