Abstract
Background Nedd4–2 is an E3 ubiquitin-protein ligase that associates with transport proteins, causing their ubiquitylation, and then internalization and degradation. Previous research has suggested a correlation between Nedd4–2 and BP. In this study, we explored the effect of intercalated cell (IC) Nedd4–2 gene ablation on IC transporter abundance and function and on BP.
Methods We generated IC Nedd4–2 knockout mice using Cre-lox technology and produced global pendrin/Nedd4–2 null mice by breeding global Nedd4–2 null (Nedd4–2−/−) mice with global pendrin null (Slc26a4−/−) mice. Mice ate a diet with 1%–4% NaCl; BP was measured by tail cuff and radiotelemetry. We measured transepithelial transport of Cl− and total CO2 and transepithelial voltage in cortical collecting ducts perfused in vitro. Transporter abundance was detected with immunoblots, immunohistochemistry, and immunogold cytochemistry.
Results IC Nedd4–2 gene ablation markedly increased electroneutral Cl−/HCO3− exchange in the cortical collecting duct, although benzamil-, thiazide-, and bafilomycin-sensitive ion flux changed very little. IC Nedd4–2 gene ablation did not increase the abundance of type B IC transporters, such as AE4 (Slc4a9), H+-ATPase, barttin, or the Na+-dependent Cl−/HCO3− exchanger (Slc4a8). However, IC Nedd4–2 gene ablation increased CIC-5 total protein abundance, apical plasma membrane pendrin abundance, and the ratio of pendrin expression on the apical membrane to the cytoplasm. IC Nedd4–2 gene ablation increased BP by approximately 10 mm Hg. Moreover, pendrin gene ablation eliminated the increase in BP observed in global Nedd4–2 knockout mice.
Conclusions IC Nedd4–2 regulates Cl−/HCO3− exchange in ICs., Nedd4–2 gene ablation increases BP in part through its action in these cells.
Keywords: hypertension, chloride, intercalated cells, cortical collecting duct, pendrin
In people and rodent models of salt-sensitive hypertension, BP elevation requires increased intake of Na+ and Cl−.1,2 One commonly used rodent model of human salt-sensitive hypertension is achieved with the administration of aldosterone and a high-NaCl diet. This treatment model produces salt-sensitive hypertension partly by stimulating renal Na+ and Cl− transporters, such as the epithelial Na+ channel (ENaC),3 the thiazide-sensitive NaCl cotransporter,4 and pendrin.5 Aldosterone modulates NaCl absorption, at least in some renal cell types, by changing the number of functional transporters in the cell membrane partly through a mechanism that involves the E3 ubiquitin-protein ligase neuronal precursor cell expressed developmentally downregulated 4–2 (Nedd4–2).6–9 When a transporter or a channel associates with Nedd4–2, it is ubiquitylated, and then, endocytosed and degraded in proteasomes or lysosomes.9,10,11 Conversely, in the absence of Nedd4–2 (i.e., in Nedd4–2 knockout mice), channel internalization and degradation fall, which increases plasma membrane abundance of channels such as ENaC, thereby contributing to the salt-sensitive hypertension observed in global Nedd4–2 null mice.8 As such, increased BP is observed in mice with embryonic, global Nedd4–2 gene ablation8; mice with inducible, kidney-specific Nedd4–2 gene ablation12; and people with certain polymorphisms of NEDD4-L, the human homolog of rodent Nedd4–2.13,14
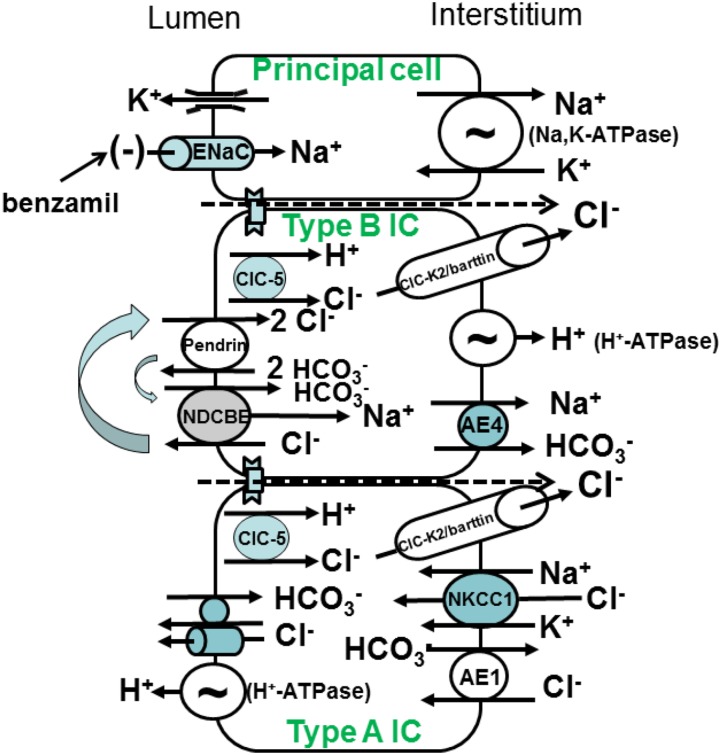
The Na+ and Cl− transporters expressed in principal cells and the various intercalated cell (IC) subtypes are displayed in Figure 1. In the cortical collecting duct (CCD), Na+ is absorbed primarily by principal cells, whereas Cl− is absorbed primarily across ICs,15 largely through electroneutral Cl−/HCO3− exchange across type B ICs.16 Apical anion exchange occurs through apical Na+-independent Cl−/HCO3− exchange mediated principally by pendrin (Slc26a4),17,18 which acts in parallel with the Na+-dependent Cl−/HCO3− exchanger, NDCBE, encoded by Slc4a819. NaCl and net H+ equivalents exit across the type B IC basolateral plasma membrane through a Cl− channel (ClC-K2/barttin or ClC-Kb),20,21 an NaHCO3 cotransporter (AE4),22 and an H+ pump (H+-ATPase)22. This NaCl and H+ exit increases the electrochemical gradient for apical anion exchange, thereby increasing Cl− absorption and HCO3− secretion. In contrast to type B ICs, type A ICs mediate net HCl secretion into the luminal fluid23–26 in series with Cl− uptake and HCO3− exit across the basolateral membrane through Cl−/HCO3− exchange (AE1), Na+-K+-2Cl− cotransporter 1, and a Cl− channel (Figure 1).20,21,26,27
Figure 1.
Ion transporters in mouse cortical collecting duct. Mouse cortical collecting duct is made up of principal cells, which mediate electrogenic Na+ absorption through the benzamil-sensitive epithelial Na+ channel (ENaC) on the apical plasma membrane. Na+ exits principal cells across the basolateral plasma membrane through the Na,K-ATPase. ENaC-mediated Na+ absorption creates a lumen-negative voltage, which provides the driving force for K+ secretion. Type B ICs mediate electroneutral NaCl absorption and HCO3− secretion through an apical plasma membrane Na+-independent electroneutral Cl−/HCO3− exchanger (pendrin) that acts in tandem with an Na+-dependent Cl−/HCO3− exchanger. NaCl exits the cell through a basolateral plasma membrane Cl− channel and an NaHCO3 cotransporter (AE4). Net H+ equivalents exit across the basolateral plasma membrane through the H+-ATPase. The type A IC mediates uptake of H+ equivalents and Cl− across the basolateral plasma membrane through Na+-K+-2Cl− cotransporter 1 (NKCC1), Cl−/HCO3− exchange (AE1), and possibly, a Cl− channel. This cell type secretes HCl through an apical H+-ATPase and an apical Cl− channel or Cl−/HCO3− exchanger.
Nedd4–2 is expressed in the aldosterone-sensitive region of the nephron,10 which includes the connecting tubule (CNT), the CCD, and the OMCD. Mouse CNT is made up of CNT cells and ICs, whereas mouse CCD is composed of principal cells and ICs.28 Nedd4–2 is highly expressed in the CCD and the CNT,10 particularly within type B and non-A, non-B ICs; CNT cells; and principal cells, with much lower abundance in type A ICs.10 Although the role of Nedd4–2 in principal cells has been well studied, little is known about its function in ICs. Our ability to generate mice in which Nedd4–2 gene ablation has occurred specifically within ICs of the CCD plus our ability to perfuse CCDs in vitro from these mice29 provide a unique opportunity by which to explore the physiologic role of IC Nedd4–2 in native tissue.
Aldosterone’s signal transduction mechanism in type B ICs is poorly understood. Because Nedd4–2 participates in aldosterone signaling in many cell types and because Nedd4–2 is expressed in ICs, we sought to determine if Nedd4–2 changes BP by altering IC function. The purpose of this study was to determine if IC Nedd4–2 gene ablation changes CCD ion transport or BP and determine the transporter(s) regulated by Nedd4–2 within ICs.
Methods
Animals
IC Nedd4–2 null mice were generated by breeding floxed Nedd4–2 mice8 with transgenic mice expressing Cre recombinase driven by the ATP6V1B1 promoter (B1-H+-ATPase Cre),30 a subunit of the H+-ATPase that is expressed in renal ICs.30 The Cre was carried through the female line. We compared IC Nedd4–2 null (Nedd4–2loxloxcre) with Cre(−) sex-matched, wild-type littermates (Nedd4–2loxlox). Unless otherwise stated, IC Nedd4–2 knockout and wild-type littermates will refer to Nedd4–2loxloxcre and Nedd4–2loxlox, respectively. Mice were genotyped by quantitative PCR (Transnetyx) and sometimes, standard PCR.8,30
Global Nedd4–2 null mice were generated as described previously8 by breeding floxed Nedd4–2 mice with mice expressing Cre recombinase globally (EIIa-Cre, Stock 003724; Jackson Labs).8 To generate Nedd4–2−/−/Slc26a4−/−; Nedd4–2+/+/Slc26a4−/−, Nedd4–2−/−/Slc26a4+/+, and wild-type littermates on a C57Bl/6 background, we first bred global pendrin null (Slc26a4−/−) on a 129 SvEv Tac background with wild-type mice on a C57Bl/6 background over ten generations. We then bred global Nedd4–2 null (Nedd4–2−/−) and pendrin null mice (Slc26a4−/−), both on a C57Bl/6 background, to generate Nedd4–2−/−/Slc26a4−/−; Nedd4–2+/+/Slc26a4−/−, Nedd4–2−/−/Slc26a4+/+, and wild-type mice, which were all Cre−/−.
Unless otherwise indicated, mice ate a balanced diet (53881300; Zeigler Brothers) prepared as a gel (0.6% agar, 74.6% water, and 24.8% mouse chow) supplemented with NaCl, which provided each mouse approximately 1.4 mEq NaCl per day (approximately 2% NaCl), which they ate for 5–7 days before being euthanized. In BP studies, mice ate a diet with 1% (LabDiet5001) or 4% NaCl (Teklad TD92034) and drank water ad libitum for 7–14 days before study.
Statistics
Results are expressed as the mean±SEM. The n represents the number of mice studied.
All other methods are given in Supplemental Material.
Results
IC Nedd4–2 Is Reduced in B1 H+-ATPase Cre; Nedd4–2loxloxcre Mice
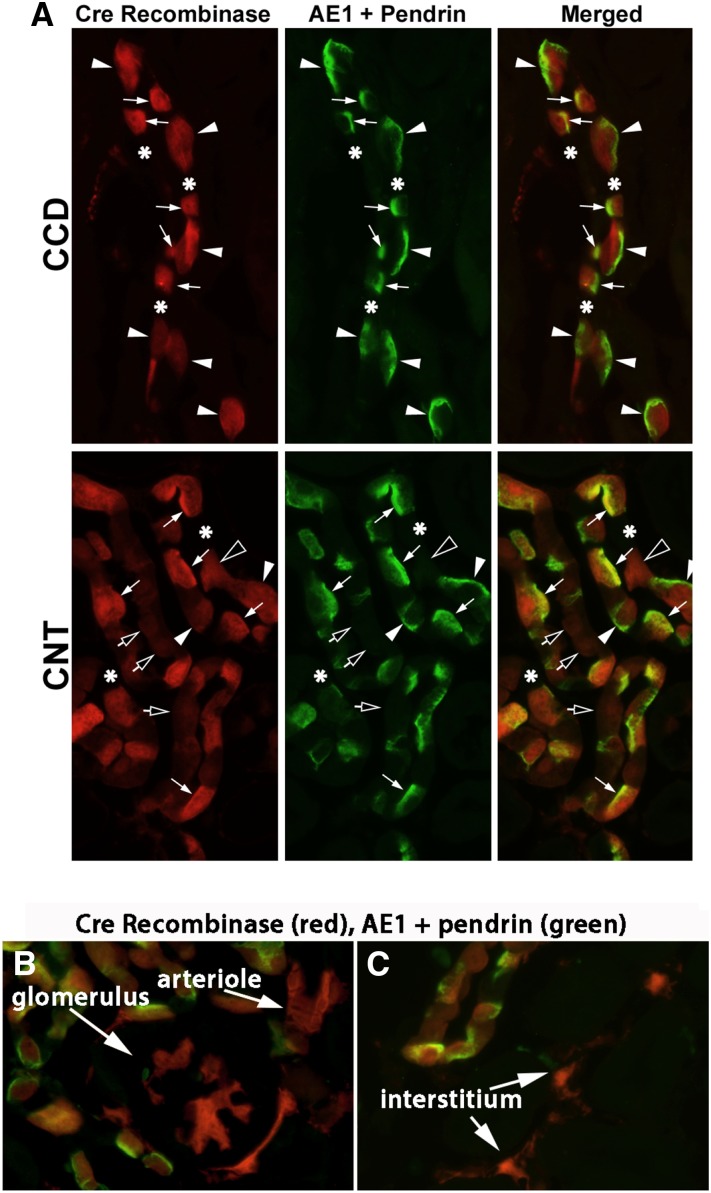
To explore the effect of Nedd4–2 on IC function, we generated IC Nedd4–2 null mice using Cre-lox technology (B1-ATPase Cre; Nedd4–2loxloxcre). To determine if Nedd4–2 knockdown is restricted to ICs, we examined Cre recombinase localization in these mice by breeding them with Cre reporter mice (tdTomato mice) and studying their offspring. In cells expressing Cre recombinase, a stop codon is deleted, resulting in tdTomato expression, which fluoresces red.31 ICs were identified by combined AE1 and pendrin labeling.32 Figure 2A shows dTomato (Cre recombinase) (red) expression in the majority of pendrin/AE1-positive cells (ICs) (Figure 2A, green) of the CCD, with only occasional expression in pendrin/AE1-negative cells (principal cells). In the CNT, dTomato labeling was also observed in the majority of pendrin/AE1-positive cells (ICs), although about 50% of pendrin/AE1-negative cells (CNT cells) had weak dTomato labeling, consistent with previous reports.30,33 dTomato labeling was also observed in occasional glomeruli, occasional blood vessels, and some cells within the interstitium (Figure 2, B and C).
Figure 2.
Cre recombinase is expressed primarily within ICs of the IC Nedd4–2 knockout mice. To determine if Cre recombinase expression is limited to ICs, IC Nedd4–2 null mice were bred to Cre reporter mice (tdTomato). The resulting B1-ATPase Cre (+), dTomato (±) offspring were studied after 7 days of the gelled diet (1.4 mEq/d NaCl). dTomato immunofluorescence (red) reflects Cre recombinase expression in that cell. Panel A shows ICs identified by combined pendrin and AE1 labeling (green) in a representative cortical collecting duct (CCD) and connecting tubule (CNT). Basolateral green immunofluorescence (white arrowheads) indicates basolateral AE1 expression, a marker of type A ICs, whereas apical green fluorescence labeling (white arrows) indicates pendrin expression, a marker of type B and non-A, non-B ICs. (A, upper panel) In the CCD, green fluorescence and red fluorescence are observed in the same cells, indicating that Cre recombinase expression is restricted to ICs. (A, lower panel) In the CNT, rare AE1/pendrin-negative cells (CNT cells) express strong dTomato/Cre recombinase (open arrowheads), although the majority of the CNT cells either have no dTomato fluorescence (asterisks) or have faint dTomato fluorescence (open arrows). We also observed occasional dTomato fluorescence outside the collecting duct and CNT. Although not all cell types could be identified in these images, dTomato was sometimes observed (B) in glomeruli, (B) in blood vessels, and in some cells, (C) within the interstitium.
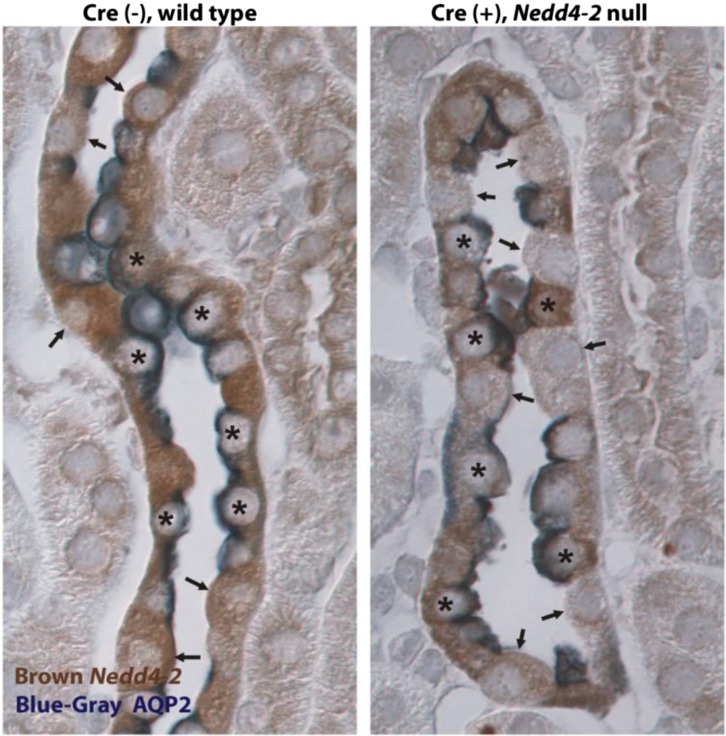
To evaluate the specificity of IC Nedd4–2 gene ablation further, we examined Nedd4–2 labeling (Figure 3, brown) in CCDs taken from IC Nedd4–2 knockout and wild-type littermates. AQP2 (Figure 3, dark blue) labeling identified principal cells. The distribution of Nedd4–2-positive and -negative cells was quantified in CCDs from mice in each group (Table 1). As shown, nearly all AQP2-positive cells (principal cells) labeled for Nedd4–2, regardless of whether they were taken from the IC Nedd4–2 null or their wild-type littermates (Nedd4–2loxlox). Nedd4–2 label was absent in 28% of ICs from wild-type CCDs, which is consistent with previous reports showing weak Nedd4–2 expression in mouse type A ICs.10 However, Nedd4–2 immunolabel was absent in 72% of ICs from IC Nedd4–2 null CCDs. Because these experiments show significant Nedd4–2 knockdown in ICs of CCDs from IC Nedd4–2 null mice with little knockdown in principal cells and because mouse CCD can be perfused in vitro, this study focused primarily on the effect of IC Nedd4–2 gene ablation on ion transport in mouse CCD.
Figure 3.
Nedd4–2 labeling is reduced in ICs taken from cortical collecting ducts of IC Nedd4–2 knockout mice. This slide shows cortical sections from an Nedd4–2 knockout mouse and a wild-type littermate labeled for AQP2 (blue; a principal cell marker) and Nedd4–2 (brown). Asterisks mark AQP2-positive (principal) cells, whereas arrows show AQP2-negative (IC) cells. In cortical sections from wild-type mice, Nedd4–2 label was observed in both ICs and principal cells. However, in the IC Nedd4–2 null mice, whereas Nedd4–2 label was seen in principal cells, Nedd4–2 label was observed in only rare ICs (AQP2-negative cells).
Table 1.
Nedd4–2 immunolabel in the mouse cortical collecting duct cell types taken from Cre (+), IC Nedd4–2 null and Cre (−), wild-type littermates after 7 days of an NaCl-rich diet (1.4 mEq/d NaCl)
| Mouse Model | Principal Cells | ICs | ||
|---|---|---|---|---|
| Nedd4–2 Positive, % | Nedd4–2 Negative, % | Nedd4–2 Positive, % | Nedd4–2 Negative, % | |
| Cre (+), IC Nedd4–2 null, n=4 | 88.4±1.6 | 11.6±1.6 | 28.5±2.5 | 71.5±2.6 |
| Cre (−), wild type, n=3 | 87.6±4.6 | 12.3±4.6 | 74.1±7.6 | 28.0±6.1 |
Each n represents counts from separate mice. IC, intercalated cell.
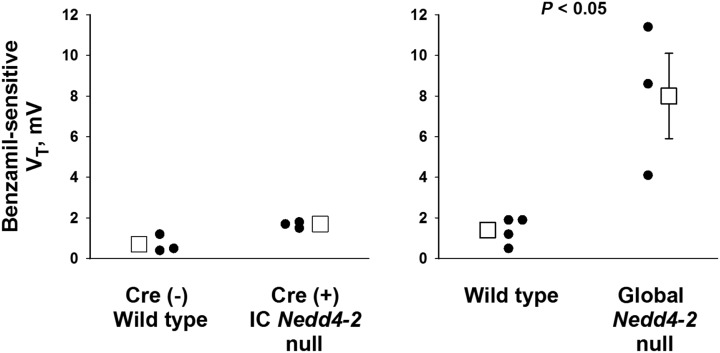
Nedd4–2 gene ablation in principal cells increases apical plasma membrane ENaC abundance, which stimulates Na+ absorption, thereby increasing the lumen-negative transepithelial voltage (Figure 1).8 With increased ENaC-mediated Na+ absorption, a greater fall in the lumen-negative VT is observed with the application of ENaC inhibitors, such as benzamil, which thereby increases benzamil-sensitive transepithelial voltage, VT. To determine if Nedd4–2 gene ablation has occurred within principal cells, we compared ENaC activity in CCDs from global Nedd4–2 null and IC Nedd4–2 null mice and their wild-type controls by measuring benzamil-sensitive VT in CCDs from mice in each group. Figure 4 shows significant benzamil-sensitive VT in CCDs from global Nedd4–2 knockout mice where Nedd4–2 gene ablation has occurred in both ICs and principal cells.8 In contrast, benzamil-sensitive VT was low in the IC Nedd4–2 null CCDs, and it was not significantly different from that measured in their wild-type littermates (Figure 4). These data show little increase in ENaC activity in principal cells of CCDs from IC Nedd4–2 knockout mice, which is consistent with minimal Nedd4–2 knockdown in this cell type.
Figure 4.
Global but not intercalated cell (IC)–specific Nedd4–2 gene ablation increases benzamil-sensitive VT. The benzamil-sensitive component of VT was calculated and used as an indicator of epithelial Na+ channel–mediated Na+ absorption. Benzamil-sensitive VT was low, and it was not significantly different in cortical collecting ducts (CCDs) from IC Nedd4–2 knockout mice (n=3) and wild-type littermates [Cre (−), floxed Nedd4–2 mice; n=3; left panel]. In contrast, benzamil-sensitive VT was much higher in CCDs from global Nedd4–2 null mice (n=3) relative to wild-type C57Bl/6 controls (n=4; right panel).
IC Nedd4–2 Gene Ablation Does Not Change Serum Electrolytes, Aldosterone, or Arterial pH
Because IC transporters are frequently modulated by changes in serum aldosterone concentration, acid-base balance, or serum electrolytes, we examined each of these in IC Nedd4–2 knockout mice and wild-type littermates. Table 2 shows that serum electrolytes and arterial blood gases are similar in both groups of mice. Because serum aldosterone is the same or lower in the IC Nedd4–2 null relative to wild-type littermates, if Nedd4–2 gene ablation increases the abundance or function of an IC transporter, it does not do so through increased circulating aldosterone.
Table 2.
Effect of intercalated cell Nedd4–2 gene ablation on serum electrolytes, arterial blood gases, and serum aldosterone
| Cre (−), Floxed Nedd4–2, Wild Type | Cre (+), IC Nedd4–2 Null | P Value | |
|---|---|---|---|
| Na+, mEq/L | 144±1 (n=4) | 144±1 (n=4) | NS |
| K+, mEq/L | 3.3±0.2 (n=4) | 3.0±0.2 (n=4) | NS |
| Cl−, mEq/L | 115±1 (n=4) | 115±1 (n=4) | NS |
| HCO3−, mEq/L | 20±1 (n=4) | 21±1 (n=4) | NS |
| Aldosterone, nM | 2.3±0.5 (n=12) | 1.6±0.4 (n=14) | NS |
| Arterial pH | 7.5±0.02 (n=4) | 7.47±0.01 (n=4) | NS |
| pCO2 | 25.2±1 | 27.7±1 | NS |
| cHCO3− | 19.4±0.5 | 19.9±0.8 | NS |
Each n represents values from separate mice. Mice consumed the NaCl-rich diet (1.4 mEq/d NaCl) for 7 d before being euthanized. IC, intercalated cell; Na+, sodium; NS, not significant; K+, potassium; Cl−, chloride; pCO2, partial pressure of CO2; cHCO3−, calculated HCO3− concentration.
IC Nedd4–2 Gene Ablation Increases Electroneutral Cl−/HCO3− Exchange in Mouse CCD
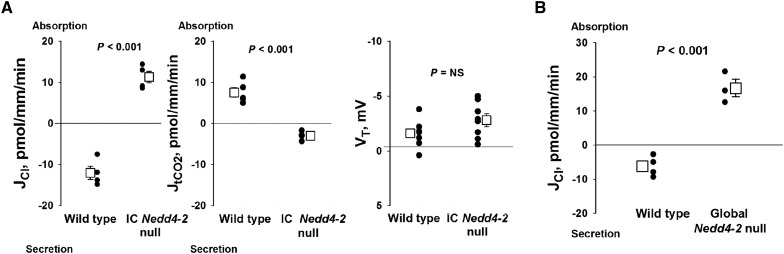
Because ICs mediate Cl− and HCO3− transport, we examined the effect of IC Nedd4–2 gene ablation on transepithelial Cl− and HCO3− transport. Figure 5A shows Cl− secretion and HCO3− absorption in CCDs taken from wild-type mice consuming the high-NaCl diet, similar to our previous observations.23,34 In contrast, CCDs from IC Nedd4–2 null mice absorb, rather than secrete, Cl− and secrete, rather than absorb, HCO3−. Transepithelial voltage was low and was not statistically different in CCDs from IC Nedd4–2 knockout mice and their wild-type littermates (Figure 5A). Therefore, IC Nedd4–2 gene ablation increases electroneutral Cl−/HCO3− exchange in mouse CCD.
Figure 5.
Intercalated cell (IC) Nedd4–2 gene ablation increases electroneutral Cl−/HCO3− exchange. (A) Cl− (JCl) and tCO2 (JtCO2) flux as well as transepithelial voltage, VT, were measured in IC Nedd4–2 knockout mice and the Cre (−), floxed Nedd4–2 littermates (wild-type littermates). As shown, cortical collecting ducts (CCDs) from wild-type littermates secrete Cl− (n=4) and absorb tCO2 (n=5). In contrast, CCDs from IC Nedd4–2 null mice absorb Cl− (n=4) and secrete tCO2 (n=4). VT, however, was unchanged with IC Nedd4–2 gene ablation (n=8 wild-type mice and n=8 IC Nedd4–2 null mice). B shows that Nedd4–2 gene ablation in both principal cells and ICs (n=3) also increased Cl− absorption, JCl, relative to that in wild-type controls (C57Bl/6; n=4). Transepithelial voltage, VT, was −2.2±0.84 mV (n=4) in C57 Bl/6, wild-type mice and −10.3±4.0 mV(n=3) in global Nedd4–2 null mice.
ENaC is an Nedd4–2-regulated channel that provides the driving force for the benzamil-sensitive Cl− absorption in mouse CCD, which may occur through paracellular Cl− transport.23,27 Therefore, we asked if Cl− absorption is higher in CCDs from global Nedd4–2 null mice, where Nedd4–2 gene ablation has occurred in both principal cells and ICs, than in IC Nedd4–2 knockout mice, where Nedd4–2 gene ablation has been restricted to ICs. Figure 5B shows, however, that global Nedd4–2 deletion resulted in changes in Cl− flux that were numerically and directionally similar to those observed in the IC Nedd4–2 null mice. We conclude that the change in CCD Cl− transport that follows global Nedd4–2 gene ablation is predominantly transcellular through ICs.
IC Nedd4–2 Gene Ablation Produces Only a Small Increment in Net H+ Flux, JtCO2, That Is Sensitive to H+-ATPase Inhibitors
Although pendrin mediates the 1:1 exchange of Cl− and HCO3−,35 IC Nedd4–2 gene ablation increased Cl− absorption more than HCO3− secretion. We, therefore, asked if IC Nedd4–2 gene ablation increases apical H+-ATPase abundance and function, thereby attenuating the increment in luminal HCO3− concentration generated with increased apical Cl−/HCO3− exchange. If so, inhibiting H+ secretion by the type A IC should increase HCO3− secretion more in CCDs from the IC Nedd4–2 knockout mice than in those from their wild-type littermates. To address this question, we measured JtCO2 before and after the application of an H+-ATPase inhibitor (bafilomycin) to the luminal fluid. Supplemental Figure 1A shows that apical H+-ATPase blockade produced a small increment in tCO2 secretion in the IC Nedd4–2 knockout mice but not in the wild-type littermates. However, H+-ATPase abundance and subcellular distribution in type A ICs were similar in kidneys from IC Nedd4–2 null mice and wild-type littermates (Supplemental Figure 1B, Supplemental Table 1). We conclude that, although Nedd4–2 gene ablation increases H+ secretion by type A ICs, this change is small and is not accompanied by an increment in H+-ATPase protein abundance in the apical region. The absence of a significant effect of Nedd4–2 gene ablation on H+-ATPase abundance and function in type A ICs is either because Nedd4–2 expression is low in type A ICs or because the apical H+-ATPase is not significantly modulated by Nedd4–2.
IC Nedd4–2 Gene Ablation Produces Little Change in Either Thiazide- or Benzamil-Sensitive Cl− Absorption and Does Not Increase Na+-dependent Cl−/HCO3− exchanger (Slc4a8) Abundance
In the rodent CCD, Cl− absorption occurs through thiazide- and amiloride (benzamil)-sensitive mechanisms.36 The former occurs through electroneutral NaCl absorption that involves the Na+-dependent Cl−/HCO3− exchanger, NDCBE, encoded by Slc4a8,19 whereas the latter occurs through an electrogenic mechanism driven by the lumen-negative voltage generated by ENaC-mediated Na+ absorption.23
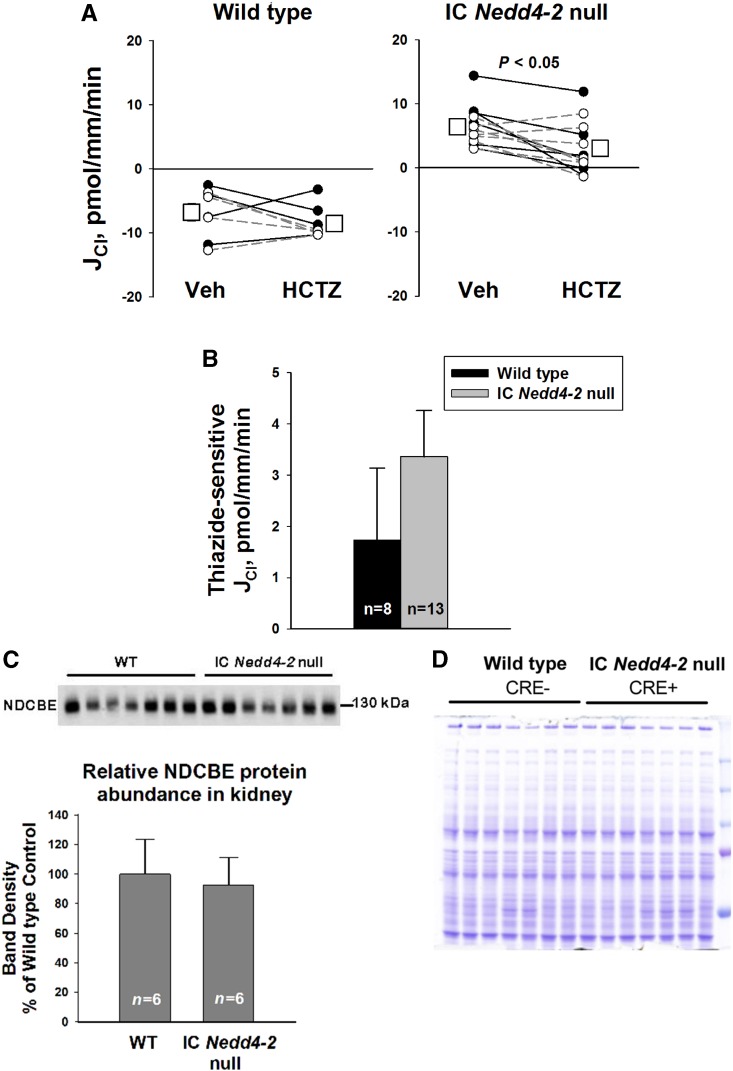
To determine if IC Nedd4–2 gene ablation increases NDCBE-mediated Cl− absorption, we compared thiazide-sensitive Cl− absorption in CCDs from IC Nedd4–2 null mice and wild-type littermates. Figure 6A shows that Cl− absorption fell slightly with the application of hydrochlorothiazide to the perfusate in CCDs from the IC Nedd4–2 null mice but not from wild-type littermates, suggesting that IC Nedd4–2 gene ablation stimulates NDCBE-mediated NaCl absorption. However, although Cl− absorption was approximately 23 pmol/mm per minute higher in CCDs from IC Nedd4–2 knockout mice than wild-type littermates, thiazide-sensitive Cl− absorption rose by only approximately 1.5 pmol/mm per minute (Figure 6B). As such, the increment in thiazide-sensitive Cl− absorption observed with IC Nedd4–2 gene ablation is relatively small.
Figure 6.
Thiazide-sensitive JCl and thiazide-sensitive Na+-dependent Cl−/HCO3− exchanger (NDCBE; Slc4a8) abundance do not increase markedly with intercalated cell (IC) Nedd4–2 gene ablation. (A) Cl− absorption, JCl, was measured in cortical collecting ducts (CCDs) from IC Nedd4–2 null mice (n=13) and wild-type (WT) littermates (n=8) with 100 μM hydrochlorothiazide (HCTZ) or vehicle (Veh) added to the perfusate. Solid lines indicate experiments where Veh was present in the first period and HCTZ was added in the second period. The dashed lines show the reverse order (i.e., HCTZ present in period 1 and then removed in period 2). B compares thiazide-sensitive JCl in CCDs from both groups. C shows NDCBE protein abundance in plasma membrane-enriched preparations from the renal cortex of IC Nedd4–2 null mice and WT littermates. Each lane was loaded with a protein sample from a different mouse; (D) 15 μg proteins were loaded per gel lane, and equal loading was confirmed by parallel Coomassie-stained gels. The anti-NDCBE antibody recognized a band at 130 kD. Densitometric values were normalized to the mean for the Cre (−), WT littermates.
Because IC Nedd4–2 gene ablation produced a small increment in the thiazide-sensitive component of Cl− absorption, further experiments examined the effect of IC Nedd4–2 gene ablation on NDCBE (Slc4a8) total protein abundance in kidney lysates from IC Nedd4–2 null mice and wild-type littermates. Figure 6C shows that NDCBE band intensity was no higher in kidney lysates from IC Nedd4–2 null mice than in those from wild-type mice. We could not examine the effect of IC Nedd4–2 gene ablation on NDCBE subcellular distribution due to the absence of an antibody suitable for immunohistochemistry or immunogold cytochemistry. We conclude that IC Nedd4–2 gene ablation does not produce a marked change in NDCBE abundance or function.
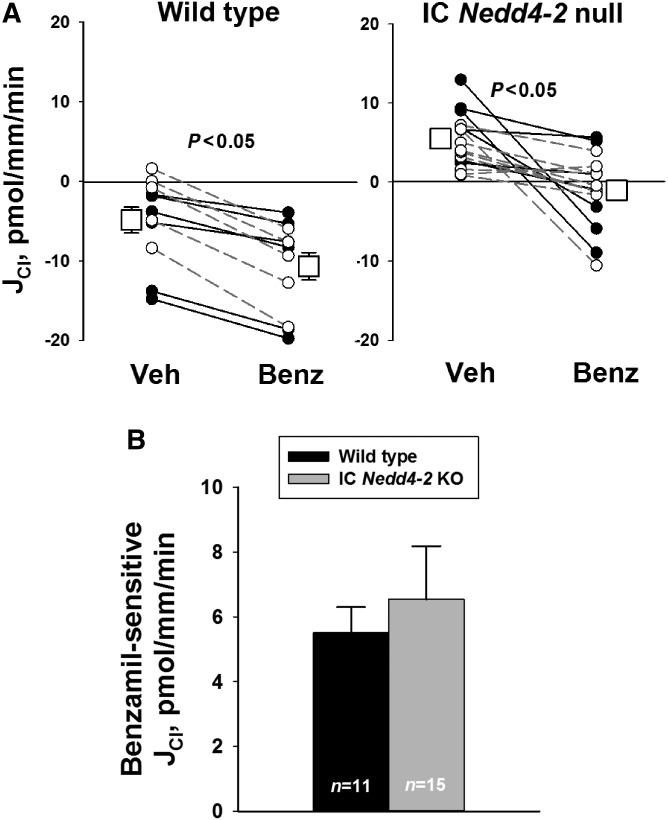
Additional experiments examined the effect of IC Nedd4–2 gene ablation on the benzamil-sensitive component of Cl− absorption, JCl (Figure 7). As shown, benzamil-sensitive JCl was similar in CCDs from Cre (+), IC Nedd4–2 knockout mice and Cre (−), wild-type littermates. We conclude that the benzamil-sensitive component of JCl is unaffected by IC Nedd4–2 gene ablation.
Figure 7.
Intercalated cell (IC) Nedd4–2 gene ablation does not increase the benzamil (Benz)-sensitive component of JCl. (A) Cl− absorption, JCl, was measured in cortical collecting ducts (CCDs) from IC Nedd4–2 null mice (n=15) and wild-type littermates (n=11) with 3 μM Benz or vehicle (Veh) in the perfusate. The solid lines indicate experiments where Veh was present in the first period and Benz was added in the second. The dashed lines show the reverse order (i.e., Benz present in period 1 and then removed in period 2). B compares Benz-sensitive JCl in CCDs from mice in each group. KO, knockout.
Nedd4–2 Gene Ablation Increases ClC-5 Abundance in Type B but Not Type A ICs
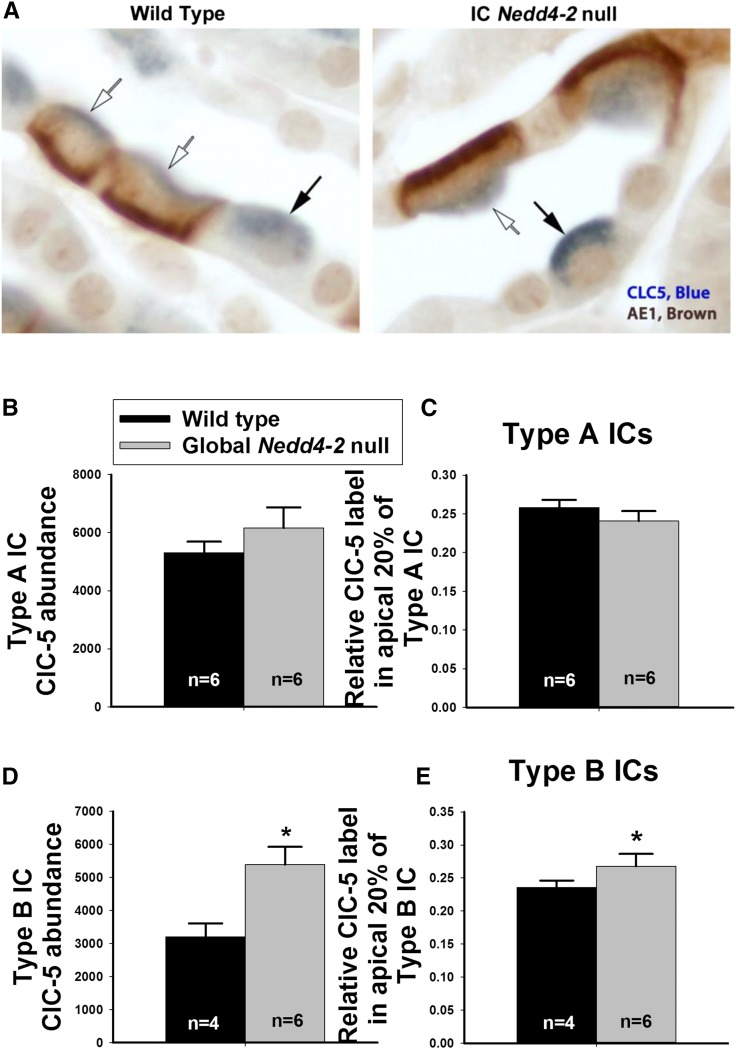
Because ClC-5 gene ablation might modulate Cl− absorption in the CCD of aldosterone-treated mice,23 we used quantitative immunohistochemistry to explore the effect of global Nedd4–2 gene ablation on ClC-5 abundance and subcellular distribution. Total ClC-5 label and ClC-5 label in the most apical 20% of the cell was quantified in type A and type B ICs from CCDs of global Nedd4–2 null mice and wild-type littermates. Figure 8A shows ClC-5 label in AE1 (+) type A and AE1 (−) type B ICs. Figure 8, B and C shows that, in type A ICs, ClC-5 label intensity per cell as well as ClC-5 label intensity in the most apical 20% of the cell were similar in IC Nedd4–2 null mice and wild-type littermates. In type B ICs, however, total label per cell and label in the most apical 20% of the cell were higher in the IC Nedd4–2 null mice (Figure 8, D and E). We conclude that, although Nedd4–2 does not alter ClC-5 abundance or subcellular distribution in type A ICs, Nedd4–2 gene ablation increases ClC-5 total protein abundance and the relative abundance of ClC-5 in the region of the apical plasma membrane of type B ICs.
Figure 8.
Nedd4–2 gene ablation increases total ClC-5 label in type B intercalated cells (ICs) and increases its relative label in the region of the apical plasma membrane. A shows ClC-5 and AE1 labeling in renal cortical sections from a wild-type mouse and a global Nedd4–2 null littermate. In other experiments not shown, cortical sections were labeled for ClC-5 and pendrin. ClC-5 abundance was quantified in both type A (AE1 positive, pendrin negative; white arrows) and type B ICs (AE1 negative, pendrin positive; black arrows). B and C show that Nedd4–2 gene ablation did not increase either total ClC-5 abundance per cell or ClC-5 label in the apical membrane region of type A ICs. (D and E) However, in type B ICs, global Nedd4–2 gene ablation increased both ClC-5 abundance per cell and the relative abundance of ClC-5 in the apical membrane region.
IC Nedd4–2 Gene Ablation Increases Pendrin Abundance in the Apical Plasma Membrane Region
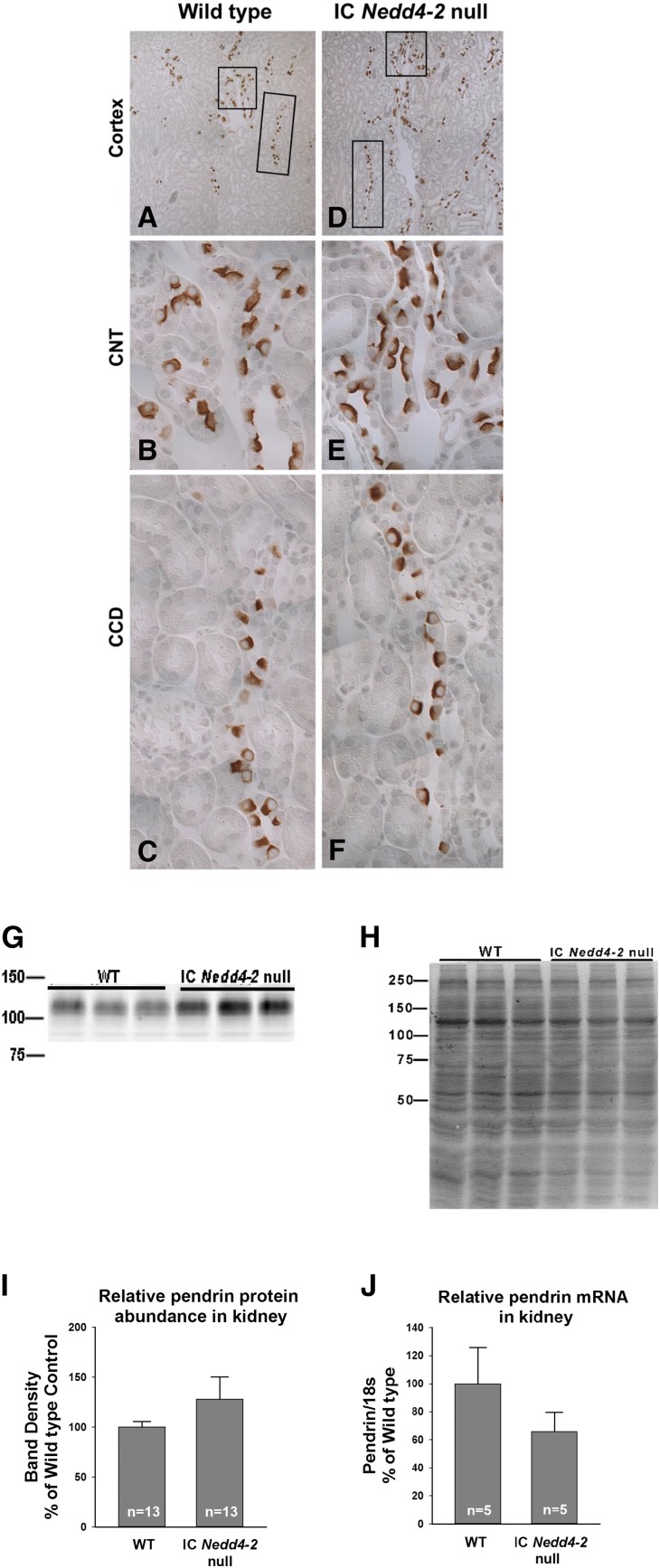
Because IC Nedd4–2 gene ablation increases apical anion exchange and because electroneutral apical anion exchange in the CCD is largely pendrin dependent, further experiments explored the effect of Nedd4–2 gene ablation on total and apical plasma membrane pendrin protein abundance and pendrin subcellular distribution. We observed that pendrin immunolabel is slightly more prominent in kidney sections from the IC Nedd4–2 null mice than in those from wild-type littermates (Figure 9, A–F). Figure 9, G–I shows that ICNedd4–2 gene ablation either produced no change or slightly increased pendrin total protein abundance. In contrast, Figure 9J shows that pendrin mRNA was either unchanged or reduced with IC Nedd4–2 gene ablation.
Figure 9.
Pendrin protein abundance is not significantly changed with intercalated cell (IC) Nedd4–2 gene ablation. Cortical sections from a representative IC Nedd4–2 knockout mouse and a wild-type (WT) littermate were labeled for pendrin. A and D show pendrin labeling at low magnification. Insets show typical (B and E) connecting tubules (CNTs) and typical (C and F) cortical collecting ducts (CCDs) at higher magnification. G shows a representative immunoblot of kidney lysates from IC Nedd4–2 knockout mice and WT littermates probed for pendrin and its respective Coomassie blue gel, which (H) confirms protein loading. I shows that pendrin band density is similar in kidney lysates from IC Nedd4–2 null mice and WT littermates. J shows that kidney pendrin (Slc26a4) mRNA when normalized to 18S mRNA is the same or reduced with IC Nedd4–2 gene ablation.
Further experiments examined the effect of IC Nedd4–2 gene ablation on apical plasma membrane and cytoplasm pendrin abundance. Immunogold cytochemistry with morphometric analysis was used to quantify pendrin total protein abundance and pendrin subcellular distribution in both type B and non-A, non-B ICs of mice from each group. Supplemental Figure 2 shows pendrin gold label in a typical type B IC taken from both an IC Nedd4–2 null mouse and a wild-type littermate. Table 3 shows apical plasma membrane and cytoplasm pendrin gold in both type B and non-A, non-B ICs from IC Nedd4–2 null mice and wild-type littermates. As shown, apical plasma membrane pendrin immunogold per type B IC was the same or slightly higher in IC Nedd4–2 knockout mice relative to their wild-type littermates. However, type B IC apical plasma membrane boundary length was 42% higher and the ratio of apical plasma membrane to cytoplasm pendrin abundance was more than twofold higher in the IC Nedd4–2 null mice relative to their wild-type littermates.
Table 3.
Effect of IC Nedd4–2 gene ablation on apical plasma membrane and cytoplasm pendrin abundance in mouse cortical collecting duct and connecting tubule
| Type B | Non-A, Non-B | |||
|---|---|---|---|---|
| Wild Type | IC Nedd4–2 Knockout | Wild Type | IC Nedd4–2 Knockout | |
| No. of mice studied | 8 | 9 | 4 | 5 |
| Apical plasma membrane gold label, gold particles per cell | 7.84±1.59 | 13.1±3.0 | 26.5±4.8 | 47.6±4.1a |
| Cytoplasmic gold, gold particles in cytoplasm per cell | 71.1±1.28 | 53.6±0.76 | 76.0±19.8 | 76.6±8.9 |
| Total gold | 79.0±13.6 | 66.7±8.8 | 102±22 | 124±11 |
| Ratio of apical plasma membrane to cytoplasm pendrin label, ×10−1 | 1.24±0.29 | 2.63±0.51a | 4.6±2.0 | 6.4±0.7 |
| Apical plasma membrane boundary length, millimeters ×10−2 | 0.72±0.07 | 1.02±0.11a | 3.04±0.49 | 4.49±0.36a |
| Apical plasma membrane label density, gold particles per 1 mm apical plasma membrane boundary length ×103 | 1.27±0.39 | 1.19±0.18 | 1.02±0.09 | 1.17±0.15 |
| Cell area, millimeters squared ×10−5 | 4.39±0.20 | 4.65±0.33 | 4.86±0.43 | 4.85±0.55 |
| Cytoplasmic label density, gold particles ×106 per 1 mm2 cytoplasmic area | 1.69±0.36 | 1.19±1.71 | 1.59±0.32 | 1.66±0.19 |
Values were determined using immunogold cytochemistry with morphometric analysis and represent the means±SEM. Values were compared with an unpaired, two-tailed t test. Mice consumed the NaCl-rich diet (1.4 mEq/d NaCl) for 7 d before being euthanized. IC, intercalated cell; Nedd4–2, neuronal precursor cell expressed developmentally downregulated 4–2.
P<0.05.
In non-A, non-B ICs, the predominant pendrin-positive cell type in the CNT, IC Nedd4–2 gene ablation increased apical plasma membrane boundary length 48% and increased apical plasma membrane pendrin (gold) label per cell by 80% (Table 3). However, differences in the ratio of pendrin (gold) label on the apical plasma membrane relative to subapical vesicles in this cell type did not reach statistical significance. These data show that IC Nedd4–2 gene ablation increases apical plasma membrane pendrin abundance in the non-A, non-B IC.
IC Nedd4–2 Gene Ablation Does Not Increase AE4, H+-ATPase, or Barttin Abundance
Nedd4–2 gene ablation may increase apical Cl−/HCO3− exchange by interacting with a basolateral plasma membrane transporter in type B ICs (Figure 1), which increases the driving force for apical anion exchange by enhancing Na+, Cl−, and H+ exit. To test this hypothesis, we examined the effect of IC Nedd4–2 gene ablation on the abundance and subcellular distribution of the type B IC transporters that localize to the basolateral membrane and mediate this Na+, Cl−, or H+ exit (Figure 1). As shown (Supplemental Figures 3 and 4), IC Nedd4–2 gene ablation did not increase AE4 or barttin total immunolabel intensity or label intensity in the region of the plasma membrane. Moreover, using immunogold cytochemistry, we observed that basolateral plasma membrane barttin gold label was not increased in type B ICs from IC Nedd4–2 null mice relative to wild-type littermates (not shown).
Because the basolateral H+-ATPase provides the driving force for apical anion exchange,22 we examined total α4–H+-ATPase immunolabel and α4–H+-ATPase subcellular distribution in type B ICs from IC Nedd4–2 null mice and wild-type littermates (Supplemental Figure 1, Supplemental Table 1). No difference in type B IC H+-ATPase abundance or subcellular distribution was detected by quantitative analysis of the H+-ATPase α4-subunit immunolabel in mice from these two groups.
These data show that while AE4, H+-ATPase, and ClC-K2/barttin all modulate apical anion exchange in mouse CCD, they do not likely do so through an interaction with Nedd4–2.
IC Nedd4–2 Modulates BP
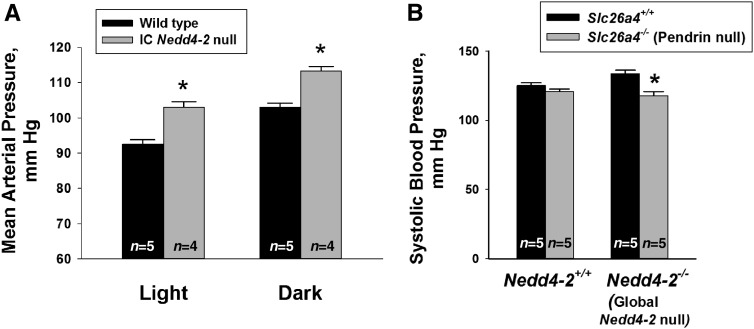
To explore the role of Nedd4–2 within ICs on BP regulation, we examined the effect of IC Nedd4–2 gene ablation on BP. By tail cuff, we observed systolic BP to be similar in IC Nedd4–2 knockout mice and wild-type littermates following a standard rodent diet (1% NaCl) (Supplemental Figure 5). However, after 14 days of a 4% NaCl diet, which increases Nedd4–2 expression in wild-type mice,10 systolic BP was higher in the IC Nedd4–2 knockout mice than in the wild-type littermates. To confirm these findings, 24-hour BP recordings were made using radiotelemetry after mice were given 7 days of a high-NaCl (4% NaCl) diet. Over a 24-hour period, mean arterial pressure was 107±1.1 mm Hg (n=4) in the IC Nedd4–2 null mice and 97±0.9 mm Hg (n=5) in the wild-type littermates (P<0.05). Therefore, BP was approximately 10 mm Hg higher in the IC Nedd4–2 knockout mice than in wild-type littermates. Moreover, during both awake (dark) and asleep (light) periods, BP was higher in the IC Nedd4–2 null mice than the wild-type mice (Figure 10A).
Figure 10.
Intercalated cells (ICs) contribute to the increment in BP observed with 2 Nedd4–2 gene ablation. A shows mean arterial pressure (MAP) measured by radiotelemetry after 7 days of a 4% NaCl diet. As shown, MAP was higher in the IC Nedd4–2 null mice (n=4) relative to wild-type littermates (n=5) during both awake (dark period; 6 pm to 6 am) and asleep (light period; 6 am to 6 pm) periods. B shows systolic BP measured by tail cuff in wild-type (Slc26a4+/+/Nedd4–2+/+) mice, pendrin null (Slc26a4−/−/Nedd4–2+/+) mice, Nedd4–2 null (Slc26a4+/+/Nedd4–2−/−) mice, and mice that were both pendrin and Nedd4–2 null (Slc26a4−/−/Nedd4–2−/−) after consuming a 4% NaCl diet ad libitum for 6 days. Measurements were made in five mice from each group. *P<0.05.
We observed some Nedd4–2 knockdown in cells from IC Nedd4–2 null kidneys that are not ICs (Figure 2). As such, we cannot exclude the possibility that off-target Nedd4–2 gene ablation contributes to the increment in BP observed in the IC Nedd4–2 null mice. Because of this, we used an additional approach to examine the effect of IC Nedd4–2 gene ablation on BP. Pendrin gene ablation not only eliminates pendrin-dependent Cl−/HCO3− exchange but also, downregulates other type B IC ion transporters that augment the driving force for apical anion exchange, such as the H+-ATPase.22,37 We hypothesized that, if global Nedd4–2 gene ablation increases BP in part by upregulating IC apical anion exchange, then eliminating apical Cl−/HCO3− exchange with pendrin gene ablation should reduce BP more in the global Nedd4–2 null mice than in mice harboring wild-type Nedd4–2. To test this hypothesis, we compared BP measured by tail cuff in Nedd4–2−/−/Slc26a4−/−; Nedd4–2+/+/Slc26a4−/−, Nedd4–2−/−/Slc26a4+/+, and wild-type mice after 7 days of a 4% NaCl diet (Figure 10B). We observed that BP rose with global Nedd4–2 gene ablation in mice harboring wild-type pendrin (Slc26a4 +/+) as reported previously.8 However, whereas pendrin gene ablation reduced systolic BP in the global Nedd4–2 null mice, it had no detectable effect on BP in mice harboring wild-type Nedd4–2. We conclude that the hypertension observed in global Nedd4–2 null mice occurs, in part, through a mechanism that depends on ICs.
Discussion
Although a human counterpart to the global Nedd4–2 knockout mice has not been observed, a number of human NEDD4–2 single-nucleotide polymorphisms highly correlate with changes in BP.13 Because Nedd4–2 is expressed in type B ICs and because IC function is highly regulated by aldosterone,5 we examined the effect of IC Nedd4–2 gene ablation on IC function and how IC Nedd4–2-affects BP. We observed that IC Nedd4–2 gene ablation in mice increases apical Cl−/HCO3− exchange in the CCD and that the increment in BP observed with global Nedd4–2 gene ablation is, in part, dependent on ICs.
Previous studies showed that the ENaC inhibitor, amiloride, eliminates the increment in BP observed with global Nedd4–2 gene ablation.8 These data might seem at odds with our observation that pendrin gene ablation also eliminates the increment in BP observed in these global Nedd4–2 knockout mice. It is possible that ENaC inhibition mediates the fall in BP seen in both models. Nedd4–2 gene ablation stimulates both apical Cl−/HCO3− exchange in ICs and ENaC-mediated Na+ absorption in principal cells,8 which increase renal NaCl absorption. Conversely, amiloride-induced ENaC blockade eliminates ENaC-mediated Na+ absorption in principal cells, while stimulating HCl secretion by type A ICs.23,24 Amiloride, therefore, reduces NaCl absorption and BP. Since pendrin gene ablation reduces ENaC-mediated Na+ absorption38, pendrin gene ablation reduces BP,39 in part, through ENaC inhibition.
The global Nedd4–2 knockout mice that we studied have a phenotype that seems limited to hypertension.8 However, perinatal lethality is observed in other global Nedd4–2 null mice41 that were developed using a floxed Nedd4–2 mouse, in which lox P sites were introduced into a sequence flanking exon 15, thereby inducing a frame shift downstream of exon 15.41 We chose not to use the latter floxed Nedd4–2 mice due to the difficulties that arise when studying mice with high perinatal mortality.41,42 Nevertheless, these data raise the possibility that the global and/or IC Nedd4–2 null mice that we used might only have a partial loss of Nedd4–2 function (i.e., hypomorphs) due to alternate splicing.6,8,12,41,42 If so, the Nedd4–2-dependent changes in ion transport that we observed would underestimate the true effect of Nedd4–2 on ion transport.
Nedd4–2 associates with the β- or γ-subunits of ENaC43 in a region of the subunit’s C terminus having a conserved sequence, known as the PY motif.11,44 Classic PY motifs, such as those observed in ENaC β- or γ-subunits, have a C-terminal PPPXYXXL sequence, where P is proline, Y is tyrosine, L is leucine, and X is any amino acid.11,44–46 ClCK-2/barttin is a Cl− channel that harbors a PPPXYXXL PY motif on the C terminus of the barttin subunit.47 This channel is expressed on the basolateral plasma membrane of both type A and type B ICs of the mouse CCD, and it is critical to the Cl− absorption observed in this segment.20,21 Specifically, the channel’s barttin subunit is required for plasma membrane channel expression and therefore, channel-mediated Cl− transport,48 which might occur through an Nedd4–2 association.47,48 When the barttin PY motif is mutated and then expressed in heterologous expression systems, increased ClCK-2/barttin–mediated Cl− channel activity is observed, possibly from the fall in channel ubiquitinylation, endocytosis, and degradation that might occur in the absence of a barttin-Nedd4–2 association.47,49 This study showed, however, that, in native ICs, Nedd4–2 gene ablation does not increase either total or plasma membrane barttin abundance. As such, the interaction of Nedd4–2 and barttin in vivo and the physiologic significance of this association remain to be determined.
ClC-5 is a Cl−/H+ exchanger expressed in the apical regions of ICs.50–52 Like barttin and ENaC, ClC-5 also harbors a PY motif with a PPLPPY sequence at its C terminus.53 When this PY motif is mutated and then expressed in Xenopus oocytes, ClC-5–mediated current and surface expression increase,53 presumably due to the absence of an interaction with Nedd4–2.54 ClC-5 associates with Nedd4–2 in heterologous expression systems through this PY motif, which reduces ClC-5–mediated current.54 However, after PY motif ablation in the mouse proximal tubule in vivo, no change in ClC-5 subcellular distribution is observed.55 As such, whether this ClC-5–Nedd4–2 association regulates ClC-5 abundance, subcellular distribution, or channel activity in vivo has been unclear. This study showed that ClC-5 total protein abundance increases with Nedd4–2 gene ablation in type B but not type A ICs. These data are consistent with previous studies showing greater Nedd4–2 expression observed in the former than in the latter cell type.10 However, the physiologic significance of IC ClC-5 remains to be determined. Although mean Cl− absorption was approximately 25% lower in CCDs from aldosterone-treated ClC-5 null mice than wild-type littermates, differences did not reach statistical significance.23 As such, whether ClC-5 contributes to the change in Cl− flux observed with Nedd4–2 gene ablation and whether it is expressed on the IC apical plasma membrane remain to be determined.23
These ClCK-2/barttin and ClC-5 studies show that the interaction of Nedd4–2 with its target proteins can differ in heterologous expression systems and native tissue. Cultured cells may lack accessory proteins that are important in protein complexes that occur in native tissue. Moreover, protein overexpression56 and fluorescent protein tags57 can lead to nonspecific, off-target effects. As such, occasional associations observed in heterologous expression systems cannot be confirmed in native tissue,55 leaving in question the physiologic significance of the initial observations. Given these limitations, we chose to first examine the physiologic role of Nedd4–2 in IC function by exploring the effect of Nedd4–2 gene ablation on transporter function in native CCDs and then using these changes in ion transport to predict specific protein targets. We then tested this hypothesis by examining the effect of IC Nedd4–2 gene ablation on IC transporter abundance and subcellular distribution in native mouse tissue.
This study shows that the increase in apical Cl−/HCO3− exchange observed with Nedd4–2 gene ablation occurs, at least in part, from increased apical plasma membrane pendrin abundance. This increment in apical plasma membrane pendrin abundance occurs primarily through changes in pendrin subcellular distribution rather than through increased pendrin total protein abundance. As such, Nedd4–2 may represent a step in the aldosterone signaling cascade by which pendrin protein abundance, subcellular distribution, and function are regulated.
Although the pendrin does not have a PY motif with a PPXY sequence, it has two PY motifs with LPXY sequences, which might provide recognition motifs for Nedd4–2 WW domains. One of these, LPKYR, corresponds to amino acids 75–79 of the mouse, rat, and human pendrin sequence and amino acids 71–75 in Xenopus, providing a potential Nedd4–2 interaction site.58 However, some proteins, such as the thiazide-sensitive NaCl cotransporter associate with Nedd4–2 independent of a PY motif.6 As such, an IC transporter, such as pendrin, might associate with Nedd4–2 through or independent of a C-terminal PY motif.
Nedd4–2 gene may target another type B IC Cl− transporter not tested in this study. For example, in addition to the type B IC transporters shown in Figure 1, Slc26a11 is expressed in ICs and acts as either a Cl−/HCO3− exchanger or a Cl− channel.59 Whether Slc26a11 modulates transepithelial ion transport, whether it is expressed on the plasma membrane, and whether it is modulated by Nedd4–2 remain to be determined. Alternatively, Nedd4–2 might associate with a receptor or a signaling molecule in type B ICs that changes apical anion exchange.
IC Nedd4–2 gene ablation led to a robust increase in Cl− absorption without a statistically significant change in transepithelial voltage. However, we cannot exclude the possibility that IC Nedd4–2 gene ablation produced a small 1- to 2-mV increase in the lumen-negative transepithelial voltage. Because the CCD transports Cl− through paracellular and transepithelial transport and because Nedd4–2 enhances paracellular conductance in collecting duct cells by associating with occludin,60 IC Nedd4–2 gene ablation might alter Cl− flux through changes in paracellular transport. However, for a change in transepithelial voltage as low as −2 mV to drive significant Cl− absorption through paracellular transport, there must be a large fall in tubule resistance. This is unlikely, because Nedd4–2 overexpression in cultured CCD cells does not change resistance at steady state.60 Moreover, if IC Nedd4–2 gene ablation stimulates occludin-mediated Cl− absorption, it should also stimulate occludin in the global Nedd4–2 null mice. Because the lumen-negative voltage is higher in CCDs from the global relative to the IC Nedd4–2 null mice, we should see a much greater increment in Cl− absorption in CCDs from the global relative to the IC Nedd4–2 null mice. Instead, we observed a similar change in Cl− flux in CCDs from global and IC Nedd4–2 null mice relative to their wild-type controls. We conclude that IC Nedd4–2 gene ablation changes Cl− flux in the mouse CCD largely through transcellular transport.
Future experiments will explore whether Nedd4–2 and pendrin associate and if this association changes apical plasma membrane pendrin ubiquitylation.11,43,61 Moreover, because Nedd4–2 exists as many isoforms due to alternative promoter usage and variable splicing,62 which isoforms mediate the Nedd4–2-dependent changes in IC ion transport also remain to be determined.
In conclusion, IC Nedd4–2 gene ablation increases electroneutral Cl−/HCO3− exchange in the mouse CCD partly from pendrin subcellular redistribution, which increases its apical plasma membrane abundance. The increment in BP observed previously in the complete absence of Nedd4–2 occurs in part through an IC-dependent mechanism.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Raoul Nelson, Dr. R. Lance Miller, Dr. John Stokes, and Dr. I. David Weiner for providing the B1-H+-ATPase Cre and the Nedd4–2 mice. We thank Dr. Greg L. Shipley (Shipley Consulting LLC, Austin, TX) for his assistance with primer design. We also thank Dr. Thomas Jentsch, Dr. Christian Hubner, and Dr. Fiona Karet for providing the barttin, AE4, and α4–H+-ATPase antibodies.K.I.L.-C. received a fellowship from the Comisión Nacional de Investigación Científica y Tecnológica. R.C. is funded by grant ANR BLANC 2012-R13011KK from l’Agence Nationale de la Recherche. This study was supported by grants DK 104125 (to S.M.W.) and AHA 15GRNT25710001 (to S.M.W.).
All authors agree to be accountable for the work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017080826/-/DCSupplemental.
References
- 1.Kurtz TW, Al-Bander HA, Morris RC Jr: “Salt-sensitive” essential hypertension in men. Is the sodium ion alone important? N Engl J Med 317: 1043–1048, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Kurtz TW, Morris RC Jr: Dietary chloride as a determinant of “sodium-dependent” hypertension. Science 222: 1139–1141, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Masilamani S, Kim G-H, Mitchell C, Wade JB, Knepper MA: Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim G-H, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA: The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 95: 14552–14557, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, et al.: Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: Role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Arroyo JP, Lagnaz D, Ronzaud C, Vázquez N, Ko BS, Moddes L, et al.: Nedd4-2 modulates renal Na+-Cl- cotransporter via the aldosterone-SGK1-Nedd4-2 pathway. J Am Soc Nephrol 22: 1707–1719, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, et al.: Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. EMBO J 20: 7052–7059, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, et al.: Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4-2. Am J Physiol Renal Physiol 295: F462–F470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores SY, Loffing-Cueni D, Kamynina E, Daidié D, Gerbex C, Chabanel S, et al.: Aldosterone-induced serum and glucocorticoid-induced kinase 1 expression is accompanied by Nedd4-2 phosphorylation and increased Na+ transport in cortical collecting duct cells. J Am Soc Nephrol 16: 2279–2287, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Loffing-Cueni D, Flores SY, Sauter D, Daidié D, Siegrist N, Meneton P, et al.: Dietary sodium intake regulates the ubiquitin-protein ligase nedd4-2 in the renal collecting system. J Am Soc Nephrol 17: 1264–1274, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Rotin D, Staub O: Role of the ubiquitin system in regulating ion transport. Pflugers Arch 461: 1–21, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, et al.: Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123: 657–665, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin H-S, Hong KW, Lim J-E, Hwang S-Y, Lee S-H, Shin C, et al.: Genetic variations in the sodium balance-regulating genes ENaC, NEDD4L, NDFIP2 and USP2 influence blood pressure and hypertension. Kidney Blood Press Res 33: 15–23, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Wen H, Lin R, Jiao Y, Wang F, Wang S, Lu D, et al.: Two polymorphisms in NEDD4L gene and essential hypertension in Chinese Hans - a population-based case-control study. Clin Exp Hypertens 30: 87–94, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Schlatter E, Greger R, Schafer JA: Principal cells of cortical collecting ducts of the rat are not a route of transepithelial Cl- transport. Pflugers Arch 417: 317–323, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Star RA, Burg MB, Knepper MA: Bicarbonate secretion and chloride absorption by rabbit cortical collecting ducts. Role of chloride/bicarbonate exchange. J Clin Invest 76: 1123–1130, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, et al.: Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, et al.: NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: Role in Cl- conservation. Hypertension 44: 982–987, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, et al.: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennings JC, Andrini O, Picard N, Paulais M, Huebner AK, Cayuqueo IK, et al.: The ClC-K2 chloride channel is critical for salt handling in the distal nephron. J Am Soc Nephrol 28: 209–217, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinelli L, Nissant A, Edwards A, Lourdel S, Teulon J, Paulais M: Dual regulation of the native ClC-K2 chloride channel in the distal nephron by voltage and pH. J Gen Physiol 148: 213–226, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambrey R, Kurth I, Peti-Peterdi J, Houillier P, Purkerson JM, Leviel F, et al.: Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A 110: 7928–7933, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanami M, Lazo-Fernandez Y, Pech V, Verlander JW, Agazatian D, Weinstein AM, et al.: ENaC inhibition stimulates HCl secretion in the mouse cortical collecting duct. I. Stilbene-sensitive Cl- secretion. Am J Physiol Renal Physiol 309: F251–F258, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nanami M, Pech V, Lazo-Fernandez Y, Weinstein AM, Wall SM: ENaC inhibition stimulates HCl secretion in the mouse cortical collecting duct. II. Bafilomycin-sensitive H+ secretion. Am J Physiol Renal Physiol 309: F259–F268, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wall SM, Fischer MP: Contribution of the Na(+)-K(+)-2Cl(-) cotransporter (NKCC1) to transepithelial transport of H(+), NH(4)(+), K(+), and Na(+) in rat outer medullary collecting duct. J Am Soc Nephrol 13: 827–835, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ: Contribution of the Na+-K+-2Cl- cotransporter (NKCC1) to Cl- secretion in rat outer medullary collecting duct. Am J Physiol 280: F913–F921, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Pech V, Thumova M, Kim Y-H, Agazatian D, Hummler E, Rossier BC, et al.: ENaC inhibition stimulates Cl- secretion in the mouse cortical collecting duct through an NKCC1-dependent mechanism. Am J Physiol Renal Physiol 303: F45–F55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wall SM, Hassell KA, Royaux IE, Green ED, Chang JY, Shipley GL, et al.: Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol 284: F229–F241, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Wall SM, Weinstein AM: Cortical distal nephron Cl(-) transport in volume homeostasis and blood pressure regulation. Am J Physiol Renal Physiol 305: F427–F438, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, et al.: The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Brown D, Hirsch S, Gluck S: Localization of a proton-pumping ATPase in rat kidney. J Clin Invest 82: 2114–2126, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, et al.: V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol 288: C1134–C1144, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Pech V, Kim Y-H, Weinstein AM, Everett LA, Pham TD, Wall SM: Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, et al. : Slc26a4 functions as an electroneutral Cl-/I-/HCO3- exchanger: Role of Slc26a4 and Slc26a6 in I- and HCO3- secretion and in regulation of CFTR in the parotid duct. J Physiol 586: 3814–3824, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terada Y, Knepper MA: Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol 259: F519–F528, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Kim Y-H, Verlander JW, Matthews SW, Kurtz I, Shin W, Weiner ID, et al.: Intercalated cell H+/OH- transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol 289: F1262–F1272, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Pech V, Wall SM, Nanami M, Bao HF, Kim YH, Lazo-Fernandez Y, et al.: Pendrin gene ablation alters ENaC subcellular distribution and open probability. Am J Physiol Renal Physiol 309: F154–F163, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Y-H, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, et al.: Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol Renal Physiol 293: F1314–F1324, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Verlander JW, Kim YH, Shin W, Pham TD, Hassell KA, Beierwaltes WH, et al.: Dietary Cl(-) restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol 291: F833–F839, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Kimura T, Kawabe H, Jiang C, Zhang W, Xiang YY, Lu C, et al.: Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc Natl Acad Sci U S A 108: 3216–3221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boase NA, Rychkov GY, Townley SL, Dinudom A, Candi E, Voss AK, et al.: Respiratory distress and perinatal lethality in Nedd4-2-deficient mice. Nat Commun 2: 287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abriel H, Loffing J, Rebhun JF, Pratt JH, Schild L, Horisberger JD, et al.: Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle’s syndrome. J Clin Invest 103: 667–673, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder PM: Down-regulating destruction: Phosphorylation regulates the E3 ubiquitin ligase Nedd4-2. Sci Signal 2: pe41, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Dahlmann A, Pradervand S, Hummler E, Rossier BC, Frindt G, Palmer LG: Mineralocorticoid regulation of epithelial Na+ channels is maintained in a mouse model of Liddle’s syndrome. Am J Physiol Renal Physiol 285: F310–F318, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger JD, Hummler E, et al.: A mouse model for Liddle’s syndrome. J Am Soc Nephrol 10: 2527–2533, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Estévez R, Boettger T, Stein V, Birkenhäger R, Otto E, Hildebrandt F, et al.: Barttin is a Cl- channel β-subunit crucial for renal Cl- reabsorption and inner ear K+ secretion. Nature 414: 558–561, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Nomura N, Tajima M, Sugawara N, Morimoto T, Kondo Y, Ohno M, et al.: Generation and analyses of R8L barttin knockin mouse. Am J Physiol Renal Physiol 301: F297–F307, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Embark HM, Böhmer C, Palmada M, Rajamanickam J, Wyatt AW, Wallisch S, et al.: Regulation of CLC-Ka/barttin by the ubiquitin ligase Nedd4-2 and the serum- and glucocorticoid-dependent kinases. Kidney Int 66: 1918–1925, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Günther W, Lüchow A, Cluzeaud F, Vandewalle A, Jentsch TJ: ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci U S A 95: 8075–8080, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Picollo A, Pusch M: Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature 436: 420–423, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Scheel O, Zdebik AA, Lourdel S, Jentsch TJ: Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436: 424–427, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Schwake M, Friedrich T, Jentsch TJ: An internalization signal in ClC-5, an endosomal Cl-channel mutated in dent’s disease. J Biol Chem 276: 12049–12054, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Hryciw DH, Ekberg J, Lee A, Lensink IL, Kumar S, Guggino WB, et al.: Nedd4-2 functionally interacts with ClC-5: Involvement in constitutive albumin endocytosis in proximal tubule cells. J Biol Chem 279: 54996–55007, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Rickheit G, Wartosch L, Schaffer S, Stobrawa SM, Novarino G, Weinert S, et al.: Role of ClC-5 in renal endocytosis is unique among ClC exchangers and does not require PY-motif-dependent ubiquitylation. J Biol Chem 285: 17595–17603, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wall JG, Plückthun A: Effects of overexpressing folding modulators on the in vivo folding of heterologous proteins in Escherichia coli. Curr Opin Biotechnol 6: 507–516, 1995 [DOI] [PubMed] [Google Scholar]
- 57.Viallet PM, Vo-Dinh T: Monitoring intracellular proteins using fluorescence techniques: From protein synthesis and localization to activity. Curr Protein Pept Sci 4: 375–388, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Staub O, Rotin D: Role of ubiquitylation in cellular membrane transport. Physiol Rev 86: 669–707, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Xu J, Barone S, Li H, Holiday S, Zahedi K, Soleimani M: Slc26a11, a chloride transporter, localizes with the vacuolar H(+)-ATPase of A-intercalated cells of the kidney. Kidney Int 80: 926–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raikwar NS, Vandewalle A, Thomas CP: Nedd4-2 interacts with occludin to inhibit tight junction formation and enhance paracellular conductance in collecting duct epithelia. Am J Physiol Renal Physiol 299: F436–F444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eaton DC, Malik B, Bao H-F, Yu L, Jain L: Regulation of epithelial sodium channel trafficking by ubiquitination. Proc Am Thorac Soc 7: 54–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Itani OA, Stokes JB, Thomas CP: Nedd4-2 isoforms differentially associate with ENaC and regulate its activity. Am J Physiol Renal Physiol 289: F334–F346, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.