Abstract
Background Although intestinal and urinary microbiome perturbations are associated with nephrolithiasis, whether antibiotics are a risk factor for this condition remains unknown.
Methods We determined the association between 12 classes of oral antibiotics and nephrolithiasis in a population-based, case–control study nested within 641 general practices providing electronic health record data for >13 million children and adults from 1994 to 2015 in the United Kingdom. We used incidence density sampling to match 25,981 patients with nephrolithiasis to 259,797 controls by age, sex, and practice at date of diagnosis (index date). Conditional logistic regression models were adjusted for the rate of health care encounters, comorbidities, urinary tract infections, and use of thiazide and loop diuretics, proton-pump inhibitors, and statins.
Results Exposure to any of five different antibiotic classes 3–12 months before index date was associated with nephrolithiasis. The adjusted odds ratio (95% confidence interval) was 2.33 (2.19 to 2.48) for sulfas, 1.88 (1.75 to 2.01) for cephalosporins, 1.67 (1.54 to 1.81) for fluoroquinolones, 1.70 (1.55 to 1.88) for nitrofurantoin/methenamine, and 1.27 (1.18 to 1.36) for broad-spectrum penicillins. In exploratory analyses, the magnitude of associations was greatest for exposure at younger ages (P<0.001) and 3–6 months before index date (P<0.001), with all but broad-spectrum penicillins remaining statistically significant 3–5 years from exposure.
Conclusions Oral antibiotics associated with increased odds of nephrolithiasis, with the greatest odds for recent exposure and exposure at younger age. These results have implications for disease pathogenesis and the rising incidence of nephrolithiasis, particularly among children.
Keywords: kidney stones, antibiotic, microbiome
Prior studies have reported associations between antibiotic exposure and diseases such as inflammatory bowel disease and asthma.1,2 These associations are thought to be mediated by disruption of the human microbiome.
It is biologically plausible that antibiotics may increase the risk of nephrolithiasis. Recent studies reported differences in the composition of the intestinal microbiome between patients with and without nephrolithiasis.3,4 Additionally, multidrug resistant nonurease-producing bacteria have been isolated from calcium-based kidney stones extracted from patients without urinary tract infection (UTI), suggesting a role for selective pressure on the urinary microbiome in kidney stone formation.5 However, despite the well established effect that antibiotics have on the microbiome, it remains unclear whether antibiotics are a risk factor for nephrolithiasis. This information would have important implications for elucidating causal pathways for kidney stone formation, understanding the increase in the prevalence of nephrolithiasis (most pronounced among children, adolescents, and young adults),6,7 and promoting antibiotic stewardship.
In this nested case–control study, we evaluated the association between oral antibiotic exposure and nephrolithiasis, and assessed its strength and temporality by antibiotic class. Because prior murine studies demonstrated a greater effect of early-life antibiotic exposure on host metabolism,8 we hypothesized that exposure to oral antibiotics at younger ages would be associated with a greater risk of nephrolithiasis.
Methods
Study Design
We conducted a population-based, nested case–control study, using the February 2015 version of The Health Improvement Network (THIN). THIN comprises data from 13.8 million individuals receiving care in 641 general practitioner (GP) practices in the United Kingdom from 1994 to 2015. These patients are representative of the United Kingdom population by age, sex, medical conditions, and death rates. Practices enter clinical data for each patient encounter, generating a longitudinal, patient-level record that includes outpatient prescriptions. Diagnoses and procedures are recorded with Read codes, the standard classification system in the United Kingdom. THIN facilitates population-based pharmacoepidemiologic studies because approximately 98% of the United Kingdom population is registered with a GP practice,9 and GPs have nearly exclusive prescribing rights within the National Health Service. This study was determined by the University of Pennsylvania Institutional Review Board to meet criteria for institutional review board exemption.
Study Population and Outcome
The outcome was nephrolithiasis diagnosis. To be considered a case, an individual had to be <90 years old and registered with his/her GP practice for 6 months before the first qualifying code for nephrolithiasis. Although this approach has been validated for ascertainment of incident diagnoses in THIN, it has not been validated for nephrolithiasis, which can be characterized by recurrent acute episodes.10 Patients who only had codes for renal colic, hypercalciuria, or nephrocalcinosis were not considered to have nephrolithiasis. The date of nephrolithiasis diagnosis (index date) was the date that the first qualifying code for nephrolithiasis was recorded (see Supplemental Table 1 for nephrolithiasis codes).11,12 Patients with codes for infectious calculi (e.g., calculous pyelonephritis) or bladder calculi before the index date were excluded. This definition of nephrolithiasis has produced estimates of associations between nephrolithiasis and hypertension and CKD12 similar to those reported in prospective cohort studies that ascertained nephrolithiasis through validated self-report.13,14
Patients <90 years old without Read codes for nephrolithiasis, bladder calculi, renal colic, hypercalciuria, or nephrocalcinosis before the index date were eligible for selection as controls. Ten controls were matched on age, sex, and GP practice to each case at their index date by incidence density sampling.
Exposure
The primary exposure was an outpatient oral antibiotic prescribed 3–12 months before the index date. This period was selected because kidney stones form over weeks to months and oral antibiotics cause changes in the quantity and composition of the microbiome for months after exposure.15 Patients who had antibiotic prescriptions within 3 months of the index date were not considered exposed to mitigate exposure misclassification (e.g., antibiotics recorded before the nephrolithiasis diagnosis were actually prescribed afterward) and because antibiotics may have been prescribed for symptoms related to stone presentations (e.g., dysuria or pyuria on urinalysis).
Antibiotics were categorized as cephalosporins, fluoroquinolones, lincosamides, macrolides, metronidazole, nitrofurantoin/methenamine, penicillins, broad-spectrum penicillins, sulfas, tetracyclines, and antimycobacterial drugs (see Supplemental Table 2 for drugs in each antibiotic class). We also examined Helicobacter pylori treatment, because it is explicitly identified in THIN and prior studies reported decreased intestinal colonization by Oxalobacter 6 months after H. pylori treatment.16 Prescriptions for each antibiotic of any duration and dosage within the exposure window was evaluated as a binary variable.
Covariates
For each individual, we identified prevalent inflammatory bowel disease, cystic fibrosis, gout, diabetes, immobility, neurogenic bladder, congenital and acquired urinary tract obstruction, and neoplasm (see Supplemental Table 3 for Read codes). We also identified UTIs during the period in which antibiotic exposure was assessed. Because of their association with nephrolithiasis, outpatient prescriptions for proton-pump inhibitors (PPIs),16 statins,17 thiazide diuretics, and loop diuretics were identified. We considered individuals exposed to these drugs if they had a prescription of any duration and dose during the period in which antibiotic exposure was assessed. For each individual, we determined the rate of health care encounters by dividing the total number of inpatient admissions, clinic visits, and emergency department visits by the period from the date of patient registration with the GP practice until the index date. This rate was divided into deciles to better approximate the relationship with each antibiotic and included as a factor variable in the models. We also recorded outpatient computed tomography scans, abdominal x-rays, and abdominal ultrasounds obtained between practice registration and the index date (categorized as zero, one, or two or more diagnostic imaging studies). Emergency department imaging was not available as only imaging studies ordered by GPs are recorded in THIN.
Statistical Analyses
Accounting for the matched design, multivariable conditional logistic regression models were fit to estimate the association between antibiotic exposure and nephrolithiasis. All models were adjusted for prevalent disease, UTI, health care encounter rate, and prescriptions for PPIs, statins, and diuretics. Model A was not adjusted for other antibiotic use. Model B was adjusted for antibiotic prescriptions other than the primary exposure within 3–12 months of the index date as a binary variable. Model C was adjusted for each antibiotic exposure other than the primary exposure within the 3–12 month exposure window as 11 separate indicator variables. A two-sided Bonferroni adjusted P value of <0.004 was the threshold for statistical significance.
In an exploratory analysis, we used generalized additive models to estimate the smoothed interaction with age at antibiotic exposure for each antibiotic associated with nephrolithiasis in the primary analysis, adjusting for sex and covariates.18 This approach fit nonparametric regression splines within the framework of a logistic regression model and estimated the odds of nephrolithiasis diagnosis for antibiotic exposures 3–12 months before the index date. We also examined additional time windows for antibiotic exposures. Exposure periods considered were 3 to <6 months, 6 to <12 months, 1 to <3 years, and 3 to <5 years before the index date. These models were adjusted as described for model C.
Five sensitivity analyses were performed. First, we excluded patients who had prior UTI. Second, we excluded patients with antibiotic prescriptions <5 days and those who were likely on continuous antibiotic prophylaxis, defined as recurrent 30-day prescriptions for ≥6 months. Third, we adjusted for obesity (body mass index [BMI] ≥30 kg/m2) and for BMI as a continuous variable among patients ≥18 years old with a recorded BMI within 2 years of the index date. Fourth, we excluded cases with nonqualifying Read codes for nephrolithiasis (e.g., renal colic, hypercalciuria, and nephrocalcinosis) before the index date. This analysis was performed because the stone event may have preceded documentation of the qualifying code for nephrolithiasis or because patients were prescribed antibiotics for symptoms from the stone that were not attributed to it. Finally, we further adjusted model C for outpatient computed tomography scans, ultrasounds, and abdominal x-rays ordered before the index date. All statistical tests were two-sided. Analyses were performed with R version 3.2.2.
Results
Population Characteristics
Our study included 25,981 patients with nephrolithiasis and 259,797 controls observed for a median of 5.4 years (Table 1; see Supplemental Table 4 for age distribution). Prescriptions for all oral antibiotics except lincosamides were greater among patients with nephrolithiasis. The most frequent indications for outpatient antibiotic prescriptions were chest infection, cough, upper respiratory tract infection, tonsillitis, and UTI.
Table 1.
Study population characteristics
| Characteristic | Cases (n=25,981) | Controlsa (n=259,797) | P Valueb |
|---|---|---|---|
| N (%) or Median (IQR) | N (%) or Median (IQR) | ||
| Women | 9070 (34.9) | 90,698 (34.9) | NA |
| Age at first stone, yr | 51.6 (40.0–63.5) | 51.6 (40.0–63.4) | <0.001 |
| BMI, kg/m2 | 27.9 (24.7–31.9)c | 27.1 (24.0–30.8)c | <0.001 |
| Observation time, yr | 5.34 (2.5–9.3) | 5.36 (2.6–9.3) | 0.07 |
| Total visits per year | 8.56 (4.4–14.9) | 5.26 (2.26–10.2) | <0.001 |
| England | 20,242 (77.9) | 202,409 (77.9) | NA |
| Northern Ireland | 1064 (4.1) | 10,640 (4.1) | |
| Scotland | 2928 (11.3) | 29,278 (11.3) | |
| Wales | 1747 (6.7) | 17,470 (6.7) | |
| Comorbid diseased | |||
| Cystic fibrosis | 9 (0.03) | 26 (0.01) | 0.002 |
| Gout | 821 (3.2) | 6216 (2.4) | <0.001 |
| Diabetes | 2765 (10.6) | 17,398 (6.7) | <0.001 |
| Immobility | 148 (0.6) | 845 (0.3) | <0.001 |
| Inflammatory bowel disease | 361 (1.4) | 1360 (0.5) | <0.001 |
| Urinary tract obstruction | 561 (2.2) | 699 (0.3) | <0.001 |
| Neoplasm | 3899 (15.0) | 31,315 (12.1) | <0.001 |
| UTI | 2386 (9.2) | 5519 (2.1) | <0.001 |
| Medications | |||
| PPIs | 4418 (17) | 29,150 (11.2) | <0.001 |
| Statins | 4485 (17.3) | 35,624 (13.7) | <0.001 |
| Thiazide diuretics | 1736 (6.7) | 18,210 (7.0) | 0.05 |
| Loop diuretics | 902 (3.5) | 7607 (2.9) | <0.001 |
| Antibiotic prescriptione | |||
| Sulfa | 2846 (10.9) | 6978 (2.7) | <0.001 |
| Cephalosporins | 1703 (6.5) | 4569 (1.8) | <0.001 |
| Fluoroquinolones | 1101 (4.2) | 3033 (1.2) | <0.001 |
| Nitrofurantoin | 916 (3.5) | 1969 (0.8) | <0.001 |
| Broad-spectrum penicillins | 1127 (4.3) | 4960 (1.9) | <0.001 |
| Metronidazole | 402 (1.6) | 2053 (0.8) | <0.001 |
| Macrolides | 1527 (5.9) | 9824 (3.8) | <0.001 |
| H. pylori treatment | 35 (0.1) | 140 (0.05) | <0.001 |
| Tetracyclines | 959 (3.7) | 6832 (2.6) | <0.001 |
| Mycobacterial treatment | 20 (0.08) | 91 (0.04) | 0.002 |
| Lincosamides | 15 (0.06) | 84 (0.03) | 0.05 |
| Penicillins | 4694 (18.1) | 34,939 (13.4) | <0.001 |
IQR, interquartile range; NA, not applicable.
For three cases, there were nine, rather than ten, matched control participants.
P values were determined through chi-square tests for binary variables and Wilcoxon rank sum tests for continuous variables.
Reduced sample sizes: n=12,490 cases, n=103,059 controls.
Comorbid disease diagnosed at any time from registration with THIN practice to index date.
Antibiotic prescription within 3–12 months before index date.
Association between Oral Antibiotics and Kidney Stones
Sulfas, cephalosporins, fluoroquinolones, nitrofurantoin/methenamine, and broad-spectrum penicillins were associated with an increased odds of nephrolithiasis diagnosis 3–12 months after antibiotic prescription (model A, Table 2). These antibiotics remained independently associated with an increased odds of nephrolithiasis diagnosis after adjustment for exposure to other antibiotics (models B and C, Table 2). Exposure to H. pylori treatment was associated with nephrolithiasis diagnosis in model C (P=0.004; Table 2), but was not statistically significant at the Bonferroni-corrected threshold in model A or B or most sensitivity analyses. The fully adjusted model C had the best fit, with an akaike information criterion approximately 2% lower than models A or B.
Table 2.
Odds of kidney stone disease according to oral antibiotic class
| Antibiotic Class | Model A | Model B | Model C | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Sulfa | 2.37 (2.23 to 2.52)a | <0.001 | 2.35 (2.22 to 2.5)a | <0.001 | 2.33 (2.19 to 2.48)a | <0.001 |
| Cephalosporins | 1.93 (1.81 to 2.07)a | <0.001 | 1.88 (1.76 to 2.01)a | <0.001 | 1.88 (1.75 to 2.01)a | <0.001 |
| Fluoroquinolones | 1.84 (1.7 to 1.99)a | <0.001 | 1.77 (1.64 to 1.91)a | <0.001 | 1.67 (1.54 to 1.81)a | <0.001 |
| Nitrofurantoin | 1.84 (1.67 to 2.02)a | <0.001 | 1.84 (1.67 to 2.02)a | <0.001 | 1.7 (1.55 to 1.88)a | <0.001 |
| Broad-spectrum penicillins | 1.37 (1.28 to 1.47)a | <0.001 | 1.31 (1.22 to 1.4)a | <0.001 | 1.27 (1.18 to 1.36)a | <0.001 |
| Metronidazole | 1.25 (1.12 to 1.4)a | <0.001 | 1.14 (1.01 to 1.27) | 0.03 | 1.09 (0.97 to 1.23) | 0.14 |
| Macrolides | 1.11 (1.04 to 1.17)a | 0.001 | 1.04 (0.98 to 1.1) | 0.22 | 1.04 (0.98 to 1.1) | 0.24 |
| H. pylori treatment | 1.7 (1.15 to 2.51) | 0.01 | 1.69 (1.14 to 2.49) | <0.01 | 1.79 (1.21 to 2.65)a | 0.003 |
| Tetracyclines | 1.03 (0.96 to 1.11) | 0.39 | 0.98 (0.91 to 1.05) | 0.53 | 0.97 (0.9 to 1.04) | 0.37 |
| Mycobacterial treatment | 1.52 (0.92 to 2.49) | 0.10 | 1.44 (0.87 to 2.37) | 0.15 | 1.35 (0.81 to 2.24) | 0.25 |
| Lincosamides | 0.94 (0.53 to 1.66) | 0.82 | 0.86 (0.48 to 1.52) | 0.59 | 0.74 (0.41 to 1.34) | 0.32 |
| Penicillins | 1 (0.97 to 1.04) | 0.97 | 0.95 (0.91 to 0.98) | 0.004 | 0.97 (0.94 to 1.01) | 0.15 |
All conditional logistic regression models were adjusted for cystic fibrosis, gout, diabetes, immobility, urinary tract obstruction, neoplasm, and inflammatory bowel disease (all prevalent); UTI within the 3–12 month exposure window; exposure to PPIs, statins, thiazide diuretics, and loop diuretics within the 3–12 month exposure window; and total rate of outpatient, inpatient and emergency department visits. Model A made no adjustment for other antibiotic use. Model B was adjusted for any other antibiotic use within the exposure window. Model C was adjusted for each other antibiotic as separate indicator variables in the model. OR, odds ratio; 95% CI, 95% confidence interval.
Below Bonferroni P value (0.05/12=0.00417).
Effect Modification by Age and Proximity of Exposure
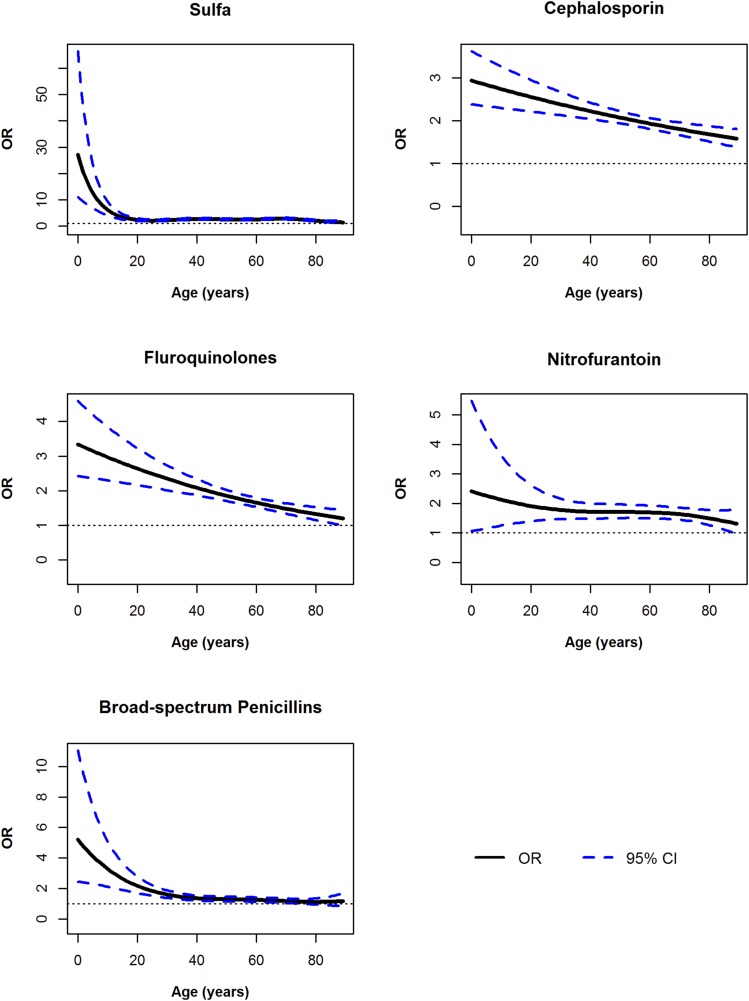
There were interactions with age for all five antibiotic classes (all P<0.001), with the highest magnitude of risk estimated for exposure to these antibiotics at younger ages. The shape of the relationship varied by antibiotic class (Figure 1; see Supplemental Table 5 for the point estimates and confidence intervals for antibiotic exposure at specific ages). Stratified analyses within matched age groups showed a similar interaction between antibiotics and age (results not shown).
Figure 1.
Odds of kidney stone disease were greatest for antibiotic exposures at younger ages. Generalized additive regression models were adjusted for cystic fibrosis, gout, diabetes, immobility, urinary tract obstruction, neoplasm, inflammatory bowel disease (all prevalent), UTI within the exposure window, rate of health care encounters, and prescriptions for PPIs, statins, thiazide diuretics, and loop diuretics, as well as exposure to other antibiotic classes within the 3–12 months exposure window. These models estimated the odds of nephrolithiasis diagnosis for antibiotic exposures 3–12 months before the index date. The dotted line represents the 95% credible intervals at each age. The R package “mgcv” was used and splines were fit with the default thin plate spline basis functions.
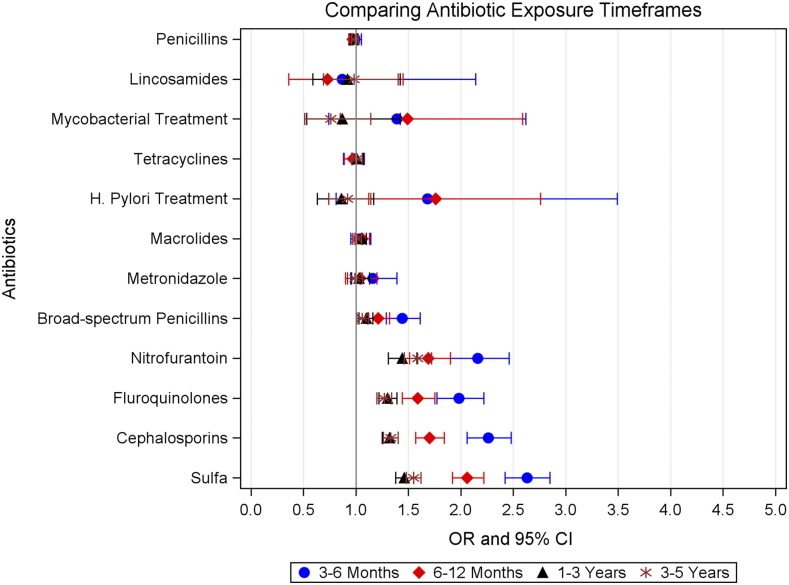
The odds of nephrolithiasis were greatest for exposure to the five antibiotic classes within 3–6 months of the index date (Figure 2, Table 3; all P<0.001). The magnitude of the association decreased with more distant exposure, but remained statistically significant from 3 to 5 years from antibiotic exposure for all classes except broad-spectrum penicillins.
Figure 2.
Odds of kidney stone disease were greatest for more recent antibiotic exposures. Conditional logistic regression models were adjusted for prevalent cystic fibrosis, gout, diabetes, immobility, urinary tract obstruction, neoplasm, and inflammatory bowel disease, and rate of health care encounters since registration. Models were also adjusted for UTI and exposure to PPIs, statins, thiazide diuretics, loop diuretics, and other antibiotic classes within each antibiotic exposure window.
Table 3.
Odds of kidney stone disease, according to recentness of oral antibiotic exposure
| Antibiotic Class | 3 to <6 mo | 6 to <12 mo | 1 to <3 yr | 3–5 yr | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Sulfa | 2.63 (2.42 to 2.85)a | <0.001 | 2.06 (1.92 to 2.22)a | <0.001 | 1.46 (1.38 to 1.55)a | <0.001 | 1.55 (1.48 to 1.62)a | <0.001 |
| Cephalosporins | 2.26 (2.06 to 2.48)a | <0.001 | 1.7 (1.57 to 1.84)a | <0.001 | 1.32 (1.25 to 1.4)a | <0.001 | 1.33 (1.26 to 1.4)a | <0.001 |
| Fluoroquinolones | 1.98 (1.77 to 2.22)a | <0.001 | 1.59 (1.44 to 1.75)a | <0.001 | 1.3 (1.22 to 1.39)a | <0.001 | 1.27 (1.2 to 1.34)a | <0.001 |
| Nitrofurantoin | 2.16 (1.9 to 2.46)a | <0.001 | 1.69 (1.51 to 1.9)a | <0.001 | 1.44 (1.31 to 1.58)a | <0.001 | 1.59 (1.46 to 1.72)a | <0.001 |
| Broad-spectrum penicillins | 1.44 (1.29 to 1.61)a | <0.001 | 1.21 (1.11 to 1.32)a | <0.001 | 1.1 (1.03 to 1.16)a | 0.002 | 1.06 (1.01 to 1.12) | 0.02 |
| Metronidazole | 1.16 (0.96 to 1.39) | 0.12 | 1.04 (0.9 to 1.2) | 0.57 | 1.03 (0.95 to 1.13) | 0.46 | 0.99 (0.92 to 1.06) | 0.69 |
| Macrolides | 1.04 (0.95 to 1.14) | 0.40 | 1.06 (0.99 to 1.13) | 0.11 | 1.05 (1 to 1.1) | 0.04 | 1.01 (0.97 to 1.05) | 0.77 |
| H. pylori treatment | 1.68 (0.81 to 3.49) | 0.17 | 1.76 (1.12 to 2.76) | 0.01 | 0.86 (0.63 to 1.17) | 0.33 | 0.92 (0.74 to 1.14) | 0.45 |
| Tetracyclines | 0.98 (0.88 to 1.08) | 0.66 | 0.97 (0.89 to 1.06) | 0.51 | 1.01 (0.96 to 1.07) | 0.69 | 1.01 (0.96 to 1.06) | 0.71 |
| Mycobacterial treatment | 1.39 (0.74 to 2.62) | 0.30 | 1.49 (0.85 to 2.59) | 0.16 | 0.87 (0.53 to 1.42) | 0.56 | 0.76 (0.51 to 1.14) | 0.18 |
| Lincosamides | 0.87 (0.36 to 2.14) | 0.76 | 0.73 (0.36 to 1.45) | 0.36 | 0.92 (0.59 to 1.42) | 0.71 | 0.98 (0.69 to 1.4) | 0.91 |
| Penicillins | 0.99 (0.94 to 1.05) | 0.83 | 0.97 (0.93 to 1.01) | 0.18 | 0.98 (0.95 to 1.01) | 0.30 | 0.99 (0.96 to 1.02) | 0.61 |
Models were adjusted for each other antibiotic as separate indicator variables in model, cystic fibrosis, gout, diabetes, immobility, urinary tract obstruction, neoplasm, inflammatory bowel disease (all prior) and rate of health care encounters since registration. Models were also adjusted for UTI and exposure to PPIs, statins, thiazide diuretics, loop diuretics, and other antibiotic classes within each antibiotic exposure window. OR, odds ratio; 95% CI, 95% confidence interval.
Below Bonferroni P value (0.05/12=0.00417).
In the sensitivity analysis excluding patients with any prior UTI, the magnitude of the association increased for sulfas and nitrofurantoin, decreased for broad-spectrum penicillins, and was similar for other classes. The most common indications for nitrofurantoin after excluding prior UTI were dysuria, abdominal pain, urinary symptoms, hematuria, and loin pain. Results did not change in the other sensitivity analyses (Table 4), including when performed on the additional exposure windows.
Table 4.
Sensitivity analyses
| Antibiotic Class | Model A: Primary Results | Model B: Excluding UTI | Model C: Excluding Treatment Duration <5 d or >6 mo | Model D: Adjusting for Obesity | Model E: Excluding Nonqualifying Stone Codes | Model F: Adjusting for Diagnostic Imaging |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Sulfa | 2.33 (2.19 to 2.48)a | 3.36 (3.07 to 3.67)a | 2.3 (2.11 to 2.5)a | 2.23 (2.04 to 2.44)a | 2.4 (2.24 to 2.56)a | 2.29 (2.15 to 2.44)a |
| Cephalosporins | 1.88 (1.75 to 2.01)a | 1.73 (1.56 to 1.92)a | 1.84 (1.69 to 2.01)a | 1.96 (1.77 to 2.18)a | 1.9 (1.76 to 2.05)a | 1.83 (1.71 to 1.96)a |
| Fluoroquinolones | 1.67 (1.54 to 1.81)a | 1.58 (1.41 to 1.77)a | 1.64 (1.47 to 1.83)a | 1.49 (1.32 to 1.68)a | 1.63 (1.49 to 1.78)a | 1.63 (1.5 to 1.77)a |
| Nitrofurantoin | 1.7 (1.55 to 1.88)a | 2.31 (1.84 to 2.9)a | 1.82 (1.59 to 2.07) a | 1.75 (1.53 to 2.01)a | 1.74 (1.57 to 1.94)a | 1.68 (1.53 to 1.86)a |
| Broad-spectrum penicillins | 1.27 (1.18 to 1.36)a | 1.14 (1.03 to 1.26) | 1.19 (1.09 to 1.3)a | 1.24 (1.12 to 1.38)a | 1.22 (1.13 to 1.33)a | 1.22 (1.13 to 1.31)a |
| Metronidazole | 1.09 (0.97 to 1.23) | 1.11 (0.95 to 1.29) | 1.13 (0.98 to 1.29) | 1.24 (1.05 to 1.46) | 1.16 (1.02 to 1.32) | 1.07 (0.95 to 1.2) |
| Macrolides | 1.04 (0.98 to 1.1) | 1.05 (0.97 to 1.13) | 1.09 (1.01 to 1.16) | 1.04 (0.95 to 1.13) | 1.07 (1 to 1.14) | 1.02 (0.96 to 1.08) |
| H. pylori treatment | 1.79 (1.21 to 2.65)a | 1.62 (1.03 to 2.55) | 0.94 (0.26 to 3.36) | 1.98 (1.08 to 3.64) | 1.25 (0.77 to 2.02) | 1.88 (1.26 to 2.8)a |
| Tetracyclines | 0.97 (0.9 to 1.04) | 0.97 (0.89 to 1.06) | 0.97 (0.89 to 1.05) | 0.95 (0.86 to 1.06) | 0.97 (0.89 to 1.06) | 0.94 (0.87 to 1.01) |
| Mycobacterial treatment | 1.35 (0.81 to 2.24) | 1.42 (0.74 to 2.71) | 1.15 (0.6 to 2.19) | 1.43 (0.69 to 2.93) | 1.14 (0.63 to 2.08) | 1.31 (0.77 to 2.21) |
| Lincosamides | 0.74 (0.41 to 1.34) | 0.55 (0.23 to 1.32) | 0.79 (0.41 to 1.53) | 0.87 (0.41 to 1.83) | 1.01 (0.54 to 1.9) | 0.74 (0.4 to 1.37) |
| Penicillins | 0.97 (0.94 to 1.01) | 0.96 (0.92 to 1) | 0.98 (0.94 to 1.02) | 0.98 (0.93 to 1.04) | 1 (0.95 to 1.04) | 0.97 (0.93 to 1.01) |
All conditional logistic regression models were adjusted for cystic fibrosis, gout, diabetes, immobility, urinary tract obstruction, neoplasm, inflammatory bowel disease (all prevalent); UTI within the exposure window from 3 to 12 months before the index date; exposure to PPIs, statins, thiazide diuretics, and loop diuretics within the exposure window from 3 to 12 months before the index date; and total rate of outpatient, inpatient, and emergency department visits. Model A (results from primary analysis, same as Model C in Table 2) was adjusted for each other antibiotic as separate indicator variables in the model. Model B only included those who did not have a UTI; if a case had a UTI, the whole match group was dropped, but if a control had a UTI, only the control was dropped (n=242,134; 22,978 cases, 219,156 controls). Model C excluded cases and controls who received antibiotics for <5 days or had sequential 30-day antibiotic prescriptions for ≥6 months (n=224,288; 21,468 cases, 202,820 controls). Model D was adjusted for obesity among those patients ≥18 years old with BMI recorded (n=115,549; 12,490 cases, 103,059 controls). Model E excluded cases with nonqualifying Read codes for nephrolithiasis (renal colic, hypercalciuria, or nephrocalcinosis) that occurred before the index date of the qualifying stone diagnosis codes (n=215,796; 19,619 cases, 196,177 controls). Model F was additionally adjusted for computed tomography scans, abdominal ultrasounds, and abdominal x-rays ordered by GPs from practice registration in THIN to the index date (n=285,778; 25,981 cases, 259,797 controls). OR, odds ratio; 95% CI, 95% confidence interval.
Below Bonferroni P value (0.05/12=0.00417).
Discussion
Exposure to sulfas, fluoroquinolones, cephalosporins, nitrofurantoin/methenamine, and broad-spectrum penicillins was associated with increased odds of nephrolithiasis. These associations remained after adjustment for multiple confounding conditions and the rate of health care encounters, and were robust to excluding patients with prior UTI. In exploratory analyses, the magnitude of these associations was greatest for exposures at younger ages. The strengths of the associations decreased with greater time from the antibiotic prescription, but the odds of nephrolithiasis diagnosis remained elevated for up to 5 years from antibiotic exposure. These results indicate that some oral antibiotics may be an important determinant of kidney stone disease.
Kidney stone disease affects people of all ages and large segments of the population worldwide. The prevalence of nephrolithiasis has increased 70% over the last 30 years with the greatest increases in incidence observed among children and young women.7,19 In 2011, 262 million antibiotic courses were prescribed in the United States, with the highest rates of administration for children <10 years old and women.20 In the United Kingdom, 30% of patients are prescribed at least one antibiotic annually.21 Our findings support the hypothesis that antibiotics may be contributing to the increasing prevalence and earlier age at onset of nephrolithiasis.22 Given that children receive more antibiotics than any other age group, and 30% of antibiotics prescribed during ambulatory care visits are inappropriate,23–25 these findings provide another reason to reduce inappropriate outpatient antibiotic use.
Our study has several strengths. First, the primary exposure was a prescription for a specific oral antibiotic, which allowed for examination of heterogeneity among antibiotic classes. Second, in the United Kingdom, GPs are the “gatekeepers” for care and have responsibility for prescribing antibiotics and recording diagnoses in the electronic health record, thereby reducing differential ascertainment of antibiotic exposure. We also matched cases and controls from the same GP practice to mitigate differences in antibiotic prescribing, diagnostic imaging, and referral practices. Third, we were able to account for potential confounding due to UTI by adjusting for prior UTI, excluding patients with infectious stones, which account for <5% of calculi,26 and in sensitivity analyses, by excluding patients with any prior UTI. The stability of the results across these analyses supports the credibility of an independent association between antibiotic exposure and nephrolithiasis. Third, the large number of children and adults included in this study allowed us to explore heterogeneity of the magnitude of the association by age at exposure and time between exposure and nephrolithiasis diagnosis. The shape of the age at antibiotic exposure curves was not monotonic, which suggests that risk at a given age may depend not only on the age at exposure, but also the particular antibiotic given. If confirmed by others, these results may help inform antibiotic selection when alternative options exist. However, the frequency of clindamycin and antimycobacterial prescriptions was low, which limits the conclusions that can be drawn about the lack of an association for these antibiotics. Fourth, the longitudinal nature of data in THIN allowed for demonstration of the temporal relationship between antibiotic prescriptions and stones.
The strength of the associations between antibiotics and nephrolithiasis diagnosis decreased with more distant antibiotic exposure. The greatest magnitude was estimated for antibiotic exposure 3–6 months before diagnosis. This relatively short time lag suggests that for individuals who are susceptible to stone formation/growth, antibiotic exposure may be sufficient to temporarily change the urinary environment to facilitate stone growth. This is consistent with simulation experiments that demonstrated short-term changes in the urinary environment induced rapid crystallization,27 and time-series studies that reported increased kidney stone presentations within days of high temperatures.28 However, the fewer patients within shorter exposure windows limited the precision of these estimates, and these results should be considered exploratory.
Because 85%–90% of individuals with nephrolithiasis in industrialized countries form calcium-based calculi, our findings likely reflect an increased risk of calcium stone formation. One potential mechanism is that antibiotics change composition of the intestinal microbiome and consequently alter macronutrient metabolism.8 Our results are consistent with prior reports that there is persistent reduction in the relative proportion of gut bacteria for months after antibiotic exposure.16,29,30 The greater magnitude of risk estimated for children, which was consistent for all five antibiotics, is also consistent with reports that antibiotic exposures at younger ages produce more profound alterations of host macronutrient metabolism than those later in life.8
Most prior research has focused on intestinal bacteria that degrade oxalate, particularly Oxalobacter formigenes.31–33 However, recent case–control studies have not found differences in the abundance of Oxalobacter in stool samples obtained from patients with and without kidney stone disease. Our results are consistent with these prior studies because Oxalobacter is resistant to many of the antibiotics associated with the increased odds of nephrolithiasis in this study, namely cephalosporins, nitrofurantoin, and broad-spectrum penicillins.34 These prior studies, however, identified differences in the abundance of other bacteria such as Eubacterium and reported that patients with kidney stones had an lower overall diversity of the gut microbiome.3,4 This lower diversity is consistent with the loss of microbiota diversity reported in other diseases, such as asthma.35 It is likely that multiple organisms would mediate the association between antibiotics and nephrolithiasis because intestinal microbes exist as a community of organisms, with the metabolic functions of one species affecting growth of others.36 The potential differential effect of antibiotic classes on these bacterial communities may explain why certain classes were not associated with nephrolithiasis (e.g., macrolides), but there was a strong signal for others. Future studies should examine the underlying microbial biomolecular mechanisms and change in intestinal metabolic products caused by different antibiotics.
It is also possible that antibiotics select for multidrug resistant bacteria in the urinary microbiome that promote stone formation.5,37 Investigators have isolated bacteria from calcium stones and reported that up to 70% of isolates were resistant to multiple antibiotics,5 raising the possibility that antibiotics place selective pressure on the urine microbiome, and that these bacteria may have a role in stone formation. We also cannot exclude direct antibiotic crystallization in the kidney, which has been described for sulfamethoxazole-trimethoprim and ciprofloxacin.38 Lastly, it is possible that these associations reflect the underlying infection for which antibiotics were prescribed (e.g., decreased fluid intake). However, prescriptions in this study were for oral antibiotics, and so it is unlikely that patients had infections that profoundly decreased fluid intake or increased insensible losses. Additionally, if underlying illness explained these associations, one would expect all antibiotic classes to be associated with nephrolithiasis, rather than only five.
We acknowledge this study’s limitations. First, we performed this study in THIN because the dataset included important confounders such as medications, comorbid conditions, and health care encounters. Nevertheless, unrecorded or misclassified UTI (as suggested by indications for nitrofurantoin prescriptions after excluding prior UTI) are possible unmeasured confounders, as are dietary intake, insensible fluid loss and/or poor fluid intake due to the underlying illness, and undiagnosed or unreported comorbid conditions. Additionally, some patients may have had undiagnosed, asymptomatic stones39 before antibiotic prescription. Second, infrequent documentation of urinary chemistries and stone composition limited our ability to define the underlying pathophysiology. Third, the odds ratios presented in the primary analysis are likely an oversimplification because of the interaction with age and time since exposure. Further research is needed to clarify the attributable risk of antibiotic exposure accounting for genetics, age at exposure, and underlying metabolic risk. Fourth, intravenous antibiotics were not captured. Nevertheless, these results are likely generalizable to most patients because the majority of therapeutic antibiotic use occurs among outpatients, although future studies are needed to verify these results in other geographic areas.40 Finally, these results reflect antibiotics prescribed. However, nonadherence to treatment courses of oral antibiotics, which is high in community settings in Europe, would bias results toward the null.41 Additionally, we did not examine for a dose-response relationship because of the low frequency of multiple prescriptions for the same antibiotic within the exposure window.
In conclusion, exposure to some oral antibiotics was associated with increased odds of nephrolithiasis with the greatest risk for more recent and younger age exposures. These findings suggest that exposure to some oral antibiotics is a novel risk factor for nephrolithiasis, one that may be modifiable for the 30% of patients who receive inappropriate outpatient prescriptions for antibiotics.25 These results have implications for the pathogenesis of the disease and for the rising incidence of nephrolithiasis, particularly among children.7,19
Disclosures
None.
Supplementary Material
Acknowledgments
G.E.T. and M.R.D. were responsible for study design. G.E.T., M.R.D., J.S.G., and D.S.G. were responsible for the literature search. M.R.D. and Q.W. handled data collection. Q.W. and T.J. performed data analysis. G.E.T., M.R.D., J.S.G., D.S.G., L.C., and T.J. performed data interpretation. G.E.T. drafted the manuscript and all authors took part in manuscript revision. T.J. created all figures.
G.E.T., M.R.D., and D.S.G. were supported by grants K23DK106428, K23DK093556, and U54DK083908, respectively, from the National Institute for Diabetes and Digestive Diseases and Kidney Diseases of the National Institutes of Health (NIH).
The NIH had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. T.J. performed all data analysis while at the University of Pennsylvania. Merck, his current employer, had no role in the study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related perspective, “Does the Receipt of Antibiotics for Common Infectious Diseases Predispose to Kidney Stones? A Cautionary Note for All Health Care Practitioners,” on pages 1590–1592.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017111213/-/DCSupplemental.
References
- 1.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE: Antibiotic exposure and IBD development among children: A population-based cohort study. Pediatrics 130: e794–e803, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Risnes KR, Belanger K, Murk W, Bracken MB: Antibiotic exposure by 6 months and asthma and allergy at 6 years: Findings in a cohort of 1,401 US children. Am J Epidemiol 173: 310–318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern JM, Moazami S, Qiu Y, Kurland I, Chen Z, Agalliu I, et al.: Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 44: 399–407, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang R, Jiang Y, Tan A, Ye J, Xian X, Xie Y, et al.: 16S rRNA gene sequencing reveals altered composition of gut microbiota in individuals with kidney stones [published online ahead of print January 20, 2018]. Urolithiasis doi: 10.1007/s00240-018-1037-y [DOI] [PubMed] [Google Scholar]
- 5.Tavichakorntrakool R, Prasongwattana V, Sungkeeree S, Saisud P, Sribenjalux P, Pimratana C, et al.: Extensive characterizations of bacteria isolated from catheterized urine and stone matrices in patients with nephrolithiasis. Nephrol Dial Transplant 27: 4125–4130, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Romero V, Akpinar H, Assimos DG: Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Rev Urol 12: e86–e96, 2010 [PMC free article] [PubMed] [Google Scholar]
- 7.Tasian GE, Ross ME, Song L, Sas DJ, Keren R, Denburg MR, et al.: Annual incidence of nephrolithiasis among children and adults in South Carolina from 1997 to 2012. Clin J Am Soc Nephrol 11: 488–496, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al.: Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158: 705–721, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Numbers of patients registered at a GP practice - July 2015, 2015. Available at: http://digital.nhs.uk/catalogue/PUB17927. Accessed February 18, 2018, 2015 [Google Scholar]
- 10.Lewis JD, Bilker WB, Weinstein RB, Strom BL: The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf 14: 443–451, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Denburg MR, Leonard MB, Haynes K, Tuchman S, Tasian G, Shults J, et al.: Risk of fracture in urolithiasis: A population-based cohort study using the health improvement network. Clin J Am Soc Nephrol 9: 2133–2140, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denburg MR, Jemielita TO, Tasian GE, Haynes K, Mucksavage P, Shults J, et al.: Assessing the risk of incident hypertension and chronic kidney disease after exposure to shock wave lithotripsy and ureteroscopy. Kidney Int 89: 185–192, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madore F, Stampfer MJ, Rimm EB, Curhan GC: Nephrolithiasis and risk of hypertension. Am J Hypertens 11: 46–53, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, et al.; Alberta Kidney Disease Network : Kidney stones and kidney function loss: A cohort study. BMJ 345: e5287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dethlefsen L, Relman DA: Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 108[Suppl 1]: 4554–4561, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharlamb V, Schelker J, Francois F, Jiang J, Holmes RP, Goldfarb DS: Oral antibiotic treatment of Helicobacter pylori leads to persistently reduced intestinal colonization rates with Oxalobacter formigenes. J Endourol 25: 1781–1785, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sur RL, Masterson JH, Palazzi KL, L’Esperance JO, Auge BK, Chang DC, et al.: Impact of statins on nephrolithiasis in hyperlipidemic patients: A 10-year review of an equal access health care system. Clin Nephrol 79: 351–355, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Hastie T, Tibshirani R: Generalized Additive Models, London, New York, Chapman and Hall, 1990 [Google Scholar]
- 19.Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH Jr, et al.: US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 60: 1308–1316, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Shallcross L, Beckley N, Rait G, Hayward A, Petersen I: Antibiotic prescribing frequency amongst patients in primary care: A cohort study using electronic health records. J Antimicrob Chemother 72: 1818–1824, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldfarb DS: The exposome for kidney stones. Urolithiasis 44: 3–7, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichichero ME: Dynamics of antibiotic prescribing for children. JAMA 287: 3133–3135, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Hicks LA, Taylor TH Jr, Hunkler RJ: U.S. outpatient antibiotic prescribing, 2010. N Engl J Med 368: 1461–1462, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr, et al.: Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA 315: 1864–1873, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Lieske JC, Rule AD, Krambeck AE, Williams JC, Bergstralh EJ, Mehta RA, et al.: Stone composition as a function of age and sex. Clin J Am Soc Nephrol 9: 2141–2146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borissova A, Goltz GE, Kavanagh JP, Wilkins TA: Reverse engineering the kidney: Modelling calcium oxalate monohydrate crystallization in the nephron. Med Biol Eng Comput 48: 649–659, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Tasian GE, Pulido JE, Gasparrini A, Saigal CS, Horton BP, Landis JR, et al.; Urologic Diseases in America Project : Daily mean temperature and clinical kidney stone presentation in five U.S. metropolitan areas: A time-series analysis. Environ Health Perspect 122: 1081–1087, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dethlefsen L, Huse S, Sogin ML, Relman DA: The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 6: e280, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly JP, Curhan GC, Cave DR, Anderson TE, Kaufman DW: Factors related to colonization with Oxalobacter formigenes in U.S. adults. J Endourol 25: 673–679, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azcarate-Peril MA, Bruno-Bárcena JM, Hassan HM, Klaenhammer TR: Transcriptional and functional analysis of oxalyl-coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes from Lactobacillus acidophilus. Appl Environ Microbiol 72: 1891–1899, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, et al.: Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol 19: 1197–1203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwak C, Kim HK, Kim EC, Choi MS, Kim HH: Urinary oxalate levels and the enteric bacterium Oxalobacter formigenes in patients with calcium oxalate urolithiasis. Eur Urol 44: 475–481, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Lange JN, Wood KD, Wong H, Otto R, Mufarrij PW, Knight J, et al.: Sensitivity of human strains of Oxalobacter formigenes to commonly prescribed antibiotics. Urology 79: 1286–1289, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al.: Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 63: 559–566, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Rakoff-Nahoum S, Foster KR, Comstock LE: The evolution of cooperation within the gut microbiota. Nature 533: 255–259, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr-Beare E, Saxena V, Hilt EE, Thomas-White K, Schober M, Li B, et al.: The interaction between enterobacteriaceae and calcium oxalate deposits. PLoS One 10: e0139575, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chopra N, Fine PL, Price B, Atlas I: Bilateral hydronephrosis from ciprofloxacin induced crystalluria and stone formation. J Urol 164: 438, 2000 [PubMed] [Google Scholar]
- 39.Bansal AD, Hui J, Goldfarb DS: Asymptomatic nephrolithiasis detected by ultrasound. Clin J Am Soc Nephrol 4: 680–684, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reports, S-S: Swedres-Svarm 2015, 2015. Available at: http://www.sva.se/en/antibiotika/svarm-reports. Accessed Dec 22, 2016
- 41.Llor C, Hernández S, Bayona C, Moragas A, Sierra N, Hernández M, et al.: A study of adherence to antibiotic treatment in ambulatory respiratory infections. Int J Infect Dis 17: e168–e172, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.