Abstract
Background Podocyte loss and effacement of interdigitating podocyte foot processes are the major cause of a leaky filtration barrier and ESRD. Because the complex three-dimensional morphology of podocytes depends on the actin cytoskeleton, we studied the role in podocytes of the actin bundling protein palladin, which is highly expressed therein.
Methods We knocked down palladin in cultured podocytes by siRNA transfection or in zebrafish embryos by morpholino injection and studied the effects by immunofluorescence and live imaging. We also investigated kidneys of mice with podocyte-specific knockout of palladin (PodoPalld−/− mice) by immunofluorescence and ultrastructural analysis and kidney biopsy specimens from patients by immunostaining for palladin.
Results Compared with control-treated podocytes, palladin-knockdown podocytes had reduced actin filament staining, smaller focal adhesions, and downregulation of the podocyte-specific proteins synaptopodin and α-actinin-4. Furthermore, palladin-knockdown podocytes were more susceptible to disruption of the actin cytoskeleton with cytochalasin D, latrunculin A, or jasplakinolide and showed altered migration dynamics. In zebrafish embryos, palladin knockdown compromised the morphology and dynamics of epithelial cells at an early developmental stage. Compared with PodoPalld+/+ controls, PodoPalld−/− mice developed glomeruli with a disturbed morphology, an enlarged subpodocyte space, mild effacement, and significantly reduced expression of nephrin and vinculin. Furthermore, nephrotoxic serum injection led to significantly higher levels of proteinuria in PodoPalld−/− mice than in controls. Kidney biopsy specimens from patients with diabetic nephropathy and FSGS showed downregulation of palladin in podocytes as well.
Conclusions Palladin has an important role in podocyte function in vitro and in vivo.
Keywords: podocyte, palladin, actin filaments, cell migration, nephrin, proteinuria
Palladin, an actin-associated protein, which was first described by Parast and Otey in fibroblasts and epithelial cells,1 plays a pivotal role in the stability and dynamics of the actin cytoskeleton. It has already been reported that different palladin isoforms are expressed in a tissue- and development-dependent way.1–3 Currently, UniProt describes seven palladin transcript variants (72–152 kD) in mice that are generated by alternative splicing and start-sites.3 However, it seems likely that additional isoforms exist.
It was shown that the knockout (KO) of palladin, which is highly and ubiquitously expressed in mouse embryos, resulted in severe tube closure defects and embryonic lethality before embryonic day 15.5.4,5 This might be caused by a disturbance of the proliferation and differentiation as well as by a reduction of cell adhesion in neuronal cells of the palladin-KO mice.5 In cell culture, it was observed that palladin downregulation leads to a reduction of robust stress fibers in rat choriocarcinoma (Rcho-1) cells and cultured mouse embryonic fibroblasts as well as to a disruption of stress fibers in human glioblastoma (U251) cells.1,6 Moreover, the neurite outgrowth of neuroblastoma cells and the growth cone formation were diminished after the knockdown of palladin.7
Recently, studies have revealed that palladin carries not only specific binding sites for F-actin and the actin-binding protein α-actinin-1,8,9 but also for other actin-associated proteins such as the focal adhesion protein Lasp-13 or proteins such as VASP or profilin, which are responsible for actin dynamics.10,11 Taken together, these results showed that palladin is a scaffolding protein regulating actin nucleation and polymerization as well as cell adhesion in different cell types. Azatov et al.12 further showed that palladin modulates force generation and mechanosensitivity in tumor-associated fibroblasts.
Another interesting finding is that the function of palladin depends on the phosphorylation status. Asano et al. demonstrated that palladin becomes phosphorylated by ERK after stimulation with the epidermal growth factor (EGF). It was speculated that the ERK pathway might be involved in the EGF–mediated cell migration.13 The data further suggest that palladin has an antimigratory function, which was also postulated by Chin and Toker6 who studied the role of palladin in breast cancer.
For a long time, it has been well known that the morphology and function of podocytes, a terminally differentiated cell type in the glomerulus which is part of the glomerular filtration barrier, are highly dependent on the actin cytoskeleton. These cells express actin specifically in a cortical net near the plasma membrane as well as in filament bundles spanning two neighboring podocyte foot processes.14 A disturbance of the actin cytoskeleton, e.g., by the loss or mutation of actin-binding proteins such as α-actinin-4 and CD2AP, severely influences the morphology and function of foot processes and the integrity of the glomerular filtration barrier.15,16
In a recent study, we showed that the expression of palladin in the kidney is essentially restricted to podocytes and arteries.17 However, nothing is known about the role of palladin in kidney function. Therefore, this study focused on the expression and function of palladin in podocytes of mouse and human kidney as well as on the behavior of epithelial cells in living zebrafish embryos, a well established animal model. Because at least seven isoforms of palladin exist that are expressed in a developmental- and tissue-dependent way, we further tried to clarify which isoforms are expressed in podocytes in vitro and in vivo. Our study reveals that palladin is functionally important for the morphology and behavior of podocytes in vitro as well as in vivo.
Methods
Cell Culture
Conditionally immortalized podocytes (CLS Cell Line Service, Germany) were handled as described previously.18 All experiments were performed using differentiated podocytes. Knockdown of palladin (PalldKD) was achieved using Silencer Select siRNA Palld1 siRNA (s90889), Palld2 siRNA (s90890), and control siRNA (4390846), respectively (Ambion; Thermo Fisher Scientific, Waltham, MA). For transfection, the K2 Transfection System (Biontex, Germany) was used according to the manufacturer’s instructions. After 72 hours cells were used for experiments, except for regulation studies of cytoskeletal genes that were performed on double-transfected PalldKD cells.
Immunocytochemistry
Cultured podocytes were fixed with 2% paraformaldehyde and incubated with the following primary antibodies: palladin (Proteintech Group, UK), synaptopodin (Progen, Germany), vinculin (Sigma-Aldrich), and α-actinin-4 (immunoGlobe, Germany). Alexa Fluor 647-conjugated (Thermo Fisher Scientific) or Cy3-conjugated secondary antibodies (Dianova/Jackson Immuno Research, Germany) were used. F-actin was stained with Alexa Fluor 488–phalloidin (Thermo Fisher Scientific). Images were taken by a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems, Germany).
Actin Dynamics and Focal Adhesion Analysis
Confluent podocytes were incubated with latrunculin A, cytochalasin D, and jasplakinolide, respectively (final concentrations 0.5 µM; Sigma-Aldrich). Podocytes were stained for F-actin, nuclei, and palladin to confirm knockdown efficiency. To investigate the actin cytoskeleton, >150 podocytes were analyzed with the software F_Seg.19
For quantification of focal adhesion area, cultured podocytes were stained for vinculin and at least 60 cells per group were studied in an automated fashion by the developed custom software “Focal Contact Segmentation and Analysis Tool,” as described previously.20
Wound Assay
Podocytes were seeded on a µ-Dish (Ibidi, Germany) and transfected with control and Palld1 siRNA, respectively. Cell migration was observed with a Leica DMI 6000B fluorescence microscope (Leica Microsystems) that was heated to 38°C by a temperature control system (Life Imaging Services, Switzerland). Images were taken every 10 minutes and processed with Volocity software (PerkinElmer).
Isolation of Glomeruli
Glomeruli were isolated with magnetic Dynabeads as described previously.21
RNA Analysis
Samples from transfected cells/glomeruli/kidneys/zebrafish larvae were processed in Tri-Reagent (Sigma-Aldrich) according to the manufacturer’s instructions. One microgram of total RNA was reverse transcribed using the QuantiTect Reverse Transcription Kit (Qiagen, Germany). Transcription of zebrafish RNA was performed using SuperScript Reverse transcription (Thermo Fisher Scientific).
For RT-PCR Taq Polymerase (Peqlab) was used for cells/mouse samples and Platinum Taq DNA Polymerase (Thermo Fisher Scientific) for zebrafish samples, respectively. RT-PCR was performed on a Mastercycler gradient (Eppendorf AG, Germany).
qRT-PCR was performed on a LightCycler Nano (Roche, Germany). Primers are listed in Supplemental Table 1.
Western Blot Analysis
Western blot was performed as already described by Kliewe et al.20 Briefly, transfected cells/glomeruli/kidneys were solved in RIPA buffer (Sigma-Aldrich) supplemented with Halt Protease Inhibitor (Thermo Fisher Scientific). Adjusted protein amounts were blotted and the membrane was incubated with primary antibodies against palladin and Gapdh (Santa-Cruz) overnight at 4°C. A goat anti-rabbit IgG-HRP (Santa Cruz) was used for 45 minutes and the signal was detected with the Clarity Western ECL Blotting Substrate (Bio-Rad). Protein expression was normalized to Gapdh as a housekeeping protein.
Human Kidney Biopsy Samples
The use of remnant kidney biopsy material was approved by the Ethics Committee of the Friedrich-Alexander University of Erlangen-Nürnberg, waiving the need for retrospective consent for the use of archived rest material. Colocalization studies were performed on biopsy samples of healthy patients (n=3), patients with FSGS (n=4), and patients with DN (n=5). Three to seven glomeruli of each biopsy sample were analyzed.
Generation of PodoPalld−/− and PodoPalld-R26R Mice
To generate a podocyte-specific palladin-KO (PodoPalld−/−) mouse, 2.5P-Cre mice (kindly provided by Dr. Moeller et al.22) and mice containing a loxP site flanked exon (GGGGTTCCCA AAGAAGTCCA GTAGAACTGC TAGAATTGCC TCTGATGAGG AGATTCAAGG CACAAAGGAT GCTGTCATCC AAGACCTGGA ACGGAAGCTT CGCTTCAAGG AGGACCTTCT GAACAATGGC CAACCG; Ensemble Gene ID: ENSMUSG00000058056 or NCBI Gene ID: 72333, generated by M.-L.B., manuscript in preparation by Mastrotoraro et al.) in the palladin gene were mated. For experiments, PodoPalld−/− mice with heterozygous Cre-recombinase expression were used. Mice without Cre-recombinase expression were used as controls (PodoPalld+/+). Experiments were done with 6-month-old male mice with C57BL/6 genetic background (at least n=3 of each group).
PodoPalld mice were mated with R26R1M mice (MPI of Immunobiology and Epigenetics, Germany) containing a lacZ gene flanked by loxP sites, to verify podocyte-specific Cre-recombinase expression.23 Experiments were performed on 2-month-old male/female C57BL/6 PodoPalld-R26R1M (cre+/−, lacZ/lacZ) and PodoPalld-R26R1M (cre−/−, lacZ/lacZ) mice (at least n=3 for each group). Mice were housed as described previously.24 Primers for genotyping are listed in Supplemental Table 1.
Mouse Model of Nephrotoxic Serum–Induced GN and Analysis of Renal Function
Two- to 3-month-old male PodoPalld+/+ and PodoPalld−/− mice were injected with nephrotoxic serum (NTS) over 2 consecutive days (n=8 per group) or with PBS as control (n=4 per group). Urine samples were collected at days 0, 4, 8, and 12 and blood samples before euthanasia. Proteinuria was measured with a Konelab analyzer (Thermo Fisher Scientific) and normalized to urine creatinine. BUN and plasma creatinine levels were measured with an enzymatic method (Konelab analyzer), respectively.
Histology
Mouse kidneys were harvested and fixed. Paraffin sections (4 µm) were performed on a Leica SM 2000R (Leica Microsystems), stained with hematoxylin and eosin, and analyzed with an Olympus BX50 microscope (Olympus Europe, Germany).
Samples were snap-frozen in liquid nitrogen using Tissue-Tek (Sakura, Germany) and cut on a Leica CM-3050-S Cryostat. Cryosections of PodoPalld mice (7 µm) were stained for F-actin using Alexa Fluor 488–phalloidin. For detection of β-galactosidase activity, sections of PodoPalld-R26R mice (9 µm) were fixed with 2% glutaraldehyde and incubated in X-gal solution.25
Immunohistochemistry
After deparaffinization, sections of mouse kidneys and human biopsy samples were rehydrated and unmasked by heating in a pressure cooker. The following primary antibodies were used for immunofluorescence staining: palladin, palladin622 (Palld622), synaptopodin, α-actinin-4, and nephrin (Progen). Alexa Fluor 488– and Cy3-conjugated secondary antibodies were used. Images were acquired using a Leica TCS SP5 confocal laser scanning microscope and super-resolution microscope (Elyra PS.1; Carl Zeiss Microscopy, Germany).
For immunohistochemistry (IHC), the Vectastain kit (Vector Laboratories) was used following manufacturer’s instructions and palladin was detected with palladin antibody.
Analysis of Glomerular Morphology on Semithin Sections
Kidneys were harvested,24 embedded in Epon 812 (SERVA, Germany), and semithin sections were cut and stained with Richardson’s (Azur II/Methylene blue). At least 50 glomeruli of PodoPalld+/+ mice and PodoPalld−/− mice were categorized into glomeruli with (1) normal morphology, (2) dilated capillaries, and (3) affected podocytes (podocytes with cyst and enlarged subpodocyte space).
For qualitative ultrastructural investigations using electron microscopy, ultrathin sections were cut and contrasted with 5% uranyl acetate and lead citrate.
Podocyte Foot Process Effacement Measurement Procedure
The slit diaphragm density was measured by structured illumination microscopy as described recently.26 Briefly, image stacks of 20 glomeruli in three individual mice per group were acquired using a Zeiss Elyra PS.1 system equipped with a 63× (NA 1.4) oil immersion objective. Z-Stacks were recorded with a size of 2430 pixel2 (78.35 µm2) with a slice-to-slice distance of 0.2 µm over approximately 3.5 µm using the 561 nm laser. 3D structured illumination microscopy reconstruction was performed with the Zeiss ZEN Software. For automatic assessment of the slit diaphragm density, multiple areas per image stack with a plan view on the slit diaphragm were segmented and measured using a custom-made FIJI-based macro.26 The slit diaphragm density was stated as length of the slit diaphragm per glomerular capillary area in µm−1. Means of both groups were compared using unpaired t test using Prism 5 (GraphPad).
Zebrafish
Zebrafish of the AB background were bred in a pH- and temperature-controlled facility as previously described.27
Injection of Morpholinos
Morpholino (MO) injection into fertilized zebrafish eggs was described by Kotb et al.28 MOs were synthesized by Gene Tools LLC (Philomath) and the following sequences were used: control MOs (CtrlMO) 5′-CCTCTTACCTCAGTTACAATTTATA-3′ and palladin MOs (PalldMO) 5′-TGTCATTCCAGCTCCCGTCCTGCAT-3′.
Staining and Live Cell Imaging of Zebrafish Larvae
Larvae were fixed in methanol/acetone (1:1) and incubated with rabbit anti-palladin622 antibody and Alexa Fluor 488–phalloidin. For palladin detection, larvae were incubated with Cy3-conjugated anti-rabbit secondary antibody.
For in vivo observation, zebrafish larvae at 30 hours postfertilization were used and images were taken every 12 minutes for 1 hour using a Leica TCS SP5 confocal LSM.
Statistical Analyses
All data are given as mean±SD or ±SEM, analyzed by unpaired t test with repeated measurements. Differences were regarded as significant at a P value <0.05.
Results
Palladin Is Expressed in Podocytes In Vivo and In Vitro
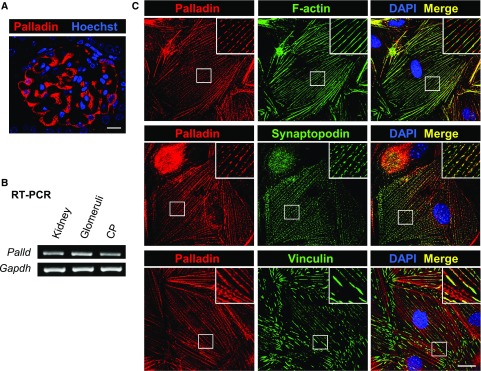
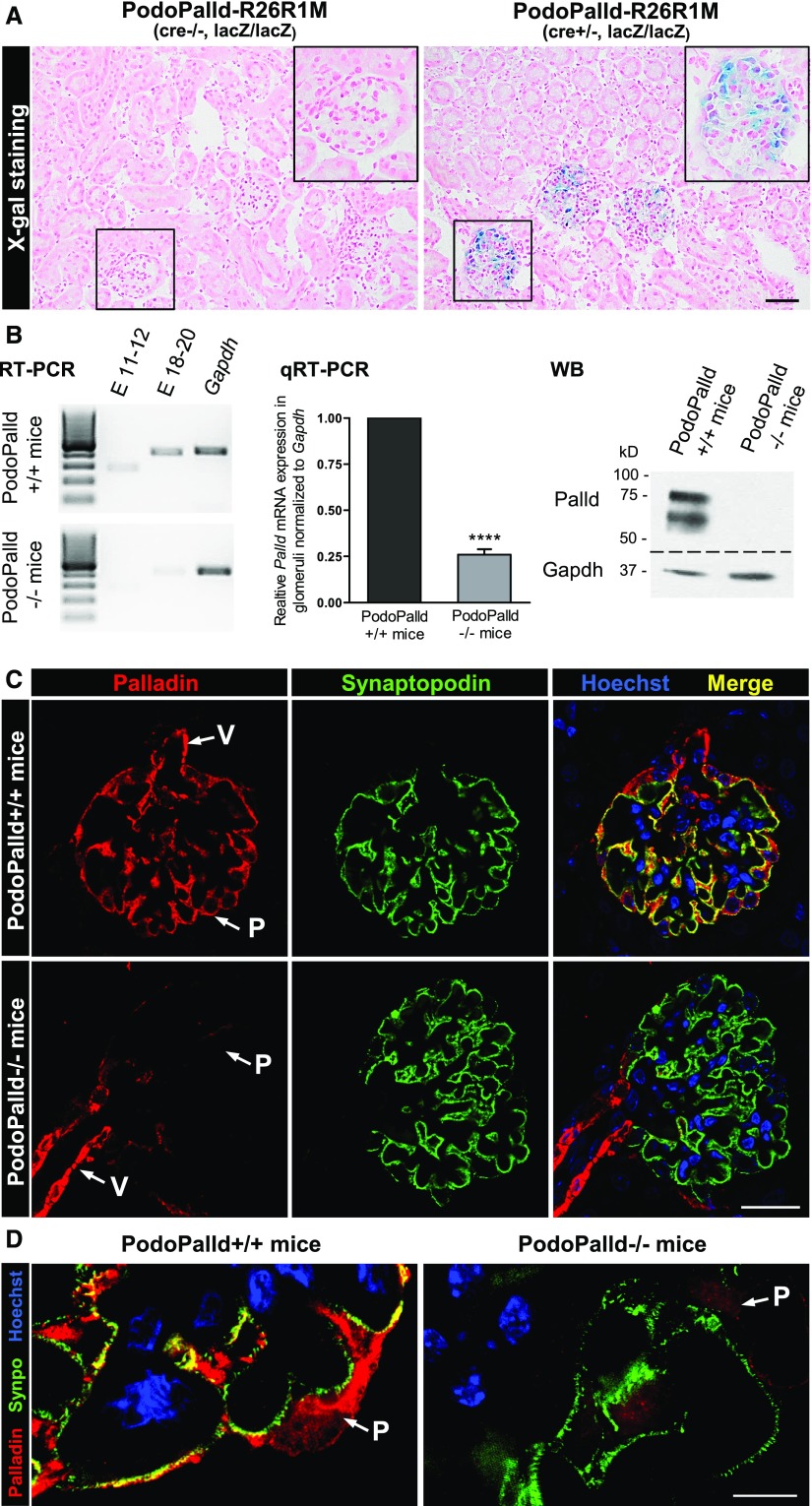
RT-PCR as well as IHC of mouse kidney sections and cultured mouse podocytes (CPs) demonstrated that podocytes express palladin (Figure 1). By double staining of cultured podocytes with antibodies against the podocyte-specific and actin-associated proteins synaptopodin and palladin, we found that both proteins are colocalized in dense bodies along actin filaments (Figure 1C). Because it was also reported that palladin is a part of focal adhesions, we stained for palladin and for the focal adhesion protein vinculin. As shown in Figure 1C, palladin is also expressed in focal adhesions.
Figure 1.
Palladin is colocalized with F-actin, synaptopodin and vinculin in podocytes. (A) Staining of murine kidney sections with anti-palladin antibody indicated a strong expression of palladin in podocytes. Scale bar represents 10 µm. (B) Additionally, palladin expression in kidney, glomeruli, and CP was confirmed by RT-PCR using palladin primers spanning exons 18–20. (C) Double staining of cultured podocytes showed that palladin is colocalized with F-actin, synaptopodin, and vinculin. Scale bar represents 20 μm. DAPI, 4′,6-diamidino-2-phenylindole.
To determine the specific isoforms (UniProt ID Q9ET54 and D3Z1J5, Supplemental Figure 1A) that are expressed in podocytes of mice in vivo and in vitro, we performed western blots of isolated glomeruli as well as cultured podocytes (Supplemental Figure 1B). Because antibodies often selectively bind specific isoforms, we further performed qRT-PCR. As shown in Supplemental Figure 1B, a strong signal was found in isolated glomeruli but not in cultured podocytes by using primers for exons 2–3. This signal may correspond to the 60-kD isoform identified in the western blots or to a new N-terminal isoform.
As shown in Supplemental Figure 1B, podocytes in vivo express 50-, 60-, and 73-kD isoforms in contrast to CP that expresses 50-, 73-, and 140-kD isoforms.
Knockdown of Palladin Affects the Actin Cytoskeleton and Focal Adhesions
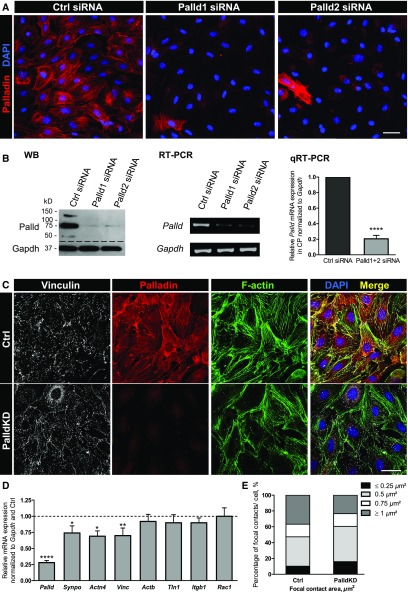
To investigate the role of palladin in the morphology and stability of the actin cytoskeleton, palladin-knockdown (PalldKD) experiments were performed. After the transfection of CPs with two different siRNAs, Palld1 and Palld2 siRNA, the almost complete loss of the protein and mRNA expression could be shown by immunofluorescence staining as well as by western blot (Figure 2, A and B) and RT-PCR/qRT-PCR (−80%±4%, n=4, P<0.001).
Figure 2.
Palladin knockdown leads to a downregulation of cytoskeletal proteins in cultured podocytes. For studying the role of palladin in vitro, CPs were transfected with siRNA. (A) To confirm the knockdown efficiency, podocytes were stained for palladin. Scale bar represents 50 µm. (B) The strong reduction of palladin expression was also shown by western blot (20 µg/lane), RT-PCR, and qRT-PCR (palladin primers spanning exons 18–20). Palladin mRNA expression of Palld1 or -2 siRNA-transfected podocytes was normalized to Gapdh and control-transfected (Ctrl) podocytes (means±SD, n=4, ****P<0.001). (C) Cultured mouse PalldKD podocytes showed weaker signals for vinculin and a marked reduction of parallel actin stress fibers in immunofluorescence staining compared with control-transfected podocytes. Scale bar represents 50 µm. (D) Additionally, a significant reduction of vinculin, synaptopodin, and α-actinin-4 mRNA was confirmed by qRT-PCR. No remarkable changes were observed for β-actin, talin-1, β1-integrin, and Rac1, respectively (mean±SEM, n=7, *P<0.05, **P<0.01, ****P<0.001). (E) Furthermore, focal adhesions were analyzed using podocytes stained for vinculin. PalldKD podocytes showed an increased number of small adhesions (≤0.5 µm2) and a decreased number of adhesions with an area of ≥1 µm2 compared with Ctrl. The percentage of focal adhesions with an area of 0.75 µm2 remained unchanged. DAPI, 4',6-diamidino-2-phenylindole; qRT-PCR, quantitative reverse transcription PCR; siRNA, small interfering RNA; WB, Western Blot.
To study the influence of palladin on the stability of the actin cytoskeleton, we compared the actin cytoskeleton stained with Alexa Fluor 488–phalloidin of PalldKD podocytes and control-transfected podocytes (Ctrl) by immunofluorescence. PalldKD podocytes developed fewer parallel actin filaments in contrast to Ctrl. The reduction of actin filaments was associated with a significantly reduced expression of the podocyte-specific and actin-binding proteins synaptopodin and α-actinin-4 mRNA (Figure 2, C and D, Supplemental Figure 2).
Moreover, we analyzed the influence of palladin on the number and size of focal adhesions. For determination of the size of the focal adhesions, we quantified the areas of the focal adhesions using custom-designed image analysis software (cf. Methods section). Our analysis revealed that PalldKD podocytes possessed more focal adhesions with an area of ≤0.5 µm2 and fewer focal adhesions with an area of ≥1 µm2 compared with Ctrl (Figure 2E). Interestingly, the percentage of focal adhesions with an area of 0.75 µm2 remained unchanged. We found that the average area of focal adhesions in PalldKD podocytes was smaller than in Ctrl (0.63 µm2 versus 0.88 µm2). Furthermore, we found by qRT-PCR that the mRNA of the focal adhesion protein vinculin was significantly downregulated by 30%±12% (n=7, P<0.05) in PalldKD podocytes. In contrast, the mRNA expression of β-actin (92%±11%), talin-1 (90%±12%), β1-integrin (90%±7%), and Rac1 (100%±13%) was unaffected in PalldKD podocytes (Figure 2D). In summary, our data show that the loss of palladin leads to a higher number of small focal adhesions and to a concomitant reduction of vinculin.
Knockdown of Palladin Influences the Migration of Podocytes and Actin Polymerization
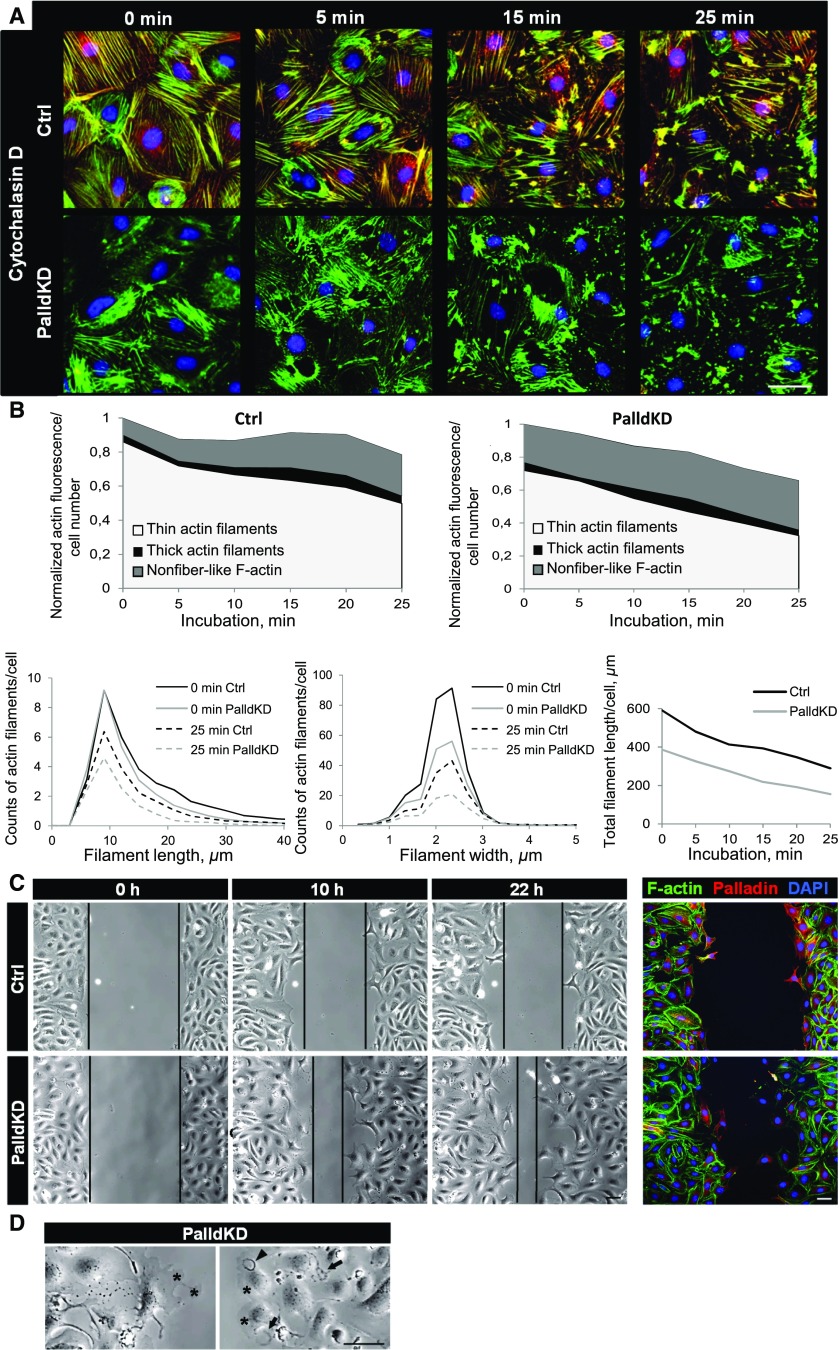
Because the actin cytoskeleton was affected by the PalldKD, we studied the dynamics of the polymerization/disassembly of actin filaments by the use of two differently acting inhibitors of actin polymerization, cytochalasin D and latrunculin A. Cytochalasin D as well as latrunculin A induced a disassembly of the actin filaments in PalldKD podocytes compared with the Ctrl (Figure 3A, Supplemental Figure 3). For quantification, we determined the filament length and size distribution with a recently developed image analysis software.19 Actin filaments were classified into three categories: thin actin filaments, thick actin filaments, and nonfiber-like F-actin. Under baseline conditions, PalldKD podocytes had fewer thin actin filaments, more nonfiber-like F-actin, a lower number of longer (>10 µm) filaments, and a reduced total filament length by about one third as compared with Ctrl (Figure 3B, Supplemental Figure 3). PalldKD podocytes that were treated with cytochalasin D or latrunculin A showed decreased numbers of thin filaments (35.2% and 32.1%) in contrast to Ctrl. The number of actin filaments with a length between 10 and 30 µm was decreased by 46.8%±13.7% after cytochalasin D and by 43.0%±13.7% after latrunculin A treatment over 25 minutes. The number of filaments with a width between 1.5 and 3 µm decreased by 44.3%±5.4% after cytochalasin D and by 46.4%±4.5% after latrunculin A treatment of 25 minutes. However, during the incubation period over 25 minutes, the reduction of the total filament length was similar in Ctrl and PalldKD in cytochalasin D– (39.4%±6.0%) and latrunculin A–treated PalldKD cells (41.3%±6.1%) (Figure 3B, Supplemental Figure 3).
Figure 3.
Palladin knockdown in cultured podocytes influences actin polymerization and stabilization as well as cell motility. (A) CPs were incubated with cytochalasin D (0.5 µM) for 25 minutes. Panels show fixed cells at different points in time, stained for F-actin (green), palladin (red), and nuclei (blue). (B) After 25 minutes, PalldKD podocytes had fewer thin actin filaments and more nonfiber-like F-actin, respectively, compared with Ctrl podocytes. Furthermore, long and wide actin filaments as well as the total actin filament length were decreased. Treatment with cytochalasin D reduced the total filament length in PalldKD and Ctrl podocytes similarly. (C) Migration assays were performed to study the migration of PalldKD podocytes over 22 hours. Images taken by brightfield microscopy show different time points (0, 10, 22 hours). The distance between the migrating areas is marked by black lines and revealed that PalldKD podocytes migrated faster into the gap than the Ctrl. After live imaging, podocytes were fixed and stained for palladin to confirm PalldKD. (D) PalldKD podocytes showed dynamic structures such as lamellipodia (asterisks), ruffles (arrowhead), and ring-like actin structures (arrows). Scale bars in (A, C, and D) represent 50 µm. DAPI, 4',6-diamidino-2-phenylindole.
Furthermore, we studied the influence of jasplakinolide, an F-actin stabilizing drug leading to a disruption of filaments, on the cytoskeleton of PalldKD and Ctrl podocytes. After an incubation of 25 minutes, the disrupted actin filaments vanished completely in PalldKD cells in contrast to the Ctrl (Supplemental Figure 4).
Furthermore, it was already reported that palladin influences cell migration.6 Therefore, we studied whether the motility of podocytes depends on palladin expression. PalldKD and Ctrl podocytes were cultured in a migration chamber as confluent layers separated into two distinct areas by a removable plastic partition in the middle of the chamber. After removing the partition, the dynamics of podocytes were followed over 22 hours by brightfield microscopy. We found that PalldKD podocytes migrated significantly faster into the gap (Figure 3C, Supplemental Material). Furthermore, PalldKD podocytes developed highly dynamic structures such as lamellipodia, ruffles, and ring-like structures, respectively (Figure 3D, Supplemental Material).
Taken together, PalldKD podocytes had fewer actin fibers, were more susceptible to inhibition of actin polymerization, and exhibited a more migratory phenotype.
Palladin Knockdown Affects the Morphology of Zebrafish Embryos
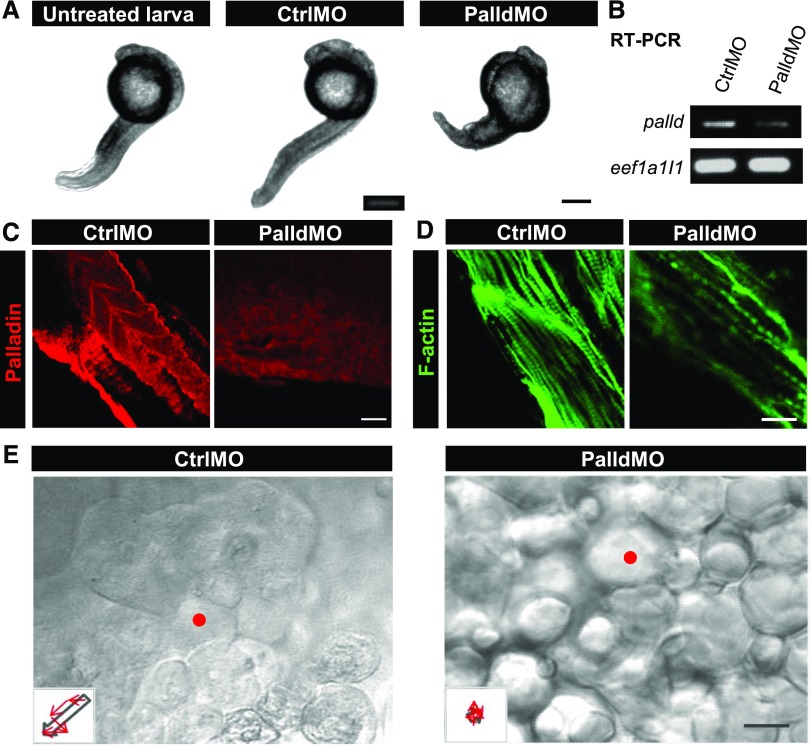
Because the KO of palladin in mice is lethal in utero,4 the zebrafish model was used as an in vivo model to study the role of palladin during development. After the injection of specific MOs against palladin (PalldMO) into fertilized zebrafish eggs at the one- to four-cell stage, we found that nearly 100% of the PalldMO embryos died after 16–18 hours.
To increase the survival rate, we injected the PalldMO into the eggs at later time points (eight- to 32-cell stage) to generate a mosaic phenotype. These PalldMO embryos survived beyond 16–18 hours (Figure 4A). The knockdown of palladin was confirmed by RT-PCR and IHC (Figure 4, B and C).
Figure 4.
Knockdown of palladin in zebrafish larvae compromises the morphology and the dynamics of epithelial cells. To study the role of palladin in vivo, fertilized zebrafish eggs were injected with MOs against palladin (PalldMO) or control MOs (CtrlMO). (A) At 2 days postfertilization, zebrafish larvae with palladin knockdown are less developed than the control and untreated larvae, respectively. (B and C) The reduced palladin expression was confirmed by RT-PCR and immunofluorescence staining. The staining shows myotomes of the middle to distal region of the zebrafish larvae. (D) Staining with phalloidin demonstrated reduced F-actin staining of myotomes after knockdown of palladin. CtrlMO larvae developed bundled actin filaments in contrast to a few filaments in PalldMO larvae. (E) Epithelial cells of CtrlMO larvae showed an intact flat epithelial layer, in contrast to PalldMO larvae showing cells with a rounded morphology. In migration studies, one selected cell (red mark) was observed over 1 hour and the cell movement is presented by trajectories (insets). In CtrlMO larvae, the cell migrates in a direction shown by the arrow, whereas in PalldMO larvae the cell does not exhibit directed migration. Scale bars represent (A) 100 µm (C–E) and 25 µm, respectively.
IHC of the embryos revealed that the actin cytoskeleton was disturbed due to the knockdown of palladin. Instead of highly organized F-actin bundles in myotomes of control embryos, PalldMO embryos developed only a few actin bundles that were disorganized (Figure 4D). Further, we observed that instead of an intact flat epithelial layer, the epithelial cells of the PalldMO embryos had a rounded morphology (Figure 4E) and instable cell-cell contacts. Exposing the embryos to very faint mechanical forces resulted in a dissociation of the whole cell layer. Beside this, the trajectories of the migration of single epithelial cells (insets of Figure 4E) demonstrated that cell migration in PalldMO embryos is undirected in contrast to that observed in control embryos (Figure 4E, Supplemental Material).
Summarizing, palladin knockdown in zebrafish embryos leads to disorganized actin filaments, impaired cell migration, and early embryonic death.
Podocyte-Specific KO of Palladin in Mice Disturbs the Morphology of Glomeruli and Podocytes
Because the KO of palladin in mice is lethal, we generated mice with a podocyte-specific KO of palladin (PodoPalld−/−) using the Cre/loxP system. We verified the expression of the NPHS2 promoter-dependent Cre-recombinase by an X-gal staining of PodoPalld-R26R1M (cre+/−, lacZ/lacZ) kidney sections (Figure 5A). Additionally, we confirmed the palladin KO by IHC, RT-PCR/qRT-PCR, and western blot analysis (Figure 5, B–D). The detection of a weak signal for palladin by RT-PCR is caused by a slight contamination of the isolated glomeruli with small vessels that still express palladin (Figure 5, B and C, Supplemental Figure 5).
Figure 5.
Confirmation of the podocyte-specific Cre-recombinase expression and palladin KO in PodoPalld−/− mice. (A) The specific expression of Cre-recombinase in podocytes was verified by the X-gal staining of PodoPalld-R26R1M (cre+/−, lacZ/lacZ) kidney cryosections. The blue staining revealed β-galactosidase–expressing podocytes and confirmed the podocyte-specific expression of Cre-recombinase. Podocytes of PodoPalld-R26R1M (cre−/−, lacZ/lacZ) mice without Cre-recombinase showed no staining. Sections were counterstained using nuclear fast red. Scale bar represents 50 µm. (B) In PodoPalld−/− mice, palladin KO was confirmed in isolated glomeruli by RT-PCR (palladin primers spanning exons [E] 11–12 and 18–20), qRT-PCR (palladin primers spanning E 18–20), and western blot (8 µg/lane). Palladin expression was normalized to Gapdh in the qRT-PCR analysis (mean±SD, n=3, ****P<0.001). (C and D) Podocyte-specific KO is shown by immunofluorescence staining of kidney paraffin sections. Pictures were taken by (C) laser scanning and (D) structured illumination microscopy. There is no palladin signal in podocytes (P) but a strong signal in vascular smooth muscle cells (V), which can be taken as a positive control. The presence of podocytes is confirmed by synaptopodin (Synpo) staining. Scale bars represent (C) 20 µm and (D) 5 µm, respectively. qRT-PCR, quantitative reverse transcription PCR; WB, Western blot.
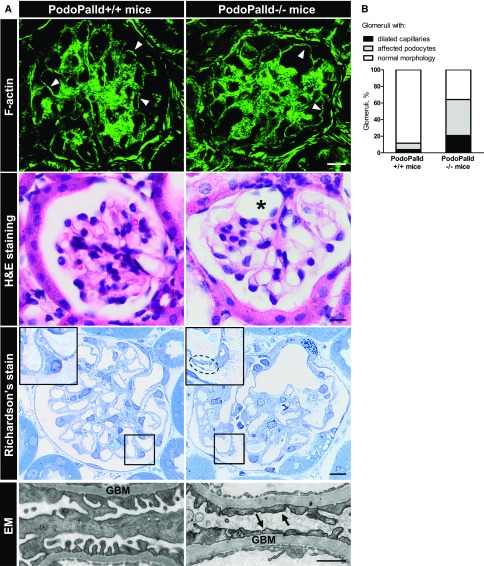
To study the morphology of PodoPalld−/− glomeruli, kidney sections were performed and stained with Alexa Fluor 488–phalloidin, hematoxylin and eosin, and Richardson’s stain, respectively. The stained sections showed a marked dilation of the capillary tuft (asterisk in Figure 6A, graph in Figure 6B) that affected 20% of the glomeruli (Figure 6B). Moreover, semithin sections of the kidneys revealed an enlarged subpodocyte space (inset in Figure 6A). For almost half of the glomeruli, noticeable deviations as an enlarged subpodocyte space and cyst formation were found in podocytes (Figure 6B). By electron microscopy, we observed a mild effacement in the PodoPalld−/− glomeruli, shown in Figure 6A.
Figure 6.
PodoPalld−/− mice develop glomeruli and podocytes with a disturbed morphology. (A) Staining of mouse kidney cryosections for F-actin did not show any difference in the cytoskeleton (arrowheads). However, hematoxylin and eosin (H&E) staining of kidney paraffin sections revealed highly dilated capillaries (asterisk) in PodoPalld−/− mice. Furthermore, podocytes with enlarged subpodocyte space (dotted line) were found in Richardson’s-stained semithin sections as illustrated in the higher magnification. Scale bars represent 10 µm. Additionally, electron microscopy (EM) revealed a mild effacement of podocyte foot processes (arrows) at the glomerular basement membrane (GBM). Scale bar represents 1 µm. (B) PodoPalld−/− mice had significantly more glomeruli with dilated capillaries and affected podocytes (podocytes with enlarged subpodocyte space and cyst) than PodoPalld+/+ mice.
This means that the podocyte-specific KO of palladin resulted in overt morphologic changes of glomeruli and podocytes in mice.
Mild Foot Process Effacement and Increased Susceptibility to Injury in PodoPalld−/− Mice
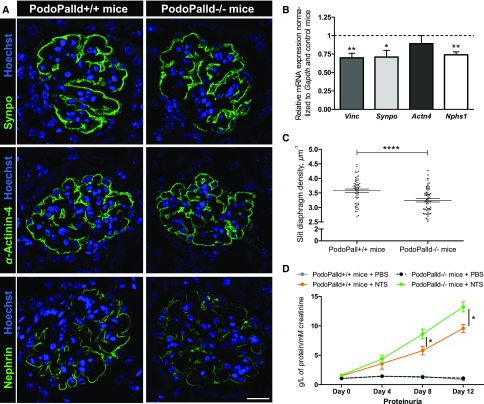
The PodoPalld−/− mice showed morphologic abnormalities. For that reason, we checked for differential expression of vinculin, α-actinin-4, nephrin, and synaptopodin by immunohistology and qRT-PCR, respectively.
As shown in Figure 7A, the protein expression of α-actinin-4 and synaptopodin was not markedly affected in PodoPalld−/− mice in contrast to the expression of nephrin. Further, the mRNA of vinculin (−30%±6%), synaptopodin (−29%±9%), and nephrin (−26%±4%) was significantly downregulated in isolated glomeruli of PodoPalld−/− mice in contrast to the controls (n=7, P<0.05, P<0.01, Figure 7B). To determine more precisely the changes of the foot process morphology using staining of the slit diaphragm by super-resolution microscopy, we employed the podocyte effacement measurement procedure on nephrin-stained kidney sections, which was recently established by Siegerist et al.26 In PodoPalld−/− mice, we found a significant reduction of the slit diaphragm density compared with the control mice (3.58±0.06 µm−1 versus 3.24±0.06 µm−1, n=58 and n=60 glomeruli of three mice per group, P<0.001) (Figure 7C).
Figure 7.
Palladin KO in podocytes leads to reduced nephrin expression in vivo and wider foot processes. (A) Immunofluorescence staining of kidney paraffin sections. There is no obvious difference in synaptopodin and α-actinin-4 expression in PodoPalld−/− mice compared with control mice, but a reduction of nephrin was observed. Scale bar represents 20 µm. (B) The significant downregulation of vinculin, synaptopodin, and nephrin mRNA in PodoPalld−/− mice by qRT-PCR (mean±SEM, n=7 per group, *P<0.05, **P<0.001). (C) The graphic shows the measurements of the slit diaphragm density of nephrin-stained paraffin sections. The slit diaphragm density was significantly lower in PodoPalld−/− mice compared with PodoPalld+/+ mice (mean±SEM, n=58 and n=60 glomeruli of three mice per group, ****P<0.001). (D) PodoPalld−/− mice showed significantly more aggravated proteinuria compared with control mice after NTS-injection at day 8 and day 12. No proteinuria was observed in mice treated with PBS (mean±SEM, PodoPalld+/+ and PodoPalld−/− mice+PBS n=4 each, PodoPalld+/+ and PodoPalld−/− mice+NTS n=8 each, *P<0.05). qRT-PCR, quantitative reverse transcription PCR; Synpo, Synaptopodin; Vinc, Vinculin.
After the injection of NTS, we found a significant increase of proteinuria in PodoPalld−/− mice (day 8: 8.61±0.82 g/L and day 12: 13.22±0.86 g/L protein per mM creatinine) compared with PodoPalld+/+ mice (day 8: 5.78±0.73 and day 12: 9.57±0.67 g/L protein per mM creatinine; n=8 per group, P<0.05) as shown in Figure 7D. PodoPalld+/+ and PodoPalld−/− mice treated with PBS as controls did not develop proteinuria.
Taken together, palladin is essential for a proper podocyte foot process morphology and therefore for an intact filtration barrier.
Palladin Is Downregulated in Glomeruli of Patients Suffering from Diabetic Nephropathy and FSGS
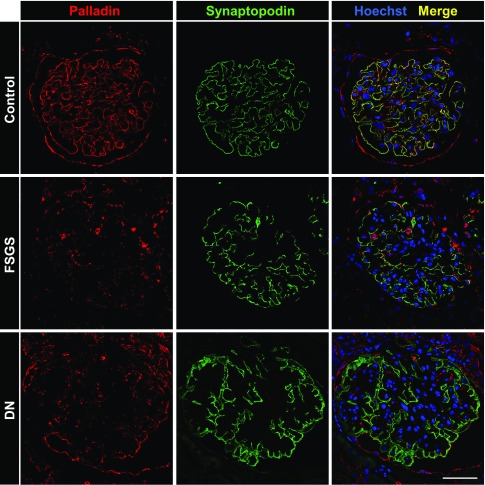
To find out whether the expression of palladin might be regulated in patients suffering from diabetic nephropathy (DN) and FSGS, renal biopsy samples were stained with an antibody against palladin. Palladin was nearly absent in podocytes of patients suffering from DN and FSGS in contrast to control kidneys (Figure 8).
Figure 8.
Palladin expression is downregulated in glomeruli of patients suffering from FSGS and DN. Human renal biopsy samples were stained for palladin and synaptopodin. In control kidneys both proteins colocalized in podocytes. In biopsy samples from patients suffering from FSGS or DN, palladin expression showed a significant reduction, whereas synaptopodin expression was unchanged. Scale bar represents 50 µm.
Discussion
Palladin, a key protein for actin bundling, nucleation, and polymerization, influences the morphology and the dynamic behavior of different cell types.1,4,8,29 Recently, we have shown that palladin is highly expressed in the kidney, especially in podocytes.8,17,30 Furthermore, it was shown that the isoform 4 of palladin is significantly upregulated in ANCA-GN.31
Because the morphology and function of podocytes are highly dependent on the actin cytoskeleton, we focused our investigation on the role of the actin-binding and regulating protein palladin. We found that the knockdown of palladin in cultured podocytes significantly reduced the number of actin fibers; in addition, the remaining filaments were less bundled in contrast to the control. This finding is in agreement with former studies done with fibroblasts and trophoblasts.1 Dixon et al.8 nicely showed that the palladin-induced actin bundling is associated with the Ig3 and Ig4 domains.
This study shows that a reduction of palladin is not only associated with a reduction of actin fibers but also with a reduced expression of the podocyte-specific and actin-binding proteins synaptopodin and α-actinin-4. This is of specific interest because it is known that mutations or the loss of α-actinin-4 in vivo lead to severe kidney diseases due to the effacement of the podocyte foot processes.32
To study the role of palladin in actin depolymerization, cultured podocytes were treated with cytochalasin D, latrunculin A, and jasplakinolide. These substances inhibit actin polymerization by different mechanisms.33–35 Cytochalasin D and latrunculin A inhibit actin polymerization by binding either to the barbed end of actin filaments or by sequestering actin monomers.33,36 Treatment of palladin-knockdown podocytes with cytochalasin D and latrunculin A significantly increased the number of short and thin filaments compared with the controls. This supports the findings of Gurung et al.29 that palladin stabilizes actin filaments and thereby prevents the dissociation of actin monomers from filaments. The increase of nonfiber-like F-actin under palladin-knockdown conditions might also be caused by the loss of the stabilization of newly formed filaments by palladin. In contrast to the results described by Niedenberger et al.,37 we did not observe a relocation of palladin to the cell nucleus after the treatment of podocytes with cytochalasin D.
In the presence of jasplakinolide, we observed a nearly complete loss of all actin fibers in palladin-knockdown cells. In contrast to cytochalasin D and latrunculin A, the depolymerization mechanism of jasplakinolide is completely different. Jasplakinolide stabilizes the actin filaments in a similar way to phalloidin, resulting in a tension-mediated rupture of the filaments. We hypothesize that palladin blocks the binding sites for jasplakinolide and therefore prevents the disruption of actin filaments in palladin-expressing cells. Furthermore, because it is described that palladin has a formin-like function,29 our results suggest that palladin is also essential to nucleate new actin filaments.
Because palladin is also expressed in focal adhesions1,17 and detachment of podocytes plays a central role in chronic glomerulopathies such as DN and FSGS, we studied the effect of palladin with regard to the expression of focal adhesion proteins such as vinculin, talin-1, and β1-integrin, all expressed in podocytes in vitro and in vivo.14,38–40 We found that vinculin was significantly downregulated in palladin-knockdown podocytes, whereas talin-1, a protein which is described to be a mechanosensor,41 and β1-integrin, one essential integrin for proper podocyte adhesion in vivo,42 were not affected.
Quantitative analysis of vinculin-positive focal adhesions revealed that the reduction of palladin led to a reduction of the focal adhesion area compared with the controls. This observation is in agreement with recent findings by Azatov et al.,12 who measured shorter focal adhesions in palladin-deficient tumor-associated fibroblasts. They found that a reduction of the focal adhesion size also influenced cellular traction forces and mechanosensitivity of the cells.12 Whether palladin influences the traction forces or mechanosensitivity of podocytes needs to be investigated in the future.
Although vinculin is such an important component of the integrin-mediated linkage of actin filaments to the extracellular matrix as well as a force-transducing and regulating protein,43 only a few studies have investigated the role of vinculin in podocytes to date.44 It was suggested that vinculin is able to recruit proteins such as α-actinin and Arp2/3 in podocytes as was described for other cell types.45 Therefore, palladin most likely modulates the adhesion and outside-in signaling in podocytes via vinculin as was reported for neuronal N1E-115 cells and NIH3T3 cells.46,47 In this context, Miao et al.48 have recently shown that puromycin aminonucleoside treatment of rats is associated with podocyte foot process effacement together with a downregulation of vinculin. Furthermore, Babayeva et al.49 observed a dispersion of vinculin-positive focal contacts in cultured podocytes after incubation with plasma of patients suffering from FSGS. These findings indicate an essential role of vinculin in the maintenance of properly formed foot processes which might be influenced by palladin.
Because of the observation that actin plays a key role in cell motility,50 we investigated the influence of palladin on podocyte migration and dynamics. Migration studies of cultured podocytes revealed that palladin-knockdown podocytes showed a lateral as well as a stationary motility,17 exhibiting highly dynamic structures such as ring-like structures in contrast to the controls. These results are in agreement with former studies presented by Chin and Toker6 and Asano et al.13 However, these results obtained in cell culture are not in agreement with the results obtained in animal models. Here, the palladin-KO cells neither migrate in lateral nor in basal directions.4 To study the influence of palladin in a living organism and to overcome the problem that palladin KO in mice is lethal at a very early time point in utero,4 we used the zebrafish larvae as a model organism for in vivo observation studies. Similar to palladin-KO mice, the KO in zebrafish embryos is also lethal at an early time point. By the injection of palladin-specific MOs at later developmental time points, we induced a viable mosaic knockdown in the zebrafish embryos. Surprisingly, we observed that the epithelial cells of the embryos have a different cell morphology. Instead of a flat epithelial morphology observed in palladin-expressing cells, we found a rounded cell shape after palladin knockdown. Additionally, in vivo observation of palladin-knockdown cells showed that these cells were highly dynamic without lateral migration (stationary motility), suggesting that the palladin-knockdown cells were unable to migrate in a specific direction. These findings are in agreement with prior studies where a palladin KO reduced the capability for invasion and migration.31,51,52
To study the role of palladin in podocytes in mice, we generated podocyte-specific palladin-KO mice (PodoPalld−/−). Morphologic analysis of these mice revealed that beside highly dilated capillaries, podocytes with an enlarged subpodocyte space were present, a phenotype previously described by Kriz and colleagues53,54 in a variety of models. Ultrastructural analysis further showed that beside areas with well developed podocyte foot processes we also found a higher number of effaced foot processes. That palladin KO affects podocyte foot processes was further validated by super-resolution microscopy, revealing a significant reduction of the slit diaphragm density, a marker inversely correlated with foot process width,26 in PodoPalld−/− mice. Moreover, the slit diaphragm protein nephrin was significantly downregulated in these mice. Surprisingly, despite these clear changes of podocyte and glomerular morphology, we did not detect albumin in the urine of PodoPalld−/− mice. This might be due to an effective reabsorption of filtered albumin by the tubules as was postulated by Dickson et al.55 However, we observed in the established model of NTS-induced GN that PodoPalld−/− mice developed a significantly increased proteinuria compared with NTS-treated controls, demonstrating an essential role of palladin for a proper glomerular filtration barrier.56,57
To find out whether palladin is differentially expressed in podocytes of patients suffering from chronic glomerulopathies, we stained human biopsy samples from patients with DN and FSGS for palladin. Interestingly, the palladin expression was significantly reduced in these biopsy samples compared with control tissue, indicating a regulation of the protein in these kidney diseases.
Taken together, this study demonstrates that palladin plays an important role in the morphology and dynamic behavior of podocytes in vivo and in vitro.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Regina Maciejewski and Henny Wegner for technical assistance. The 2.5P-Cre mice and primers for genotyping were kindly provided by Dr. Marcus J. Moeller (Department of Internal Medicine II, Nephrology and Clinical Immunology, Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen University Hospital, Aachen, Germany).
Part of the work was supported by an Emerging Fields Initiative for Cell Cycle in Disease and Regeneration from the Friedrich-Alexander-Universität Erlangen-Nürnberg (Germany). This study was supported by a grant from the German Research Foundation (DFG, grant INST 2026/131, FUGG) to K.E. and N.E. and by a grant from the Federal Ministry of Education and Research (BMBF, grant 01GM1518B, STOP-FSGS) to N.E. This work was also partially supported by grants from the Italian Space Agency (ASI) (grant number: DC-DTE-2011-2013) and the Italian Ministry of Education, Universities and Research (PRIN 2010–2011 grant number 2010R8JK2X_006) to M.-L.B. The work was funded by the Forschungsverbund Molekulare Medizin, University Medicine Greifswald, Greifswald, Germany, to N.E.
The study was designed by N.E., K.E., and N.A.; cell culture experiments and morphologic analysis of mouse kidneys were conducted by N.A.; biopsy samples were handled and analyzed by N.A.; T.A.L. performed the zebrafish experiments; induction of GN by nephrotoxic serum injection and analysis of mouse urine samples were performed by P.K., C.E.C., and C.C.; H.R. programmed the software F_Seg and the focal adhesion analysis software; F.S. performed super-resolution analysis of the slit diaphragm density; electron microscopy was conducted by A.B.; M.-L.B. generated floxed Palld mice; J.v.d.B. provided PodoPalld mice; C.A.O. and K.A. generated the palladin antibody and handled human kidney biopsy samples, respectively; all other experiments were performed by N.A.; experimental data were analyzed by N.A.; N.A., N.E., and K.E. wrote the main manuscript text. N.A. prepared figures. All authors reviewed the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017091039/-/DCSupplemental.
References
- 1.Parast MM, Otey CA: Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol 150: 643–656, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H-V, Moser M: Comparative expression analysis of the murine palladin isoforms. Dev Dyn 237: 3342–3351, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Rachlin AS, Otey CA: Identification of palladin isoforms and characterization of an isoform-specific interaction between Lasp-1 and palladin. J Cell Sci 119: 995–1004, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Luo H, Liu X, Wang F, Huang Q, Shen S, Wang L, et al.: Disruption of palladin results in neural tube closure defects in mice. Mol Cell Neurosci 29: 507–515, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Tan J, Chen X-J, Shen C-L, Zhang H-X, Tang L-Y, Lu S-Y, et al.: Lacking of palladin leads to multiple cellular events changes which contribute to NTD. Neural Dev 12: 4, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin YR, Toker A: The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell 38: 333–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boukhelifa M, Parast MM, Valtschanoff JG, LaMantia AS, Meeker RB, Otey CA: A role for the cytoskeleton-associated protein palladin in neurite outgrowth. Mol Biol Cell 12: 2721–2729, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon RDS, Arneman DK, Rachlin AS, Sundaresan NR, Costello MJ, Campbell SL, et al.: Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem 283: 6222–6231, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Rönty M, Taivainen A, Moza M, Otey CA, Carpén O: Molecular analysis of the interaction between palladin and alpha-actinin. FEBS Lett 566: 30–34, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Boukhelifa M, Moza M, Johansson T, Rachlin A, Parast M, Huttelmaier S, et al.: The proline-rich protein palladin is a binding partner for profilin. FEBS J 273: 26–33, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Boukhelifa M, Parast MM, Bear JE, Gertler FB, Otey CA: Palladin is a novel binding partner for Ena/VASP family members. Cell Motil Cytoskeleton 58: 17–29, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Azatov M, Goicoechea SM, Otey CA, Upadhyaya A: The actin crosslinking protein palladin modulates force generation and mechanosensitivity of tumor associated fibroblasts. Sci Rep 6: 28805, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asano E, Maeda M, Hasegawa H, Ito S, Hyodo T, Yuan H, et al.: Role of palladin phosphorylation by extracellular signal-regulated kinase in cell migration. PLoS One 6: e29338, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drenckhahn D, Franke RP: Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 59: 673–682, 1988 [PubMed] [Google Scholar]
- 15.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, et al.: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, et al.: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Endlich N, Schordan E, Cohen CD, Kretzler M, Lewko B, Welsch T, et al.: European Renal cDNA Bank Consortium : Palladin is a dynamic actin-associated protein in podocytes. Kidney Int 75: 214–226, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, et al.: Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Rogge H, Artelt N, Endlich N, Endlich K: Automated segmentation and quantification of actin stress fibres undergoing experimentally induced changes. J Microsc 268: 129–140, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Kliewe F, Scharf C, Rogge H, Darm K, Lindenmeyer MT, Amann K, et al.: Studying the role of fascin-1 in mechanically stressed podocytes. Sci Rep 7: 9916, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kindt F, Hammer E, Kemnitz S, Blumenthal A, Klemm P, Schlüter R, et al.: A novel assay to assess the effect of pharmaceutical compounds on the differentiation of podocytes. Br J Pharmacol 174: 163–176, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Moeller MJ, Kovari IA, Holzman LB: Evaluation of a new tool for exploring podocyte biology: Mouse Nphs1 5′ flanking region drives LacZ expression in podocytes. J Am Soc Nephrol 11: 2306–2314, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Schordan S, Grisk O, Schordan E, Miehe B, Rumpel E, Endlich K, et al.: OPN deficiency results in severe glomerulosclerosis in uninephrectomized mice. Am J Physiol Renal Physiol 304: F1458–F1470, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol 13: 1561–1567, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Siegerist F, Ribback S, Dombrowski F, Amann K, Zimmermann U, Endlich K, et al.: Structured illumination microscopy and automatized image processing as a rapid diagnostic tool for podocyte effacement. Sci Rep 7: 11473, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller T, Rumpel E, Hradetzky S, Bollig F, Wegner H, Blumenthal A, et al.: Non-muscle myosin IIA is required for the development of the zebrafish glomerulus. Kidney Int 80: 1055–1063, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Kotb AM, Simon O, Blumenthal A, Vogelgesang S, Dombrowski F, Amann K, et al.: Knockdown of ApoL1 in zebrafish larvae affects the glomerular filtration barrier and the expression of nephrin. PLoS One 11: e0153768, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurung R, Yadav R, Brungardt JG, Orlova A, Egelman EH, Beck MR: Actin polymerization is stimulated by actin cross-linking protein palladin. Biochem J 473: 383–396, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck MR, Dixon RDS, Goicoechea SM, Murphy GS, Brungardt JG, Beam MT, et al.: Structure and function of palladin’s actin binding domain. J Mol Biol 425: 3325–3337, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang EH, Gasim AH, Kerber ML, Patel JB, Glaubiger SA, Falk RJ, et al.: Palladin is upregulated in kidney disease and contributes to epithelial cell migration after injury. Sci Rep 5: 7695, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kos CH, Le TC, Sinha S, Henderson JM, Kim SH, Sugimoto H, et al.: Mice deficient in alpha-actinin-4 have severe glomerular disease. J Clin Invest 111: 1683–1690, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casella JF, Flanagan MD, Lin S: Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature 293: 302–305, 1981 [DOI] [PubMed] [Google Scholar]
- 34.Coué M, Brenner SL, Spector I, Korn ED: Inhibition of actin polymerization by latrunculin A. FEBS Lett 213: 316–318, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED: Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269: 14869–14871, 1994 [PubMed] [Google Scholar]
- 36.Morton WM, Ayscough KR, McLaughlin PJ: Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol 2: 376–378, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Niedenberger BA, Chappell VA, Otey CA, Geyer CB: Actin dynamics regulate subcellular localization of the F-actin-binding protein PALLD in mouse Sertoli cells. Reproduction 148: 333–341, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Reiser J, Kriz W, Kretzler M, Mundel P: The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11: 1–8, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Li JJ, Kwak SJ, Jung DS, Kim J-J, Yoo T-H, Ryu D-R, et al.: Podocyte biology in diabetic nephropathy. Kidney Int Suppl 72: S36–S42, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Kihara I, Tsuchida S, Yaoita E, Yamamoto T, Hara M, Yanagihara T, et al.: Podocyte detachment and epithelial cell reaction in focal segmental glomerulosclerosis with cellular variants. Kidney Int Suppl 63: S171–S176, 1997 [PubMed] [Google Scholar]
- 41.Giannone G, Jiang G, Sutton DH, Critchley DR, Sheetz MP: Talin1 is critical for force-dependent reinforcement of initial integrin-cytoskeleton bonds but not tyrosine kinase activation. J Cell Biol 163: 409–419, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, et al.: Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atherton P, Stutchbury B, Jethwa D, Ballestrem C: Mechanosensitive components of integrin adhesions: Role of vinculin. Exp Cell Res 343: 21–27, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedrich C, Endlich N, Kriz W, Endlich K: Podocytes are sensitive to fluid shear stress in vitro. Am J Physiol Renal Physiol 291: F856–F865, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Carisey A, Tsang R, Greiner AM, Nijenhuis N, Heath N, Nazgiewicz A, et al.: Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr Biol 23: 271–281, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C: Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol 179: 1043–1057, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izard T, Brown DT: Mechanisms and functions of vinculin interactions with phospholipids at cell adhesion sites. J Biol Chem 291: 2548–2555, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miao J, Fan Q, Cui Q, Zhang H, Chen L, Wang S, et al.: Newly identified cytoskeletal components are associated with dynamic changes of podocyte foot processes. Nephrol Dial Transplant 24: 3297–3305, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Babayeva S, Miller M, Zilber Y, El Kares R, Bernard C, Bitzan M, et al.: Plasma from a case of recurrent idiopathic FSGS perturbs non-muscle myosin IIA (MYH9 protein) in human podocytes. Pediatr Nephrol 26: 1071–1081, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Pollard TD, Borisy GG: Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Goicoechea SM, Bednarski B, García-Mata R, Prentice-Dunn H, Kim HJ, Otey CA: Palladin contributes to invasive motility in human breast cancer cells. Oncogene 28: 587–598, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goicoechea SM, García-Mata R, Staub J, Valdivia A, Sharek L, McCulloch CG, et al.: Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene 33: 1265–1273, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagata M, Kriz W: Glomerular damage after uninephrectomy in young rats. II. Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int 42: 148–160, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann S, Podlich D, Hähnel B, Kriz W, Gretz N: Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15: 1475–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Dickson LE, Wagner MC, Sandoval RM, Molitoris BA: The proximal tubule and albuminuria: Really! J Am Soc Nephrol 25: 443–453, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.