Abstract
Cardiovascular disease (CVD) is a major cause of mortality and morbidity in patients with CKD. In the past decade, intestinal dysbiosis and altered gut epithelial barrier function are increasingly recognized in CKD. Uremic patients have slow intestinal transit time, impaired protein assimilation, and decreased consumption of dietary fiber. The use of multiple medications also may contribute to the proliferation of dysbiotic bacteria, which affect the barrier function of intestinal epithelium. In addition, fluid overload and uremic toxins per se directly reduce the gut barrier function. The major consequence of these alterations, the translocation of bacterial fragments from bowel lumen to systemic circulation, can lead to diverse biologic effects and probably represents an important nontraditional CVD risk factor in CKD. Among all bacterial fragments, endotoxin is the most well studied. Plasma endotoxin levels are markedly elevated in both patients with CKD and those on dialysis, and are associated with the systemic inflammatory state, accelerated atherosclerosis, and clinical CVD in patients on dialysis. Optimization of BP control and the use of ultrapure dialysate can reduce plasma endotoxin levels, with probable metabolic and cardiovascular benefits. The benefit of synbiotic therapy is not confirmed, although results from animal studies are impressive. The biologic effects and clinical relevance of other bacterial fragments, such as bacterial DNA fragments, are less well defined. Further studies are needed to delineate the pathogenic relation between circulating bacterial fragments and CVD, and to define the role of the plasma bacterial fragment level as a prognostic indicator of CKD.
Keywords: Dialysis, cardiovascular disease, infection, survival
The association between CKD and cardiovascular disease (CVD) has been noted for over 150 years.1 In patients with CKD, CVD is more frequent and more severe than in the general population, but is often not recognized and is undertreated.2 CVD accounts for 35% and 50% of all deaths among patients with stage 3 and 4 CKD, respectively.3,4 At the time of initiation of dialysis, 70% of patients with CKD have significant coronary atherosclerosis, 40% have symptomatic ischemic heart disease, 40% have heart failure, 20% have peripheral vascular disease, and 10% have had a previous stroke or transient ischemic attack.2
Cardiovascular risk factors in CKD are often classified as traditional or nontraditional. The increase in cardiovascular risk in CKD is partly because of the high prevalence of traditional cardiovascular risk factors, such as hypertension and diabetes.2 However, even after adjustment for traditional cardiovascular risk factors, the risk of cardiovascular mortality increases linearly once the eGFR falls below 75 ml/min per 1.73 m2.3,4 Therefore, a number of kidney-specific nontraditional risk factors have been proposed,2 including volume overload,5,6 sympathetic hyperactivity,7 calcium phosphate imbalance,8,9 hyperhomocystinemia,10,11 and the effect of other organic uremic toxins.12,13 In addition, dialysis-specific risk factors, such as dialysis membrane flux and biocompatibility, may also contribute.14
In the past decade, the clinical effect of the gut microbiome is increasingly recognized in various diseases.15,16 Symbiosis is usually defined, in the field of microbiome study, as the interaction between human host and microbiota in the context of maintaining homeostasis and beneficial effect to the human host, whereas dysbiosis is the term that describes microbial imbalance or maladaptation inside the body. In CKD, the symbiotic relationship between host and intestinal microbiome is disturbed because of the proliferation of dysbiotic bacteria.17,18 The causes of intestinal dysbiosis in CKD include slow intestinal transit time,19 impaired protein assimilation,20 decreased consumption of dietary fiber,21 iron therapy,22 and frequent use of antibiotics.23,24 The results of gut dysbiosis are two-fold. First, fermentation of protein and amino acids by dysbiotic bacteria leads to the generation of toxic compounds such as ammonia, amines, thiols, phenols, and indoles,17 which trigger a systemic inflammatory state. Notably, trimethylamine N-oxide is a gut-derived amine oxide that has been implicated in the etiology of CVD in the normal population25 and in patients with CKD.26 Second, intestinal dysbiosis partly accounts for the impaired barrier function of the intestinal epithelium, resulting in the translocation of bacterial fragments through the bowel wall. In the past few years, numerous studies have shown that translocated bacterial fragments play important roles in the pathogenesis of uremic toxicity, inflammation, insulin resistance, protein-energy wasting, and progression of CKD, and are an important nontraditional cardiovascular risk factor in CKD.17
Circulating Bacterial Fragments in Renal Failure
Intestinal Epithelial Barrier in Health and Diseases
The intestinal epithelium is a single layer of columnar epithelial cells that separates the intestinal lumen from the underlying lamina propria.27 In good health, the intestinal barrier is highly effective, with the luminal side heavily colonized with gut bacteria and the basolateral side remaining sterile. Intestinal epithelial cells are bound together by tight junctions.28 Commensal gut microbes maintain functional integrity of gut by several mechanisms, including maintenance of tight junction protein structure,29,30 induction of epithelial heat-shock proteins,31,32 upregulation of mucin genes,33 competition with pathogenic bacteria for binding to intestinal epithelial cells,34 and secretion of antimicrobial peptides.35 Notably, commensal bacteria help maintain the intestinal epithelial barrier by suppressing intestinal inflammation via activation of the Toll-like receptor 2 with cell wall lipoteichoic acid.36 Toll-like receptor 2 stimulation preserves tight junction–associated barrier assembly against stress-induced damage through promotion of phosphatidylinositol 3-kinase/protein kinase B–mediated cell survival via myeloid differentiation factor 88.37 On the other hand, the gut innate immune system controls the overgrowth of pathobiotic bacteria inside the gut lumen.38
Bacterial translocation through the intestinal wall has long been recognized.39 Exposure to toxins, drugs, pathogens, or local inflammation may affect the integrity of gut mucosa, leading to translocation of bacteria or their fragments into the systemic circulation.39 The classic scenario is the spontaneous bacterial peritonitis of liver cirrhosis.40 Most peritonitis episodes caused by Enterobacteriaceae species in patients on peritoneal dialysis (PD) are also caused by bacteria translocation.41
Because intact bacteria can translocate through the intestinal barrier, it is logical to predict that nonviable but biologically active bacterial fragments could pass through even more readily. Notably, bacterial endotoxin, the cell wall component of Gram-negative bacteria, has been shown to be bioactive in the systemic circulation.42 An early study reported that plasma endotoxin levels were higher in patients with edematous heart failure than in patients without edematous heart failure and healthy volunteers. Short-term diuretic treatment led to a reduction in plasma endotoxin, although it did not affect other inflammatory cytokines.43 More recently, microbial translocation through the gut has been found to be a major cause of immune activation in patients with HIV type 1 (HIV-1)42; the plasma endotoxin level in patients with HIV-1 correlates with the degree of immune activation.42
Bacterial Fragments Present in the Systemic Circulation
Among all possible bacterial fragments, bacterial endotoxin is most commonly measured in the systemic circulation, partly because the limulus amoebocyte lysate assay is readily available for quality assurance of the water supply in hemodialysis units. Other bacterial fragments that are identifiable in the systemic circulation include bacterial DNA fragments, peptidoglycan, and bacterial polysaccharide A.
Mechanisms of Bacterial Fragment Translocation in CKD
There are several causes of increased circulating levels of bacterial fragments in CKD patients, including gut dysmotility, bowel wall edema, overgrowth of pathogenic bacteria, and loss of epithelial barrier integrity.17 Massive diffusion of urea into the gut and its conversion to ammonia and ammonium hydroxide plays a critical role.44,45 The metabolic changes in CKD alter the ecological balance in the gut, favoring the overgrowth of pathobiotic bacteria.38,46 Several metabolic changes in CKD may be responsible for inducing gut dysbiosis, including metabolic acidosis, organic uremic toxins, intestinal ischemia, volume overload, dietary changes, polymer phosphate binder, iron therapy, and frequent use of antibiotics.38 Excessive urease-possessing bacteria and a decline in the production of short-chain fatty acids, which are the principal nutrients for colonic epithelial cells, also contribute to the leaky gut in cases of uremia.47 In addition, CKD is associated with prolonged gastric emptying, reduced fasting, and postprandial small bowel water content, indicating abnormal gastrointestinal motility and absorption.48 Reduced postprandial small bowel water content is correlated with plasma endotoxin level, suggesting that bowel wall edema leads to impaired gut barrier function.48 Taken together, the current evidence suggests that disruption of gut barrier function in CKD is the major mechanism that allows for the translocation of bacterial fragments to the systemic circulation.49
Mechanisms of Bacterial Fragment Translocation in Dialysis
Circulating endotoxin levels are markedly more elevated in patients on dialysis with CKD than those predialysis with CKD, suggesting that dialysis treatment per se contributes to endotoxemia. Circulating endotoxin level was observed to rise three-fold after dialysis was initiated.50 Recurrent hemodynamic stress and cardiac dysfunction, leading to altered intestinal perfusion and mucosal permeability, have been implicated in hemodialysis.50,51 In patients with AKI, hemodialysis and fluid removal result in a reduction of splanchnic blood flow.52 In patients on chronic hemodialysis, the predialysis endotoxin level is correlated with dialysis-induced hemodynamic stress, myocardial stunning, serum cardiac troponin T, and C-reactive protein (CRP) levels.50
However, not all studies show that dialysis has these effects. Grant et al.53 found that superior mesenteric artery blood flow was similar between patients on PD and healthy controls. Although plasma endotoxin levels were significantly higher in the PD group, endotoxin levels did not correlate with baseline or postprandial superior mesenteric artery blood flow, body volume status, left ventricular mass index, or end-diastolic volume,53 suggesting that gut ischemia and edema were not directly related to the translocation of bacterial endotoxin in this setting.
Bacterial Endotoxin
Pathogenic Mechanisms
Endotoxin is a phospholipid and a major component of the outer membranes of Gram-negative bacteria. It is partly transported into intestinal capillaries through a mechanism dependent on Toll-like receptor 4 (TLR4),54 and is then taken up by liver and mononuclear phagocytic cells.55 In healthy individuals, endotoxin can be detected in the systemic circulation at low concentrations (<200 pg/ml).56,57
Endotoxin translocation from the gut has long been suggested as a cause of systemic inflammation in CKD.17,58 Circulating endotoxin binds LPS-binding protein (LBP); this complex interacts with MD-2, which forms a complex with TLR4 and is anchored by CD14.59 TLR4 is a traditional pathogen-associated molecular pattern, the activation of which results in a downstream inflammatory cascade. Endotoxin concentration as low as 1 pg/ml can induce cellular activation and expression of CD14, which is a 55 kD glycosylphosphatidylinositol-anchored membrane protein (mCD14) that is also found as a soluble serum protein (sCD14). At low concentrations, LBP catalyzes the transfer of endotoxin to mCD14 on the immune cells, resulting in cytokine release.58 At higher concentrations, LBP transfers endotoxin to lipoproteins, which is eventually cleared from the circulation.
Endotoxin may trigger the initiation and progression of atherosclerosis by mediating endothelial cell injury, boosting monocyte recruitment, transforming macrophages to foam cells, and activating coagulant activity.60,61 Previous studies showed that sCD14 level is associated with the progression of renal function decline, CVD, and the mortality of patients with CKD.62–64 In addition, endotoxin contributes to the development of insulin resistance, obesity, and diabetes in mice.65 In patients on hemodialysis, predialysis plasma endotoxin levels are correlated with erythropoietin dosage and erythropoietin resistance index.66 Patients receiving conventional thrice weekly hemodialysis had higher predialysis serum endotoxin level than those receiving short daily hemodialysis or nocturnal hemodialysis, even after adjusting for age and diabetic status.67
Relation with CVD in CKD
Two studies have reported that plasma endotoxin level correlates with ultrafiltration rate in patients on hemodialysis67 and the drop in systolic BP during hemodialysis treatment.66 Plasma endotoxin level of patients on hemodialysis also correlates with cardiac troponin T and CRP levels.67 Another study showed gradated increases in circulating endotoxin level with higher CKD stage.50 However, there was no association between circulating endotoxin level and vascular calcification, carotid–femoral pulse-wave velocity (PWV), or other factors relating to peripheral cardiovascular structure or function.50 Circulating endotoxin level was associated with an increased risk of mortality, but the association disappeared when corrected for cardiovascular risk factors,50 suggesting that the effect on mortality was mediated through its cardiovascular effects.
Patients on PD had higher plasma endotoxin level than predialysis patients with CKD, which was in turn higher than healthy controls.68 Plasma endotoxin level was significantly correlated with serum CRP and albumin levels.68 Patients with preexisting CVD had higher plasma endotoxin levels than those without CVD, and plasma endotoxin levels correlated with carotid intima media thickness.68 In another study on patients on PD, plasma endotoxin level correlated with the number of hospital admission and duration of hospitalization for cardiovascular reasons.69 In patients on hemodialysis, a cohort study found that plasma endotoxin level had a modest but significant correlation with serum CRP level and is an independent predictor of patient death within 3 years.70 In another study, plasma endotoxin level significantly correlated with serum soluble CD14 level, which is associated with inflammation and protein-energy wasting.63 Taken together, the results suggest that circulating endotoxin may contribute to the systemic inflammatory state, accelerated atherosclerosis, and clinical CVD in patients on dialysis.
However, the results of published studies are not always consistent. A study reported that high plasma endotoxin level was associated with better technique survival in patients on PD.71 The reason for this paradoxical observation is unknown, but could be because of the lack of a standardized method for measuring plasma endotoxin level, or the rapid clearance of endotoxin from the systemic circulation despite the systemic effect being sustained. In this regard, soluble CD14 could be a valuable marker of circulating endotoxin load.
Interventional Studies
Several strategies have been proposed for the manipulation of the intestinal microbiome, including probiotics, prebiotics, synbiotics, oral adsorbents, and genetically engineered bacteria.17 However, few have examined the effect on plasma endotoxin level. Treating human epithelial cell with metabolites secreted by Bifidobacterium infantis causes an increase in tight junction proteins zonula occludens-1 and occludin and reduces claudin-2, suggesting that intestinal barrier function is restored.29 Similarly, probiotic bacteria enhance intestinal epithelial barrier function in murine models of colitis and in human Crohn disease.72,73 Administration of either pasteurized Akkermansia muciniphila or its outer membrane protein increases the expression of tight-junction proteins claudin 3 and occludin in murine intestine, alleviating endotoxemia in mice fed a high-fat diet.
Strategies to restore the biochemical milieu of the gut may also be beneficial. For example, consumption of a diet supplemented with an indigestible, fermentable complex carbohydrate leads to significant improvements in gut microbiome and plasma endotoxin level.74,75 Rossi et al.51 noted that plasma endotoxin levels of patients with CKD was marginally lower during synbiotic therapy, but the difference was not statistically significant. In this study, synbiotic therapy also reduced serum indoxyl sulfate but not p-cresyl sulfate level.51
Other human trials show reductions in endotoxin with established clinical treatments. For example, circulating endotoxin level significantly decreased with the introduction and optimization of antihypertensive therapy in both patients with and without CKD.76 The reduction in endotoxin level was greater in patients without CKD despite similar drug usage and BP.76 However, the reduction in endotoxin level did not correlate with the change in arterial PWV or hemodynamic status.76 In patients on hemodialysis, plasma endotoxin level fell by one third within 4 weeks of conversion to ultrapure dialysate.77 In this study, the time-averaged plasma endotoxin level correlated with serum CRP level, carotid–femoral PWV, and malnutrition inflammation score.77 In essence, this study strongly supports the notion that circulating endotoxin has direct effects on systemic inflammatory state, and ultrapure dialysate is effective in reducing circulating endotoxin in patients on hemodialysis.77 However, ultrapure dialysate has already become standard practice in most developed countries. Because endotoxin level was not measured in the spent dialysate, it remains unclear whether the clearance of circulating endotoxin is increased or the gastrointestinal leak of endotoxin is reduced by the use of ultrapure dialysate.
Taken together, there are few approaches that may effectively reduce plasma endotoxin level. Although some strategies report a significant reduction of plasma endotoxin level and associated cardiovascular or metabolic benefits, the magnitude of reduction in the endotoxin level is generally modest. More importantly, it remains uncertain whether the cardiovascular or metabolic benefits are actually causally linked to the reduction in endotoxemia.
Bacterial DNA Fragments
Endotoxin may not be the only source of microbial inflammatory trigger. Other types of bacterial fragments are also present in the systemic circulation and probably contribute to the pathogenesis of systemic inflammation and CVD. Among all microbial components, a bacterial-derived DNA fragment is the most readily detectable. Most bacterial genomes contain the highly conserved 16S ribosomal RNA gene. DNA fragments of bacterial origin can be easily detected and discerned from human DNA.78,79 Plasma bacterial DNA level may be a superior marker of the circulating load of bacterial fragments because endotoxin is the cell wall component of Gram-negative bacteria, whereas bacterial DNA assay detects both Gram-positive and Gram-negative bacteria. Previous studies showed a significant but modest correlation between plasma bacterial DNA and endotoxin levels.69,80 Both bacterial DNA and endotoxin levels are correlated with serum CRP but not serum procalcitonin level,69 suggesting that bacterial fragments are related to systemic inflammation but not ongoing infection.
Pathogenic Mechanisms
Toll-like receptor 9 (TLR9) is a well characterized sensor for bacterial DNA, although TLR9-independent DNA recognition mechanisms may also exist.81 TLR9 is another classic pathogen-associated molecular pattern, and its stimulation results in the activation of intracellular signaling pathways, such as the mitogen-activated protein kinase, PI3-kinase, and Jun N-terminal kinase pathways NF-κB and AP-1.81 In PMN, bacterial DNA has profound effect on cellular trafficking, induces chemokine expression, regulates expression of adhesion molecules, enhances phagocyte activity, and rescues PMN from constitutive apoptosis.81 Bacterial DNA fragments induce IL-6 in mononuclear cells82 and promote the survival of inflammatory cells in patients with CKD.78 The induction of systemic inflammation by bacterial DNA may aggravate atherosclerotic plaque instability and trigger cardiovascular events.
In addition to its proinflammatory properties, bacterial DNA may directly affect the cardiovascular system. For example, Paladugu et al.83 showed that bacterial DNA causes dose-dependent suppression of rat cardiac myocyte contraction in vitro. Further, the signaling pathway of direct myocardial depression is not well established.
Observational Studies
There are few published studies on the relationship between circulating bacterial DNA fragments and CVD. Patients on chronic dialysis have significantly higher plasma bacterial DNA levels than those with stage 1–2 CKD, marginally higher levels than those with stage 3–4 CKD, and similar levels to patients receiving hemodialysis or PD.80 Plasma bacterial DNA level of patients on incident PD was significantly correlated with serum CRP level, malnutrition inflammation score, and subjective global assessment score, but not Charlson Comorbidity Index score.69,80 In addition, plasma bacterial DNA levels predict cardiovascular events. In a prospective study of patients on incident PD, plasma bacterial DNA level was an independent predictor of the composite cardiovascular end point in 24 months.69 Plasma bacterial DNA level also correlated with the number of hospital admissions and duration of hospitalization for cardiovascular reasons.69 In contrast, plasma endotoxin level had only a marginally significant effect in predicting cardiovascular events, and the correlation with hospitalization was less substantial.69 Furthermore, baseline plasma bacterial DNA level was significantly correlated with change in carotid–radial PWV in 12 months.69 In another study, plasma bacterial DNA quartile was associated with patient survival and peritonitis-free survival by univariate analysis,80 but the associations became insignificant after multivariate analysis to adjust for clinical confounding factors. Taken together, circulating bacterial DNA fragment may contribute to the hemodynamic changes and CVD of patients on PD, but the effect on patient survival is not confirmed. There are, however, no published data regarding patients on hemodialysis or predialysis with CKD.
Interventional Studies
Few studies examined the effect of intervention on serum bacterial DNA levels. In the study on ultrapure dialysate described previously, serum bacterial DNA fragment level (contrary to plasma endotoxin level) showed no significant change after ultrapure dialysate.77 Although the time-averaged serum bacterial DNA level correlated with malnutrition inflammation score and the subjective global assessment score, it did not correlate with serum CRP level or arterial PWV.77 The results of this study suggest that ultrapure dialysate has little effect on serum bacterial DNA fragment levels.77 To the best of our knowledge, there is no published study on the change in serum bacterial DNA fragment levels after the manipulation of intestinal microbiome.
Other Bacterial Fragments
In addition to endotoxin and bacterial DNA, other bacterial fragments could be found in the systemic circulation and may be of clinical relevance. For example, peptidoglycan, an essential component of the bacterial cell wall, stimulates the innate immune system via signaling through the pattern-recognition receptor nucleotide-binding oligomerization domain-containing protein 1 pathway.84 Polysaccharide A, produced by Bacteroides fragilis, induces the accumulation of forkhead box protein 3-positive regulatory T cells and production of IL10, which potentially modulate cellular immunity.85 The role of these and other bacterial fragments in CKD and their relation with CVD warrants further study.
Conclusions
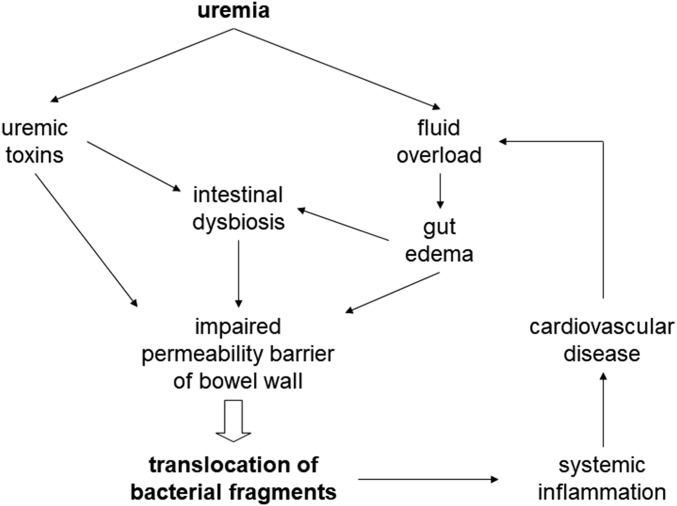
Intestinal dysbiosis is increasingly recognized in CKD. Multiple mechanisms contribute to the proliferation of pathobionts in the gut. Intestinal dysbiosis, fluid overload, and uremic toxins per se all impinge on the gut barrier function (Figure 1). As a result, translocation of bacterial fragments to the systemic circulation is enhanced, which leads to multiple biologic effects and represents an important nontraditional cardiovascular risk factor in CKD. Plasma endotoxin levels are markedly elevated in CKD, and are associated with the systemic inflammatory state, accelerated atherosclerosis, and clinical CVD in patients on dialysis. Several strategies have been tested to reduce plasma endotoxin levels, but the clinical benefit is still not established. Published data on the biologic effects and clinical relevance of other bacterial fragments, such as bacterial DNA, are promising but not confirmed. Further studies are also needed to delineate the pathogenic relation between circulating bacterial fragments and CVD, and to define the role of plasma bacterial fragment levels as a prognostic indictor of patients with CKD.
Figure 1.
Pathogenic mechanism and consequence of bacterial fragment translocation in uremia. Organic uremic toxins may affect intestinal barrier function directly or via the proliferation of dysbiotic bacteria in the gut. In addition, renal failure results in fluid overload and gut edema, which also affects the intestinal barrier function. The end result is translocation of bacterial fragments to the systemic circulation, which leads to systemic inflammation and cardiovascular disease, resulting in a vicious cycle by further aggravating fluid overload.
Disclosures
None.
Acknowledgments
This study was supported in part by Chinese University of Hong Kong research accounts 6901031 and 7101215. The results presented in this article have not been published previously in whole or part.
C.-C.S. was responsible for literature review and preparation of the first draft. C.W.M. was responsible for additional literature review and input on endotoxin and its cardiac effect in hemodialysis. P.K.-T.L. was responsible for overall coordination, manuscript preparation, and final editing.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Bright R: Cases and observations illustrative of renal disease accompanied with the secretion of albuminous urine. Guy’s Hospital Trans 1: 338–379, 1836 [PMC free article] [PubMed] [Google Scholar]
- 2.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al.: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al.; Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al.; Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Ateş K, Nergizoğlu G, Keven K, Sen A, Kutlay S, Ertürk S, et al.: Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int 60: 767–776, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Kwan BC, Szeto CC, Chow KM, Law MC, Cheng MS, Leung CB, et al.: Bioimpedance spectroscopy for the detection of fluid overload in Chinese peritoneal dialysis patients. Perit Dial Int 34: 409–416, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J: Cardiovascular risk in chronic kidney disease: Role of the sympathetic nervous system. Cardiol Res Pract 2012: 319432, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shanahan CM: Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat Rev Nephrol 9: 661–670, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Paloian NJ, Giachelli CM: A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol 307: F891–F900, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang FR, Fang JT, Chen JB, Lin CL, Chen HY, Lee CN, et al.: Hyperhomocystinemia and the prevalence of symptomatic atherosclerotic vascular disease in Taiwanese chronic hemodialysis patients: A retrospective study. Ren Fail 25: 765–774, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Lee CH, Chang HW, Wang IK, Lin CL, Chen TC, Wang PH, et al.: Diabetes mellitus, hyperhomocystinemia and atherosclerotic vascular disease in Taiwanese chronic hemodialysis patients: A retrospective study. Ren Fail 26: 317–323, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Lin CJ, Wu V, Wu PC, Wu CJ: Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One 10: e0132589, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himmelfarb J, Stenvinkel P, Ikizler TA, Hakim RM: The elephant in uremia: Oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int 62: 1524–1538, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Palmer SC, Rabindranath KS, Craig JC, Roderick PJ, Locatelli F, Strippoli GF: High-flux versus low-flux membranes for end-stage kidney disease. Cochrane Database Syst Rev 9: CD005016, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrlich SD: The human gut microbiome impacts health and disease. C R Biol 339: 319–323, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Ahmadmehrabi S, Tang WHW: Gut microbiome and its role in cardiovascular diseases. Curr Opin Cardiol 32: 761–766, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS: Role of the gut microbiome in uremia: A potential therapeutic target. Am J Kidney Dis 67: 483–498, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampaio-Maia B, Simões-Silva L, Pestana M, Araujo R, Soares-Silva IJ: The role of the gut microbiome on chronic kidney disease. Adv Appl Microbiol 96: 65–94, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Wu MJ, Chang CS, Cheng CH, Chen CH, Lee WC, Hsu YH, et al.: Colonic transit time in long-term dialysis patients. Am J Kidney Dis 44: 322–327, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P: Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int 64: 2196–2203, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, et al.: High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81: 300–306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wandersman C, Delepelaire P: Bacterial iron sources: From siderophores to hemophores. Annu Rev Microbiol 58: 611–647, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Jernberg C, Löfmark S, Edlund C, Jansson JK: Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 156: 3216–3223, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Dethlefsen L, Relman DA: Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 108[Suppl 1]: 4554–4561, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al.: Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368: 1575–1584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomlinson JAP, Wheeler DC: The role of trimethylamine N-oxide as a mediator of cardiovascular complications in chronic kidney disease. Kidney Int 92: 809–815, 2017 [DOI] [PubMed] [Google Scholar]
- 27.van der Flier LG, Clevers H: Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71: 241–260, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Farquhar MG, Palade GE: Junctional complexes in various epithelia. J Cell Biol 17: 375–412, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, et al.: Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 295: G1025–G1034, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R: Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Fujiya M, Musch MW, Nakagawa Y, Hu S, Alverdy J, Kohgo Y, et al.: The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe 1: 299–308, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, et al.: Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 290: C1018–C1030, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Mattar AF, Teitelbaum DH, Drongowski RA, Yongyi F, Harmon CM, Coran AG: Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int 18: 586–590, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA: Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun 73: 5183–5188, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlee M, Harder J, Köten B, Stange EF, Wehkamp J, Fellermann K: Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin Exp Immunol 151: 528–535, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeda K, Kaisho T, Akira S: Toll-like receptors. Annu Rev Immunol 21: 335–376, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Cario E, Gerken G, Podolsky DK: Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132: 1359–1374, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Anders HJ, Andersen K, Stecher B: The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 83: 1010–1016, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Nagpal R, Yadav H: Bacterial translocation from the gut to the distant organs: An overview. Ann Nutr Metab 71[Suppl 1]: 11–16, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Wiest R, Lawson M, Geuking M: Pathological bacterial translocation in liver cirrhosis. J Hepatol 60: 197–209, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Szeto CC, Chow VC, Chow KM, Lai RW, Chung KY, Leung CB, et al.: Enterobacteriaceae peritonitis complicating peritoneal dialysis: A review of 210 consecutive cases. Kidney Int 69: 1245–1252, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al.: Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12: 1365–1371, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Niebauer J, Volk HD, Kemp M, Dominguez M, Schumann RR, Rauchhaus M, et al.: Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 353: 1838–1842, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS: Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol Dial Transplant 27: 2686–2693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaziri ND, Goshtasbi N, Yuan J, Jellbauer S, Moradi H, Raffatellu M, et al.: Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrol 36: 438–443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, et al.: Chronic kidney disease alters intestinal microbial flora. Kidney Int 83: 308–315, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND: Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39: 230–237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant CJ, Harrison LE, Hoad CL, Marciani L, Gowland PA, McIntyre CW: Patients with chronic kidney disease have abnormal upper gastro-intestinal tract digestive function: A study of uremic enteropathy. J Gastroenterol Hepatol 32: 372–377, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Ramezani A, Raj DS: The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25: 657–670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, et al.: Circulating endotoxemia: A novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, et al.: Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A randomized trial. Clin J Am Soc Nephrol 11: 223–231, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jakob SM, Ruokonen E, Vuolteenaho O, Lampainen E, Takala J: Splanchnic perfusion during hemodialysis: Evidence for marginal tissue perfusion. Crit Care Med 29: 1393–1398, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Grant C, Harrison L, Hoad C, Marciani L, Cox E, Buchanan C, et al.: Endotoxemia in peritoneal dialysis patients: A pilot study to examine the role of intestinal perfusion and congestion. Perit Dial Int 37: 111–115, 2017 [DOI] [PubMed] [Google Scholar]
- 54.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, et al.: Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol 176: 3070–3079, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Caridis DT, Reinhold RB, Woodruff PW, Fine J: Endotoxaemia in man. Lancet 1: 1381–1385, 1972 [DOI] [PubMed] [Google Scholar]
- 56.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, et al.: Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: Prospective results from the Bruneck Study. J Am Coll Cardiol 34: 1975–1981, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Goto T, Edén S, Nordenstam G, Sundh V, Svanborg-Edén C, Mattsby-Baltzer I: Endotoxin levels in sera of elderly individuals. Clin Diagn Lab Immunol 1: 684–688, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freudenberg MA, Tchaptchet S, Keck S, Fejer G, Huber M, Schütze N, et al.: Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: Benefits and hazards of LPS hypersensitivity. Immunobiology 213: 193–203, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Pugin J, Heumann ID, Tomasz A, Kravchenko VV, Akamatsu Y, Nishijima M, et al.: CD14 is a pattern recognition receptor. Immunity 1: 509–516, 1994 [DOI] [PubMed] [Google Scholar]
- 60.Eggesbø JB, Hjermann I, Ovstebø R, Joø GB, Kierulf P: LPS induced procoagulant activity and plasminogen activator activity in mononuclear cells from persons with high or low levels of HDL lipoprotein. Thromb Res 77: 441–452, 1995 [DOI] [PubMed] [Google Scholar]
- 61.Reidy MA, Bowyer DE: Distortion of endothelial repair. The effect of hypercholesterolaemia on regeneration of aortic endothelium following injury by endotoxin. A scanning electron microscope study. Atherosclerosis 29: 459–466, 1978 [DOI] [PubMed] [Google Scholar]
- 62.Poesen R, Ramezani A, Claes K, Augustijns P, Kuypers D, Barrows IR, et al.: Associations of soluble CD14 and endotoxin with mortality, cardiovascular disease, and progression of kidney disease among patients with CKD. Clin J Am Soc Nephrol 10: 1525–1533, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raj DS, Carrero JJ, Shah VO, Qureshi AR, Bárány P, Heimbürger O, et al.: Soluble CD14 levels, interleukin 6, and mortality among prevalent hemodialysis patients. Am J Kidney Dis 54: 1072–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raj DS, Shah VO, Rambod M, Kovesdy CP, Kalantar-Zadeh K: Association of soluble endotoxin receptor CD14 and mortality among patients undergoing hemodialysis. Am J Kidney Dis 54: 1062–1071, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al.: Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Harrison LE, Burton JO, Szeto CC, Li PK, McIntyre CW: Endotoxaemia in haemodialysis: A novel factor in erythropoetin resistance? PLoS One 7: e40209, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jefferies HJ, Crowley LE, Harrison LE, Szeto CC, Li PK, Schiller B, et al.: Circulating endotoxaemia and frequent haemodialysis schedules. Nephron Clin Pract 128: 141–146, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, et al.: Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol 3: 431–436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szeto CC, Kwan BC, Chow KM, Kwok JS, Lai KB, Cheng PM, et al.: Circulating bacterial-derived DNA fragment level is a strong predictor of cardiovascular disease in peritoneal dialysis patients. PLoS One 10: e0125162, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feroze U, Kalantar-Zadeh K, Sterling KA, Molnar MZ, Noori N, Benner D, et al.: Examining associations of circulating endotoxin with nutritional status, inflammation, and mortality in hemodialysis patients. J Ren Nutr 22: 317–326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szeto CC, Kwan BC, Chow KM, Lai KB, Pang WF, Chung KY, et al.: Endotoxemia is associated with better clinical outcome in incident Chinese peritoneal dialysis patients: A prospective cohort study. Perit Dial Int 30: 178–186, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, et al.: Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580–591, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Gupta P, Andrew H, Kirschner BS, Guandalini S: Is lactobacillus GG helpful in children with Crohn’s disease? Results of a preliminary, open-label study. J Pediatr Gastroenterol Nutr 31: 453–457, 2000 [DOI] [PubMed] [Google Scholar]
- 74.Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, et al.: High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 9: e114881, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, et al.: Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Renal Physiol 310: F857–F871, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.John SG, Owen PJ, Harrison LE, Szeto CC, Lai KB, Li PK, et al.: The impact of antihypertensive drug therapy on endotoxemia in elderly patients with chronic kidney disease. Clin J Am Soc Nephrol 6: 2389–2394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwan BC, Chow KM, Ma TK, Cheng PM, Leung CB, Li PK, et al.: Effect of using ultrapure dialysate for hemodialysis on the level of circulating bacterial fragment in renal failure patients. Nephron Clin Pract 123: 246–253, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Navarro MD, Carracedo J, Ramírez R, Madueño JA, Merino A, Rodríguez M, et al.: Bacterial DNA prolongs the survival of inflamed mononuclear cells in haemodialysis patients. Nephrol Dial Transplant 22: 3580–3585, 2007 [DOI] [PubMed] [Google Scholar]
- 79.Cazzavillan S, Ratanarat R, Segala C, Corradi V, de Cal M, Cruz D, et al.: Inflammation and subclinical infection in chronic kidney disease: A molecular approach. Blood Purif 25: 69–76, 2007 [DOI] [PubMed] [Google Scholar]
- 80.Kwan BC, Chow KM, Leung CB, Law MC, Cheng PM, Yu V, et al.: Circulating bacterial-derived DNA fragments as a marker of systemic inflammation in peritoneal dialysis. Nephrol Dial Transplant 28: 2139–2145, 2013 [DOI] [PubMed] [Google Scholar]
- 81.El Kebir D, József L, Filep JG: Neutrophil recognition of bacterial DNA and Toll-like receptor 9-dependent and -independent regulation of neutrophil function. Arch Immunol Ther Exp (Warsz) 56: 41–53, 2008 [DOI] [PubMed] [Google Scholar]
- 82.Schindler R, Beck W, Deppisch R, Aussieker M, Wilde A, Göhl H, et al.: Short bacterial DNA fragments: Detection in dialysate and induction of cytokines. J Am Soc Nephrol 15: 3207–3214, 2004 [DOI] [PubMed] [Google Scholar]
- 83.Paladugu B, Kumar A, Parrillo JE, Der S, Osman J, Mensing J, et al.: Bacterial DNA and RNA induce rat cardiac myocyte contraction depression in vitro. Shock 21: 364–369, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN: Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16: 228–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Round JL, Mazmanian SK: Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A 107: 12204–12209, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]