Abstract
Background The development of antibodies specific to HLA expressed on donor tissue (donor-specific antibodies [DSAs]) is a prominent risk factor for kidney graft loss. Non-HLA antibodies with pathogenic potential have also been described, including natural antibodies (Nabs). These IgG Nabs bind to immunogenic self-determinants, including oxidation-related antigens.
Methods To examine the relationship of Nabs with graft outcomes, we assessed Nabs in blinded serum specimens collected from a retrospective cohort of 635 patients who received a transplant between 2005 and 2010 at Necker Hospital in Paris, France. Serum samples were obtained immediately before transplant and at the time of biopsy-proven rejection within the first year or 1 year after transplant. Nabs were detected by ELISA through reactivity to the generic oxidized epitope malondialdehyde.
Results Univariate Cox regression analysis identified the development of post-transplant Nabs (defined as 50% increase in reactivity to malondialdehyde) as a significant risk factor for graft loss (hazard ratio, 2.68; 95% confidence interval, 1.49 to 4.82; P=0.001). Post-transplant Nabs also correlated with increased mean Banff scores for histologic signs of graft injury in post-transplant biopsy specimens. Multivariable Cox analyses confirmed Nabs development as a risk factor independent from anti-HLA DSAs (hazard ratio, 2.07; 95% confidence interval, 1.03 to 4.17; P=0.04). Moreover, patients with Nabs and DSAs had a further increased risk of kidney graft loss.
Conclusions These findings reveal an association between Nabs, kidney graft injury, and eventual graft failure, suggesting the involvement of Nabs in immune mechanisms of rejection.
Keywords: non-HLA antibodies, DSA, oxidation-specific epitopes, natural polyreactive antibodies, natural antibodies
Antibodies reactive to kidney allografts are associated with rejection episodes and graft failure. Since the pioneering work by Paul Terasaki, most of the attention has focused on IgG antibodies binding specifically to donor HLA class 1 and class 2 molecules (donor-specific antibodies [DSAs]). Yet, other antibodies with pathogenic potential have also been described, including antibodies to AT1R,1 ETAR,2 and LG3.3 The generation and pathogenic mechanism of these non-HLA antibodies are still being investigated. In recent years, our laboratory examined another type of non-HLA antibody known as natural antibodies (Nabs). Nabs react to multiple, distinct self- and exogenous antigenic structures4,5 as well as apoptotic cells.6,7 We previously reported the association between IgG Nabs, antibody-mediated rejection, and poorer long-term graft outcome after kidney transplantation in single-center, retrospective studies.7,8 Nabs were also associated with primary graft dysfunction in heart transplant recipients, especially in patients who received mechanical support before transplantation.9
Oxidation-related antigens are additional immunogenic targets of Nabs resulting from lipid peroxidation as a response to oxidative stress.10 Peroxidation generates highly reactive products, such as malondialdehyde (MDA), that covalently bind to proteins or lipids. These adducts create neoepitopes recognized by Nabs.11 Reactivity to MDA has been observed in atherosclerosis12 and autoimmune conditions, such as rheumatoid arthritis13 and SLE.14 After transplantation, oxidative stress and local production of MDA have been described amid graft injury15 and chronic rejection16 as well as graft dysfunction.17 Here, we sought to assess the generation of IgG Nabs reactive to MDA in a large cohort of well characterized patients with kidney transplants and examine their relation to histopathology, graft function, and long-term survival.
Methods
Patient Population
All 635 consecutive patients who underwent kidney transplantation at Necker Hospital between 2005 and 2010 with assessment of reactivity to MDA were enrolled in this population-based study. The transplantation allocation system followed the rules of the French national agency for organ procurement (Agence de la Biomédecine). All of the transplants were compatible on the basis of ABO blood group. Negative IgG T cell and B cell complement–dependent cytotoxicity crossmatching was required for all of the recipients. Clinical data on the donors and recipients were obtained from two national registries: Données Informatiques Validées en Transplantation (http://www.divat.fr/) and Agence de la Biomédecine (http://www.sipg.sante/fr/portail/).
Histologic Assessment and Renal Function
Histologic assessments of protocol biopsies collected at 1 year post-transplant or indication biopsies within the first year after transplantation were performed. The biopsies were graded from zero to three according to the Banff histologic parameters for peritubular capillary inflammation, glomerulitis, transplant glomerulopathy, tubulitis, interstitial inflammation, interstitial fibrosis, tubular atrophy, and vascular fibrous intimal thickening.18 C4d staining was performed by immunohistochemistry on paraffin sections using the human C4d polyclonal antibody. Renal function was assessed by eGFR and proteinuria assays.
Detection of DSAs
All of the patients were tested for the presence of circulating donor-specific anti-HLA antibodies in banked pre- and post-transplant serum samples (at the Jean Dausset Histocompatibility Laboratory). The presence of circulating DSAs against HLA-A, HLA-B, HLA-Cw, HLA-DR, HLA-DQ, and HLA-DP was retrospectively determined using single-antigen flow bead assays (One Lambda, Inc., Canoga Park, CA) on a Luminex platform. Beads with a normalized mean fluorescence intensity >500 arbitrary units were judged positive as previously described.19 This is the standard cutoff for DSA positivity in our laboratory. HLA typing of the transplant recipients was performed (Innolipa HLA Typing Kit; Innogenetics, Ghent, Belgium). Donor HLA typing for HLA-Cw and HLA-DP was only performed if recipients had circulating anti–HLA-Cw and/or anti–HLA-DP. The tissue typing was conducted using the microlymphocytotoxicity technique using One Lambda, Inc. tissue-typing trays; traditional molecular biology controls were included. Serum samples were collected immediately pretransplant and at the time of biopsy.
Purification of Serum IgG
IgG from patient serum specimens was purified using the Melon Gel IgG Purification Kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. Briefly, serum samples were diluted 1:10 in Melon purification buffer and incubated with the Melon gel for 5 minutes. IgG was then eluted from the gel by centrifugation.
Assessment of Reactivity to MDA
MDA-modified BSA was generated by incubating acid-hydrolyzed 1,1,3,3-tetramethoxypropane (Sigma-Aldrich, St. Louis, MO) with BSA as previously described. Briefly, 1 M 1,1,3,3-tetramethoxypropane was hydrolyzed in 96 mM HCl for 10 minutes at 37°C and then neutralized with NaOH. BSA (2 mg/ml) with 0.2 M MDA was incubated for 3 hours at 37°C followed by extensive dialysis against PBS at 4°C for 36 hours. High-binding 96-well plates (Corning, Kennebunk, ME) were coated with 12.5 μg/ml MDA-modified BSA overnight at 4°C. Plates were blocked with Tris-buffered saline supplemented with 0.5% nonfat dry milk for 1 hour at room temperature. Purified IgG was diluted fourfold in Tris-buffered saline supplemented with 0.5% nonfat dry milk for 2 hours at room temperature. Pooled healthy sera were included as a standard control. Horseradish peroxidase–conjugated donkey anti-human IgM (Jackson ImmunoResearch Labs, West Grove, PA) or donkey anti-human IgG (Jackson ImmunoResearch Labs) was then incubated for 1 hour at room temperature. The ELISA was developed using 3,3′,5,5′-tetramethylbenzidine (Life Technologies, Carlsbad, CA), and the OD was measured at 450 nm and expressed as arbitrary units. The development of post-transplant Nabs (Nabs+) was defined as an increase of serum reactivity to MDA (as arbitrary units) of at least 50% between sera collected post-transplant and those collected pretransplant. The reproducibility of the MDA ELISA was assessed by intra- and interassay variation testing performed every 2 weeks over 10 weeks for a total of six assays. Supplemental Table 1 shows the results of the intra- and inter-assay testing on a representative control sample. Five to ten replicates of the representative sample were included in each assay plate. Both intra- and inter-assay coefficients of variation (percentage) were <6%, indicating high reproducibility and minimal drift. Furthermore, the reproducibility of the assay was confirmed by testing all samples in our cohort in duplicate assays performed on 2 different days. Correlations between the results from the duplicate assays were calculated using Spearman's rank correlation coefficient. Supplemental Figure 1 shows the significant correlation (r=0.96; P<0.001) measured between the duplicate assessment of all cohort samples (n=1462).
Statistical Methods
Continuous variables are described using means and SDs or medians and interquartile ranges. We compared means and proportions between groups using the t test, ANOVA (Mann–Whitney U test for mean fluorescence intensity), or the chi-squared test (or the Fisher's exact test where appropriate). The kidney survival analysis was performed from the time of transplantation to a maximum follow-up of 7 years, with kidney graft loss as the event of interest, which was defined as the patient’s return to dialysis. For the patients who died with a functioning graft, graft survival was censored at the time of death.20 We confirmed that the differences in graft survival between the four groups (Nabs−/DSA−, Nabs−/DSA+, Nabs+/DSA−, and Nabs+/DSA+) were not affected by competition with patient death using competing risks regression modeling (Supplemental Figure 2).
Kidney allograft survival according to Nabs and DSA status was plotted using Kaplan–Meier curves and compared using the log rank test. Cox proportional hazards models were applied to quantify the hazard ratios (HRs) and the 95% confidence intervals (95% CIs) for kidney graft loss. The associations of donor, recipient, and transplant parameters; immunologic factors; and histologic factors with graft loss were first assessed in univariate regression analyses. All variables with P value of 0.20 or less were then included in one multivariable Cox model. The proportionality assumption for the Cox model was verified using the log graphic method. The final model was adjusted for extended criteria donor status, graft rank, DSA (pre- and/or post-transplant), development of Nabs, eGFR, proteinuria, C4d graft deposition, glomerular and peritubular inflammation, transplant glomerulopathy, and interstitial inflammation and tubulitis. We used STATA (version 14; Data Analysis and Statistical Software) and R (version 3.2.1; R Foundation for Statistical Computing) for the descriptive and survival analyses. Probability values <0.05 were considered significant.
Results
Baseline Characteristics and Generation of Nabs
In this study, we assessed the generation of IgG Nabs reactive to MDA during the first year after transplantation in 635 patients transplanted at Necker Hospital in Paris, France. Nabs assessment was carried out at Columbia University Medical Center blinded from clinical data. Patient and donor characteristics at time of transplantation are summarized in Table 1. We defined the development of post-transplant Nabs (Nabs+) by an increase of serum reactivity to MDA of at least 50% between sera collected pretransplant and sera collected post-transplant. There were 66 Nabs+ patients in the first year after transplant. Median follow-up time was 7.636±2.791 years. Mean recipient age, recipient sex, cause of ESRD, HLA mismatch, and cold ischemia time were similar between Nabs+ patients and those who did not develop post-transplant Nabs (Nabs−). The Nabs− group had a greater proportion of deceased donors (n=463; 81.37%; P=0.05) compared with the Nabs+ group (n=47; 71.21%). A higher number of Nabs+ were extended criteria donors (n=31; 46.97%; P=0.04), had DSA at time of transplant (n=26; 39.39%; P<0.001), or had received a prior transplant (n=25; 37.88%; P<0.001) compared with Nabs− (n=194; 34.09%, n=82; 14.41%, and n=112; 19.68%, respectively). Additionally, there was a trend toward a greater proportion of women in the group of Nabs+ patients (n=34; 52.52%; P=0.06) compared with the group of Nabs− patients (n=255; 39.54%).
Table 1.
Baseline characteristics of the patient cohort
| Parameter | N | All Patients | N | Nabs− Patients | N | Nabs+ Patients | P Value |
|---|---|---|---|---|---|---|---|
| Recipient characteristics | |||||||
| Age, yr, mean (SD) | 635 | 48.49 (13.41) | 569 | 48.52 (13.19) | 66 | 48.25 (15.16) | 0.89 |
| Men, no. (%) | 635 | 376 (59.21) | 569 | 344 (60.46) | 66 | 32 (48.48) | 0.06 |
| ESRD causes, no. (%) | 635 | 569 | 66 | ||||
| GN | 175 (27.56) | 156 (27.41) | 19 (28.79) | ||||
| Diabetes | 41 (6.46) | 38 (6.68) | 3 (4.55) | ||||
| PKD | 52 (8.19) | 45 (7.91) | 7 (10.60) | ||||
| Interstitial | 111 (17.48) | 101 (17.75) | 10 (15.15) | ||||
| Vascular | 17 (2.68) | 14 (2.46) | 3 (4.55) | ||||
| Toxic | 7 (1.10) | 7 (1.23) | 0 | ||||
| Other | 91 (14.33) | 81 (14.24) | 10 (15.15) | ||||
| Unknown | 141 (22.20) | 127 (22.32) | 14 (21.21) | 0.88 | |||
| Donor characteristics | |||||||
| Age, yr, mean (SD) | 635 | 52.79 (16.03) | 569 | 52.29 (16.17) | 66 | 57.11 (14.20) | 0.01 |
| Men, no. (%) | 635 | 363 (57.17) | 569 | 326 (57.29) | 66 | 37 (56.06) | 0.85 |
| Hypertension, no. (%) | 633 | 174 (27.49) | 568 | 148 (25.70) | 65 | 26 (40.00) | 0.01 |
| Creatinine, mean (SD) | 635 | 90.09 (55.61) | 569 | 91.75 (57.83) | 66 | 75.83 (26.65) | <0.001 |
| Deceased donor, no. (%) | 635 | 510 (80.31) | 569 | 463 (81.37) | 66 | 47 (71.21) | 0.05 |
| Extended criteria donor, no. (%) | 635 | 225 (35.43) | 569 | 194 (34.09) | 66 | 31 (46.97) | 0.04 |
| Transplant baseline characteristics | |||||||
| Prior kidney transplant, no. (%) | 635 | 137 (21.57) | 569 | 112 (19.68) | 66 | 25 (37.88) | <0.001 |
| Cold ischemia time, h, mean (SD) | 633 | 18.57 (10.45) | 568 | 18.77 (10.39) | 65 | 16.83 (10.89) | 0.18 |
| Delayed graft function, no. (%) | 575 | 180 (31.30) | 524 | 166 (31.68) | 51 | 14 (27.45) | 0.53 |
| No. of HLA-A/-B/-DR mismatches, mean (SD) | 635 | 3.52 (1.41) | 569 | 3.52 (1.42) | 66 | 3.52 (1.33) | 0.98 |
| Anti-HLA DSA at transplantation, no. (%) | 635 | 108 (17.01) | 569 | 82 (14.41) | 66 | 26 (39.39) | <0.001 |
| Anti-HLA DSA class at transplantation, no. (%) | 635 | 569 | 66 | ||||
| Class 1 | 46 (42.59) | 38 (46.34) | 8 (30.77) | ||||
| Class 2 | 62 (57.41) | 44 (53.66) | 18 (69.23 | 0.16 | |||
| Anti-HLA DSA at transplantation, MFI, median (IQR) | 635 | 2658 (1246–6300) | 2209 (1160–6210) | 3564 (2002–8484) | 0.28 |
Nabs, natural antibodies; PKD, polycystic kidney disease; DSA, donor-specific antibody; MFI, mean fluorescence intensity; IQR, interquartile range.
Nabs Development Is Associated with Poorer Graft Survival and Worse Microvascular Injury and Graft Function
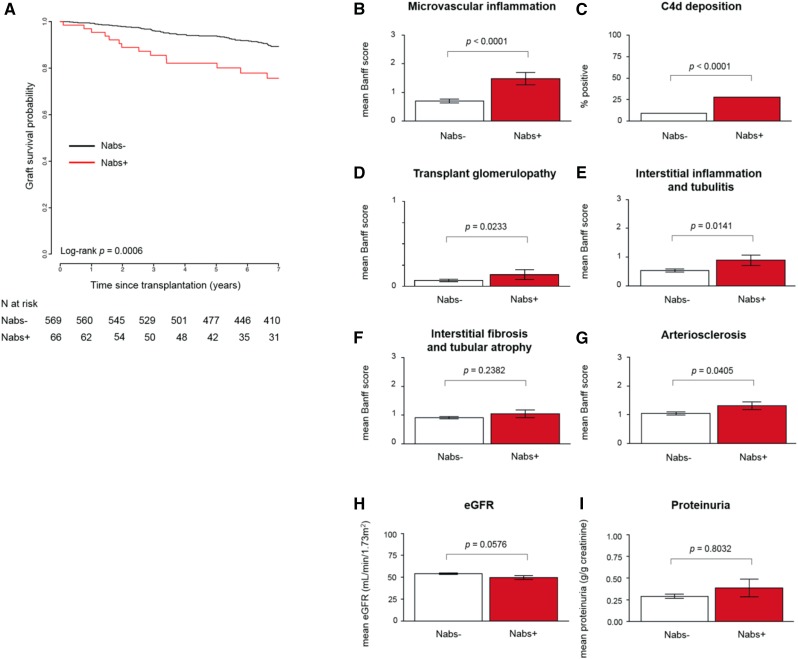
As reported in Figure 1A, Kaplan–Meier survival analysis revealed that Nabs development within the first year was significantly associated with worse graft survival (log rank P<0.001). Graft survival at 7 years was 75.7% for Nabs+ patients compared with 89.3% for patients without Nabs, highlighting the negative effect of Nabs on long-term outcomes. The significant association between Nabs development and poorer graft survival was also observed when the threshold of Nabs development was defined as >25% (log rank P<0.01), >75% (P<0.01), >100% (P=0.02), or >200% (P<0.01) increase in reactivity to MDA in post-transplant compared with pretransplant sera (Supplemental Figure 3). All patients included in the cohort underwent protocol biopsy at 1 year post-transplant or had biopsies performed at the time of rejection within the first post-transplant year. A comprehensive histologic assessment of these biopsies had been carried out as previously reported,21 allowing us to examine the association between Nabs and evidence of graft injury. Patients who developed Nabs during the first year post-transplant had significantly elevated mean Banff scores for nearly all histologic markers of graft deterioration, such as microvascular inflammation (glomerular and peritubular capillaritis; P<0.001) (Figure 1B), C4d deposition (P<0.001) (Figure 1C), transplant glomerulopathy (P=0.02) (Figure 1D), interstitial inflammation and tubulitis (P=0.01) (Figure 1E), and arteriosclerosis (P=0.04) (Figure 1G), compared with those who did not develop Nabs. Interstitial fibrosis and tubular atrophy was the only histologic marker that did not differ between the two groups (Figure 1F). Renal function measured by eGFR was decreased in Nabs+ patients compared with Nabs− patients, although the difference did not reach significance (P=0.06) (Figure 1H). Lastly, no difference in proteinuria was observed between the two groups (Figure 1I), and there were no differences for other tubular injuries, such as BK virus–associated nephropathy, between the Nabs+ (one of 66; 1.52%; P values is NS) and Nabs− (eight of 569; 1.41%) patients (results not shown).
Figure 1.
The development of natural antibodies (Nabs) is associated with poorer graft survival and worse microvascular injury and graft function. (A) Kaplan–Meier curve depicting 7-year graft survival of Nabs+ and Nabs− kidney transplant recipients. Histologic examinations of biopsies performed at 1 year post-transplant or the time of rejection within the first year are shown as mean Banff score for (B) microvascular inflammation, (C) C4d deposition, (D) transplant glomerulopathy, (E) interstitial inflammation and tubulitis, (F) interstitial fibrosis and tubular atrophy, and (G) arteriosclerosis. Renal function was assessed and is shown as (H) mean eGFR and (I) mean proteinuria.
Nabs Development Is Not Related to Donor Type or Recipient’s Sex
Differences in donor type (living/deceased or extended/standard criteria) and a trend of more women in the group of Nabs+ patients (Table 1) prompted analysis of Nabs development according to donor type and recipient’s sex. There were no differences in graft survival between Nabs+ patients who were men and those who were women (Supplemental Figure 4A) (log rank P=0.82) or Nabs− patients who were men and those who were women (P=0.41). Among the Nabs+ patients, there was no difference between recipients on the basis of sex sensitized for class 1 (P=0.97) or class 2 (P=0.58; results not shown). Similarly, graft survival in Nabs+ patients was not significantly different between living or deceased donors (Supplemental Figure 4B) (P=0.89) or in extended criteria donors compared with standard criteria donors (Supplemental Figure 4C).
Nabs Development Is an Independent Risk Factor for Worse Graft Survival
Pre- and post-transplant risk factors associated with graft loss were further determined in univariate analyses. Pretransplant factors with significant effect on graft loss included extended criteria donors and previous transplant, whereas significant post-transplant factors included increase in reactivity to MDA >50% (Nabs+), eGFR, proteinuria, microvascular inflammation, C4d deposition, and interstitial inflammation and tubulitis (Table 2). The presence of DSA, detected pre- and/or post-transplant, was also significantly associated with graft loss. These significant variables were then assessed in a multivariable Cox regression model, the results of which are shown in Table 3. Only four variables were identified as independent risk factors of graft loss, including two variables related to the graft function (eGFR: HR, 0.97; 95% CI, 0.96 to 0.99; P<0.001 and proteinuria at 1 year: HR, 1.70; 95% CI, 1.35 to 2.36; P<0.001) as well as two immunologic variables (DSA with mean fluorescent intensity ≥6000: HR, 2.61; 95% CI, 1.25 to 5.45; P=0.03 and development of Nabs: HR, 2.07; 95% CI, 1.03 to 4.17; P=0.04).
Table 2.
Risk factors associated with graft loss in univariate analyses
| Univariate Analysis Parameters | HR | 95% CI | P Value |
|---|---|---|---|
| Baseline recipient characteristics | |||
| Age per 1-yr increment | 0.99 | 0.98 to 1.01 | 0.49 |
| Sex | |||
| Women | 1 | — | |
| Men | 1.15 | 0.71 to 1.87 | 0.57 |
| Baseline donor characteristics | |||
| Age per 1-yr increment | 1.00 | 0.99 to 1.02 | 0.41 |
| Sex | |||
| Women | 1 | — | |
| Men | 1.05 | 0.67 to 1.70 | 0.83 |
| Donor HTA | |||
| No | 1 | — | |
| Yes | 1.47 | 0.90 to 2.41 | 0.13 |
| Deceased donor | |||
| No | 1 | — | |
| Yes | 1.37 | 0.72 to 2.61 | 0.34 |
| Extended criteria donor | |||
| No | 1 | — | |
| Yes | 1.69 | 1.06 to 2.71 | 0.03 |
| Baseline transplant characteristics | |||
| Prior kidney transplant | |||
| No | 1 | — | |
| Yes | 2.14 | 1.31 to 3.50 | 0.002 |
| Cold ischemia time, h | |||
| <12 | 1 | — | |
| 12–24 | 1.58 | 0.83 to 3.01 | |
| ≥24 | 1.25 | 0.60 to 2.59 | 0.35 |
| Baseline immunologic characteristics | |||
| No. of HLA-A/-B/-DR mismatches | 0.91 | 0.78 to 1.08 | 0.27 |
| Pre- and/or post-transplant anti-HLA DSA | |||
| DSA | |||
| No | 1 | — | |
| MFI | |||
| <6000 | 1.57 | 0.87 to 2.83 | |
| ≥6000 | 3.78 | 1.89 to 7.53 | <0.001 |
| Immunologic characteristics at biopsy | |||
| >50% Increase in reactivity to MDA in post- compared with pretransplant (Nabs+) | |||
| No | 1 | — | |
| Yes | 2.68 | 1.49 to 4.82 | 0.001 |
| Function at biopsy | |||
| eGFR, ml/min per 1.73 m2 | 0.96 | 0.94 to 0.97 | <0.001 |
| Proteinuria, log transformation | 1.94 | 1.56 to 2.41 | <0.001 |
| Histology at biopsy | |||
| Glomerular and peritubular inflammation | |||
| Low score: 0 | 1 | — | |
| Transplant glomerulopathy | |||
| High score: ≥1 | 2.13 | 1.31 to 3.47 | 0.002 |
| Interstitial fibrosis and tubular atrophy | |||
| Low score: 0 | 1 | — | |
| High score: ≥2 | 1.13 | 0.76 to 2.16 | 0.36 |
| C4d graft deposition | |||
| Low score: 0 | 1 | — | |
| High score: ≥1 | 2.48 | 1.37 to 4.47 | 0.003 |
| Interstitial inflammation and tubulitis | |||
| Low score: 0 | 1 | — | |
| High score: ≥2 | 2.01 | 1.15 to 3.52 | 0.02 |
| Arteriosclerosis | |||
| 0–1 | 1 | — | |
| ≥2 | 1.21 | 0.74 to 1.97 | 0.45 |
| Arteriolar hyalinosis | |||
| 0–1 | 1 | — | |
| ≥2 | 0.96 | 0.50 to 1.83 | 0.90 |
HR, hazard ratio; 95% CI, 95% confidence interval; —, not applicable; HTA, hypertension; DSA, donor-specific antibody; MFI, mean fluorescence intensity; MDA, malondialdehyde; Nabs, natural antibodies.
Table 3.
Risk factors associated with graft loss in multivariable analyses
| Multivariable Analysis Parameters | HR | 95% CI | P Value |
|---|---|---|---|
| eGFR, ml/min per 1.73m2 | 0.97 | 0.96 to 0.99 | <0.001 |
| Proteinuria, g/g creatinine, log | 1.70 | 1.35 to 2.36 | <0.001 |
| >50% Increase in reactivity to MDA in post- compared with pretransplant (Nabs+) | |||
| No | 1 | — | |
| Yes | 2.07 | 1.03 to 4.17 | 0.04 |
| Pre- and/or post-transplant anti-HLA DSA | |||
| No | 1 | — | |
| MFI | |||
| <6000 | 1.12 | 0.59 to 2.12 | |
| ≥6000 | 2.61 | 1.25 to 5.45 | 0.03 |
HR, hazard ratio; 95% CI, 95% confidence interval; MDA, malondialdehyde; Nabs, natural antibodies; —, not applicable; DSA, donor-specific antibody; MFI, mean fluorescence intensity.
The Effect of Nabs on Graft Survival and Injury in the Absence or Presence of DSA
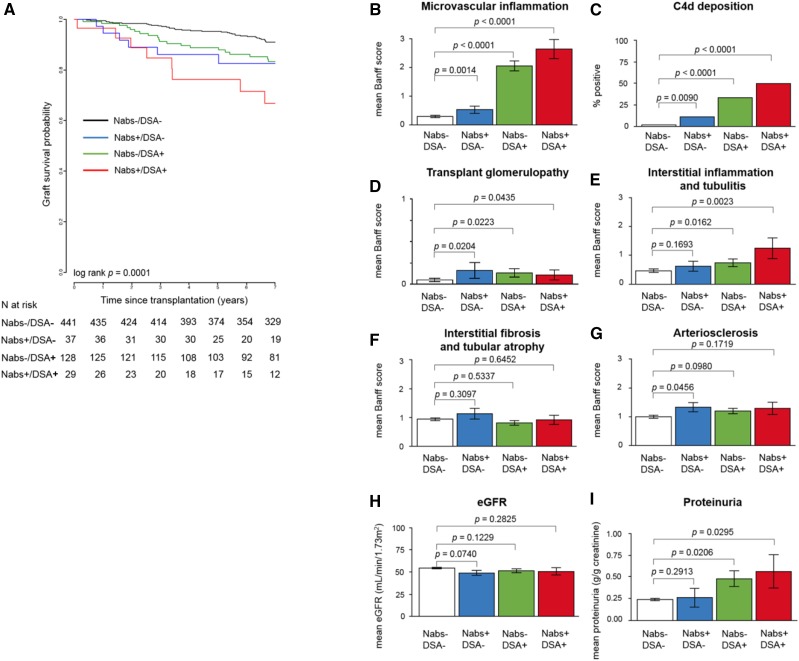
Previous studies have reported that antibodies to non-HLA targets, such as AT1R, can act in synergy with DSA, resulting in worse overall graft survival.22,23 To investigate whether a similar phenomenon could be observed with Nabs, we compared the outcomes of patients divided into four distinct groups according to their Nabs and DSA status: Nabs−/DSA−, Nabs+/DSA−, Nabs−/DSA+, and Nabs+/DSA+. Patients who were DSA+ included those with DSA detected at the time of transplant and/or post-transplant (n=157). Figure 2A depicts the 7-year graft survival for the four groups. Patients with neither antibody experienced the best outcome, with 91.1% graft survival at 7 years. In contrast, patients with either DSA or Nabs had significantly decreased graft survival compared with those in the Nabs−/DSA− group. No significant difference was found between Nabs+/DSA− and Nabs−/DSA+ groups, suggesting that both types of antibodies were equally detrimental. However, Nabs+/DSA+ patients experienced the poorest outcome, with 66.8% survival at 7 years. The difference between this latter group and all other groups was significant (log rank P<0.001), suggesting a possible additive effect between both antibody types in their contribution to graft loss. A subsequent comparison between Nabs+/DSA− and Nabs−/DSA− groups revealed that Nabs generation alone during the first year post-transplant in the absence of DSA was significantly associated with four of six histologic markers of graft injury: microvascular inflammation (P=0.001) (Figure 2B), C4d deposition (P<0.01) (Figure 2C), transplant glomerulopathy (P=0.02) (Figure 2D), and arteriosclerosis (P=0.05) (Figure 2G). Mean biopsy scores for interstitial inflammation and tubulitis (Figure 2E) and interstitial fibrosis and tubular atrophy (Figure 2F) along with graft function (eGFR [Figure 2H] and proteinuria [Figure 2I]) were not significantly associated with Nabs.
Figure 2.
The development of natural antibodies (Nabs) in the absence or presence of donor-specific antibodies (DSA) is associated with poorer graft survival and worse microvascular injury. (A) Kaplan–Meier curve depicting 7-year graft survival of Nabs−/DSA−, Nabs−/DSA+, Nabs+/DSA−, and Nabs−+/DSA+ kidney transplant recipients. Histologic examination of biopsies performed at 1 year post-transplant or the time of rejection within the first year are shown as mean Banff scores for (B) microvascular inflammation, (C) C4d deposition, (D) transplant glomerulopathy, (E) interstitial inflammation and tubulitis, (F) interstitial fibrosis and tubular atrophy, and (G) arteriosclerosis. Renal function was assessed and is shown as (H) mean eGFR and (I) mean proteinuria.
Discussion
The role of non-HLA antibodies is increasingly recognized in antibody-mediated rejection of solid organ transplants. Nabs are non-HLA antibodies previously linked to poor outcomes in kidney and heart transplantation.8,9 This blinded study using a large, well characterized cohort explored the association of early Nabs development with long-term outcomes and graft injury in the year after transplant. Collectively, our results established a link between the generation of early post-transplant Nabs, immune-mediated injury in the graft, and long-term graft failure. The results of our multivariable analysis underscored the relevance of IgG Nabs as an important risk factor of graft survival independent of DSA.
The mechanisms of Nabs generation and their mode of action remain uncertain. It has been suggested that DSA-mediated graft damage exposes endothelial cell neoepitopes, resulting in the production of autoantibodies toward the graft.24 However, our results show that Nabs can develop in the absence of DSA. Additionally, studies in mice have shown that ischemia-reperfusion injury unmasked cryptic self-antigens that contributed to tissue destruction.25 It is possible that such early damage to human kidney graft stimulates endothelial lipid peroxidation, leading to oxidation-specific epitope exposure and subsequent Nabs development. It is also plausible that local inflammation and graft tissue injury resulting from the surgical trauma, the residual T cell alloresponse, or even the nephrotoxicity of some immunosuppressors could trigger the generation of Nabs. Furthermore, Nabs to oxidation-specific epitopes are known to crossreact to bacterial antigens, such as those of Streptococcus pneumoniae.26 Exposure to bacteria may, therefore, be a potential mechanism of Nabs generation. We could not verify this hypothesis in our cohort because prior pneumococcal infection status was unknown and pneumococcal vaccination was not performed before 1 year post-transplantation.
The pathogenic potential of Nabs is also unclear. These antibodies may directly damage the endothelium through a complement-dependent mechanism comparable with that mediated by DSA27 and certain non-HLA antibodies (anti-LG328). This view is supported by the detection of more abundant C4d deposition in post-transplant biopsies collected from Nabs+/DSA− patients compared with that in post-transplant biopsies collected from Nabs−/DSA− patients. Furthermore, we had previously observed that IgG Nabs are predominantly of the complement-fixing IgG3 and IgG1 subclasses8,9 and can efficiently activate complement in vitro.8 However, both HLA and non-HLA antibodies have been reported to cause graft damage via complement-independent means, including antibody-dependent cell cytotoxicity and endothelial cell activation.1,29,30 Nabs may also activate the graft endothelium in a similar fashion. Although we could not formally show an additive effect between Nabs and DSA due to the limited size of our cohort, the significant decrease in graft survival observed in patients with both types of antibodies is striking. This observation may result from the summation of the contribution of the two types of antibodies operating through distinct mechanisms. Alternatively, there may be biologic synergy between both antibodies, such as has been observed for DSA and AT1R antibodies.22,23 Remarkably, some monoclonal Nabs produced by immortalized B cell clones generated in our laboratory displayed a strong rheumatoid factor activity (E. Zorn, unpublished data). We are currently investigating whether Nabs with such rheumatoid factor properties can crosslink DSA, exacerbating their effect on graft endothelial cells.
Overall, our blinded, retrospective study conducted on a carefully characterized cohort of over 600 kidney transplant recipients underscores the relevance of Nabs as an independent immunologic risk factor of graft loss. The strong association between Nabs and histologic markers of tissue damage at 1 year post-transplant points to their involvement in a graft deterioration process that leads to long-term graft failure. Our assessment was limited to 1 year. A more extensive study examining the longitudinal dynamics of Nabs in a larger, more diverse cohort is now warranted.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grant R01-A1123342 from the National Institutes of Health and a fellowship grant from Enduring Hearts (to S.B.S.). A.L. and D.A. are supported by the Emmanuel Boussard Foundation and the Centaure Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017111157/-/DCSupplemental.
References
- 1.Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, et al.: Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 352: 558–569, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Banasik M, Boratyńska M, Kościelska-Kasprzak K, Mazanowska O, Bartoszek D, Zabińska M, et al.: Long-term follow-up of non-HLA and anti-HLA antibodies: Incidence and importance in renal transplantation. Transplant Proc 45: 1462–1465, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Cardinal H, Dieudé M, Brassard N, Qi S, Patey N, Soulez M, et al.: Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant 13: 861–874, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, Burastero SE, Notkins AL, Casal P: Human monoclonal rheumatoid factor-like antibodies from CD5 (Leu-1)+ B cells are polyreactive. J Immunol 140: 4180–4186, 1988 [PubMed] [Google Scholar]
- 5.Notkins AL: Polyreactivity of antibody molecules. Trends Immunol 25: 174–179, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Tuominen A, Miller YI, Hansen LF, Kesäniemi YA, Witztum JL, Hörkkö S: A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler Thromb Vasc Biol 26: 2096–2102, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Porcheray F, Fraser JW, Gao B, McColl A, DeVito J, Dargon I, et al.: Polyreactive antibodies developing amidst humoral rejection of human kidney grafts bind apoptotic cells and activate complement. Am J Transplant 13: 2590–2600, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao B, Moore C, Porcheray F, Rong C, Abidoglu C, DeVito J, et al.: Pretransplant IgG reactivity to apoptotic cells correlates with late kidney allograft loss. Am J Transplant 14: 1581–1591, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.See SB, Clerkin KJ, Kennel PJ, Zhang F, Weber MP, Rogers KJ, et al.: Ventricular assist device elicits serum natural IgG that correlates with the development of primary graft dysfunction following heart transplantation. J Heart Lung Transplant 36: 862–870, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, et al.: Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest 119: 1335–1349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, et al.: Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med 200: 1359–1370, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salonen JT, Ylä-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, et al.: Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet 339: 883–887, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Grönwall C, Amara K, Hardt U, Krishnamurthy A, Steen J, Engström M, et al.: Autoreactivity to malondialdehyde-modifications in rheumatoid arthritis is linked to disease activity and synovial pathogenesis. J Autoimmun 84: 29–45, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Frostegård J, Svenungsson E, Wu R, Gunnarsson I, Lundberg IE, Klareskog L, et al.: Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum 52: 192–200, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Djamali A: Oxidative stress as a common pathway to chronic tubulointerstitial injury in kidney allografts. Am J Physiol Renal Physiol 293: F445–F455, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Cristol JP, Vela C, Maggi MF, Descomps B, Mourad G: Oxidative stress and lipid abnormalities in renal transplant recipients with or without chronic rejection. Transplantation 65: 1322–1328, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Fonseca I, Reguengo H, Almeida M, Dias L, Martins LS, Pedroso S, et al.: Oxidative stress in kidney transplantation: Malondialdehyde is an early predictive marker of graft dysfunction. Transplantation 97: 1058–1065, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al.; Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, et al.: Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21: 1398–1406, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamb KE, Lodhi S, Meier-Kriesche HU: Long-term renal allograft survival in the United States: A critical reappraisal. Am J Transplant 11: 450–462, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al.: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi M, Rebellato LM, Cai J, Hopfield J, Briley KP, Haisch CE, et al.: Higher risk of kidney graft failure in the presence of anti-angiotensin II type-1 receptor antibodies. Am J Transplant 13: 2577–2589, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Philogene MC, Bagnasco S, Kraus ES, Montgomery RA, Dragun D, Leffell MS, et al.: Anti-angiotensin II type 1 receptor and anti-endothelial cell antibodies: A cross-sectional analysis of pathological findings in allograft biopsies. Transplantation 101: 608–615, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nath DS, Angaswamy N, Basha HI, Phelan D, Moazami N, Ewald GA, et al.: Donor-specific antibodies to human leukocyte antigens are associated with and precede antibodies to major histocompatibility complex class I-related chain A in antibody-mediated rejection and cardiac allograft vasculopathy after human cardiac transplantation. Hum Immunol 71: 1191–1196, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, et al.: Identification of the target self-antigens in reperfusion injury. J Exp Med 203: 141–152, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binder CJ, Hörkkö S, Dewan A, Chang MK, Kieu EP, Goodyear CS, et al.: Pneumococcal vaccination decreases atherosclerotic lesion formation: Molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med 9: 736–743, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, et al.: Complement activation in acute humoral renal allograft rejection: Diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol 10: 2208–2214, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Yang B, Dieudé M, Hamelin K, Hénault-Rondeau M, Patey N, Turgeon J, et al.: Anti-LG3 antibodies aggravate renal ischemia-reperfusion injury and long-term renal allograft dysfunction. Am J Transplant 16: 3416–3429, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Le Bas-Bernardet S, Hourmant M, Coupel S, Bignon JD, Soulillou JP, Charreau B: Non-HLA-type endothelial cell reactive alloantibodies in pre-transplant sera of kidney recipients trigger apoptosis. Am J Transplant 3: 167–177, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Jackson AM, Sigdel TK, Delville M, Hsieh SC, Dai H, Bagnasco S, et al.: Endothelial cell antibodies associated with novel targets and increased rejection. J Am Soc Nephrol 26: 1161–1171, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.